Abstract
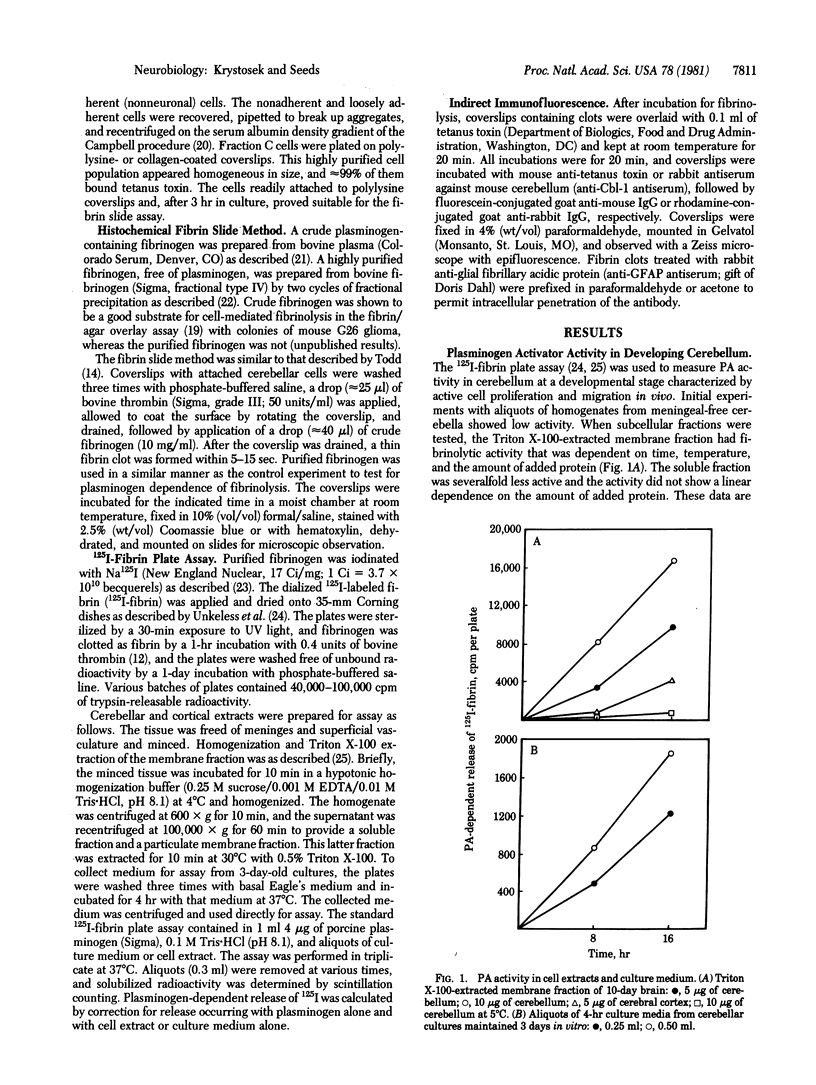
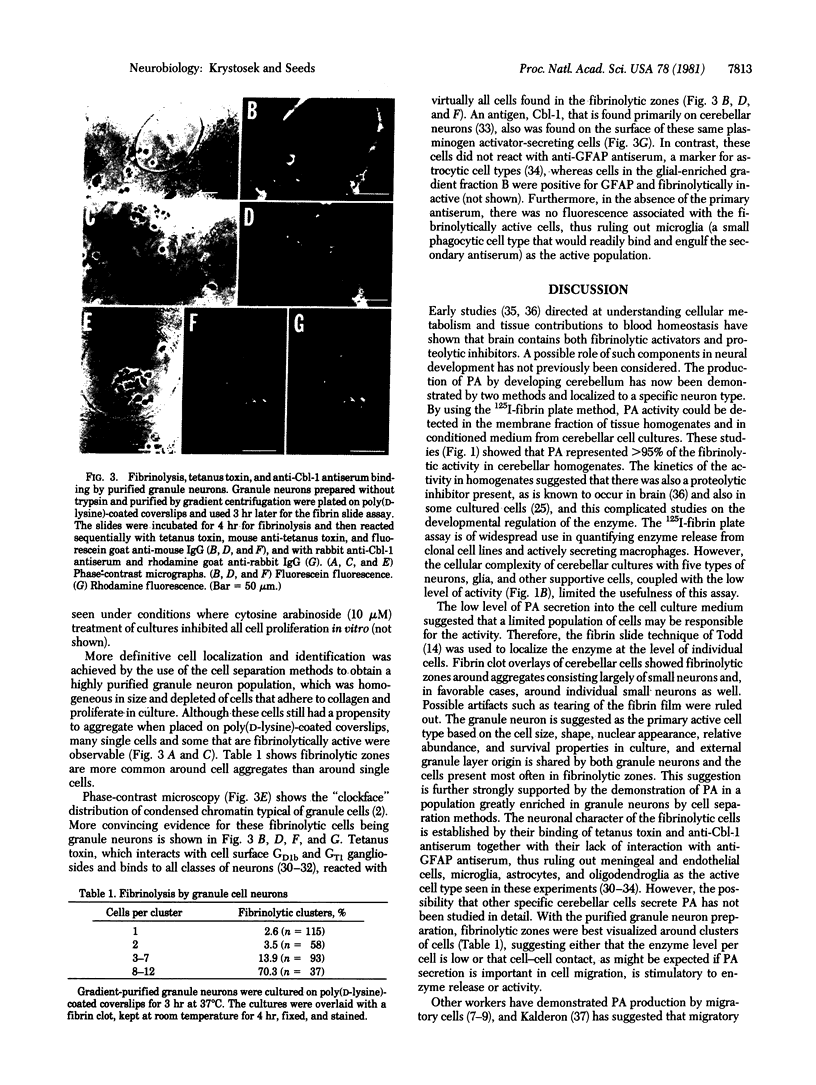
Dispersed cell cultures were established from 7- to 9-day postnatal mouse cerebellum. The fibrin slice method was used to obtain a localization of plasminogen activator production to specific cells. Fibrinolytically active cells were small (5- to 8-micrometer diameter), round, and occurred singly or in aggregates. Fibrinolysis was both plasminogen and time dependent, inhibitable by epsilon-aminocaproic acid and soybean trypsin inhibitor and did not occur when cells were fixed in formalin prior to the fibrin overlay. Strong fibrin degradation occurred only when granule neurons were abundant in the cultures. These plasminogen activator secreting cells were identified as granule neurons by cell separation methods, nuclear morphology, and their ability to bind tetanus toxin and rabbit antiserum against mouse cerebellum (anti-Cbl-1 antiserum). Plasminogen activator also could be quantified in fractionated tissue homogenates or in cell culture medium by the 125I-labeled fibrin plate assay. Fibrinolysis in cerebellar extracts was 95% dependent on the presence of added plasminogen; furthermore, the activity was greater in cerebellar extracts as compared to cerebral cortex of the same age. At the age examined, the cerebellum contains many migratory neurons, and plasminogen activator production may be involved in the process of cell movement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969 Jul;136(3):269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- Astedt B., Holmberg L. Immunological identity of urokinase and ovarian carcinoma plasminogen activator released in tissue culture. Nature. 1976 Jun 17;261(5561):595–597. doi: 10.1038/261595a0. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Brecher A. S., Quinn N. M. The occurrence of a trypsin inhibitor in brain. Biochem J. 1967 Jan;102(1):120–121. doi: 10.1042/bj1020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. L., Schanchner M., Sharrow S. O. Isolation of glial cell-enriched and -depleted populations from mouse cerebellum by density gradient centrifugation and electronic cell sorting. Brain Res. 1977 May 20;127(1):69–86. doi: 10.1016/0006-8993(77)90380-8. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunogenic properties of the glial fibrillary acidic protein. Brain Res. 1976 Oct 29;116(1):150–157. doi: 10.1016/0006-8993(76)90257-2. [DOI] [PubMed] [Google Scholar]
- Dimpfel W., Huang R. T., Habermann E. Gangliosides in nervous tissue cultures and binding of 125I-labelled tetanus toxin, a neuronal marker. J Neurochem. 1977 Aug;29(2):329–334. doi: 10.1111/j.1471-4159.1977.tb09626.x. [DOI] [PubMed] [Google Scholar]
- FANTL P., FITZPATRICK M. Fibrinolysis induced by brain extracts. Br J Exp Pathol. 1950 Apr;31(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967 Feb;32(2):277–287. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Benedict W. F. Fibrinolytic activity in a human fibrosarcoma cell line and evidence for the induction of plasminogen activator secretion during tumor formation. Cell. 1975 Oct;6(2):245–252. doi: 10.1016/0092-8674(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Jones P., Benedict W., Strickland S., Reich E. Fibrin overlay methods for the detection of single transformed cells and colonies of transformed cells. Cell. 1975 Jul;5(3):323–329. doi: 10.1016/0092-8674(75)90108-7. [DOI] [PubMed] [Google Scholar]
- Kalderon N. Migration of Schwann cells and wrapping of neurites in vitro: a function of protease activity (plasmin) in the growth medium. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5992–5996. doi: 10.1073/pnas.76.11.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIALE I. L., SIDMAN R. L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961 Oct;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Messer A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 1977 Jul 8;130(1):1–12. doi: 10.1016/0006-8993(77)90838-1. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Paranjpe M., del Ande Eaton S., Boone C. W. Neoplasms produced from C3H/10T 1/2 cells attached to plastic plates; saturation density, anchorage dependence and serum requirement of in vitro lines correlated with growth aggressiveness in vivo. J Cell Physiol. 1978 Jul;96(1):63–71. doi: 10.1002/jcp.1040960108. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W. Cerebellar cell surface antigens of mouse brain. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4110–4114. doi: 10.1073/pnas.72.10.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Langman J. Repair of the external granular layer after postnatal treatment with 5-fluorodeoxyuridine. Am J Anat. 1970 Nov;129(3):247–259. doi: 10.1002/aja.1001290302. [DOI] [PubMed] [Google Scholar]
- Shimada M., Langman J. Repair of the external granular layer of the hamster cerebellum after prenatal and postnatal administration of methylazoxymethanol. Teratology. 1970 May;3(2):119–133. doi: 10.1002/tera.1420030204. [DOI] [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- TODD A. S. The histological localisation of fibrinolysin activator. J Pathol Bacteriol. 1959 Jul;78:281–283. doi: 10.1002/path.1700780131. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Thomson J., Magnani J. L., Steinberg M. S. Two distinct adhesion mechanisms in embryonic neural retina cells. III. Functional specificity. Dev Biol. 1981 Jan 30;81(2):379–385. doi: 10.1016/0012-1606(81)90304-3. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Sidman R. L. Histogenesis of mouse cerebellum in microwell cultures. Cell reaggregation and migration, fiber and synapse formation. J Cell Biol. 1977 Dec;75(3):915–940. doi: 10.1083/jcb.75.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W. S., Kirsch W. M., Martinez-Hernandez A., Fink L. M. In vitro plasminogen activator activity in human brain tumors. Cancer Res. 1978 Feb;38(2):297–302. [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]