Abstract
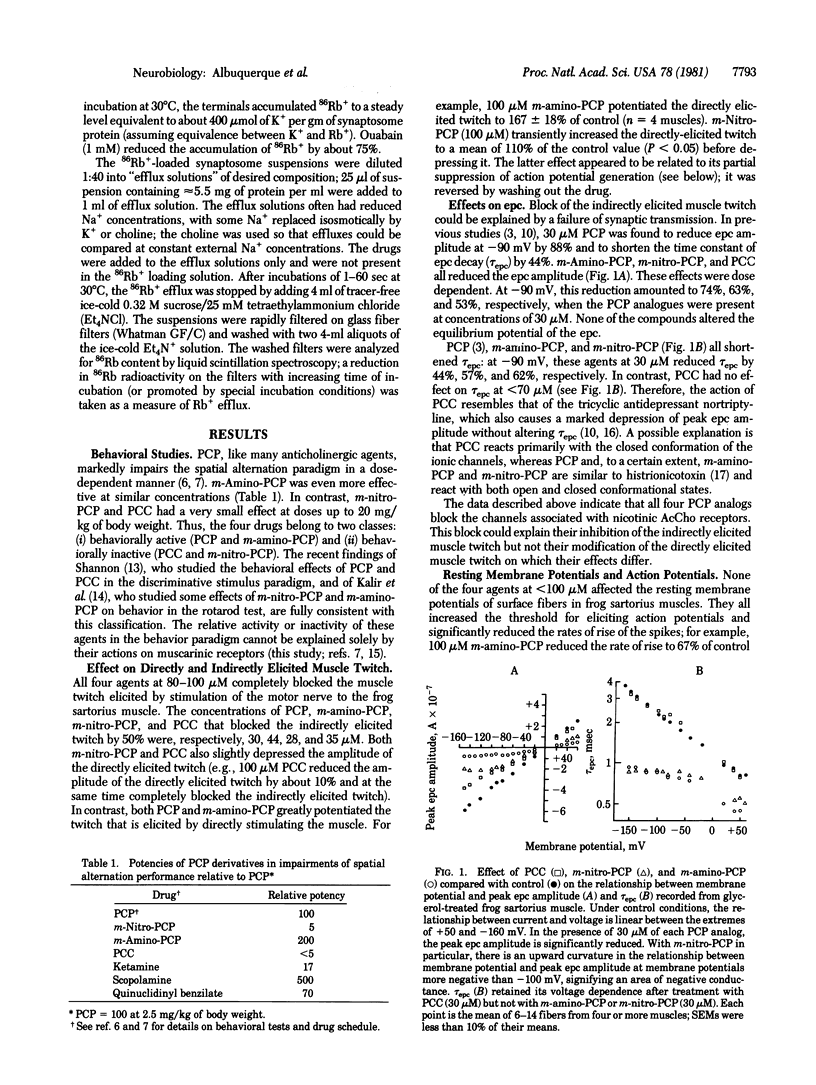
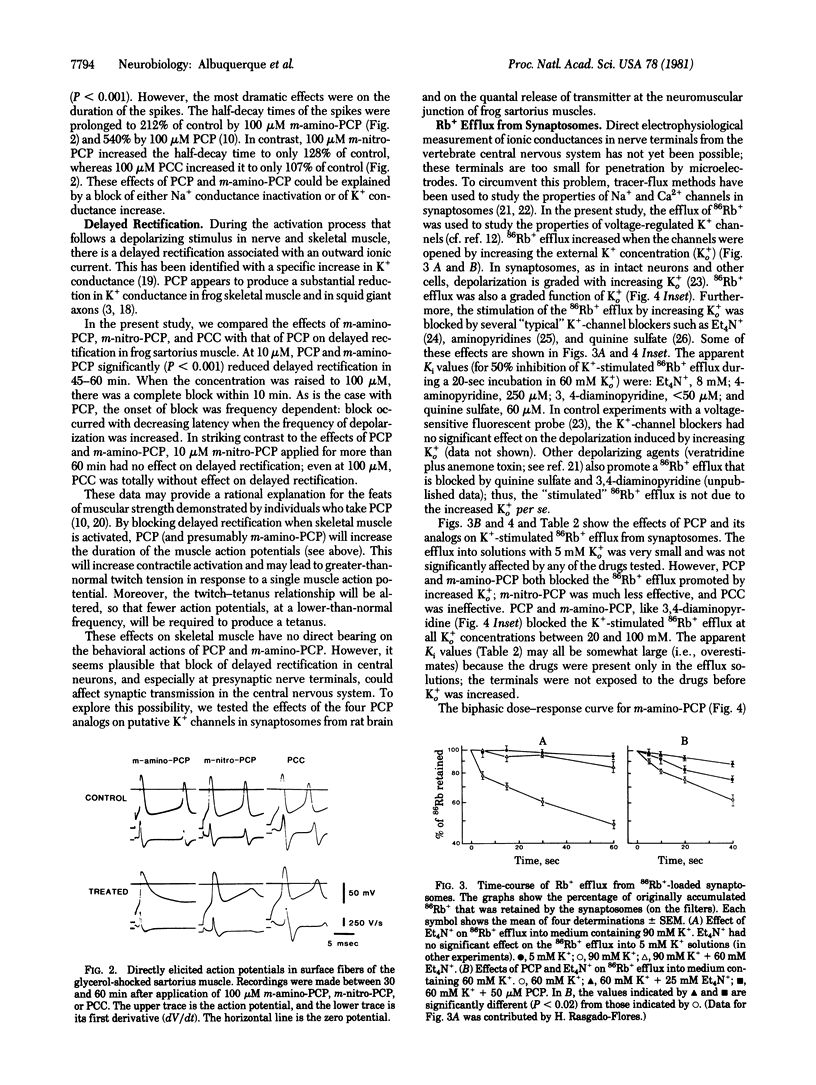
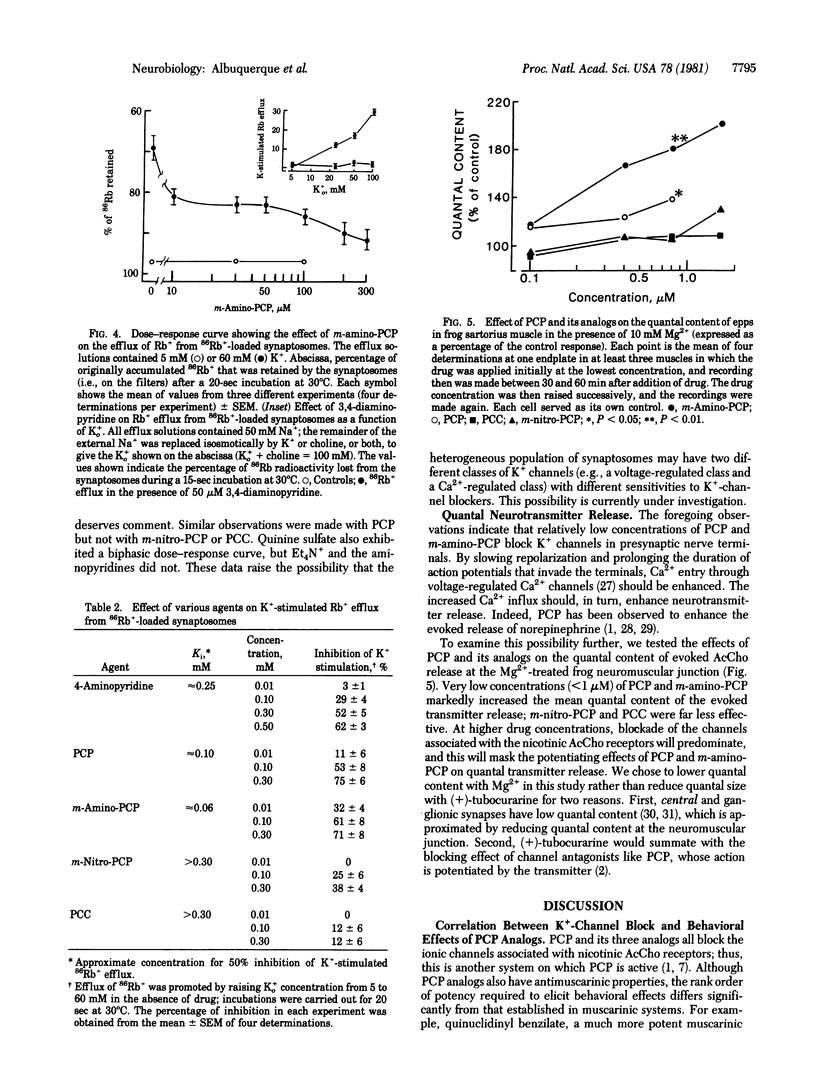
The action of phencyclidine [1-(1-phenylcyclohexyl)piperidine; PCP] and its behaviorally active analog (m-amino-PCP) and of two behaviorally inactive analogs [m-nitro-PCP and 1-piperidinocyclohexanecarbonitrile (PCC)] were examined in this study. In a test of spatial alternation performance in rats, PCP and m-amino-PCP were much more potent behavior modifiers than were PCC and m-nitro-PCP. We studied the effects of the drugs on the ionic channels of the electrically excitable membrane and of the nicotinic acetylcholine (AcCho) receptors at the neuromuscular junction of frog skeletal muscle. All four compounds blocked the indirectly elicited muscle twitch and depressed the amplitude and rate of rise of directly elicited muscle action potentials. They also caused a voltage- and concentration-dependent decrease in the peak amplitude of the endplate current but did not react with the nicotinic AcCho receptor. These observations indicate that the four compounds have comparable blocking effects on the ionic channels associated with the nicotinic AcCho receptor. In contrast, the behaviorally active agents could be distinguished from behaviorally inactive ones by their effects on K+ conductance. PCP and m-amino-PCP blocked delayed rectification in frog sartorius muscles, prolonged the muscle action potential more than 2-fold, and markedly potentiated the directly elicited muscle twitch. The behaviorally active compound also blocked depolarization-induced 86Rb+ efflux from rat brain synaptosomes (presumably a measure of K+ conductance) and increased quantal content at the frog neuromuscular junction. In these actions, m-nitro-PCP was much less effective, and PCC was relatively ineffective. Because PCP and m-amino-PCP are much more potent behavior modifiers than PCC and m-nitro-PCP, we suggest that the behavioral effects of PCP and m-amino-PCP, may be due to a block of K+ conductance and enhancement of transmitter release at central neurons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E. Sites of action of phencyclidine. II. Interaction with the ionic channel of the nicotinic receptor. Mol Pharmacol. 1980 Sep;18(2):167–178. [PubMed] [Google Scholar]
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Witkop B., Eldefrawi A. T., Eldefrawi M. E. Phencyclidine interactions with the ionic channel of the acetylcholine receptor and electrogenic membrane. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1224–1228. doi: 10.1073/pnas.77.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Arner L. S., Stallcup W. B. Rubidium efflux from neural cell lines through voltage-dependent potassium channels. Dev Biol. 1981 Apr 15;83(1):138–145. doi: 10.1016/s0012-1606(81)80016-4. [DOI] [PubMed] [Google Scholar]
- Aronstam R. S., Eldefrawi M. E., Eldefrawi A. T., Albuquerque E. X., Jim K. F., Triggle D. J. Sites of action of phencyclidine. III. Interactions with muscarinic receptors. Mol Pharmacol. 1980 Sep;18(2):179–184. [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S. D., Cox R. D., Maayani S., Meibach R. D. Anticholinergic behavioral effect of phencyclidine. Eur J Pharmacol. 1979 Oct 26;59(1-2):103–106. doi: 10.1016/0014-2999(79)90029-3. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., Oeffinger K. C. The effect of phencyclidine on dopamine metabolism in the mouse brain. Life Sci. 1981 Jan 26;28(4):361–369. doi: 10.1016/0024-3205(81)90080-1. [DOI] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Blaustein M. P., Ratzlaff R. W. Sodium channels in presynaptic nerve terminals. Regulation by neurotoxins. J Gen Physiol. 1980 Sep;76(3):287–313. doi: 10.1085/jgp.76.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Ratzlaff R. W., Strichartz G. R., Blaustein M. P. Saxitoxin binding to synaptosomes, membranes, and solubilized binding sites from rat brain. J Membr Biol. 1979 Nov 30;50(3-4):287–310. doi: 10.1007/BF01868894. [DOI] [PubMed] [Google Scholar]
- Lapa A. J., Albuquerque E. X., Sarvey J. M., Daly J., Witkop B. Effects of histrionicotoxin on the chemosensitive and electrical properties of skeletal muscle. Exp Neurol. 1975 Jun;47(3):558–580. doi: 10.1016/0014-4886(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Lundh H., Leander S., Thesleff S. Antagonism of the paralysis produced by botulinum toxin in the rat. The effects of tetraethylammonium, guanidine and 4-aminopyridine. J Neurol Sci. 1977 May;32(1):29–43. doi: 10.1016/0022-510x(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Some properties of potassium-stimulated calcium influx in presynaptic nerve endings. J Gen Physiol. 1980 Dec;76(6):709–728. doi: 10.1085/jgp.76.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolt R. T., Sr, Gay G. R., Farris R. D. Phencyclidine (PCP) intoxication: diagnosis in stages and algorithms of treatment. Clin Toxicol. 1980 Jun;16(4):509–529. doi: 10.3109/15563658008989980. [DOI] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Witkop B., Warnick J. E., Albuquerque E. X. Differentiation of the open and closed states of the ionic channels of nicotinic acetylcholine receptors by tricyclic antidepressants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5240–5244. doi: 10.1073/pnas.78.8.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon H. E. Evaluation of phencyclidine analogs on the basis of their discriminative stimulus properties in the rat. J Pharmacol Exp Ther. 1981 Mar;216(3):543–551. [PubMed] [Google Scholar]
- Spyker D. A., Lynch C., Shabanowitz J., Sinn J. A. Poisoning with 4-aminopyridine: report of three cases. Clin Toxicol. 1980 Jun;16(4):487–497. doi: 10.3109/15563658008989978. [DOI] [PubMed] [Google Scholar]
- Taube H. D., Montel H., Hau G., Starke K. Phencyclidine and ketamine: comparison with the effect of cocaine on the noradrenergic neurones of the rat brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):47–54. doi: 10.1007/BF00510820. [DOI] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Hudson C. S., Rash J. E. Neostigmine-induced alterations at the mammalian neuromuscular junction. I. Muscle contraction and electrophysiology. J Pharmacol Exp Ther. 1978 May;205(2):326–339. [PubMed] [Google Scholar]
- Tsai M. C., Albuquerque E. X., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E., Triggle D. J. Sites of action of phencyclidine. I. Effects on the electrical excitability and chemosensitive properties of the neuromuscular junction of skeletal muscle. Mol Pharmacol. 1980 Sep;18(2):159–166. [PubMed] [Google Scholar]
- Weinstein H., Maayani S., Srebrenik S., Cohen S., Sokolovsky M. Psychotomimetic drugs as anticholinergic agents. II. Quantum-mechanical study of molecular interaction potentials of 1-cyclohexylpiperidine derivatives with the cholinergic receptor. Mol Pharmacol. 1973 Nov;9(6):820–834. [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


