Abstract
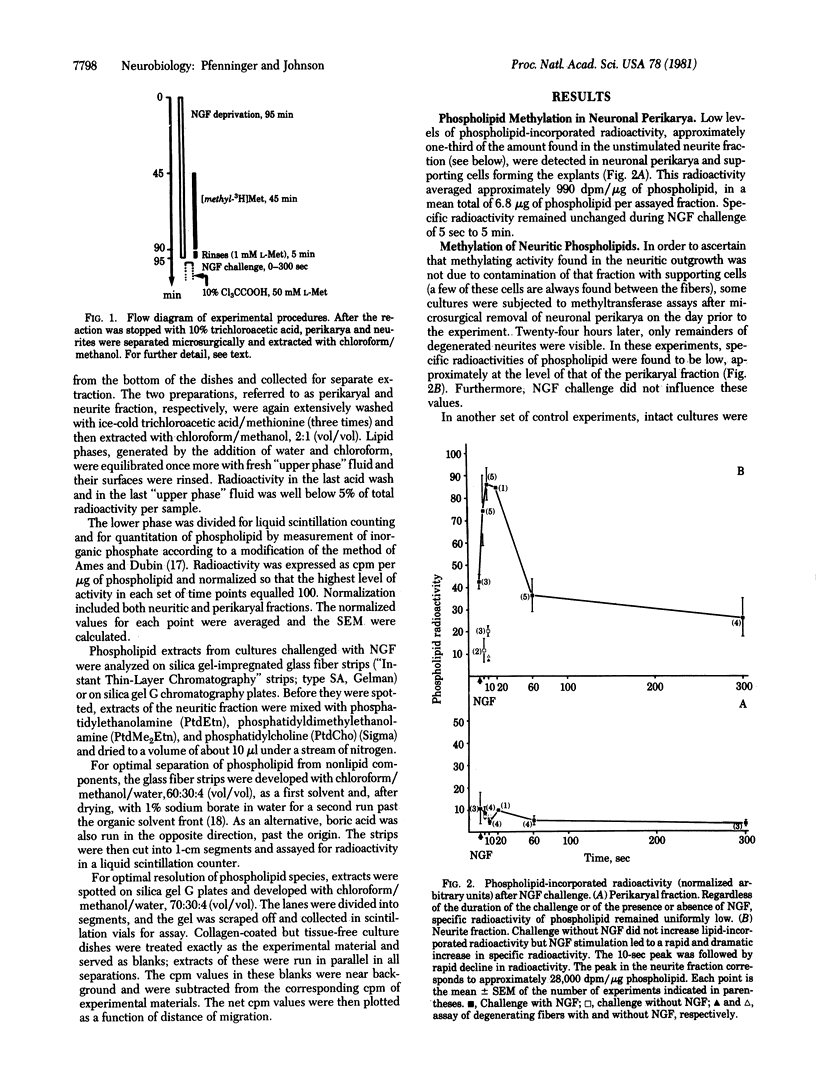
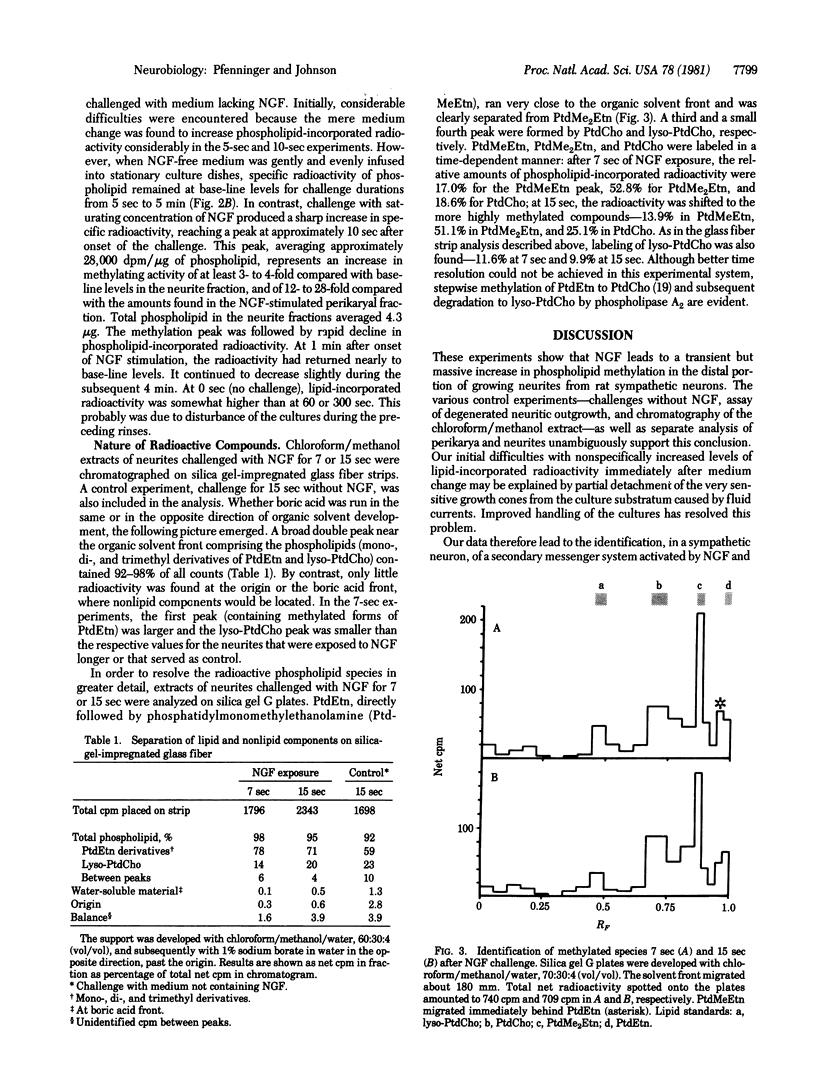
Cultures of neurons from rat superior cervical ganglia were deprived of nerve growth factor, loaded with [methyl-3H]methionine, and then challenged with nerve growth factor for different periods of time. Growing neurites and perikarya were separated microsurgically and extracted with chloroform/methanol. Lipid-incorporated radioactivity in the extracts was measured and expressed on the basis of the amount of phospholipid present. The methylated species in the neurite fraction were identified by thin-layer chromatography as mono-, di-, and trimethylphosphatidylethanolamine (phosphatidylcholine). Furthermore, a small peak of lysophosphatidylcholine was detected. In the neurites, but not in the perikarya, phospholipid methylation was found to reach a peak at 10 sec after onset of stimulation. Stimulated levels were at least 4 times higher than levels of unstimulated controls. The peak was followed by rapid decline of phospholipid-incorporated radioactivity. Our result indicates that phospholipid methylation is part of a nerve-growth-factor-activated secondary messenger system in growing sympathetic neurites. The potential significance of this conclusion for directed neuritic growth and membrane expansion is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Banerjee S. P., Snyder S. H., Cuatrecasas P., Greene L. A. Binding of nerve growth factor receptor in sympathetic ganglia. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2519–2523. doi: 10.1073/pnas.70.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Branching patterns of individual sympathetic neurons in culture. J Cell Biol. 1973 Mar;56(3):702–712. doi: 10.1083/jcb.56.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Gundersen R. W., Barrett J. N. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980 Dec;87(3 Pt 1):546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H., Otten U., Thoenen H. Nerve growth factor-mediated selective induction of ornithine decarboxylase in rat pheochromocytoma; a cyclic AMP-independent process. FEBS Lett. 1978 Aug 15;92(2):313–316. doi: 10.1016/0014-5793(78)80777-7. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Schwab M., Thoenen H. A second messenger required for nerve growth factor biological activity? Nature. 1981 Aug 27;292(5826):838–840. doi: 10.1038/292838a0. [DOI] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth G., Cohen P., Shooter E. M. Ca2+ transmembrane fluxes and nerve growth factor action on a clonal cell line of rat phaeochromocytoma. Nature. 1980 Jan 10;283(5743):202–204. doi: 10.1038/283202a0. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Chemotactic response of nerve fiber elongation to nerve growth factor. Dev Biol. 1978 Sep;66(1):183–196. doi: 10.1016/0012-1606(78)90283-x. [DOI] [PubMed] [Google Scholar]
- Meiri H., Spira M. E., Parnas I. Membrane conductance and action potential of a regenerating axonal tip. Science. 1981 Feb 13;211(4483):709–712. doi: 10.1126/science.7455707. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Maylié-Pfenninger M. F. Lectin labeling of sprouting neurons. II. Relative movement and appearance of glycoconjugates during plasmalemmal expansion. J Cell Biol. 1981 Jun;89(3):547–559. doi: 10.1083/jcb.89.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Howell J. I., Lucy J. A. Lysolecithin and cell fusion. Nature. 1970 Aug 22;227(5260):810–814. doi: 10.1038/227810a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Whitlock C. Alteration of cellular adhesion by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4055–4058. doi: 10.1073/pnas.74.9.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbany A. A., Ambron R. T., Schwartz J. H. Membrane glycolipids: regional synthesis and axonal transport in a single identified neuron of Aplysia californica. Science. 1979 Jan 5;203(4375):78–80. doi: 10.1126/science.83001. [DOI] [PubMed] [Google Scholar]
- Stöckel K., Paravicini U., Thoenen H. Specificity of the retrograde axonal transport of nerve growth factor. Brain Res. 1974 Aug 23;76(3):413–421. doi: 10.1016/0006-8993(74)90818-x. [DOI] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. W., Tolson N. W., Guroff G. Increased phosphorylation of specific nuclear proteins in superior cervical ganglia and PC12 cells in response to nerve growth factor. J Biol Chem. 1980 Nov 10;255(21):10481–10492. [PubMed] [Google Scholar]



