Abstract
The progression of atherosclerosis involves complex changes in the structure, composition and biology of the artery wall. Currently, only anatomical plaque burden is routinely characterized in living patients, whereas compositional and biological changes are mostly inaccessible. However, anatomical imaging alone has proven to be insufficient for accurate diagnostics of the disease. Multispectral optoacoustic tomography offers complementary data to anatomical methods and is capable of imaging both tissue composition and, via the use of molecular markers, the biological activity therein. In this paper we review recent progress in multispectral optoacoustic tomography imaging of atherosclerosis with specific emphasis on intravascular applications. The potential capabilities of multispectral optoacoustic tomography are compared with those of established intravascular imaging techniques and current challenges on the road towards a clinically viable imaging modality are discussed.
Keywords: atherosclerosis, intravascular imaging, molecular imaging, multispectral imaging, optical imaging, optoacoustic imaging, photoacoustic imaging, ultrasound
Atherosclerosis is a vascular disease of medium- and large-sized arteries that is characterized by inflammation and lipid deposition, leading to arterial stenosis and thrombosis formation [1–3]. Atherosclerotic vascular disease can cause sudden death, myocardial infarction (heart attack) and stroke, the leading causes of death worldwide. The pathological remodeling of an artery is accompanied by certain alterations of its composition and by changes in its biological profile. Patients harboring atherosclerotic plaque are in danger of myocardial infarction and sudden cardiac death in the case of plaque rupture. Although much is known about the composition of rupture-prone plaque and the biological activity that promotes destabilization, physicians do not currently have the tools to fully assess these important components in patients. Advanced imaging methods could facilitate early detection and possibly treatment of unstable plaque before it ruptures [3].
Significant activity in imaging atherosclerosis is focused on intravascular methods for the coronary arteries (i.e., catheter-based procedures in which imaging is performed from within the blood vessel). In the clinic, intravascular imaging is widely accepted and remains the only reliable method for imaging plaque in the coronary artery [4–6]. While a number of noninvasive imaging approaches (ultrasonography, MRI, CT and fludeoxyglucose PET) can provide clinical, structural and molecular insights into atherosclerosis in large arteries, such as the carotid arteries or the aorta, their resolution and sensitivity are insufficient for imaging the coronary arteries [6].
The main clinical intravascular coronary arterial imaging modality is intravascular ultrasound (IVUS) [7]. The proximity of the acoustic components (<5 mm) to the imaged plaque enables structural imaging with far greater detail than achieved by any current noninvasive method, and enables detection and quantification of plaque burden and stenosis. Further increases in image resolution have been realized using optical coherence tomography (OCT) [8], a rapidly growing clinical intracoronary imaging approach, where optical scattering, rather than ultrasound scattering, is used to form arterial images via interferometry. However, OCT has limited penetration depths of 1–2 mm, and therefore it is not appropriate for deeper imaging of blood vessels.
Despite continuing improvements in the performance of both IVUS and OCT, their use has so far been mostly limited to structural imaging. A different class of imaging methods with sensitivity to biochemical parameters has also been developed to read biochemical information relevant to the progression of atherosclerosis. Near-infrared spectroscopy (NIRS) has been used to measure absorption spectra from the blood vessel tissue to assess lipid content [9]. Due to the complex behavior of light propagation in tissue and the complex spectra involved, the measured spectrum is typically correlated to biochemical tissue parameters using a training set of data obtained from a blood vessel that exhibited high lipid content. At present, additional clinical data are required before the reliability of the method can be determined, and precise clinical applications utilizing NIRS remain to be defined. Moreover, NIRS-based detection does not distinguish between various subtypes of lipid (e.g., oxidized low-density lipoprotein).
Near-infrared fluorescence (NIRF) molecular imaging has recently been introduced as a new imaging approach, where fluorescent probes are used to reveal biological activity in the blood vessel wall [10]. The use of exogenous probes offers an array of specific molecular and cellular targeting. A major limitation of this approach has been the clinical availability of fluorocromes with molecular specificity. However the recent translation of fluorescence molecular imaging to humans opens exciting possibilities for the application of targeted fluorochromes in imaging atherosclerosis and other cardiovascular diseases [11].
Advances in optoacoustic (or photoacoustic) technology may also prove important for the detection and management of atherosclerosis. Optoacoustic imaging is a potent optical imaging approach that has gained significant attention in recent years since it is insensitive to photon scattering in tissue and offers high-resolution optical images within several millimeters to centimeters tissue depth [12,13]. The technique is based on the transformation of transient optical energy absorbed in tissues to acoustic waves through thermoelastic expansion. Since the carriers of image information are acoustic waves, resolution and penetration depths similar to ultrasonography may be achieved in tissue, which is generally superior to optical resolution for distances over 1 mm [14]. However, the image contrast is optical, which often has more significance in the characterization of biological tissue. In particular, multispectral optoacoustic tomography (MSOT) allows the detection of various photo-absorbing molecules and NPs distributing in tissues, by resolving their absorption spectrum signatures [14–17]. While earlier implementations of the technology focused on small animal imaging [18,19], breast cancer imaging [20] and blood vessel anatomy [19,21,22], optoacoustic imaging has been more recently considered for atherosclerosis imaging applications [23–25]. MSOT offers potentially unique characteristics to atherosclerosis imaging since it can resolve important molecules involved in atherosclerosis including lipids [24], oxygenated and deoxygenated hemoglobin content [21], as well as extrinsically administered imaging agents that enable molecular imaging, including various photo-absorbing molecules spanning from NPs [17] to organic dyes and fluoro chromes [15]. One of the major challenges of optoacoustic imaging in general and MSOT in particular is the low efficiency in which the optical energy is converted to acoustic waves. As a result, intravascular applications of optoacoustic technology have suffered from considerably lower imaging speed than purely optical or acoustical techniques [23,24]. Table 1 presents a comparison between the potential capabilities of MSOT and those of IVUS, OCT and NIRF. Throughout the paper, the term MSOT will be restricted to optoacoustic imaging where more than a single wavelength is used.
Table 1.
A comparison between the various intracoronary imaging modalities discussed in this article.
| Modality | Resolution | Penetration | Contrast mechanism |
Pullback rate | Flushing |
|---|---|---|---|---|---|
| IVUS | 100–200 μm | Whole artery | Acoustic scattering | 0.5 mm/s | Not required |
| OCT/OFDI | 20 μm | 1–2 mm | Optical scattering | OCT: 1 mm/s OFDI: 20–40 mm/s |
Required |
| NIRF | 250–1000 μm | Whole artery | Fluorescence | 1 mm/s | Not required |
| MSOT | 100–200 μm | Whole artery | Optical absorption spectrum |
0.1 mm/min | May not be required in specific wavelength bands (e.g., the near- infrared window) |
The values in the table are typical for in vivo applications. For MSOT, the values are estimates based on state-of-the-art technology. The pullback rate was calculated for four wavelengths, a pulse repetition rate of 10 Hz, 300 A-scans per slice and a step size of 200 μm.
IVUS: Intravascular ultrasound; MSOT: Multispectral optoacoustic tomography; NIRF: Near-infrared fluorescence; OCT: Optical coherence tomography; OFDI: Optical frequency domain imaging.
Since MSOT is a hybrid optical-acoustic method, it shares similarities with IVUS, OCT and NIRF in both performance and in hardware implementation. An MSOT catheter could potentially be integrated with any of these modalities, as has already been shown for the case of a hybrid optoacoustic–IVUS catheter [23]. Additionally, the potential capabilities of MSOT could be best understood in the context of existing techniques. For these reasons, we first briefly review the leading coronary-artery-targeted intravascular-imaging approaches and assess their performance relative to intravascular MSOT. We discuss advantages and limitations of each technology and summarize next steps towards preclinical and clinical intravascular MSOT applications.
IVUS
IVUS is a pulse-echo technique that maps the strength of acoustic back-scattering in the blood-vessel wall, which is the result of heterogeneity in the acoustic properties of tissue. IVUS imaging is performed using a miniaturized ultrasound transducer mounted on the tip of a catheter inserted into the blood vessel imaged. The transducer generates a directional beam of short acoustic pulses, which propagate into the artery, and detects the waves that are subsequently reflected from the tissue and reach the transducer. In these so-called A-scans, the amplitude of the detected wave signal depends on the strength of the acoustic scatterers, whereby the time of arrival corresponds to their depth. 3D imaging is enabled by scanning the blood vessel along its circumference and length. With recent technological advances, IVUS may reach resolutions of approximately 100–200 μm with typical longitudinal pullback scanning rates of 0.5 mm/s.
IVUS systems resolve typical ultrasonic contrast, which is determined by the density and elastic moduli of the tissue, and can detect interfaces between components that have different acoustic properties (e.g., fluids, soft tissue, calcified tissue or metal in coronary stents). IVUS is typically used for measuring the degree of stenosis, assessing plaque burden and a few compositional features (e.g., calcium), as well as for optimizing stent deployment [26]. While some morphological features are significantly more common in vulnerable plaque than in stable plaque, plaque classification based on morphological (grayscale) IVUS images has not yet demonstrated the reliability required for clinical prognosis. Although plaque composition is clearly linked to plaque instability, grayscale IVUS provides limited discriminatory contrast for atherosclerotic plaque composition. In recent years, advanced mathematical analyses of IVUS raw signals have expanded IVUS ability in discerning the composition of atherosclerotic plaque. Most notable is the ability of virtual-histology (VH)-IVUS to detect the lipid-rich necrotic core in plaques, which has been validated in human coronary arteries [27]. Despite these promising findings, recent studies in a porcine model suggest that VH-IVUS might have limited accuracy in the case of complex lesions [28,29]. In addition, a landmark clinical trial (PROSPECT) demonstrated that VH-IVUS measures of high-risk plaques were not as predictive as grayscale IVUS burden measures [4].
OCT
OCT may be viewed as the optical counterpart of IVUS, but uses light instead of sound to detect heterogeneity in tissue via optical back-scattering. The use of light dictates different detection principles and leads to substantially different image characteristics. In intravascular OCT, the catheter is composed of a single-mode optical fiber that guides light to the blood-vessel wall and collects scattered light due to microscopic variations in the refractive index of the tissue. The collected light is interfered with a reference beam enabling detection of both phase and amplitude. The combined phase–amplitude data is used to form an A-scan signal similar to that of IVUS. The transition from A-scans to 3D imaging is obtained by rotating and translating the catheter within the blood vessel. Originally, A-scan data was obtained using so-called time-domain OCT [30], where mechanical scanning was used to detect the signal strength at each depth level individually. The later transition to frequency domain OCT, or optical frequency domain imaging (OFDI) [31], where scanning is performed in the laser wavelength, has led to significant improvement in imaging speed. In both cases, the bandwidth of the optical source determines the axial resolution of the images.
Frequency domain OCT has a characteristic resolution of 20 μm and pull-back speeds of 20–40 mm/s. Penetration depth is typically limited to 1–2 mm, and is thus insufficient for imaging the entire arterial wall in the case of coronary arteries. To achieve maximum penetration depth using OCT, both optical scattering and absorption should be as low as possible. OCT is thus usually performed with wavelengths around 1300 nm, where water absorption is acceptable and tissue scattering is low. However, the strong signal attenuation through blood at these wavelengths requires flushing to be performed during the imaging session. However, even when flushing is performed, residual blood may occasionally obscure parts of the blood vessel and may lead to image misinterpretations.
The high resolution offered by OCT enables better detection of superficial structural features in blood vessels as compared with IVUS, (e.g., lumen geometry, fibrous cap thickness and stent structure/coverage). In the case of atherosclerosis, OCT has shown potential to detect thin-cap fibroatheroma, a leading pathologic subtype of rupture-prone plaque, characterized by a large necrotic core and a thin fibrous cap (<65 μm) [32]. The high resolution of OCT enables a reliable estimation of the thickness of the fibrous cap, an insurmountable task for current IVUS systems. Macrophages are also visible on OCT images and dedicated algorithms based on the normalized standard deviation parameter have shown promise in their identification [33], although further investigation is required for assessing the reliability of this method in real-time operations. Full visualization of the large necrotic core, eccentric remodeling and plaque burden measurements, however, are beyond the reach of OCT because of its limited penetration depth.
NIRF imaging
Fluorescence imaging is a well-established method for molecular imaging that has found various applications in preclinical research. In vivo applications often involve the injection of a fluorescent probe that can target disease biomarkers in vivo. Numerous fluorescent probes have been considered for detecting different characteristics of vascular disease [34]. In the case of atherosclerosis, inflammation associated proteases, including cathepsins and matrix metalloproteinase (MMP) have been visualized using activatable probes [10,35–37]. In a study by Jaffer et al., a cathepsin enzyme-activatable probe was used, which was generally quenched until cleaved by an inflammation regulated protease such as cathepsin B [10]. In a study by Vinegoni et al., the US FDA-approved NIRF agent indocyanine green (ICG) was shown to target lipids and macrophages in atherosclerotic plaques, and to have minimal accumulation in non-inflamed tissue [38]. In both cases, the probes had their excitation and emission bands in the near-infrared (NIR) imaging window (700–950 nm [14]), where blood absorption is relatively low, enabling imaging without flushing.
Intravascular NIRF imaging was originally performed using a 1D pull-back fiber [39] and more recently with a 2D pull-back rotating fiber [10]. The principle of operation of the 2D pull-back system resembles that of intravascular OCT; however, the NIRF catheter utilizes a multimode fiber for illumination and fluorescence collection. In particular, the NIRF catheter is based on an optical fiber that guides laser light into the tissue. The light emitted by the fluorescent probes is collected by the same fiber and is sent through an appropriate filter to a highly sensitive optical detector that measures its intensity. Since fluorescent light is incoherent, the depth of the probes cannot be detected by the technique used in OCT. Thus, translation and rotation of the fiber leads to a 2D, surface-weighted image. The use of NIR light has enabled in vivo imaging in the presence of blood with a good signal-to-noise ratio (SNR). The typical axial resolution demonstrated in the study by Jaffer et al. was estimated at 500–1000 μm in blood and 250–500 μm in saline, and may be improved by the use of focusing optics [10]. The pull-back speed demonstrated in a study by Jaffer et al. is comparable to that of IVUS and was mainly a limitation of the motors and not of the system sensitivity [10].
Current challenges in NIRF imaging include robust quantification of the NIRF signal and in image interpretation. Light attenuation and scattering lead to a depth-dependent weighting in the images. As a result, NIRF images depend on the location of the catheter inside the lumen, where areas of the blood vessel that are closer to the fiber appear stronger owing to surface weighting. Since NIRF imaging does not discern depth, fully correcting for bias in the images is not possible with standalone NIRF imaging. However, coregistration of NIRF images with structural images obtained from IVUS or OCT [40] could enable better quantification and interpretation of NIRF images.
MSOT
In optoacoustic imaging, an optically absorbing object is illuminated with modulated light, whose absorption creates a distribution of temperature variations in the object proportional to the absorbed energy. Through the process of thermal expansion, this leads to pressure variations in the object, which propagate outwards as acoustic waves. The efficiency of the conversion of the thermal energy to pressure waves is commonly described by the Grüneisen coefficient, which is a thermodynamic property of the tissue and depends on the tissue type [41] and temperature [42].
Commonly, the modulated light is provided by a pulsed laser, where the pulse repetition rate determines the rate at which the acoustic fields are formed. By measuring all the acoustic waves emanating from the object, a 3D map of the absorbed optical energy may be constructed, which is generally proportional to the light fluence and optical absorption coefficient. Commonly, the tissue is assumed to be acoustically homogeneous and lossless [41], enabling a simple mathematical description the optoacoustic effect and accurate image formation schemes. However, local variations in acoustic properties, losses, dispersion and acoustic scattering may lead to signal distortion and image artifacts. In the case of extreme acoustic distortion (e.g., when imaging through the lungs, some parts of the specimen might not be accessible via optoacoustic imaging). In that sense, optoacoustic imaging shares the same limitations with conventional medical ultrasonography.
In MSOT, measurements are performed using laser light at several wavelengths to obtain a 3D map of the absorption spectrum of the specimen [12]. Optical contrast agents that possess absorption resonances within the measurement span may be mapped through the processing of the multispectral data [16]. The map of a contrast agent, referred to as an MSOT image, may be superimposed on the structural single-wavelength optoacoustic image to enable coregistered molecular and structural imaging. One of the challenges of multispectral analysis is the wavelength-dependent attenuation of light in tissue, which alters the spectrum of the light as it diffuses deeper in the tissue [41]. As a result, identical objects that are situated in different positions in tissue may appear to have different absorption spectra in the multispectral image dataset. Nonetheless, in many cases, MSOT has the potential to supplant fluorescence imaging, as fluorescent probes may be detected by MSOT based on their distinct absorption spectrum [15]. Another advantage is that MSOT, like NIRF, could potentially be performed in blood, without flushing or occlusion by utilizing the NIR imaging window [14].
The imaging capabilities of MSOT are determined by both the optical and acoustical components used in the system design. Resolution is commonly determined by the acoustic design, though in some cases the optical design may have a major contribution to it as well. Specifically, when imaging is performed at depths in which total light diffusion has yet to occur, optical focusing can be the decisive factor in the lateral resolution of the MSOT image. This regime relates mostly to optoacoustic microscopy [21], although it is potentially relevant to the first millimeter of artery wall in intravascular imaging when flushing is performed. At deeper parts, light diffusion may become dominant and the lateral resolution would be determined by the acoustic focusing. The axial resolution is determined solely by the acoustic bandwidth of the detector, in analogy to OCT where axial resolution is determined by the optical bandwidth of the source. The SNR of the image is determined by the amount of optical energy deposited in the region of interest, the Grüneisen coefficient and by the sensitivity of the detector. Since light scattering and absorption lead to attenuation of the light fluence (i.e., the energy that can be potentially deposited in the tissue), image SNR decreases with depth. Highly absorbing substances (e.g., blood) usually enjoy a high SNR and are often visible in the single-wavelength optoacoustic images. Injected fluorescent probes commonly produce weaker absorption than the tissue background when their concentration in tissue is less than 100 nM and become visible only in the MSOT image [14,43]. As the MSOT image is produced by the often subtle differences between the optoacoustic images at different wavelengths, MSOT may require higher detector sensitivity than what is required for single-wavelength optoacoustic imaging [14,43,44].
Generally, the optoacoustic effect is characterized by low efficiency, which requires high detection sensitivities, typically better than 1 kPa. Additionally, the frequency content of the acoustic fields is inversely proportional to the size of the illuminated object, where submillimeter objects emit acoustic fields of several MHz. Commonly, piezoelectric materials, which convert pressure changes into voltage, are used for the acoustic detection. The optimal design of the piezoelectric detector is highly dependent on the imaging geometry. While the SNR is generally scaled with the detector size, increasing the size of the detector might lead to a reduced detection bandwidth, and thus resolution, owing to the effect of spatial averaging [45]. Optical interferometric detectors for ultrasound have also been demonstrated for optoacoustic imaging [46–48]. In such detection schemes, ultrasound is detected by the effect it has on the optical properties of the medium in which it propagates. By using various interferometric techniques, an interrogating optical beam can sense these changes and thus measure the acoustic field. One of the advantages of optical schemes is that they can often be miniaturized without hindering their sensitivity [47]. Finally, micromachined ultrasonic transducers have been suggested for optoacoustic imaging as their fabrication capabilities enable manufacturing highly dense 2D detector arrays [49–51]. However, the use of this technology for optoacoustic imaging of biological tissue has so far been limited [51].
Imaging application for atherosclerosis
In atherosclerosis, MSOT has shown potential for imaging both endogenous and exogenous contrast factors. Two promising exogenous contrast agents have been demonstrated so far with MSOT technology: gold NPs [52] and a MMP-activatable fluorescent probe [25]. In the former, it has been shown that the intake of the gold NPs by macrophages leads to a substantial change in the spectrum of the NPs. This effect was used for detecting gold NP-loaded macrophages injected to a diseased rabbit aorta, as shown in the ex vivo intravascular MSOT images in Figure 1. In a second example, MMP activity was detected using MMP-activatable fluorescent imaging agent in conjunction with an extravascular MSOT system. Detection of the probe via MSOT was possible because of the alteration in the probe’s absorption spectrum owing to its activation. The experiment was performed in resected human carotid plaques, which were incubated in a suspension of the fluorescent MMP agent. An MSOT image was formed based on the spectrum of the probe in activated form, and revealed MMP activity in the plaque, a known indicator of plaque instability. Figure 2 shows the MSOT image laid over the single-wavelength optoacoustic image and compared with epifluorescent images obtained after dissecting the plaque. In contrast to the intravascular images shown in Figure 1, the images in Figure 2 were obtained using a small-animal imaging apparatus that entailed broad illumination of an entire section of the plaque and a large ultrasound transducer [43]. This design, which was not constrained by the confined geometry of intravascular setups, generally enables a higher detection sensitivity than an intravascular setup, though at the price of reduced spatial resolution owing to the large size of the detector.
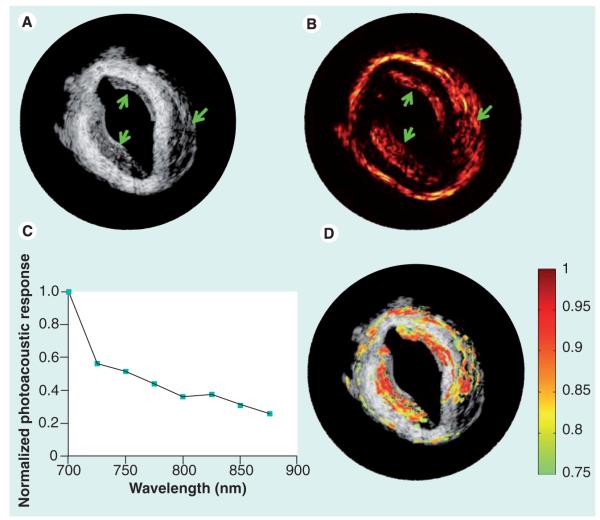
Figure 1. Intravascular multispectral optoacoustic tomography of gold nanoparticle-bearing macrophages in rabbit aorta.
(A) Intravascular ultrasound image and (B) optoacoustic image acquired at 700 nm of an atherosclerotic rabbit aorta injected with gold nanoparticle-bearing macrophages. The arrows indicate the locations where injection was performed. (C) The normalized spectral optoacoustic response obtained in a small section on the aorta, where injection was performed. (D) Multispectral optoacoustic tomography image corresponding to the recovered spectrum overlaid onto the intravascular ultrasound image revealing the injected regions.
Reproduced with permission from [52].
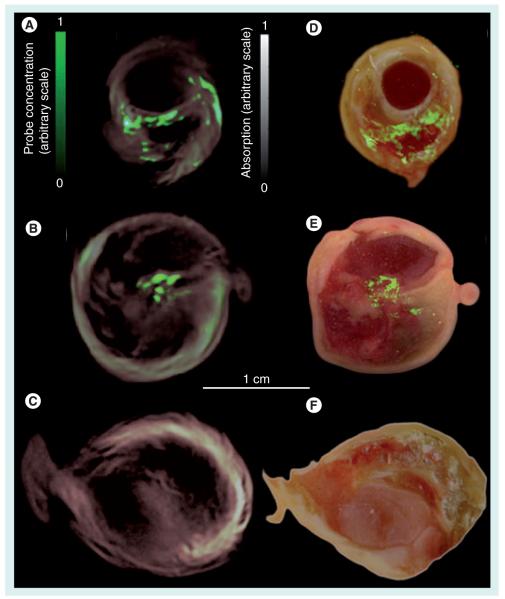
Figure 2. Localization of matrix metalloproteinase activity in three carotid specimens.
Samples 1 and 2 were incubated in MMPsense™ 680, a fluorescent probe activated by matrix metalloproteinase, while sample 3 (control) was incubated in PBS. (A–C) Imaging results from intact plaques made with MSOT. Cross-sectional multispectral reconstruction, revealing the location of MMPSense680 activity in the slice, is shown by the bright signal (green color online) that is superimposed onto the morphological single-wavelength (635 nm) optoacoustic images. (D–F) The corresponding epifluorescence images from dissected plaque (in green online) superimposed onto the color images of cryosections from the three carotid plaque specimens.
Reproduced from [25] with permission.
In the case of endogenous contrast, the complex composition of atherosclerotic plaque is expected to lead to spectrally rich MSOT data that can be used for its identification. Indeed, a preliminary study in a rabbit model has shown that MSOT can potentially distinguish between several components of atherosclerotic plaque [53]. Specific attention has been devoted for imaging lipid content in plaque owing to its importance in characterizing plaque vulnerability. Lipids possess a relatively strong absorption band centered on 930 nm [24] whose maximum absorption band is comparable to that of connective tissue. Thus, the application of MSOT around this wavelength could be used resolve lipid content in plaque. At 1210 nm lipids have an additional peak, whose absorption coefficient is significantly higher than that of the connective tissue and becomes the most dominant component in the optoacoustic images. Recently, intravascular MSOT imaging of lipids has been demonstrated in an excised atherosclerotic human coronary artery [24]. Figure 3a & b show the optoacoustic images of the artery obtained at wavelengths corresponding to the 1210 nm absorption resonance of lipids. The match between the spectrum obtained from the optoacoustic images and the known absorption spectrum of lipids (Figure 3C) indicates the presence of lipids in the specimen.
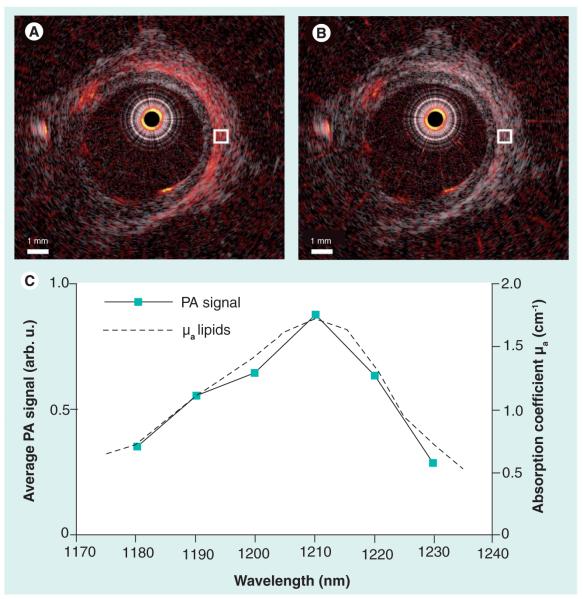
Figure 3. Intravascular optoacoustic imaging of lipids in human aorta using the 1210 nm wavelength.
An intravascular ultrasound image of a diseased explanted human aorta with overlaid pseudocolor optoacoustic images obtained at (A) 1210 nm and (B) 1230 nm. (C) The spectrum of the optoacoustic signal strength averaged in the area indicated in (A & B). The optoacoustic spectrum fits the reference absorption spectrum of lipids, indicating the presence of lipids in the examined area.
Reproduced with permission from [24].
Recently, ex vivo investigations have been made into imaging lipids also via their absorption band at 1720 nm, which is significantly higher than absorption in the 1210 nm band. Additionally, it was found that at wavelengths around 1720 nm, lipids constitute the strongest absorption in tissue [54]. Around this wavelength, the absorption spectrum of water has a local minimum which, although higher than blood absorption in the NIR imaging window, enables a reasonable light transmission through at least several millimeters [55]. Despite the higher absorption of water, the improved optoacoustic signal from the lipids led overall to a significant improvement in image SNR. Since the absorption of water and blood around 1720 nm is similar, the presence of blood did not severely limit the imaging performance [54,55].
One of the most important developments in the application of MSOT to atherosclerosis is its adaptation to coronary artery geometry. Commonly, MSOT is performed noninvasively with free-space optical beams and large-area transducers located at a distance from the imaged object. Early work by Sethuraman et al. has demonstrated acoustic detection within blood vessels using an IVUS catheter, where optical illumination was provided from the exterior of the blood vessel [56]. Although the method was limited to excised blood vessels, it gave an initial impression of the image quality to be expected from MSOT and showcased its potential for imaging atherosclerosis. In subsequent studies, integrated catheters were constructed, which performed both optical illumination and acoustic detection. The illumination was performed by coupling the laser beam to an optical fiber, which guided the light into the interior of the blood vessel. Several strategies were used for performing the illumination and acoustic detection: in studies by Karpiouk et al. [23] and Jansen et al. [24], a piezoelectric transducer with a limited detection angle was used, leading to a partial overlap between the acoustic detection field and the cone profile of the illumination. In studies by Yang et al. [57] and Wei et al. [58], a coaxial design was utilized that enabled a full overlap between the acoustic detection field and the illumination. In a study by Sethuraman et al. [56] the illumination was uniform over the cross-section of the blood vessel, whereas the directionality of the detector determined the angular resolution. In all cases, rotation was performed to obtain a full cross-sectional view. Despite the disparity in designs, the axial and lateral resolutions achieved in these studies were comparable to that of IVUS.
Similarly to purely optical methods, the high optical absorption and scattering of blood poses a challenge to the in vivo application of intravascular MSOT. However, since the illumination travels only one way in MSOT – from the catheter to the target – whereas in purely optical techniques the photons must complete a round-trip and return to the catheter, signal attenuation due to blood is reduced. Nonetheless, at wavelengths for which blood absorption is extremely high, flushing must be used. In the NIR imaging window, the relatively low absorption of blood could potentially enable performing MSOT without flushing. Indeed, it has already been shown that the effect of blood is not detrimental for intravascular NIRF imaging when performed in vivo at wavelengths around 800 nm. Thus, MSOT could potentially be used for intravascular imaging of NIR biomarkers through blood. The exact span of the NIR imaging window for MSOT will depend on the catheter design, namely the illumination power and detector sensitivity, and on the imaging frame rate. Ex vivo demonstrations of MSOT at 1720 nm suggest it could be used for imaging lipid content in atherosclerotic plaque without flushing.
As MSOT is a hybrid technology that combines ultrasound and optical hardware, MSOT catheters could have IVUS and OCT capabilities added to them. Examples of integrated IVUS-opotoacoustic catheters can be found in studies by Karpiouk et al. [23] and Jansen et al. [24], and typical integrated images are shown in Figures 1 & 3. Integration of MSOT with OCT may be performed by using double-clad fibers, which can provide a single-mode operation for the OCT beam with a small cross-section and a multi-operation for the MSOT illumination with a large cross-section. This approach has been previously used for integrating NIRF with OCT with low cross-talk between the modalities [40]. Thus, integration with OCT can probably be performed without increasing the total diameter of the MSOT catheter. The imaging abilities of MSOT may offer complementary information to existing modalities. By combining MSOT’s ability to detect endogenous lipids and targeted fluorochromes/NPs reporting on plaque inflammation, angiogenesis or apoptosis, with structural features provided by IVUS or OCT, more accurate identification of high-risk plaques may be possible. Additionally, the results from different modalities may be used in a corroborative manner: images of macrophages detected by MSOT via the use of contrast agents may be compared with macrophage quantification obtained by OCT [33]; lipid detection by VH-IVUS [27] or NIRS may be corroborated with that of MSOT; and quantitative plaque burden could potentially be assessed by both MSOT and IVUS.
Integrating MSOT with existing structural-imaging catheter technologies may offer several advantages over NIRF-based integration. The inherent 3D data of MSOT allows for a natural coregistration with existing modalities. Specifically, because an integrated catheter would constitute a single unit in which all imaging modalities rotate and translate synchronously, the relative position and orientation of the A-scans performed by MSOT would be the same as those performed by IVUS or OCT. Common morphological structures for MSOT and IVUS/OCT, such as the luminal area, vessel area, or position of the stent struts, may potentially be used to further enhance the accuracy of the coregistration. Depth information in MSOT, which NIRF lacks, could be used to account for light attenuation in blood and tissue, thus enabling better interpretation of the images.
Clinical considerations & challenges
Despite progress in recent years, many challenges remain before intravascular MSOT can become a viable method for imaging atherosclerosis. These challenges pertain to the technology involved as well as to the complex biology of atherosclerosis. Envisioned clinical applications will have to conform to the standards employed in the field, while offering additional information on the state of the disease over established technologies.
Arguably the biggest challenges to intracoronary application of MSOT are miniaturization (<1.2 mm diameter) and speed (>0.5 mm/s pull-back rates through blood, or >10 mm/s during saline flushing). Although both the optical fibers and the ultrasound detectors come in coronary artery-compatible sizes, miniaturization comes at a cost. When piezoelectric technology is used for acoustic detection, miniaturization often means lower sensitivity. On the optical side, fibers with small diameters are more difficult to couple light to than fibers with large diameters. Miniaturization of the optics could enable better focusing of the fiber output, which could potentially lead to a higher (optical) resolution in the case when flushing is performed. Additionally, because the transducer can detect signals originating outside its prescribed sensitivity field – albeit with lower efficiency – when the illumination is broader than the acoustic detection field, the accumulative effect of signals that originate outside the detection field could lead to increased noise in the image or artifacts. Due to the tradeoff between catheter size and image SNR, miniaturization inhibits measurement speed. The smallest reported catheter [24], having a diameter of 1.25 mm, has been demonstrated to obtain approximately two cross-sectional single-wavelength images per second. Such frame rates (approximately two orders of magnitude slower than what is achieved by IVUS) are unacceptable in clinical cardiac catheterizations. Acquisition of multispectral data would lead to even longer procedure times because of the increased size of the data set.
In the case of ex vivo extravascular imaging of plaque, for example in a study by Razansky et al., considerably faster imaging may be performed by increasing the number of acoustic detection elements [25]. In this case, a large portion of the object is uniformly illuminated and is fully reconstructed from the sets of projection acquired from a single laser shot. This technique has been previously demonstrated for whole-body small-animal imaging systems, where a 10-Hz frame rate was demonstrated with a 10-Hz pulse repletion rate. Although this approach may also be used in intravascular applications, the confined geometry of the blood vessel would require significant miniaturization of the acoustic transducers, which would reduce their SNR. Additionally, such an implementation would require illuminating a larger area of the blood vessel, for example in an entire slice in a study by Hsieh et al., necessitating higher coupling efficiency of the pulsed laser to the fiber and potentially hindering the efforts for miniaturization [59]. Alternatively, imaging speed may be enhanced by switching to lasers with repetition rates higher than those reported in recent studies [23–25]. However, because laser power should be kept below the tissue damage threshold, more pulses per second would mean weaker pulses, and thus weaker acoustic signals. As a result, to increase the frame rate while maintaining image quality necessitates increasing the sensitivity of the ultrasound detector. This challenge may be met by improving the design of the piezoelectric transducers employed, or by considering alternative technologies such as capacitive micromachined ultrasonic transducers [49–51] and optical detectors [46–48].
Conclusion
MSOT is an emerging technology for 3D imaging of optical molecular markers and for analyzing tissue composition. The unique contrast offered by MSOT makes it a promising technology for intravascular imaging and could complement IVUS and OCT/OFDI. Specifically, as MSOT is a hybrid modality that combines both ultrasound and optical components, it could be integrated with either IVUS or OCT to provide multimodality imaging. In recent years, investigations have been made into applying MSOT for intravascular imaging, namely for characterizing atheroslecrotic plaque. This involved the technical development of several coronary artery-targeted catheter designs, as well as the investigation of MSOT’s ability to discern plaque composition and to detect protease and macrophage activity.
Future perspective
Although MSOT has yet to become a clinically viable tool for intravascular imaging in vivo, recent developments in catheter miniaturization suggest that imaging a small number of cross-sections is currently possible in a catheterization procedure. Considering the accelerated development of optoacoustic catheters and versatility of recently proposed designs, it is fair to assume that small-scale preclinical in vivo studies will be performed in the near future. However, current designs cannot be used for more than proof-of-principle studies, because the largely inhomogeneous structure of atherosclerotic plaque requires imaging numerous cross-sections and 3D analysis of the data recovered – an insurmountable task at current imaging speeds.
Whether MSOT becomes a practical tool for intravascular imaging will depend mostly on technological advancement in lasers and acoustic detectors. High-repetition-rate lasers with sufficient power and tunability in the NIR imaging window already exist (e.g., Ti-sapphire lasers) and can be used for imaging lipids using their 930-nm absorption band as well as various NIR fluorescent probes. Assuming a sufficiently sensitive acoustic detector, the kilohertz repetition rate of Ti-sapphire lasers could enable MSOT imaging at frame rates comparable to that of IVUS, whereas the use of the NIR imaging window could enable imaging through blood – making such a frame rate clinically acceptable. Recent achievements in the miniaturization and sensitivity enhancement of optical detectors of ultrasound make them already an attractive alternative to piezoelectric transducers for intravascular applications. A fiber-only MSOT could be fabricated using two fibers – one for illumination and one for detection – or more elegantly out of a single double-clad fiber. In either case, the small diameter of optical fiber may eliminate the need for further miniaturization.
An additional crucial factor in the evolution of intravascular MSOT is the identification of useful atherosclerosis-targeted imaging agents. Although endogenous contrast from lipid is useful for plaque characterization, a more detailed picture of disease progression could be painted by imaging molecular agents targeting specific biological processes (e.g., inflammation, angiogenesis, oxidative stress and apoptosis). Early preclinical studies with intravascular MSOT could profit from the availability of targeted fluorescent probes already useful for imaging atherosclerosis. More advanced studies could involve MSOT-specific contrast agents characterized by high optoacoustic efficiencies and distinctive absorption spectra (e.g., NPs).
Successful performance in preclinical studies would probably pave the way for clinical applications. The recent clinical approval of OCT/OFDI and NIRS for coronary arterial imaging in patients should facilitate translation of intravascular MSOT into the clinic, although limitations on illumination power may require higher detection sensitivities than those acceptable in preclinical studies. The currently low selection of clinically approved contrast agents is expected to pose a much greater limitation to potential clinical use. Nonetheless, the recent translation of fluorescence targeting for intraoperative imaging of ovarian cancer [11], the recently described capabilities of indocyanine green [38], and the clinically viable performance of intravascular NIRF create the incentive already for translating fluorescent probes specific for atherosclerosis despite the immaturity of intravascular MSOT. Additionally, MSOT’s ability to image endogenous tissue contrast such as lipids is likely to be sufficient to justify a clinical MSOT trial, even without the approval of additional contrast agents.
Executive summary.
Intravascular ultrasound
▪ Intravascular ultrasound (IVUS) imaging is based on acoustic contrast, is useful for assessing plaque burden and stent apposition, but is limited in its ability for characterizing the composition of atherosclerotic plaque.
▪ Capable of whole-artery imaging in coronary artery geometries.
▪ 3D grayscale images; tissue characterization via virtual-histology-IVUS.
▪ No flushing is required.
Optical coherence tomography
▪ Optical coherence tomography (OCT) is capable of structural imaging with a higher resolution than IVUS, which enables better visualization of blood-vessel morphology. The high resolution achieved by OCT enables assessing the thickness of the thin fibrous cap – a hallmark of rupture-prone plaque.
▪ Penetration depth is limited to 1–2 mm.
▪ 3D grayscale images; macrophage density assessments via the normalized standard deviation parameter.
▪ Flushing is required.
Near-infrared fluorescence
▪ Can image fluorescent probes, which are administered prior to imaging and can target specific biological activities relevant for diagnosing atherosclerosis (e.g., inflammation or angiogenesis).
▪ Capable of whole-artery imaging in coronary artery geometries.
▪ 2D, surface-weighted images (no depth information). Single wavelength at present, multiwavelength feasible.
▪ No flushing is required.
Multispectral optoacoustic tomography
▪ Multispectral-optoacoustic tomography (MSOT) images the optical absorption spectra of tissue with a typical resolution similar to that of ultrasound methods.
▪ Still in initial experimental stages in intravascular applications.
▪ Capable of whole-artery imaging in coronary-artery geometries.
▪ 3D multispectral images with slower acquisition speeds compared with IVUS, OCT and near-infrared fluorescence.
▪ Detection of indigenous (lipids, blood) and exogenous (fluorescent probes, nanoparticles) chromophores.
▪ Flushing may not be required when imaging at specific wavelengths (e.g., in the near-infrared window of 700–950 nm).
Conclusion
▪ Due to the versatile contrast offered by MSOT, its intravascular implementation could offer a more complete picture of the blood-vessel condition and lead to new diagnosis and treatment strategies for atherosclerosis.
▪ With recent technological progress, intravascular MSOT specifications can currently enable limited proof-of-principle in vivo demonstrations.
▪ A substantial increase in imaging speed is still required to achieve a performance level acceptable for in vivo imaging in preclinical studies or clinical use.
Acknowledgments
F Jaffer is funded by an NIH grant: NIH R01 HL-108229. V Ntziachristos acknowledges financial support from the European Research Council Advanced Investigator Award, and the BMBF’s Innovation in Medicine Award.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(15):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque part I: evolving concept. J. Am. Coll. Cardiol. 2005;46(6):937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010;30(7):1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E. What do clinicians expect from imagers? J. Am. Coll. Cardiol. 2006;47(8):101–103. doi: 10.1016/j.jacc.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 6.Suh WM, Seto AH, Margey RJP, et al. Advances in cardiovascular imaging. Circ. Cardiovasc. Imaging. 2011;4:169–178. doi: 10.1161/CIRCIMAGING.110.958777. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Yock P. Intravascular ultrasound novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–616. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. J. Am. Coll. Cardiol. Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waxman S, Dixon SR, L’Allier P, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques. J. Am. Coll. Cardiol. 2009:858–868. doi: 10.1016/j.jcmg.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Jaffer FA, Calfon MA, Rosenthal A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J. Am. Coll. Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 12.Ntziachristos V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, Wang LV. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006;77:041101. [Google Scholar]
- 14.Ntziachristos V, Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT) Chem. Rev. 2010;110:2783–2794. doi: 10.1021/cr9002566. ▪ Provides a thorough review of the capabilities of multispectral optoacoustic tomography.
- 15.Razansky D, Distel M, Vinegoni C, et al. Multi-spectral optoacoustic tomography of deep-seated fluorescent proteins in vivo. Nat. Photon. 2009;3:412–417. [Google Scholar]
- 16.Glatz J, Deliolanis NC, Buehler A, et al. Blind source unmixing in multi-spectral optoacoustic tomography. Opt. Express. 2011;19:3175–3184. doi: 10.1364/OE.19.003175. [DOI] [PubMed] [Google Scholar]
- 17.Taruttis A, Herzog E, Razansky D, et al. Real-time imaging of cardiovascular dynamics and circulating gold nanorods with multispectral optoacoustic tomography. Opt. Express. 2010;18:19592–19602. doi: 10.1364/OE.18.019592. [DOI] [PubMed] [Google Scholar]
- 18.Kruger RA, Kiser WL, Reinecke DR, et al. Thermoacoustic computed tomography using a conventional linear. Med. Phys. 2003;30:856–860. doi: 10.1118/1.1565340. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Pang W, Ku G, et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 20.Oraevsky AA, Karabutov AA, Andreev VG, et al. Laser opto-acoustic imaging of the breast: detection of cancer angiogenesis. Proc. SPIE. 1999;3597:352–363. [Google Scholar]
- 21.Zhang H, Maslov K, Stoica G, et al. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 22.Zhang E, Laufer J, Beard P. Backward-mode multiwavelength photoacoustic scanner using a planar Fabry–Perot polymer film ultrasound sensor for high-resolution three-dimensional imaging of biological tissues. Appl. Optics. 2008;47:561–577. doi: 10.1364/ao.47.000561. [DOI] [PubMed] [Google Scholar]
- 23.Karpiouk AB, Wang B, Emelianov SY. Development of a catheter for combined intravascular ultrasound and photoacoustic imaging. Rev. Sci. Instrum. 2010;81:014901. doi: 10.1063/1.3274197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen K, van der Steen AFW, van Beusekom HMM, et al. Intravascular photoacoustic imaging of human coronary atherosclerosis. Opt. Lett. 2011;36:597–599. doi: 10.1364/OL.36.000597. ▪▪ First (and only) intravascular optoacoustic imaging of a human atheroslecrotic coronary artery.
- 25.Razansky D, Harlaar NJ, Hillebrands JL, et al. Multispectral optoacoustic tomography of matrix metalloproteinase activity in vulnerable human carotid plaques. Mol. Imaging Biol. 2012;14(3):277–285. doi: 10.1007/s11307-011-0502-6. ▪▪ First (and only) multispectral optoacoustic tomography imaging of matrix metalloproteinase (MMP) activity in human atherosclerotic plaque. MMP activity plays a key role in plaque destabilization and the ability to detect its activity via an activatable imaging agent serves as a strong motivation for enabling intravascular versions of this technology.
- 26.Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. J. Eur. Heart. 2008;29:1851–1857. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 27.Hong MK, Mintz GS, Lee CW, et al. A three-vessel virtual histology intravascular ultrasound ana lysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. J. Am. Coll. Cardiol. 2007;101:568–572. doi: 10.1016/j.amjcard.2007.09.113. [DOI] [PubMed] [Google Scholar]
- 28.Thim T, Hagensen MK, Wallace-Bradley D, et al. Unreliable assessment of necrotic core by virtual histology intravascular ultrasound in porcine coronary artery disease. Circ. Cardiovasc. Imaging. 2010;3:384–391. doi: 10.1161/CIRCIMAGING.109.919357. [DOI] [PubMed] [Google Scholar]
- 29.Granada JF. In vivo plaque characterization using intravascular ultrasound–virtual histology in a porcine model of complex coronary lesions. Arterioscler. Thromb. Vasc. Biol. 2007;27:387–393. doi: 10.1161/01.ATV.0000253907.51681.0e. [DOI] [PubMed] [Google Scholar]
- 30.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun SH, Tearney GH, Vakoc BJ, et al. Comprehensive volumetric optical microscopy in vivo. Nat. Med. 2006;12:1429–1433. doi: 10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolodgie FD, Burke A, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001;16:285–292. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Tearney GJ. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 34.Klohs J, Wunder A, Licha K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res. Cardiol. 2008;103:144–151. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim DE, Kim JY, Schellingerhout D, et al. Protease imaging of human atheromatacaptures molecular information of atherosclerosis, complementing anatomic imaging. Arterioscler. Thromb. Vasc. Biol. 2010;30:449–456. doi: 10.1161/ATVBAHA.109.194613. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Tung CH, Mahmood U, et al. In vivo imaging of proteolyticactivity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 37.Jaffer F, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J. Am. Coll. Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Vinegoni C, Botnaru I, Aikawa E, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 2011;3:84ra45. doi: 10.1126/scitranslmed.3001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffer FA, Vinegoni C, John MC, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo H, Kim JW, Shishkov M, et al. Intra-arterial catheter for simultaneous microstructural andmolecular imaging in vivo. Nat. Med. 2011;17:1680–1684. doi: 10.1038/nm.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox BT, Laufer JG, Beard PC. The challenges for quantitative photoacoustic imaging. Proc. SPIE. 2009;7177:717713. [Google Scholar]
- 42.Wang B, Emelianov S. Thermal intravascular photoacoustic imaging. Biomed. Opt. Express. 2011;2(11):3072–3078. doi: 10.1364/BOE.2.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma R, Taruttis A, Ntziachristos V. Multispectral optoacoustic tomography (MSOT) scanner for whole-body small animal imaging. Opt. Express. 2009;17(24):21414–21426. doi: 10.1364/OE.17.021414. [DOI] [PubMed] [Google Scholar]
- 44.Razansky D, Baeten J, Ntziachristos V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT) Med. Phys. 2009;36(3):939–945. doi: 10.1118/1.3077120. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal A, Ntziachristos V, Razansky D. Model-based optoacoustic inversion with arbitrary-shape detectors. Med. Phys. 2011;38(7):4285–4295. doi: 10.1118/1.3589141. [DOI] [PubMed] [Google Scholar]
- 46.Zhang EZ, Beard PC. A miniature all-optical photoacoustic imaging probe. Proc. SPIE. 2011;7899:78991F. [Google Scholar]
- 47.Rosenthal A, Razansky D, Ntziachristos V. High-sensitivity compact ultrasonic detector based on a pi-phase-shifted fiber Bragg grating. Opt. Lett. 2011;36:1833–1835. doi: 10.1364/OL.36.001833. [DOI] [PubMed] [Google Scholar]
- 48.Chen SL, Ling T, Guo LJ. Low-noise small-size microring ultrasonic detectors for high-resolution photoacoustic imaging. J. Biomed. Opt. 2011;16(5):056001. doi: 10.1117/1.3573386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li ML, Wang PH, Liao PL, et al. Three-dimensional photoacoustic imaging by a CMOS micromachinedcapacitive ultrasonic sensor. IEEE Elec. Dev. Lett. 2011;32(8):1149–1151. [Google Scholar]
- 50.Yeh DT, Oralkan O, Wygant IO, et al. 3-D ultrasound imaging using a forward-looking CMUT ring array for intravascular/ intracardiac applications. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2006;53:1202–1211. doi: 10.1109/tuffc.2006.1642519. [DOI] [PubMed] [Google Scholar]
- 51.Cheng X, Chen J, Li C. A miniature capacitive micromachined ultrasonic transducer array for minimally invasive photoacousticimaging. J. Microelectromech. Syst. 2010;19(4):1002–1011. [Google Scholar]
- 52.Wang B, Su JL, Karpiouk AB, et al. Intravascular photoacoustic imaging. IEEE J. Sel. Top. Quant. Electron. 2010;18:8867–8878. doi: 10.1109/JSTQE.2009.2037023. ▪▪ Provides a thorough review of advancements in the technology of intravascular optoacoustics.
- 53.Wang B, Su JL, Amirian J, et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt. Express. 2010;18:4889–4897. doi: 10.1364/OE.18.004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B, Karpiouk A, Yeager D, et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Opt. Lett. 2012;37(7):1244–1246. doi: 10.1364/OL.37.001244. ▪ Provides an experimental demonstration of ex vivo intravascular optoacoustic imaging through blood with blood-vessel dimensions corresponding to that of the human coronary arteries.
- 55.Wang P, Wang HW, Sturek M, et al. Bond-selective imaging of deep tissue through the optical window between 1600 and 1850 nm. J. Biophoton. 2012;5(1):25–32. doi: 10.1002/jbio.201100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sethuraman S, Aglyamov SR, Amirian JH, et al. Intravascular photoacoustic imaging using an IVUS imaging catheter. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2007;54:978–986. doi: 10.1109/tuffc.2007.343. ▪ First optoacoustic imaging of an artery with intravascular acoustic detection.
- 57.Yang JM, Maslov K, Yang HC, et al. Photoacoustic endoscopy. Opt. Lett. 2009;34:1591–1593. doi: 10.1364/ol.34.001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei W, Li X, Zhou Q, et al. Integrated ultrasound and photoacoustic probe for co-registered intravascular imaging. J. Biomed. Opt. 2011;16:106001. doi: 10.1117/1.3631798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh BY, Chen SL, Ling T, et al. Integrated intravascular ultrasound and photoacoustic imaging scan head. Opt. Lett. 2010;35:2892–2894. doi: 10.1364/OL.35.002892. [DOI] [PubMed] [Google Scholar]