Abstract
Malignant melanoma is the deadliest form of skin cancer, known for its drug resistance and high metastatic potential. Deregulated PI3 and MAP kinase pathways promote early melanocytic lesion development and confer drug resistance. No agent exists to target these deregulated pathways to prevent cutaneous non-invasive melanocytic cells or invasive melanomas from developing into more aggressive widely disseminated metastatic disease. In this study, a selenium containing isosteric analogue of PBIT [S,S′-1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea] called PBISe [Se,Se′-1,4-phenylenebis(1,2-ethanediyl)bis-isoselenourea] is shown to moderate these two major signaling pathways to prevent cutaneous melanocytic lesion or melanoma development. Topical application of PBISe retarded melanocytic lesion development in laboratory-generated skin by 70-80% and in animal skin by ∼50%. Mechanistically, prevention of lesion development occurred due to decreased Akt3 signaling, which increased MAP kinase pathway activity to inhibitory levels. The combined effect of targeting these pathways led to decreased cell proliferation and increased apoptotic cell death thereby preventing melanoma development. Thus, topically applied PBISe treatment has potential to prevent non-invasive melanocytic lesion and invasive metastatic melanoma development in skin.
Keywords: Melanoma, PBISe, Akt3, chemoprevention, apoptosis, proliferation
Introduction
Malignant melanoma remains the most deadly and invasive form of skin cancer leading to >80% of all skin cancer deaths (1, 2). Despite the use of surgery to excise early non-invasive melanocytic lesions to prevent progression to metastatic disease, incidence and mortality rates for metastatic melanoma continue to rise (2, 3). Accumulating evidence have demonstrated protective effects of sunscreens from damaging UV rays, which otherwise can potentially induce the development of melanomas from melanocytes located at the base of the skin epidermis (4). However, the utility of sunscreens for protecting skin from sunburn damage is raising concerns that its use might encourage sun exposure, which could have the opposite effect and increase rates of skin cancers (5).
No agent targeting Akt3 or V600EB-Raf signaling involved in early melanoma development is available clinically to prevent the disease or retard early stage non-invasive melanocytic lesion cells from progressing into metastatic melanoma (6). Preventing melanoma development in its early stages using chemopreventive agents is a viable strategy to decrease premalignant lesions from progressing into lethal advanced stages in humans. If an agent of this type were available, it could have significant clinical potential to decrease mortality rates and reduce costs associated with treatment and management of advanced-stage disease (7). Therefore, novel compounds are needed that could be used to augment existing preventive strategies and one approach is the development of topical agents, which could moderate key pathways involved in development of melanocytic lesions or melanoma in skin.
Development of melanomas from melanocytes is a multi-stage complex process involving changes in expression and activities of a number of genes and signaling pathways regulating cellular differentiation, growth, senescence, survival and migration (8). B-Raf is a member of the MAP kinase pathway whose activity is deregulated in melanomas by mutation to a constitutively active form called V600EB-Raf in ∼90% of benign nevi or normal moles (9, 10). However, the presence of V600EB-Raf alone does not cause melanoma as this aberrant protein activates the downstream MAP kinase pathway to inhibitory levels, leading to cellular senescence (10-16). Therefore, very few V600EB-Raf containing moles ever develop into melanoma and remain in a senescent stage (14, 15). Other factors such as loss of tumor suppressors PTEN, p16INK4a or activation of oncogenes such as Akt3 are needed to moderate V600EB-Raf activity and the downstream MAP kinase pathway in order to drive tumor progression (14-17). Elevated Akt3 activity, occurs in ∼70% of melanomas and inhibits V600EB-Raf protein activity by phosphorylating negative regulatory sites, which moderates protein activity to levels promoting melanoma development rather than driving cells into senescence (10, 15, 17-20). Therefore, therapeutics targeting Akt3 activity has potential to increase MAP kinase activity to inhibitory levels to promote cell senescence. Furthermore, melanoma cells containing V600EB-Raf treated with PLX 4032, a specific inhibitor of this mutant protein, were found to develop resistance to the drug by activating Akt3 signaling (21). Finally, combined targeting of Akt3 and V600EB-Raf has been reported to synergistically inhibit melanoma (22). Thus, agents regulating these two signaling pathways could have significant potential to prevent melanocytic lesion development (10, 15, 17).
PBISe [S,S′-1,4-phenylenebis(1,2-ethanediyl)bis-isoselenourea] is a selenium containing analog of the iNOS inhibitor PBIT [S,S′-1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea] that has been evaluated as a therapeutic agent for treatment of systemically spread melanoma; however, its chemopreventive potential has not been examined (23-25). In this study, the chemopreventive efficacy of topically applied PBISe has been tested on laboratory generated skin reconstructs containing melanocytic lesions and xenografted cutaneous melanoma tumors in mice (26, 27). Mechanistically, PBISe decreased Akt3 signaling resulting in increased MAP kinase activity to inhibitory levels, which promoted cell senescence and apoptosis (23). Thus, topically applied PBISe could have significant clinical potential for preventing early melanocytic lesion development in skin.
Materials and Methods
PBISe and PBIT synthesis
Bromide salts of PBISe (Mol. Wt. 538.80) and PBIT (Mol. Wt. 444.44) were synthesized and identity as well as purity verified by NMR and mass spectrometry as described previously (24). Aliquots of 10 mM PBISe and PBIT (>95% purity by HPLC) stock solutions made in PBS were stored at −20° C for in vitro use. For in vivo experiments PBISe and PBIT were dissolved in DMSO to make a 48× stock solution and diluted to 120 μl in acetone prior to use.
Cell lines and culture conditions
WM35 radial growth phase melanocytic lesion cell line expressing green fluorescence protein (GFP) and WM115 vertical growth phase cells were grown as described previously (28). Normal human primary melanocytes were cultured in 1× MCDB 153 (Sigma), 2% FBS, 10% Chelated FBS (Hyclone), 100 nM ET3 (VWR), 10 ng/ml SCF (R&D), 20 pM Cholera Toxin (Sigma), 4.5 ng/ml bFGF (Promega) and 2 mM L-Glutamine (Mediatech) as described previously (29). Normal human FF2441 fibroblasts and GFP-expressing human metastatic melanoma cell lines UACC 903 and 1205 Lu were cultured in high glucose DMEM with glutamax (Invitrogen) supplemented with 10% FBS. Passage 2 to 5 human foreskin keratinocyte cells, were isolated and cultured in EpiLife E-medium (a serum-free HEPES based medium) containing 1× HKGS consisting of bovine pituitary extract, bovine insulin, hydrocortisone, bovine transferrin, and human EGF (Cascade Biologics) as detailed previously (17).
Creation of laboratory generated skin
Laboratory generated skin was made from human cell lines (average size measurements: length - 14 mm, breadth – 21 mm, height – 1 mm) by suspending normal human FF2441 fibroblast cells in 10% reconstitution buffer, (10% DMEM (Mediatech), 2.4 μl/ml of 10 M NaOH, and 80% collagen I (Becton Dickinson) at a cell density of 3.75 × 105 cells/ml on ice followed by incubating 1.5 ml aliquots in 12-well culture plates at 37°C tissue culture incubator for 3 h to form a dermal matrix (17). 1 ml aliquot of E-medium was added to each well containing a dermis and allowed to grow for 2 days. A mixture of GFP expressing WM35 or UACC 903 cells and normal human keratinocytes at a ratio of 1:10 were resuspended in 1 ml E-medium and added on top of the dermal matrix to produce a keratinized layer containing non-invasive melanocytic lesions or invasive melanomas. After 2 days of incubation, skin reconstructs were transferred onto wire grids to form a complete keratinized layer for 7-8 days in a tissue culture incubator (17). During this period, developing skin reconstructs were fed via diffusion from E-medium (replaced on alternate days) below the wire grids.
Topical drug treatment of laboratory generated skin
Skin reconstructs containing non-invasive melanocytic lesions or invasive melanoma tumor nodules that were similar in number and size were grouped into control vehicle PBS or 5, 10, 15, and 20 μM PBISe treatment groups (n=3 skin reconstructs in each group) and exposed to each agent applied once per day for 8 days. At the end of treatment, 6 images from each skin reconstruct were photographed using a Nikon SMZ 1500 fluorescent microscope (Nikon Instruments) to quantify number and area of melanoma tumor nodules expressing GFP using IP Lab software (BD Biosciences). Average area occupied by melanoma tumor nodules from each treatment group was measured and plotted against each drug concentration.
Histological and morphological characterization of laboratory generated skin
Morphology and architecture of skin reconstructs prior to, and at the end of each treatment regime were analyzed by fixation with 10% paraformaldehyde (Electron Microscopy Science) followed by storage in 0.5 M EDTA. Skin reconstructs were trimmed into strips, frozen in O.C.T compound and sectioned. Formalin fixed paraffin embedded sections were stained with H&E to examine skin architecture, whereas frozen sections were used to photograph GFP expressing tumor nodules.
Melanoma tumor xenograft studies using topical PBISe treatment
Animal experiments were conducted according to protocols approved by Institutional Animal Care and Use Committee at Penn State University. Twelve, four weeks old female athymic nude mice having an average weight of 20 g (Harlan Sprague Dawley) were injected with 2.5 × 105 cells in 200 μl DMEM-containing 10% FBS into the subcutaneous skin layer on the right and left flanks above the rib cages to conduct tumor kinetics studies. After 24 h, mice were randomly assigned to 3 groups (n=4 mice/group) before starting topical treatment. Vehicle control (Acetone–120 μl), 0.095 μmoles PBISe or PBIT (corresponding to 15 μg selenium each side or 1.5 ppm selenium/animal) in 120 μl vehicle were topically applied at the site of tumor cells injection every day for 29 days. Average tumor size and body weights from each treatment group were measured and plotted against days.
For mechanistic studies, 5 × 106 UACC 903 cells were injected into the subcutaneous skin layer of nude mice. Mice were treated with vehicle, PBIT or PBISe, as detailed above. Size and time matched tumors were harvested at days 9, 11, 13, and 15 to assess changes in cell proliferation and apoptosis (23). Cell proliferation and apoptosis rates in tumor cells were measured in formalin-fixed, paraffin-embedded tumor sections using purified mouse anti-human Ki-67 (PharMingen) and TUNEL staining (Roche Diagnostics). A minimum of 6 different tumors with 4-6 fields per tumor was analyzed and results reported as the average ± SEM.
Analysis of caspase-3/7 activity in tumor lysates
A small portion of flash frozen tumor was pulverized into powder followed by isolation of protein lysates using protein lysis buffer (600-800 μl per 50 mg powder, 50 mM Tris-HCl, pH 7.5 containing 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 10 mM sodium β-glycerol phosphate, 5 mM sodium pyrophosphate, 1 mM activated sodium orthovanadate, protease inhibitor cocktail from Sigma and 0.1% (v/v) 2-mercaptoethanol). Whole tumor lysates were centrifuged at 10,000 × G thrice to remove cell debris. Protein concentrations were quantitated using Bio-Rad protein assay reagent (Bio-Rad laboratories) and analyzed for caspase-3/7 activity. Caspase-3/7 activity was determined by incubating 100 μg of protein lysate with caspase-3/7 substrate as described previously (23, 30).
Toxicity assessments
4-6 weeks old female nude mice (Harlan Sprague Dawley) were treated with either vehicle control, PBISe or PBIT (n=5) as described in tumor kinetics studies. At the end of treatment, blood was collected from each sacrificed animal in a plasma separator tube with lithium heparin (BD Microtainer) following cardiac puncture and analyzed for SGOT (AST - aspartate aminotransferase), SGPT (ALT - alanyl aminotransferase), alkaline phosphatase, glucose, blood urea nitrogen, total protein and creatinine levels to ascertain possible liver, heart, kidney and pancreas related toxicity. Harlan has provided values of serum enzymes and metabolites representing range of normal values for female athymic nude mice. A portion of vital organs - liver, heart, kidney, intestine pancreas and adrenal - from each animal was formalin fixed and paraffin-embedded to examine for toxicity-associated changes in cell morphology and tissue organization following H&E staining (23)
Introduction of siRNAs in to UACC 903 cells by nucleofection
1 × 106 UACC 903 cells were nucleofected (Amaxa Nucleofector-I) with 100 and 200 picomoles of scrambled control or Akt3 targeting siRNA, using reagent-R and K-17 program as described previously (15). Scramble and Akt3 siRNAs (Invitrogen) sequences used in this study were reported previously (15, 18). After two days recovery in DMEM supplemented with 10% FBS, cells were transferred to media containing no serum and 2 h later, stimulated with 10% FBS containing DMEM for 60 m. Cell lysates collected and analyzed by Western blotting to measure the expression of Akt3, pAkt (S473), pErk-1/2 (T202/Y204) and α-enolase, which served as a control for protein loading. The intensities of protein bands were quantitated using Image-J and normalized against α-enolase.
In vitro drug treatment and collection of cell lysates
1.5 × 106 GFP expressing cells WM35, WM115 or UACC 903 in 10 ml DMEM containing 10% FBS were grown in a 100 mm culture dish for ∼36 h. Exponentially growing cells were treated with 3, 5, 9, 10 or 15 μM PBISe in 10 ml DMEM supplemented with 10% FBS for 6, 12 and 24 h. Total cell lysates from adherent and floating cells were collected from PBS and PBISe treated plates. Floating cells were transferred to a 50 ml conical tube, centrifuged at 1,500 rpm for 5 m and cell pellet washed twice with PBS before being lysed using protein lysis buffer. Adherent cells were washed twice with PBS and incubated with 100 μl protein lysis buffer for 30 m on ice. Lysates collected from adherent and non-adherent cells were then combined, centrifuged at 10,000 rpm for 10 m at 4°C, and clear supernatant transferred to a pre-chilled 1.5 ml centrifuge tube (31). Protein concentration in the samples was quantified using the BCA assay (Thermo Scientific).
Western blotting
15-30 μg of protein derived from cells treated with different agents were analyzed by Western blotting on NuPAGE Gels (Invitrogen). Following electrophoresis, samples were transferred to polyvinylidene difluoride membrane (Pall Corporation), and analyzed for expression and activity of PI3 kinase and MAP kinase signaling pathway members. Primary antibodies: Total Akt, pAkt(S473), pPRAS40(T246), total Erk1/2, pErk1/2(T202/Y204), cleaved PARP, and caspase-3 antibodies (Cell Signaling Technologies); PRAS40 (Invitrogen); and α-enolase, cyclinD1, p27(Kip1), p21(CIP1); and secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology). Immunoblots were developed using either Enhanced Chemi-luminescence or SuperSignal West Femto Maximum Sensitivity substrate (Thermo Scientific) (17, 23, 31).
Analysis of caspase-3/7 activity in total cell lysates
Caspase-3/7 activity in the cell lysates collected for Western blot analysis was determined using Apo-ONE homogenous caspase-3/7 activity assay kit (Promega Corporation). In brief, 20–30 μg in 40 μl lysis buffer was incubated with caspase-3/7 substrate (R110-Z-DEVD dissolved in caspase-3/7 assay buffer) for 2 h at 37°C with constant shaking in a light protected container. Amount of R110 released was determined using a SPECTRA max-M2 plate reader (Molecular Devices Corporation) set at a 485 nm excitation and 520 nm emission wavelengths. Average relative fluorescence units values from triplicate wells were plotted as a bar graph with ± SEM (23, 30, 32).
Cell viability, IC50, proliferation, and cell cycle analysis
The viability and IC50 of melanoma and normal human fibroblast FF2441 exposed to 1–28 μM PBISe or PBIT were measured using the MTS assay (Promega Corporation). A total of 5 × 103 FF2441 or melanoma cells/well in 100 μl of DMEM containing 10% FBS were grown in a 96-well plate for 36 or 72 h to reach ∼70% confluence and treated with either PBS vehicle, PBIT or PBISe for 24 h and numbers of viable cells compared with PBS controls. Similarly, 20 × 103 melanocytes/well in 100μl of melanocyte medium were grown in a 96-well plate for 72 h to reach ∼70% confluence and treated with 1-100 μM PBISe for 24 h. IC50 values for each compound were determined for each cell lines from three independent experiments using GraphPad Prism version 4.01 and averages represented with ± SEM (GraphPad Software).
Cellular proliferation rate was measured by seeding 5 × 103 cells in a 96-well plate, followed by treatment with PBISe, PBIT or PBS for 24 h. Cells were labeled with BrdU, using a Cell Proliferation ELISA kit, 4 h prior to end of treatment (Roche Diagnostics) (18). Amount of BrdU taken up by cells was measured by incubating with a peroxidase conjugated anti-BrdU binding antibody and read in a 96-well plate reader set at 370 nm and compared to PBS treated control cells. Values were determined from three independent experiments.
Cell cycle analysis was undertaken by growing 1.5 × 106 melanoma cells in 100-mm culture dishes for 36 h, followed by treatment with PBISe or PBIT for 24 h. Adherent and non-adherent cells were collected and stained using 1 ml of 100 μg/ml propidium iodide (Sigma), 20 μg/ml of RNase A (Roche Diagnostics), and 3 μg/ml of Triton X-100 dissolved in 0.1% (W/V) sodium citrate for 30 m at 4°C(33). Stained cells were analyzed using the FACScan analyzer (BD Biosciences). Data was processed utilizing ModFit LT software (Verity Software House).
Statistical analysis
Statistical analysis was undertaken using the One-way ANOVA followed by Dunn's multiple comparisons test or Student's t-test. Results were considered significant at P < 0.05.
Results
PBISe but not control PBIT inhibits melanocytic lesion and melanoma cell growth in culture
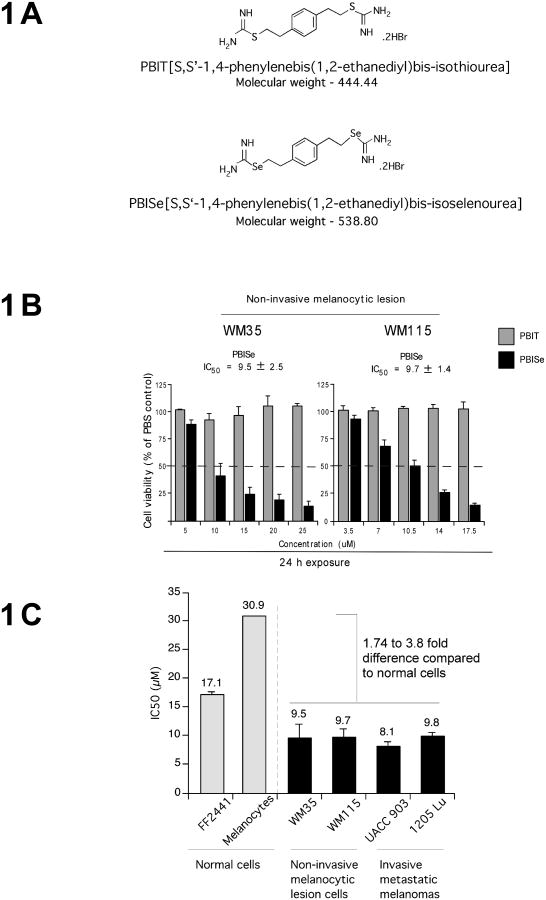
PBISe and PBIT are iNOS inhibitors containing selenium and sulfur respectively, which are shown diagrammatically in Fig. 1A (23, 34). PBISe but not PBIT was effective at reducing the growth of non-invasive melanocytic lesion WM35 and WM115 cells. IC50 values of PBISe for WM35 and WM115 cells were 9.5 and 9.7 μM respectively (Fig. 1B). Prior studies have demonstrated anti-melanoma activity of PBISe against invasive metastatic melanoma UACC 903 and 1205 Lu cells (23). In contrast, PBIT had no effect on cell viability at these concentrations suggesting a possible cancer preventive potential for PBISe (Fig. 1B). Specificity of PBISe for killing melanocytic lesion and melanoma cells but not normal melanocytes or fibroblast cells was determined by comparing the IC50 values to normal human epidermal melanocytes (NHEM) and fibroblasts (FF2441) that had a value of 30.7 and 17 μM respectively (Fig. 1C). Fibroblasts and melanocytes had an IC50 value that was 1.7-3.8 fold higher than that of melanocytic lesion or melanoma cell lines. Thus, PBISe can inhibit survival of cultured non-invasive melanocytic lesion as well as invasive melanoma derived cell lines and has a lesser effect on normal cells.
Figure 1. PBISe inhibits melanoma cells growth in culture.
A. Structures of PBIT and PBISe. PBISe is an isosteric analog of PBIT and was synthesized by replacing sulfur with selenium. B. PBISe inhibits growth of cultured melanoma cells. Cell lines representing non-invasive melanocytic lesions (WM35 and WM115) were treated with increasing concentrations of PBIT or PBISe for 24 h. Cell viability was determined by MTS assay and IC50 values calculated using GraphPad Prism. Results of three independent experiments were plotted; bars, mean ± S.E.M. C. PBISe has negligible effect on normal cells at concentrations killing melanocytic and melanoma cells. Human normal epidermal melanocytes, fibroblasts and melanocytic lesion or melanoma cell lines (WM35, WM115, 1205 Lu, and UACC 903) were treated with PBISe for 24 h and IC50 values compared; bars, average IC50 from 3 independent experiments ± S.E.M.
PBISe is effective at preventing melanocytic lesion development in laboratory-generated skin
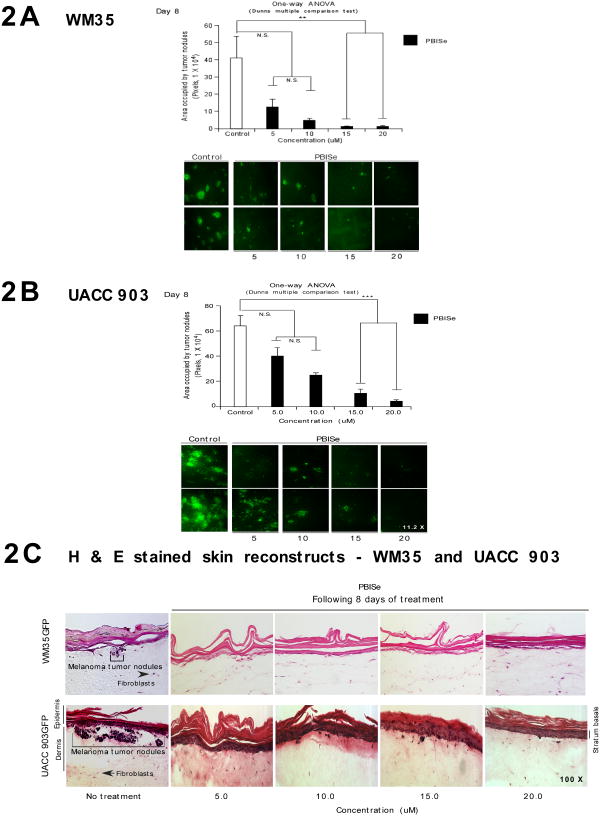
The potency of PBISe for killing cutaneous non-invasive melanocytic lesion or invasive melanoma cells was examined by seeding these cells into laboratory generated skin and topically treating with PBISe (17). Skin reconstructs used for this study contain GFP-tagged WM35 melanocytic lesion or UACC 903 melanoma cells and the amount of GFP following treatment was quantified. This approach is an accepted organotypic skin melanoma model for evaluating the efficacy of topically added chemopreventive agents (17). PBISe decreased total area occupied by GFP tumor lesions present in the organotypic skin model in a dose dependent manner compared to PBIT, which had a negligible effect (Figs. 2A and 2B; P < 0.05, One-way ANOVA).
Figure 2. Topical application of PBISe inhibits growth of melanoma tumors in laboratory generated skin reconstructs.
A and B. Topically applied PBISe inhibited the growth of melanocytic lesions and melanoma tumors developing in skin reconstructs. Laboratory generated skin containing melanocytic lesions or melanoma tumors were treated with PBISe or vehicle for 8 days and sizes of area occupied by developing GFP tumors quantified. C. PBISe causes negligible damage to the cells present in skin. H & E stained skin reconstructs containing melanocytic lesion of melanoma cells were treated topically with PBISe and compared to untreated controls. No change in skin morphology or morphology of keratinocytes or fibroblasts was observed.
Skin architecture and morphology of constituent cells morphology containing radial WM35 and invasive UACC 903 melanoma tumors were compared in H&E stained sections from PBISe versus control treated skins (Fig. 2C). Similar histological and morphological features were observed under all treatment regimes showing an intact keratinized layer and dermal fibroblasts having a similar size, shape and distribution, suggesting that PBISe does not damage skin or the cells present in it (Fig. 2C). Thus, PBISe can decrease melanocytic lesion and melanoma tumor development in laboratory-generated skin with negligible effect on skin architecture or morphology of fibroblasts or keratinocytes.
Topical application of PBISe is not toxic to mice and inhibits melanoma tumor development in animal skin
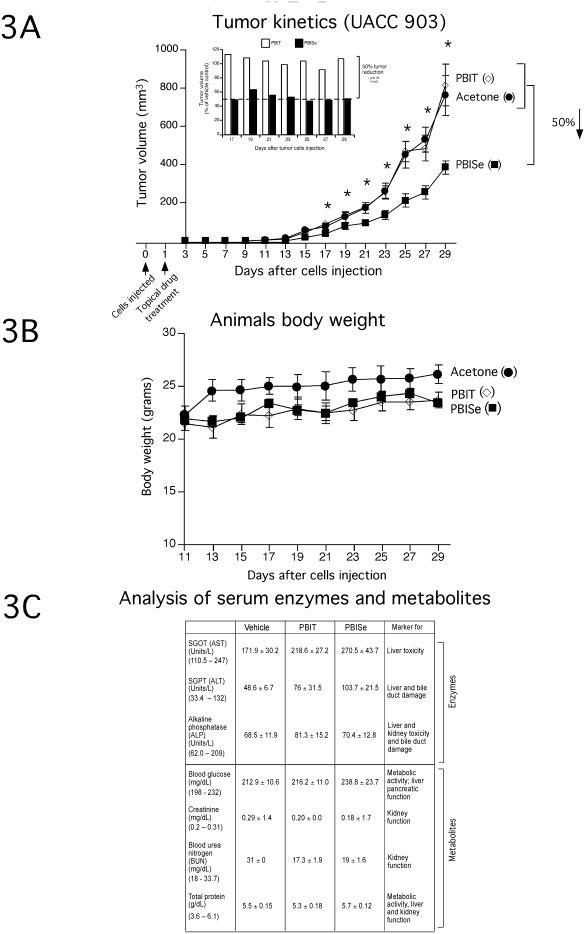
To determine the inhibitory efficacy of PBISe on tumor inhibition in animal skin, 2.5 × 105 cutaneously invasive UACC 903 cells were subcutaneously injected above the left and right rib cages of 4 to 6 weeks old female nude mice, and skin above the tumor treated topical with PBISe, PBIT or vehicle acetone. Non-invasive WM35 melanocytic lesion cells lines could not be used for this study since they do not form tumors in animal skin. Animals containing cutaneous UACC 903 tumors were exposed to PBISe and found to consistently have ∼50% smaller lesions starting at day 17 compared to those treated with either PBIT or vehicle control (Fig. 3A, P < 0.05 two tailed students t-test).
Figure 3. Topically applied PBISe inhibit development of cutaneous melanomas with negligible systemic toxicity.
A. Topically applied PBISe decreases development of subcutaneous melanocytic lesions in mice. Following injection of UACC 903 cells subcutaneously into mice, area above sites was topically treated daily with acetone, PBISe or PBIT and sizes of developing tumors measured on alternate days. B. Daily topical treatment with PBISe did not significantly alter animal body weight. Body weights of animals treated topically with acetone, PBISe or PBIT were measured on alternate days to establish possible toxicity. C. Daily topical treatment with PBISe did not significantly alter blood biomarkers indicative of major organ related toxicity. Blood collected from animals treated topically with PBISe, PBIT or acetone was collected from animals at day 29 and analyzed for enzyme activities of liver (SGOT, SGPT), kidney (Alkaline phosphatase) and heart (SGOT, SGPT and alkaline phosphatase). Concentrations of metabolites (glucose, creatinine, urea nitrogen and total protein) in serum were also measured. Control values for this mouse species are listed below each factor in brackets for comparison.
No statistically signigicant differences in animal body weights between PBISe, PBIT or control treated groups was observed, suggesting a lack of systemic toxicity at drug concentrations used (Fig. 3B). This was subsequently confirmed by examining serum biomarkers for blood parameters indicative of major organ related toxicity at the end of treatment regime, which showed negligible differences between groups and fell within normal parameter for this mouse species (Fig. 3C) (35). Thus, PBISe can retard cutaneous tumor formation in animals when applied topically without significant systemic toxicity.
PBISe exposure triggered apoptosis in melanoma cells growing in culture and in xenografted tumors
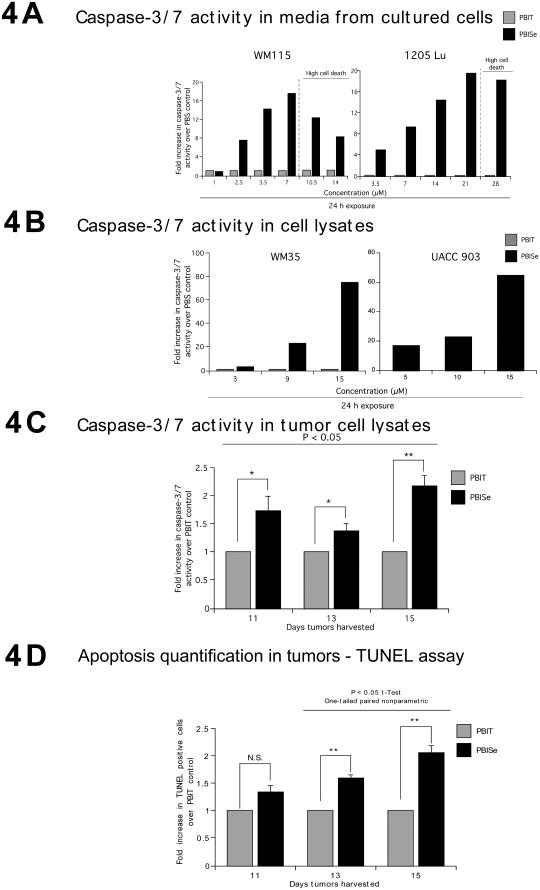
The mechanism preventing cutaneous melanocytic lesion development following PBISe treatment was established by analyzing apoptosis and proliferation rates using lysates derived from of cultured melanocytic lesion or melanoma cells or xenografted tumors treated with agents. Since elevated caspase-3/7 activity in cultured cells is an indicator of apoptotic cell death, levels in cells exposed to PBISe or PBIT were measured using Apo-ONE homogenouse caspase-3/7 activity kit. A dose dependent increase in caspase-3/7 activity in cultured non-invasive WM115 and invasive 1205 Lu cells was observed only when cells were treated with PBISe but not with PBIT (Fig. 4A). For both cell lines, higher concentrations of PBISe caused significant cell death, which consequently reduced caspase-3/7 activity. Similar results were observed for protein lysates derived from cultured non-invasive WM35 and invasive UACC 903 cells treated with PBISe and analyzed for caspase-3/7 activity (Fig. 4B). Analysis of caspase-3/7 activity in protein lysates collected from size and time matched tumors harvested at days 11, 13, and 15 likewise showed a 1.5-2.2-fold increase in apoptosis (Fig. 4C, P < 0.05, Student's t-test). In contrast, PBIT or acetone vehicle treatment did not increase apoptosis rates. Confirming these observations, PBISe treatment increased number of TUNEL positive apoptotic cells observed following immunohistological analysis of xenografted size and time matched tumors isolated at days 11, 13, and 15 in a dose dependent manner (Fig. 4D). Compared to controls, a consistent increase in TUNEL positive cells was observed following PBISe treatment (Fig. 4D, P < 0.05, Student's t-test). Thus, PBISe increases apoptosis rates in melanoma cells growing in culture or in animals.
Figure 4. PBISe induces apoptosis to inhibit survival of melanocytic lesion and melanoma cells.
A and B. PBISe treatment increased Caspase-3/7 activity in cultured melanocytic lesion and melanoma cell lines. Cell lines representing non invasive melanocytic lesions (WM35 and WM115) and invasive melanomas (1205 Lu and UACC 903) were treated with increasing concentrations of PBIT or PBISe for 24 h and caspase-3/7 activity from culture media or cell lysates measured. Bar graph represents fold increase over PBS treated controls. C and D. Tumors from mice treated with PBISe showed increased Caspase-3/7 activity in tumor lysates and had more TUNEL positive cells compared to PBIT treated controls. Protein lysates collected from PBISe or PBIT treated size and time matched tumors harvested at days 11, 13 and 15 were incubated with R110 conjugated caspase-3/7 substrate (R110-Z-DEVD) for 1 h and released Rhodamine-110 measured in a plate reader. TUNEL positive cells from formalin fixed paraffin embedded size and time matched tumors from PBISe or PBIT were scored for percentage of positive cells from a minimum of 3 tumors (3 to 5 fields/tumor); bar, average ± S.E.M.
PBISe decreases cellular proliferation rates and halts cell cycle progression in cultured melanocytic lesion as well as in melanoma cells
Since topically applied PBISe had potential to prevent non-invasive melanocytic lesion as well as invasive melanoma cell growth in laboratory generated skin and decrease xenografted tumor development, effect of PBISe exposure on proliferation rates and cell cycle progression were examined. Compared to PBS vehicle or PBIT, a significant decrease in proliferation measured by BrdU incorporation was observed at 1-5 μM PBISe for non-invasive melanocytic lesion WM35 or WM115 cells (Fig. 5A). In contrast, metastatic melanoma 1205 Lu cells, required ∼14 μM PBISe to decrease cell proliferation by ∼60% (Fig. 5A).
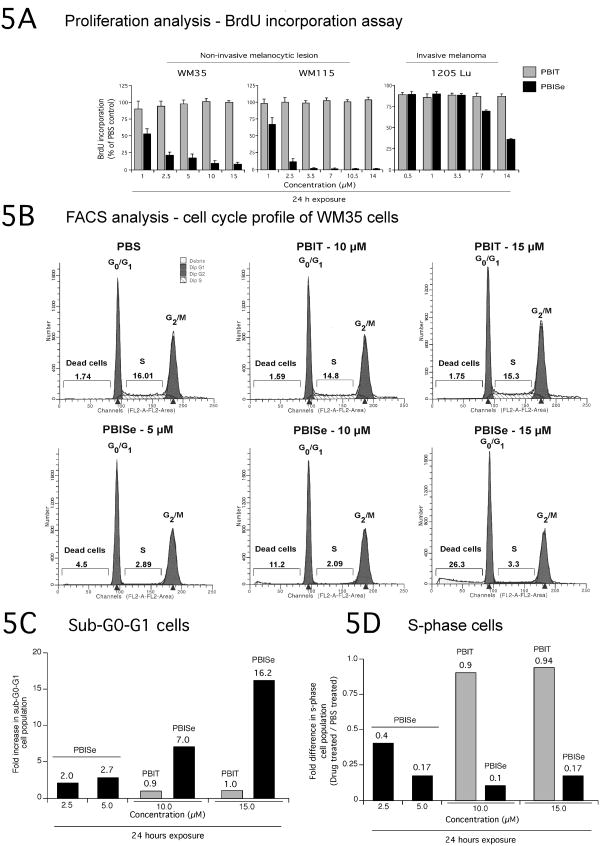
Figure 5. PBISe treatment of cultured melanoma cells increases the subG0/G1 and decreases the S-phase cell population.
A. PBISe inhibits the proliferative potential of cultured melanocytic lesion and melanoma cells. A BrdU ELISA kit was used to assess the proliferative potential of cells following treatment with PBISe, PBIT and PBS; bar mean ± S.E.M. B, C, and D. PBISe treatment decreased population of cells in S-phase and increased sub G0/G1 phase of the cell cycle. Cultured non-invasive WM35 cells were treated with increasing concentrations of PBIT, PBISe or PBS for 24 h. Adherent and detached cells were collected, stained with propidium iodide and cell cycle analyzed using a FACScan.
Basis for decreased cellular proliferation following PBISe treatment was examined next by analyzing percentage of cell population in the different phases of the cell cycle. Compared to control PBS or PBIT, PBISe treatment increased the sub-G0-G1 WM35 cell population and decreased S-phase cell population in a dose dependent manner (Fig. 5B). At 10-15 μM PBISe, a 7-16 fold increase in sub-G0-G1 WM35 cells were observed compared to PBIT indicating increased apoptosis (Fig. 5C). Compared to controls, a significant decrease in S-phase cells was observed at 5-15 μM PBISe (Fig. 5D). Thus, PBISe treatment inhibited non-invasive melanocytic lesion and metastatic melanoma cell proliferation by decreasing the S-phase cell population and increasing the sub-G0-G1 component indicating elevated apoptotic cell death.
Targeting Akt3 using siRNA or PBISe increased MAP kinase activity reducing the proliferative potential and promoting apoptosis of cultured cells
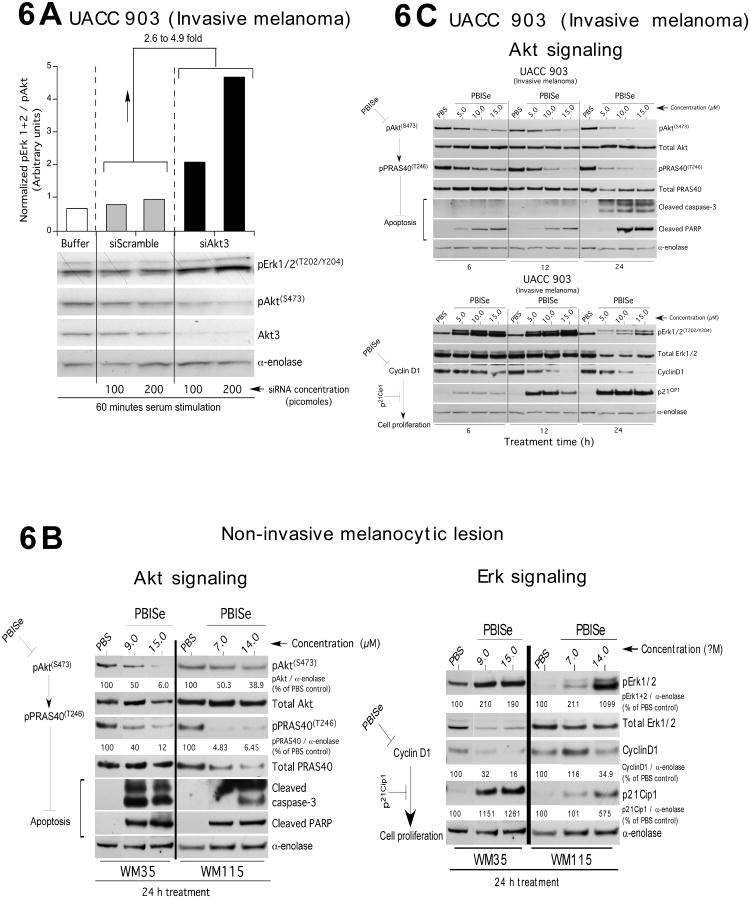
Western blotting was used to identify the protein signaling events decreasing cell survival following Akt3 inhibition in invasive metastatic cells using siRNA or following PBISe treatment. Compared to UACC 903 cells nucleofected with scrambled siRNA, Akt3 siRNA led to 2-5 fold higher levels of pErk1/2 in a dose dependent manner (Fig. 6A). This result confirmed prior reports showing that decreasing pAkt3 levels led to increased V600EB-Raf activity resulting in rising downstream MAP kinase pathway signaling to levels that are inhibitory, which induced cell senescence (14, 15). For non-invasive WM35 and WM115 cells, pErk1/2 levels indicating elevated MAP kinase pathway activity, rose with increasing PBISe treatment in a time and dose dependent manner corresponding directly with decreasing pAkt levels and downstream pPRAS40 levels leading to elevated pErk1/2 in a time and dose dependent manner (Fig. 6B). Similar effects on Akt and Erk signaling pathways were observed when invasive UACC 903 cells were treated with PBISe (Fig. 6C). For WM35, WM115 and UACC 903 cell lines, cyclin D1 levels decreased and p21 levels increased consistently in a dose and time dependent manner following PBISe treatment corresponding to increasing pErk1/2 (Fig. 6B & 6C). Thus, PBISe reduced Akt3 activity thereby decreasing the inhibitory effect on V600EB-Raf, which led to high inhibitory MAP kinase pathway activity down regulating cyclin D1 and increasing p21 levels thereby promoting apoptosis and cell senescence.
Figure 6. PBISe targets Akt3 signaling to regulate non-invasive melanocytic and invasive melanoma cell survival.
A. Inhibition of Akt3 signaling increases MAP kinase pathway signaling. SiRNA mediated inhibition of Akt3 protein levels led to increased pErk1/2 levels in UACC 903 cells. UACC 903 cells were nucleofected with 100 and 200 picomoles of siRNA targeting Akt3 or a control scrambled siRNA and cell lysates collected after 2 h serum starvation followed by 1 h serum stimulation. Expression of pAkt and pErk1/2 were measured by Western blotting and the band intensities measured using Image-J software. Protein levels were normalized against α-enolase and the pErk1/2 to pAkt ratio measured. B. and C. PBISe treatment of non-invasive melanocytic lesion and invasive melanoma cell decreased Akt3 signaling and increased MAP kinases pathway activity to inhibitory levels. PBISe treatment in all cell lines decreased the pAkt and downstream pPRAS40 levels, which consequently increased levels of pErk1/2. Combined targeting of these pathways promoted decreased cell growth indicated by lowered cyclin-D1 and increased p21 levels as well as increasing rates of apoptosis observed as increased levels of cleaved caspase-3 and PARP.
Discussion
Despite skin-cancer prevention programs, availability of UV protecting sunblocks, and surgical procedures for removing pre-invasive melanocytic skin lesions, incidence of metastatic melanoma and mortality rates resulting from disseminated metastatic disease continue to rise (1, 36). Therefore, additional agents to prevent the disease from developing or progressing past its earliest stages, which could be added to creams, lotions or sunblocks, are needed. Preventive agents of this type would have potential to decrease disease incidence and mortality rates (6).
Chemopreventive effects of inorganic and organic selenium derivatives have been evaluated for colon, lung, prostate and esophageal cancers (37-39). Selenium containing compounds can induce the activities of phase-II enzymes and inhibit phase-I enzymes to prevent the progression as well as reduce growth of tumor cells (23). Selenium has also been shown to increase the therapeutic efficacy of drugs in combination treatment regimes, and to increase the potency of chemopreventive and therapeutic agents when selenium is substituted for sulfur in drugs by increasing rates of cellular apoptosis (23). Furthermore, low selenium levels have been reported in the serum of patients suffering from cancers including melanoma (40, 41). Various preclinical and clinical studies have shown anti-cancer activity of inorganic and organic selenium containing agents (37, 39, 40, 42) and the mechanistic basis of tumor inhibition was studied (43-45). Our study demonstrates that substitution of sulfur in PBIT with selenium provided the compound with novel properties to enhance its anti-cancer activity. Promoting enhanced anti-cancer activity by replacing sulfur in various chemotherapeutic agents with selenium has been reported (37, 42). PBISe could be metabolized in to alkyl selenol or incorporated in to proteins to confer this activity, but the possibility was not explored in this study.
While selenium-containing compounds can enhance the cancer inhibitory activity of agents, recent preclinical and clinical studies have demonstrated that administration of selenium compounds might have no effect or induce serious side effects(46, 47). For example, combining selenium and vitamin E in a Cancer Prevention Trial (SELECT), the largest phase III randomized placebo-controlled study, found that oral administration of selenomethionine did not prevent prostate cancer and might increase rates of diabetes (47). Another study found that dietary supplementation of antioxidant mixture containing selenium increased melanoma risk in women (46). Lack of chemopreventive efficacy of selenomethionine for inhibiting tumor growth has also been reported in preclinical rodent models (43, 44).
Although several concerns and controversies abound regarding the use of selenium for preventing cancers, the present study demonstrates a strategy to improve the efficacy of pharmaceutical agents by substituting sulfur with selenium. In addition, several recent studies have shown that the dose and form of selenium are the key factors that influence the outcome of selenium treatment (43, 44). Therefore, care must be taken when deciding the dose and form of selenium for clinical use. Methylselenocysteine was effective at inhibiting tumor growth compared to selenomethionine suggesting requirement for particular structural features necessary for anticancer activity (48). Based on our observations, PBISe topical treatment successfully retarded the development of early WM35 as well as metastatic UACC 903 in laboratory generated skin reconstruct models. These observations suggest PBISe might be useful for inhibiting the progression of dysplastic nevi in to pre-malignant lesions as well as metastatic melanomas. Also, where a cutaneous melanoma has been removed, PBISe might prevent recurrence which would be a scenario analogous to the use of tamoxifen as a breast cancer prevention agent where any risks incurred by using the agent are outweighed by the high risk of cancer recurrence (49).
Prior studies have shown that intra-peritoneal administration of PBISe but not PBIT retarded melanoma tumor growth without causing systemic toxicity (23). This study extends this initial discovery showing that daily topical application of PBISe retards cutaneous non-invasive melanocytic lesion or invasive melanoma development. Topical PBISe decreased tumor development in laboratory generated skin reconstructs by 70-80% and the development of tumors in the skin of animals by ∼50% compared to controls, thereby demonstrating the chemopreventive potential of PBISe.
Similar to our data, prior studies have shown that substituting sulfur with selenium could increase the potency of pharmaceutical agents to inhibit oncogene function (37, 45). Similar to other selenium containing chemotherapeutic agents, PBISe triggered cellular apoptosis by inhibiting Akt3/PRAS40 signaling, thereby increasing caspase-3/7 activity in melanoma cells (30). Recent studies have also demonstrated that selenium can decrease active and total Akt protein levels by destabilization of this protein in cells; however, decreased total Akt levels were not observed in this study (50). PBISe can also trigger cell death mediated by activation of caspases. Caspases are activated during the process of cell apoptosis and caspase-3 is a key factor responsible for either partial or complete triggering of this cascade. PBISe elevated caspase-3/7 activity of cells by inducing cleaved caspase-3 and PARP. TUNEL assay performed on tumors taken from mice treated with PBISe showed a two-fold increase of apoptosis compared to controls, indicating reduced tumor volume is in part due to increased cellular apoptosis.
In addition to triggering apoptosis and increasing the sub-G0-G1 cell population, PBISe decreased cell proliferation by inhibiting the proportion of cells in the S-phase of the cell cycle (23). Mechanistically, this occurred by PBISe regulating MAP kinase signaling through inhibition of the Akt3 pathway. By decreasing the Akt3 pathway activity, mutant V600EB-Raf could not be phosphorylated by Akt3 to decrease the activity of the MAP kinase pathway (23). Akt3 has been shown to phosphorylate V600EB-Raf in order to release the senescence block induced by high MAP kinase signaling pathway activity, which in turn can promote early melanoma development (8, 31).
In conclusion, topical application of PBISe inhibited cutaneous non-invasive melanocytic lesion development and invasive melanoma development in skin reconstructs and retarded the growth of sub-cutaneous xenografted tumors with negligible systemic toxicity. Since no targeted chemopreventive agents are available for melanoma, PBISe is a promising candidate for preventing early melanocytic lesion development with insignificant side effects. Thus, PBISe has potential for use as a topical chemopreventive agent to retard development of cutaneous melanocytic lesions or melanoma development.
Acknowledgments
We thank Drs. Sung Jin Huh, Arati Sharma and Mitchell Cheung for technical assistance with animal experimentation and Drs. Melissa Tran, Samina Alam and Craig Meyers for help with the laboratory generated skin technique.
Grant support: The American Cancer Society (RSG-04-053-01-GMC), NIH CA-127892-01A, NIH NCI contract (NO2-CB-56603), The Foreman Foundation for Melanoma Research.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Oliveria SA, Hay JL, Geller AC, Heneghan MK, McCabe MS, Halpern AC. Melanoma survivorship: research opportunities. J Cancer Surviv. 2007;1:87–97. doi: 10.1007/s11764-007-0009-y. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–83. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 4.Diffey BL. Sunscreens as a preventative measure in melanoma: an evidence-based approach or the precautionary principle? The British journal of dermatology. 2009;161(3):25–7. doi: 10.1111/j.1365-2133.2009.09445.x. [DOI] [PubMed] [Google Scholar]
- 5.Gorham ED, Mohr SB, Garland CF, Chaplin G, Garland FC. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Annals of epidemiology. 2007;17:956–63. doi: 10.1016/j.annepidem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Francis SO, Mahlberg MJ, Johnson KR, Ming ME, Dellavalle RP. Melanoma chemoprevention. Journal of the American Academy of Dermatology. 2006;55:849–61. doi: 10.1016/j.jaad.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 9.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 10.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 11.Gill M, Celebi JT. B-RAF and melanocytic neoplasia. J Am Acad Dermatol. 2005;53:108–14. doi: 10.1016/j.jaad.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Haluska FG, Ibrahim N. Therapeutic targets in melanoma: map kinase pathway. Curr Oncol Rep. 2006;8:400–5. doi: 10.1007/s11912-006-0065-x. [DOI] [PubMed] [Google Scholar]
- 13.Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer research. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 17.Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer research. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 19.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–85. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 20.Madhunapantula SV, Robertson GP. The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma. Pigment cell & melanoma research. 2009;22:400–19. doi: 10.1111/j.1755-148X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer research. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14:3571–81. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 23.Madhunapantula SV, Desai D, Sharma A, Huh SJ, Amin S, Robertson GP. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol Cancer Ther. 2008;7:1297–308. doi: 10.1158/1535-7163.MCT-07-2267. [DOI] [PubMed] [Google Scholar]
- 24.Desai D, Madhunapantula SV, Gowdahalli K, Sharma A, Chandagaludoreswamy R, El-Bayoumy K, et al. Synthesis and characterization of a novel iNOS/Akt inhibitor Se,Se′-1,4-phenylenebis(1,2-ethanediyl)bisisoselenourea (PBISe)--against colon cancer. Bioorg Med Chem Lett. 2010;20:2038–43. doi: 10.1016/j.bmcl.2009.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–44. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen N, Sharma A, Nguyen N, Sharma AK, Desai D, Huh SJ, et al. Cancer prevention research. Philadelphia, Pa: Melanoma Chemoprevention in Skin Reconstructs and Mouse Xenografts using Isoselenocyanate-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maksymowych AB, Robertson NM, Litwack G. Efficacy of pyridoxal treatment in controlling the growth of melanomas in cell culture and an animal pilot study. Anticancer research. 1993;13:1925–37. [PubMed] [Google Scholar]
- 28.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, et al. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma research. 1997;7(2):S35–42. [PubMed] [Google Scholar]
- 29.Quong RY, Bickford ST, Ing YL, Terman B, Herlyn M, Lassam NJ. Protein kinases in normal and transformed melanocytes. Melanoma research. 1994;4:313–9. doi: 10.1097/00008390-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer research. 2007;67:3626–36. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer research. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, et al. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. Journal of medicinal chemistry. 2008;51:7820–6. doi: 10.1021/jm800993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. The Journal of cell biology. 1975;66:188–93. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–19. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Heron S, Yarnell E. Journal of alternative and complementary medicine. Vol. 7. New York, NY: 2001. The safety of low-dose Larrea tridentata (DC) Coville (creosote bush or chaparral): a retrospective clinical study; pp. 175–85. [DOI] [PubMed] [Google Scholar]
- 36.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Advances in experimental medicine and biology. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 37.Pinto JT, Sinha R, Papp K, Facompre ND, Desai D, El-Bayoumy K. Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. International journal of cancer. 2007;120:1410–7. doi: 10.1002/ijc.22500. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, Mosca P, et al. Targeting Akt3 Signaling in Malignant Melanoma Using Isoselenocyanates. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finley JW, Davis CD. BioFactors. Vol. 14. Oxford, England: 2001. Selenium (Se) from high-selenium broccoli is utilized differently than selenite, selenate and selenomethionine, but is more effective in inhibiting colon carcinogenesis; pp. 191–6. [DOI] [PubMed] [Google Scholar]
- 40.Klein EA. Selenium and vitamin E cancer prevention trial. Annals of the New York Academy of Sciences. 2004;1031:234–41. doi: 10.1196/annals.1331.023. [DOI] [PubMed] [Google Scholar]
- 41.Reinhold U, Biltz H, Bayer W, Schmidt KH. Serum selenium levels in patients with malignant melanoma. Acta Derm Venereol. 1989;69:132–6. [PubMed] [Google Scholar]
- 42.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, Mosca P, et al. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin Cancer Res. 2009;15:1674–85. doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick DL, Rao KV. Chemoprevention of hormone-dependent prostate cancer in the Wistar-Unilever rat. European urology. 1999;35:464–7. doi: 10.1159/000019880. [DOI] [PubMed] [Google Scholar]
- 44.Li GX, Lee HJ, Wang Z, Hu H, Liao JD, Watts JC, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–12. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen KM, Sacks PG, Spratt TE, Lin JM, Boyiri T, Schwartz J, et al. Modulations of benzo[a]pyrene-induced DNA adduct, cyclin D1 and PCNA in oral tissue by 1,4-phenylenebis(methylene)selenocyanate. Biochemical and biophysical research communications. 2009;383:151–5. doi: 10.1016/j.bbrc.2009.03.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098–105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 47.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutation research. 2005;591:224–36. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Dent SF, Gaspo R, Kissner M, Pritchard KI. Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1351-3. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Shin SH, Kang S, Lee YS, Bae S. A novel activation-induced suicidal degradation mechanism for Akt by selenium. International journal of molecular medicine. 2008;21:91–7. [PubMed] [Google Scholar]