Abstract
Objective To investigate whether varenicline is associated with an increased risk of serious cardiovascular events compared with another drug used for smoking cessation, bupropion.
Design Nationwide historical cohort study.
Setting Denmark, 2007-10.
Participants New users of varenicline (n=17 926) and bupropion (n=17 926).
Main outcome measures Individual level data on dispensed drug prescriptions, cardiovascular events, and potential confounders were linked between registries. Cox regression was used to estimate hazard ratios of cardiovascular events in analyses matched for propensity score. The primary outcomes at six months after start of treatment were acute coronary syndrome, ischaemic stroke, and cardiovascular death analysed individually and as a composite of any major event.
Results There were 57 major cardiovascular events among varenicline users (6.9 cases per 1000 person years) compared with 60 events among bupropion users (7.1 cases per 1000 person years); the hazard ratio for any major event was 0.96 (95% confidence interval 0.67 to 1.39). Varenicline use was not associated with an increased risk of acute coronary syndrome (1.20, 0.75 to 1.91), ischaemic stroke (0.77, 0.40 to 1.48), and cardiovascular death (0.51, 0.13 to 2.02). In subgroup analyses, the risk of any major cardiovascular event was not significantly different between patients with and without a history of cardiovascular disease (1.24 (0.72 to 2.12) and 0.83 (0.51 to 1.36), respectively; P=0.29).
Conclusions This cohort study found no increased risk of major cardiovascular events associated with use of varenicline compared with bupropion for smoking cessation. On the basis of the upper confidence limit, the data allowed the exclusion of a 40% increased risk of the composite outcome of any major cardiovascular event. While the estimates were less precise for specific outcomes, any differences would be small in absolute terms.
Introduction
Smoking is a major threat to public health globally and represents the number one preventable cause of mortality worldwide.1 Consequently, any intervention that helps people to stop smoking will have a huge impact on mortality by reducing the burden of associated disease.
Varenicline, a partial agonist at the α4β2 nicotinic acetylcholine receptor, is more efficacious for smoking cessation than placebo and bupropion, and at least equally efficacious as nicotine replacement products.2 Recent findings, however, have raised concerns about its cardiovascular safety. A randomised controlled trial examining efficacy and safety of varenicline in patients with stable cardiovascular disease found somewhat higher rates of non-fatal myocardial infarction, need for coronary revascularisation, and peripheral vascular disease among patients receiving varenicline compared with placebo.3 Although the differences were not significant, these findings prompted the United States Food and Drug Administration to issue a drug safety communication about a possible increased risk of certain adverse cardiovascular events associated with varenicline.4 A subsequent meta-analysis of 14 randomised controlled trials found a significantly increased risk of adverse cardiovascular events in users of varenicline compared with placebo (odds ratio 1.72, 95% confidence interval 1.09 to 2.71), although absolute differences between the groups were small (event rate 1.06% in the varenicline group and 0.82% in the placebo group).5 In contrast, a more recent meta-analysis of randomised controlled trials found no significantly increased risk of cardiovascular events (relative risk 1.40, 0.82 to 2.39; event rate 0.63% in the varenicline group and 0.47% in the placebo group; risk difference 0.27%, −0.10 to 0.63).6 Potential mechanisms for an association between varenicline and cardiovascular events include modulation of parasymphathetic output from the brainstem to the heart, release of catecholamines, or a prothrombotic effect.5 7
An increased risk of cardiovascular events associated with varenicline would have important implications for the care of the many patients who want to stop smoking and specifically for the millions of patients who are prescribed varenicline each year. Concerns about cardiovascular risk would add to previous safety concerns regarding neuropsychiatric adverse events, as indicated by spontaneous reporting.8 The reports suggesting an increased cardiovascular risk from varenicline are based on limited data; while the randomised trial of patients with cardiovascular disease was underpowered to detect specific cardiovascular events3 and the meta-analysis was a post hoc analysis of efficacy trials and relied on a broad non-specific definitions of cardiovascular events.5 9 The meta-analysis that did not find an increased risk of cardiovascular events had relatively low power and, given the upper confidence limits, was able to exclude only an increase in risk of 140% or more.6 To date, no controlled observational studies of adverse cardiovascular events among real world varenicline users have been published. With adequate sources of data and by applying comprehensive confounder control, observational studies can provide clinically useful evidence regarding concerns about drug safety, not least because they reflect effects of drugs in real world users outside the controlled environment of clinical trials.10 Within the setting of a large nationwide registry based cohort study in Denmark, we investigated whether varenicline use was associated with increased risk of serious cardiovascular events compared with use of another drug used for smoking cessation, bupropion.
Methods
We conducted a historical prospective cohort study among participants who started treatment with varenicline or bupropion in 2007-10. The primary outcomes were the composite of any major cardiovascular event and its individual components acute coronary syndrome (myocardial infarction and unstable angina), ischaemic stroke, and cardiovascular death. The secondary outcomes were other serious cardiovascular events, the individual end points being ischaemic heart disease (including angina pectoris, ischaemic heart disease, and coronary revascularisation), heart failure, peripheral arterial disease, transient ischaemic attack, and cardiac arrhythmia. In most of the trials included in the meta-analysis that had found an increased cardiovascular risk, the treatment lasted for 12 weeks whereas the follow-up times during which cardiovascular events were recorded ranged from 24 to 52 weeks.5 Because this indicated that varenicline might also increase risk after the treatment finished, we set our primary time point of follow-up at six months after the start of treatment. Our secondary analyses included different follow-up times, ranging from six weeks to 24 months. We also carried out subgroup analyses by sex, duration of use, and in participants with and without pre-existing cardiovascular disease.
The source population was defined from the Danish Civil Registration System,11 comprising all Danish people aged ≥18 during the study period. Using the participants’ unique civil registration numbers, we linked individual level information on drug use, hospital contacts, causes of death, and potential confounders.
Users of varenicline and bupropion were identified from the National Prescription Registry.12 This nationwide registry holds information on all prescriptions filled at all Danish pharmacies from 1995, including the anatomic therapeutic chemical (ATC) code, date of filling the prescription, number of tablets, and tablet strength. We established a cohort of new users of varenicline (code N07BA03) and bupropion (code N06AX12), including those who filled a first prescription for either drug during the study period. We excluded individuals who had filled a prescription before 2007. In Denmark, bupropion is not approved for the treatment of depression. Although varenicline was marketed in Denmark in September 2006, the study did not start until 1 January 2007. This allowed the exclusion of participants who were early users as the first users of a newly marketed drug might be highly selected individuals who differ from later and more representative users of the drug.13 For study inclusion, participants had to have been registered in Denmark for at least two years.
Information on cardiovascular outcomes was obtained from the National Patient Registry.14 This nationwide registry holds information on all hospital contacts in Denmark, including all diagnoses and procedures, classified according to ICD-10 (international classification of diseases, 10th revision) and the Nordic Classification of Surgical Procedures (NCSP), respectively. Major cardiovascular events were identified from primary and secondary diagnoses registered during hospital admissions or at emergency departments. Other serious cardiovascular events were identified from registered primary diagnoses only and from records of surgical procedures. Cardiovascular deaths were identified from the Cause of Death Registry,15 which records all deaths in Denmark classified according to ICD-10. Table e1 in the appendix lists the ICD-10 and NCSP codes used for all outcomes.
The National Patient Registry has high validity in the identification of myocardial infarction, with estimated positive predictive value and sensitivity both >90% for a registered diagnosis.16 17 For ischaemic stroke, the positive predictive value has been estimated as 88-90%.17 18 19 For diagnoses of heart failure, peripheral vascular disease, transient ischaemic attack, and atrial fibrillation, the values were >90%, >90%, 60%, and >90%, respectively.17 18 20
Information on potential confounders (age, sex, place of birth and place of living; medical history and healthcare use; and use of other selected drugs) was obtained from the Civil Registration System, the National Patient Registry, and the National Prescription Registry, respectively (ICD-10 and ATC codes, table e2 in appendix). Missing values were replaced with mode imputation. The proportion of missing values was <1% for all potential confounders (table e3 in appendix).
After estimation of propensity score, users of varenicline were propensity score matched 1:1 to bupropion users,21 with greedy 5-to-1 matching technique.22 The propensity score was estimated with logistic regression, with all variables listed in tables 1and 2 , and additionally, all estimable two way interactions between demographic and healthcare use variables, included as predictors.
Table 1.
Baseline demographic characteristics* of people using varenicline and bupropion to help with tobacco use cessation in nationwide registry based cohort study in Denmark, with follow-up from January 2007 to December 2010. Figures are numbers (percentage) of participants unless stated otherwise
| Variable | Varenicline (n=17 926) | Bupropion (n=17 926) |
|---|---|---|
| Men | 8639 (48) | 8674 (48) |
| Mean (SD) age (years) | 48.4 (12.8) | 48.5 (12.8) |
| Calendar year: | ||
| 2007 | 4089 (23) | 6818 (38) |
| 2008 | 4512 (25) | 4154 (23) |
| 2009 | 4322 (24) | 3524 (20) |
| 2010 | 5003 (28) | 3430 (19) |
| Region of residence: | ||
| Greater Copenhagen | 4836 (27) | 4911 (27) |
| Zealand | 3081 (17) | 3066 (17) |
| Southern Denmark | 4851 (27) | 4795 (27) |
| Central Denmark | 3474 (19) | 3468 (19) |
| North Denmark | 1684 (9) | 1686 (9) |
| Degree of urbanisation: | ||
| Population density (inhabitants/km2): | ||
| ≤49 | 1056 (6) | 1022 (6) |
| 50-99 | 5105 (28) | 5024 (28) |
| 100-199 | 4462 (25) | 4493 (25) |
| ≥200 | 1764 (10) | 1759 (10) |
| Copenhagen suburbs | 3919 (22) | 3984 (22) |
| Copenhagen | 1620 (9) | 1644 (9) |
| Country of birth: | ||
| Denmark | 16 842 (94) | 16 847 (94) |
| Europe | 488 (3) | 462 (3) |
| Rest of world | 596 (3) | 617 (3) |
*Matched for propensity score.
Table 2.
Baseline medical characteristics* of people using varenicline and bupropion to help with tobacco use cessation in nationwide registry based cohort study in Denmark, with follow-up from January 2007 to December 2010. Figures are numbers (percentage) of participants unless stated otherwise
| Variable | Varenicline (n=17 926) | Bupropion (n=17 926) |
|---|---|---|
| Medical history†: | ||
| Acute coronary syndrome | 395 (2) | 404 (2) |
| Other ischaemic heart disease | 761 (4) | 815 (5) |
| Heart failure/cardiomyopathy | 178 (1) | 184 (1) |
| Valve disorders | 77 (<1) | 79 (<1) |
| Cardiac surgery/invasive cardiac procedure | 367 (2) | 377 (2) |
| Peripheral arterial disease | 327 (2) | 348 (2) |
| Procedure for peripheral arterial disease | 167 (1) | 166 (1) |
| Cerebrovascular disease | 548 (3) | 568 (3) |
| Arrhythmia | 333 (2) | 369 (2) |
| Renal disease | 69 (<1) | 81 (<1) |
| Chronic lung disease | 1239 (7) | 1268 (7) |
| Rheumatic disease | 237 (1) | 236 (1) |
| Venous thromboembolism | 205 (1) | 199 (1) |
| Cancer | 569 (3) | 588 (3) |
| Drugs used in past year: | ||
| β blockers | 1289 (7) | 1339 (7) |
| ARB/ACE-I | 2125 (12) | 2193 (12) |
| Calcium channel blockers | 1246 (7) | 1276 (7) |
| Diuretics | 1827 (10) | 1862 (10) |
| Nitrates | 298 (2) | 309 (2) |
| Lipid lowering drugs | 2322 (13) | 2397 (13) |
| Platelet inhibitors | 1755 (10) | 1836 (10) |
| Anticoagulants | 183 (1) | 198 (1) |
| Anti-arrhythmic drugs | 18 (<1) | 19 (<1) |
| Antidiabetic drugs | 646 (4) | 665 (4) |
| Antiobstructive pulmonary inhalants | 2650 (15) | 2714 (15) |
| Antidepressants | 2298 (13) | 2415 (13) |
| Anti-anxiety drugs | 1533 (9) | 1592 (9) |
| Corticosteroids, oral | 1074 (6) | 1160 (6) |
| NSAIDs | 4987 (28) | 5067 (28) |
| Healthcare use: | ||
| Admissions to hospital in past year: | ||
| 0 | 15 389 (86) | 15 349 (86) |
| 1-2 | 1634 (9) | 1668 (9) |
| 3-4 | 438 (2) | 456 (3) |
| ≥5 | 465 (3) | 453 (3) |
| Outpatient hospital contacts in past year: | ||
| 0 | 10 537 (59) | 10 480 (58) |
| 1-2 | 5017 (28) | 5022 (28) |
| 3-4 | 1406 (8) | 1434 (8) |
| ≥ 5 | 966 (5) | 990 (6) |
| Drugs used in past year: | ||
| 0 | 2581 (14) | 2506 (14) |
| 1-2 | 5142 (29) | 5082 (28) |
| 3-4 | 3700 (21) | 3691 (21) |
| 5-9 | 4528 (25) | 4567 (25) |
| ≥10 | 1975 (11) | 2080 (12) |
ARB=angiotensin receptor blocker; ACE-I=angiotensin converting enzyme inhibitor; NSAID=non-steroidal anti-inflammatory drug.
*Matched for propensity score.
†As registered in past 10 years.
Study participants were followed from the date of filling the first prescription for varenicline or bupropion. Treatment status was defined by the initial drug, and study participants were considered always exposed to the respective drug for the entire duration of follow-up. For the primary analyses, participants were followed until the date of censoring (death, disappearance, or emigration), end of study (31 December 2010), switching to the other drug, six months after start of treatment, or event, whichever occurred first. In the analyses of other serious cardiovascular events, an additional censoring criterion was the occurrence of a major event.
We used the Kaplan-Meier method to generate survival curves according to treatment status and compared the groups using the log rank test. We used Cox proportional hazards regression to estimate hazard ratios with 95% confidence intervals, with days since start of treatment as the time scale. The proportional hazards assumption was assessed by a Wald test for the interaction between treatment status and the underlying time scale. P values for comparisons between subgroups were similarly based on the Wald test. All statistical tests were two sided with P<0.05 indicating significance. The statistical analyses were performed with SAS software (version 9.3; SAS, Cary, NC).
Results
From a source population of 4 781 228 individuals, we identified 92 540 people who had used varenicline or bupropion in 2007-10. Among these, 14 814 participants were excluded because they started treatment before 2007 or were not registered in Denmark two years before the start of treatment. Baseline characteristics for the remaining 77 726 new users of varenicline and bupropion are shown in table e3 in the appendix. After estimation of propensity score, we were able to 1:1 match 17 926 users of varenicline to users of bupropion and hence had a total study cohort of 35 852 participants. A detailed account of the enrolment of participants is shown in figure e1 in the appendix.
Tables 1 and 2 show participants’ characteristics matched for propensity score at the time of start of treatment; the two groups were well balanced on demographic characteristics, medical history, drug use, and healthcare use.
During 16 679 person years of follow-up, we identified 117 cases of any major cardiovascular event, 72 cases of acute cardiovascular syndrome, 37 cases of ischaemic stroke, and nine cardiovascular deaths. The mean follow-up time was 168 days (SD 37) among varenicline users and 171 days (SD 34) among bupropion users. Follow-up was ended prematurely in 499 participants because of death (n=112), emigration (n=20), or switching to the other drug (n=367). The estimated median number of days covered by filled prescriptions was 28 days (interquartile range 14-60) among varenicline users and 53 days (53-53) among bupropion users. Assessment of the interaction between treatment status and the underlying time scale showed that the proportional hazards assumption was met for all primary and secondary outcomes.
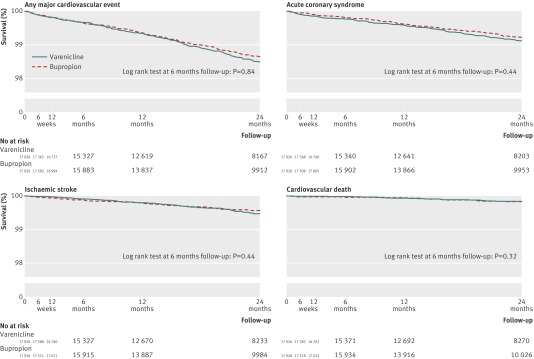
The Kaplan-Meier curves for major cardiovascular events were similar in users of varenicline and bupropion up to 24 months of follow-up after start of treatment (fig 1). The log rank tests showed no differences between users of varenicline and bupropion for any major cardiovascular event (P=0.84), acute coronary syndrome (P=0.44), ischaemic stroke (P=0.44), or cardiovascular death (P=0.32) at the primary time point of follow-up (six months).
Fig 1 Kaplan-Meier curves of major cardiovascular events among users of varenicline and bupropion. Major cardiovascular event was defined as any of acute coronary syndrome, ischaemic stroke, or cardiovascular death. Primary analysis was major cardiovascular events at six months
Table 3 shows the hazard ratios for major cardiovascular events in users of varenicline and bupropion. The incidence rate for any major cardiovascular event per 1000 person years was 6.9 among varenicline users and 7.1 among bupropion users. Use of varenicline was not associated with an increased risk of the composite endpoint of any major cardiovascular event (hazard ratio 0.96, 95% confidence interval 0.67 to 1.39) compared with bupropion use. Similarly, there were no significant associations between use of varenicline and any of the individual major cardiovascular events: acute coronary syndrome (1.20, 0.75 to 1.91), ischaemic stroke (0.77, 0.40 to 1.48), and cardiovascular death (0.51, 0.13 to 2.02).
Table 3.
Risk of major cardiovascular events* at six months’ follow-up in people using varenicline and bupropion to help with tobacco use cessation in nationwide registry based cohort study in Denmark, with follow-up from January 2007 to December 2010
| Outcome event† | Person years | Events | Rate/1000 person years | Hazard ratio (95% CI) |
|---|---|---|---|---|
| Any major cardiovascular event‡ | ||||
| Varenicline | 8268 | 57 | 6.9 | 0.96 (0.67 to 1.39) |
| Bupropion | 8411 | 60 | 7.1 | 1 (ref) |
| Acute coronary syndrome | ||||
| Varenicline | 8270 | 39 | 4.7 | 1.20 (0.75 to 1.91) |
| Bupropion | 8416 | 33 | 3.9 | 1 (ref) |
| Ischaemic stroke | ||||
| Varenicline | 8278 | 16 | 1.9 | 0.77 (0.40 to 1.48) |
| Bupropion | 8419 | 21 | 2.5 | 1 (ref) |
| Cardiovascular death§ | ||||
| Varenicline | 8281 | 3 | 0.4 | 0.51 (0.13 to 2.02) |
| Bupropion | 8425 | 6 | 0.7 | 1 (ref) |
*Matched for propensity score including all variables listed in table 1 and all estimable two way interactions between demographic and healthcare use variables. Study cohort included 35 858 patients, with new users of varenicline and bupropion matched 1:1 on propensity score and followed up to six months after start of treatment. Outcomes reported here were defined as primary outcomes.
†Ascertained with data from National Patient Registry (inpatient admissions and emergency department visits) and National Cause of Death Registry.
‡Any of acute coronary syndrome, ischaemic stroke, or cardiovascular death.
§Includes cardiac death and death from ischaemic stroke.
Table 4 shows the hazard ratios for other serious cardiovascular in users of varenicline and bupropion. Varenicline use was not significantly associated with ischaemic heart disease (0.89, 0.66 to 1.20), heart failure (0.82, 0.39 to 1.70), peripheral arterial disease (1.11, 0.81 to 1.54), transient ischaemic attack (1.60, 0.62 to 4.13), or cardiac arrhythmia (0.64, 0.36 to 1.11).
Table 4.
Risk of serious cardiovascular events* (other than major cardiovascular events) at six months follow-up in people using varenicline and bupropion to help with tobacco use cessation in nationwide registry based cohort study in Denmark, with follow-up from January 2007 to December 2010
| Outcome event† | Person years | Events | Rate/1000 person years | Hazard ratio (95% CI) |
|---|---|---|---|---|
| Angina/ischaemic heart disease‡ | ||||
| Varenicline | 8247 | 82 | 9.9 | 0.89 (0.66 to 1.20) |
| Bupropion | 8386 | 93 | 11.1 | 1 (ref) |
| Heart failure | ||||
| Varenicline | 8264 | 13 | 1.6 | 0.82 (0.39 to 1.70) |
| Bupropion | 8405 | 16 | 1.9 | 1 (ref) |
| Peripheral arterial disease§ | ||||
| Varenicline | 8247 | 78 | 9.5 | 1.11 (0.81 to 1.54) |
| Bupropion | 8392 | 71 | 8.5 | 1 (ref) |
| Transient ischaemic attack | ||||
| Varenicline | 8265 | 11 | 1.3 | 1.60 (0.62 to 4.13) |
| Bupropion | 8409 | 7 | 0.8 | 1 (ref) |
| Cardiac arrhythmia¶ | ||||
| Varenicline | 8263 | 20 | 2.4 | 0.64 (0.36 to 1.11) |
| Bupropion | 8404 | 32 | 3.8 | 1 (ref) |
*Matched for propensity score including all variables listed in table 1 and all estimable two way interactions between demographic and healthcare use variables. Study cohort included 35 858 patients, with new users of varenicline and bupropion matched 1:1 on propensity score and followed up to six months after start of treatment. Outcomes reported here were defined as secondary outcomes.
†Ascertained with data from National Patient Registry (inpatient admissions and emergency department visits).
‡Defined as diagnosis of angina/ischaemic heart disease or admission for coronary artery bypass grafting or percutaneous coronary intervention.
§Defined as diagnosis of peripheral arterial disease or procedure to treat peripheral arterial disease.
¶Includes atrial and ventricular arrhythmias but not conduction block.
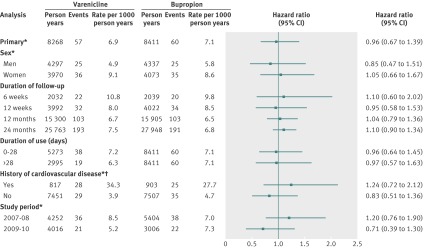
Figure 2 shows additional analyses for any major cardiovascular event in different subgroups of patients and at different time points of follow-up. The risk associated with varenicline compared with bupropion was similar in men and women (P=0.57). The risk of major cardiovascular events was similar independently of the duration of follow-up; use of varenicline was not associated with increased risk of any major cardiovascular event within six weeks (hazard ratio 1.10, 95% confidence interval 0.60 to 2.02), 12 weeks (0.95, 0.58 to 1.53), 12 months (1.04, 0.79 to 1.36), or 24 months (1.10, 0.90 to 1.34). Duration of varenicline use, estimated by filled prescriptions, did not alter the risk of any major cardiovascular event (0.96 (0.64 to 1.45) among those with 0 to 28 days of use; 0.97, (0.57 to 1.63) among those with >28 days of use; P=0.99) Overall, participants with a history of cardiovascular disease had much higher rates of any major cardiovascular event than participants with no such history. The risk of any major cardiovascular event associated with use of varenicline was not significantly different between participants with (1.24, 0.72 to 2.12) and without (0.83, 0.51 to 1.36) a history of cardiovascular disease (P=0.29). Finally, the risk of any major cardiovascular event associated with varenicline did not differ significantly according to study period (1.20, (0.76 to 1.90) for years 2007-08; 0.71 (0.39 to 1.30) for years 2009-10; P=0.17).
Fig 2 Additional analyses of association between varenicline and risk of major cardiovascular events compared with bupropion. Major cardiovascular event was defined as any of acute coronary syndrome, ischaemic stroke, or cardiovascular death. *Six months’ follow-up. †Includes acute coronary syndrome, other ischaemic heart disease, cardiac surgery/invasive cardiac procedure, heart failure/cardiomyopathy, peripheral arterial disease or procedure to treat this condition, and cerebrovascular disease
In sensitivity analysis, we excluded participants with missing values in a complete case analysis for any major cardiovascular event; restriction to complete cases had no impact on the result (hazard ratio 0.96, 95% confidence interval 0.67 to 1.39). To examine the impact of the potential confounders after matching, each of the potential confounders was separately included as adjustment factors in an analysis for any major cardiovascular event. The change in estimate was small (<2.5%) for all potential confounders (see table e4 in appendix).
Discussion
In this large nationwide cohort study, compared with bupropion, use of varenicline to help in smoking cessation was not associated with an increased risk of the composite outcome of any major cardiovascular event and its components acute coronary syndrome, ischaemic stroke, and cardiovascular death. There was no increased risk of major cardiovascular events at any time point of follow-up, which included analyses from six weeks to 24 months after the start of treatment. Additionally, there was no significantly increased risk of any major cardiovascular event in a subgroup of participants with a history of cardiovascular disease. Furthermore, there was no significantly increased risk of other serious cardiovascular events evaluated as secondary outcomes. These results contrast with a recent meta-analysis of randomised controlled trials that reported a 72% increased risk of serious cardiovascular events among users of varenicline compared with placebo.5 There are several possible reasons for the different results in our study and the meta-analysis. Firstly, nearly all the trials in the meta-analysis were not primarily designed to investigate the risk of cardiovascular events. In particular, all but one of the 14 included trials lacked a specific definition of cardiovascular events and the meta-analysis relied on a non-standard post hoc definition of cardiovascular outcomes.5 9 Furthermore, along with the lack of clarity regarding the method used for the quantification of risk,23 24 25 the meta-analysis was limited by shortcomings of individual trials, such as higher drop out rates in patients receiving placebo.26 27 Finally, the participants included in the trials were largely selected (for example, most trials excluded patients with cardiovascular disease) and might have differed from the population of real world tobacco users included in our study. A trial specifically examining the cardiovascular safety and efficacy of varenicline in patients with stable cardiovascular disease found a small excess of individual cardiovascular events (non-fatal myocardial infarction (difference between groups 1.1%, 95% confidence interval −0.6 to 2.9; total 10 events), need for coronary revascularisation (difference between groups 1.4%, −0.4 to 3.2; total 11 events), and peripheral vascular disease (difference between groups 0.6%, −1.0 to 2.1; total eight events)); there were no significant differences, but the sample size was limited to 353 participants exposed to varenicline and 350 exposed to placebo.3 Another recently published meta-analysis of data from randomised controlled trials found no increased risk of serious cardiovascular events, including a broad range of ischaemic and arrhythmic cardiovascular events; while the absolute differences in event rates were small, the study was able to exclude only a 2.4-fold increase in the relative risk (relative risk 1.40, 95% confidence interval 0.82 to 2.39).6 Our report expands on the available knowledge on the cardiovascular safety of varenicline by providing the first large scale population based observational study with detailed analyses of specific cardiovascular outcomes, individual level data of high completeness, and timing of events. Our study had a much higher number of participants exposed to varenicline than the meta-analyses (17 926 in our study compared with 4908 in the meta-analysis by Singh et al5 and 5431 in the meta-analysis of Prochaska and Hilton6). Our study also included by far the highest number of cardiovascular events; with a broad definition of cardiovascular events, including myocardial infarction, unstable angina, coronary artery disease, arrhythmias, transient ischaemic attacks, stroke, sudden death or cardiovascular related death, or heart failure, the meta-analyses included 52 events (follow-up 24-52 weeks)5 and 34 events (median follow-up 16 weeks).6 It contrast, for our primary outcome, we used a specific definition of any major cardiovascular event that included acute coronary syndrome, ischaemic stroke, and cardiovascular death and identified 57 events in participants exposed to varenicline during six months of follow-up. The meta-analyses were unable to analyse specific events, whereas we additionally estimated hazard ratios for the three specific events and for an additional five serious cardiovascular events defined as secondary outcomes. Because of the large sample size, the study had sufficient power to exclude a 40% relative increase in the risk of any major cardiovascular event associated with varenicline use. Whereas the estimates were less precise for the specific events, it should be noted that any differences between the groups would probably be small in absolute terms and, although not examined in this study, would be outweighed by the risk conferred by continuing smoking.
Strengths and weaknesses
This study has several strengths. Firstly, we identified use of varenicline and bupropion from a comprehensive nationwide registry on filled prescriptions, which improved the precision on timing of use and thus reduced the potential for treatment misclassification. The comparative head to head design, with bupropion as the reference, allowed us to compare varenicline with a drug with the same indication and no known cardiovascular risk.28 29 30 31 32 In pharmacoepidemiology, a study design that compares two active treatment groups has several advantages compared with designs with non-users as the comparison group—for example, it reduces the potential of selection bias can reduce confounding by unmeasured baseline characteristics.33 34 To further reduce the potential for confounding, users of varenicline and bupropion were matched on the individual propensity for starting treatment with varenicline. The propensity scores were derived from a non-parsimonious model including a large number of potential confounders. The propensity score matching yielded two well balanced treatment groups and removed potentially influential participants with no comparable controls.21 The rate of 6.9 major cardiovascular events per 1000 person years among varenicline users was similar to the rate of acute myocardial infarction and stroke reported in a population based study in the United Kingdom among people who started nicotine replacement therapy (incidence rate 7.1 per 1000 person years).35 We also recognise limitations to this study. The filling of the first prescription was taken to represent the actual start of treatment. If varenicline were in fact associated with an increased risk of cardiovascular events, non-adherence to treatment would bias the results toward the null. Furthermore, the study outcomes were derived from hospital data and therefore focused on serious events treated in a hospital setting. For the primary outcomes, major cardiovascular events, use of data from the National Patient Registry for the ascertainment of cases is known to be sensitive and specific.16 17 18 19 For individual secondary outcomes (such as some cardiac arrhythmias and transient ischaemic attack), however, validity and sensitivity might be limited. Additionally, despite propensity matching, we cannot rule out residual confounding from unmeasured baseline differences in health between the groups. We had no data on smoking; differences between groups in smoking cessation rates during follow-up might possibly balance out adverse effects of varenicline, although this is unlikely at six months’ follow-up given that beneficial effects from smoking cessation take time to develop.36 37 38 Differences in smoking intensity at baseline might have influenced results. Because evidence indicates that varenicline is more efficacious than bupropion,2 it might have been prescribed to patients who had more intense tobacco exposure, were more addicted, or had tried other treatments in the past (although not bupropion because of the new user study design). If so, patients exposed to varenicline would have had a higher baseline risk of cardiovascular events, thus introducing a bias towards increased risk associated with varenicline. On the other hand, this might be less likely because prescription of varenicline was much more common than bupropion throughout the study period. Finally, the median length of time covered by varenicline prescriptions was 28 days, which is markedly less than the recommended duration of treatment of 12 weeks; if any cardiovascular effects of varenicline depended on cumulative dose, our results might not be directly comparable with trial data, in which 12 week treatment regimens were used. On the other hand, shorter than recommended duration of treatment might be more representative of real world use of varenicline, possibly reflecting the fact that a considerable proportion of patients do not succeed in stopping smoking and therefore do not refill prescriptions; a similar average duration of treatment was observed in a previous observational study.39
In conclusion, this large nationwide cohort study found no significantly increased risk of any major cardiovascular event, including acute coronary syndrome, ischaemic stroke, and cardiovascular death, or other serious cardiovascular events associated with use of varenicline compared with bupropion.
What is already known on this topic
Varenicline is used to help people with smoking cessation
Recent studies, including a meta-analysis of randomised trials, have indicated that varenicline could be associated with increased risk of cardiovascular events, though another meta-analysis of randomised trials reported contradictory findings
What this study adds
This large register based study investigated the risk of cardiovascular events in a population based cohort of real world varenicline users
Compared with users of bupropion, another drug used for smoking cessation, varenicline users were not at increased risk of serious cardiovascular events
Contributors: All authors contributed to conception and design of the study, the analysis and interpretation of the study results, and critically revised the manuscript. HS and BP drafted the manuscript. HS conducted the statistical analyses and acquired the data. AH supervised the study and is guarantor. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript for submission.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the Danish Data Protection Agency. Ethical approval is not required for register based research in Denmark.
Data sharing: No additional data available.
Cite this as: BMJ 2012;345:e7176
Web Extra. Extra material supplied by the author
Appendix: Supplementary tables and figure [posted as supplied by author]
References
- 1.WHO report on the global tobacco epidemic. World Health Organization, 2009. http://whqlibdoc.who.int/publications/2009/9789241563918_eng_full.pdf.
- 2.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2011;2:CD006103. [DOI] [PubMed] [Google Scholar]
- 3.Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010;121:221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA Drug Safety Communication: Chantix (varenicline) may increase the risk of certain cardiovascular adverse events in patients with cardiovascular disease. Issued 22 July 2011. www.fda.gov/Drugs/DrugSafety/ucm264436.htm.
- 5.Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ 2011;183:1359-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prochaska J, Hilton J. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ 2012;344:e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison-Woolrych M, Maggo S, Tan M, Savage R, Ashton J. Cardiovascular events in patients taking varenicline: a case series from intensive postmarketing surveillance in New Zealand. Drug Saf 2012;35:33-43. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Information for healthcare professionals: varenicline (marketed as Chantix) and bupropion (marketed as Zyban, Wellbutrin, and generics). www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa-tientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.
- 9.Samuels L. Varenicline: cardiovascular safety. CMAJ 2011;183:1407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 2011;90:777-90. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39(7 suppl):22-5. [DOI] [PubMed] [Google Scholar]
- 12.Kildemoes HW, Sorensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39(7 suppl):38-41. [DOI] [PubMed] [Google Scholar]
- 13.Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 2011;90:777-90. [DOI] [PubMed] [Google Scholar]
- 14.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7 suppl):30-3. [DOI] [PubMed] [Google Scholar]
- 15.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39(7 suppl):26-9. [DOI] [PubMed] [Google Scholar]
- 16.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol 2003;56:124-30. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsen SP, Overvad K, Sorensen HT, Tjonneland A, Husted SE. Predictive value of stroke and transient ischemic attack discharge diagnoses in the Danish National Registry of Patients. J Clin Epidemiol 2002;55:602-7. [DOI] [PubMed] [Google Scholar]
- 19.Krarup LH, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology 2007;28:150-4. [DOI] [PubMed] [Google Scholar]
- 20.Frost L, Vestergaard P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med 2004;164:1993-8. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parson LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Proceedings of the 26th Annual SAS Users Group International Conference. SAS, 2001;214-6.
- 23.Alper BS. Varenicline: quantifying the risk. CMAJ 2011;183:1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squire EN. Varenicline: quantifying the risk. CMAJ 2011;183:1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi H, Umemoto T. Varenicline: quantifying the risk. CMAJ 2011;183:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency confirms positive benefit-risk balance for Champix. Benefits as a smoking cessation medicine outweigh slight reported increase in cardiovascular events. Issued 21 July 2011. www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/07/news_detail_001314.jsp&mid=WC0b01ac058004d5c1&jsenabled=true.
- 27.Brophy JM. ACP Journal Club. Review: varenicline increases risk for serious adverse cardiovascular events in tobacco users. Ann Intern Med 2011;155:JC4-5. [DOI] [PubMed] [Google Scholar]
- 28.Beyens MN, Guy C, Mounier G, Laporte S, Ollagnier M. Serious adverse reactions of bupropion for smoking cessation: analysis of the French Pharmacovigilance Database from 2001 to 2004. Drug Saf 2008;31:1017-26. [DOI] [PubMed] [Google Scholar]
- 29.Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 2011;20:903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med 2011;171:1055-60. [DOI] [PubMed] [Google Scholar]
- 31.Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med 2006;119:1080-7. [DOI] [PubMed] [Google Scholar]
- 32.Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J 2003;24:946-55. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf 2010;19:858-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturmer T, Jonsson FM, Poole C, Brookhart MA. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology 2011;22:298-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard R, Lewis S, Smith C, Godfrey C, Smeeth L, Farrington P, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control 2005;14:416-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med 2002;137:494-500. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg L, Palmer JR, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. N Engl J Med 1990;322:213-7. [DOI] [PubMed] [Google Scholar]
- 38.Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 2006;368:647-58. [DOI] [PubMed] [Google Scholar]
- 39.Harrison-Woolrych M, Ashton J. Utilization of the smoking cessation medicine varenicline: an intensive post-marketing study in New Zealand. Pharmacoepidemiol Drug Saf 2010;19:949-53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Supplementary tables and figure [posted as supplied by author]