Abstract
Objectives
The aim of this study was to identify the molecular mechanisms underlying high-fat and high-cholesterol (HFC) diet-induced steatohepatitis and associated liver fibrosis progression in a novel stroke-prone, spontaneously hypertensive 5/Dmcr (SHRSP5/Dmcr) rat model.
Methods
SHRSP5/Dmcr rats were given the control or HFC-diet for 2, 8, and 16 weeks. Plasma and hepatic gene expression of key molecules involved in fatty acid oxidation, inflammation, oxidative stress, and fibrosis were subsequently analyzed.
Results
Rats fed the HFC-diet showed increased plasma tumor necrosis factor-α (TNF-α) and hepatic p50/p65 signals, but reduced hepatic Cu2+/Zn2+-superoxide dismutase across the treatment period and reduced plasma total adiponectin at 8 weeks. In HFC-diet-fed rats, transforming growth factor-β1 (TGF-β1) was elevated prior to the appearance of obvious liver fibrosis pathology at 2 weeks, followed by elevations in platelet-derived growth factor-B (PDGF-B) and α-smooth muscle actin (α-SMA), corresponding to evident liver fibrosis, at 8 weeks and by α1 type I collagen production at 16 weeks. The HFC-diet increased hepatic total cholesterol accumulation, although hepatic triglyceride declined by 0.3-fold from 2 to 16 weeks due to reduced hepatic triglyceride synthesis, as suggested by the diacylglycerol acyltransferase 1 and 2 measurements.
Conclusions
TNF-α and p50/p65 molecular signals appeared to be major factors for HFC-diet-induced hepatic inflammation and oxidative stress facilitating liver disease progression. While the up-regulation of TGF-β1 prior to the appearance of any evident liver fibrosis could be an early signal for progressive liver fibrosis, elevated PDGF-B and α-SMA levels signified evident liver fibrosis at 8 weeks, and subsequent increased α1 type I collagen production and reduced triglyceride synthesis indicated extensive liver fibrosis at 16 weeks in this novel SHRSP5/Dmcr model.
Electronic supplementary material
The online version of this article (doi:10.1007/s12199-012-0273-y) contains supplementary material, which is available to authorized users.
Keywords: Steatohepatitis, Inflammation, TNF-α, NF-κb, Fibrosis
Introduction
The number of patients with fatty liver, nonalcoholic steatohepatitis (NASH) and associated fibrosis and cirrhosis, generically referred to as nonalcoholic fatty liver disease (NAFLD), is increasing worldwide due to excess fat intake coupled with less physical activity, both of which are hallmarks of modern-day lifestyle [1–4]. While fat infiltration of hepatocytes is the common characteristic of all stages of NAFLD, including NASH and associated liver fibrosis, the biochemical mechanisms underlying disease progression in humans have not been fully established [2]. Research on these mechanisms is constrained by ethical issues with respect to repeated liver biopsies and by a limited ability to delineate cause and effect from complex interactive metabolic disease pathogenesis, with the results that it remains difficult to distinguish NASH within NAFLD patients simply by clinical laboratory assessment [4, 5].
While various animal models with genetic, chemically induced (e.g., carbon tetrachloride), or dietary [e.g., methionine and choline-deficient (MCD) diet] alterations show progression to the inflammatory condition of NASH [5], only a few rodent models appear to mimic the NASH pathology and the over-nutritional metabolic abnormality contexts of disease progression relevant to patients with liver diseases [5, 6]. In addition, many animal studies only characterize a limited experimental condition without sequentially following a time-course of liver disease progression in detail. Indeed, these problems are partly due to the lack of good animal models with dietary-induced NASH and associated liver fibrosis that would allow a time-course evaluation of liver disease progression within a practically short period.
In an earlier publication, we reported the development of the stroke-prone spontaneously hypertensive 5/Dmcr rat (SHRSP5/Dmcr) strain, historically called arteriolipidosis-prone rats. This rat is registered at the National BioResource Project for the Rat in Japan [7] and represents the fifth substrain of the original SHRSP rat descended from the normotensive Wistar-Kyoto rat in Japan [8]. During an investigation of the effects of a high-fat and high-cholesterol-containing (HFC) diet on arteriosclerosis rats, carried out within the framework of a nutritional study, we incidentally found that the fibrotic liver of the SHRSP5/Dm rat showed extensive enlargement and a whitish color, with extensive lipid accumulation [8]. The HFC-diet-induced steatohepatitis and liver fibrosis in the SHRSP5/Dmcr rat reflected pathological features closely resembling the steatohepatitis and liver fibrosis progression in patients despite the absence of apparent hyperglycemia, insulin resistance, and obesity [8]. Based on these pathological findings, we expected to find perturbed lipid metabolism and increased inflammatory and pro-fibrogenic reactions when these rats were fed the HFC-diet.
In the initial study reported here, we focused on revealing how a few key underlying molecular mechanisms, particularly the genes associated with peroxisome proliferator-activated receptor α (PPARα), tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), and platelet-derived growth factor-B (PDGF-B), would change at each stage of liver disease progression as bland steatosis advanced through progressive inflammatory, oxidative stress, and fibrotic conditions, in this newly recognized SHRSP5/Dmcr model. Our findings indicate rather dynamic interplays and simultaneous changes in the biochemical balance of the liver and the roles played by each gene associated with hepatic inflammation, fibrosis, and fatty acid oxidation in this novel SHRSP5/Dmcr model.
Materials and methods
Animals
This animal study was approved by the Committee of Animal Experimentation (Approval No. 10) and conducted at Kinjo Gakuin University in accordance with its Animal Experiment Guidelines [8]. We obtained 30 male rat offspring at the age of 10 weeks that were used throughout our experiment; mating and housing has been described elsewhere [8]. They were provided with stroke-prone control chow (SP-diet) (Funabashi Farm, Chiba, Japan) [9] and water ad libitum. The nutrient components (weight %) of the SP-diet and the HFC-diet have been described earlier [8]. At 10 weeks of age, 30 male rats were randomized to six groups, of which three groups were fed SP-diet (control) and the remaining groups were fed HFC-diet for 2, 8, and 16 weeks, respectively. During the feeding period, two of the five rats in the HFC-diet group died at 16 weeks (n = 3), due possibly to spontaneous stroke of SHRSP5/Dmcr rats [7]. At each time point, blood was collected from the abdominal aorta via a syringe with the rat under pentobarbital anesthesia and directed into chilled tubes; the plasma was separated by centrifugation at 3,000 rpm for 10 min. All animals in each group were then sacrificed under pentobarbital anesthesia and the livers quickly removed and weighed. A small liver section was fixed in 4% buffered paraformaldehyde. The remaining liver tissue and plasma were stored at −80°C until use [8].
Biochemical measurements in plasma and liver
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured by the colorimetric method using a Transaminase C II Test kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma triglyceride and total cholesterol were quantified by the TG E and TC E WAKO kit, and liver triglyceride and total cholesterol concentrations were obtained from aliquots of lipid extracts prepared by the Folch method [10]. Plasma total adiponectin concentration was determined by an enzyme-linked immunosorbent assay (ELISA) kit (Otsuka Pharmaceutical, Tokyo, Japan). Similarly, plasma TNF-α concentrations were quantitatively measured by the Quantikine® ELISA kit (R&D Systems, Minneapolis, MN).
Preparation of liver homogenate and nuclear fraction
Sections of whole rat liver samples were homogenized with threefold (vol/wt) 10 mM phosphate buffer (pH 7.4) containing 0.25 M sucrose. The nuclear fraction from the frozen liver of each rat was prepared using a CelLyticTM NuCLEARTM Extraction kit (Sigma Aldrich, St. Louis, MO). Protein concentrations of the whole liver homogenate and nuclear fraction were measured using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) [11, 12].
Western blot analyses
Samples of whole liver homogenate were subjected to 10 or 12.5% polyacrylamide gel electrophoresis as described elsewhere [11–14]. The primary polyclonal antibodies against adiponectin receptor 1 (AdipoR1) and 2 (AdipoR2) (Alpha Diagnostic International, San Antonio, TX), or α-smooth muscle actin (α-SMA) (Sigma Aldrich) were purchased from the source shown herein. The band was analyzed by densitometry, using the Lane and Spot Analyzer ver. 5.0 (ATTO Corp, Tokyo, Japan). Proteins measured from the whole liver tissue homogenate or nuclear fraction were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or histone H1, respectively, within the same gel preparation.
Quantitative real-time PCR
mRNA concentration monitoring, total RNA isolation, and complementary DNA synthesis, as well as the PCR primer mixture and PCR amplification cycle steps were as described elsewhere [11]. The primers were designed by using Primer Express software (Applied Biosystems, Foster City, CA), based on the sequence of accession and GI numbers shown in Electronic Supplementary Material (ESM) Table 1. Each mRNA level was normalized to the level of GAPDH mRNA expression in the same preparation [11, 12].
Statistical analysis
Results are expressed as a mean ratio value ± standard deviation (SD) to show the variance of original data. The Wilcoxon rank-sum test with the two-sided exact-test [15, 16] was performed at each time point of 2, 8, and 16 weeks (Windows SAS software ver. 8.2; SAS Institute, Cary, NC) in order to statistically and precisely test the difference between the means of two diet treatments (SP- and HFC-diet) for the plasma, Western blot, and mRNA data at a significance of p < 0.05.
Results
Body and liver weight
Body weight was not statistically significantly different between the two diet groups at each treatment period (Table 1). Liver weight and ratio of liver and body weight did not change in the SP-diet group throughout the treatment period, whereas it increased significantly during each of the treatment periods (2, 8, and 16 weeks) in the HFC-diet group.
Table 1.
Effects of high-fat and cholesterol-containing diet on body and liver weight and on biochemical characteristics in plasma and liver at each treatment period
| Diet treatment group: | SP-diet group | HFC-diet group | ||||
|---|---|---|---|---|---|---|
| Treatment period: | 2 weeks | 8 weeks | 16 weeks | 2 weeks | 8 weeks | 16 weeks |
| Body and liver weight | ||||||
| Body weight (g) | 224 ± 17 | 256 ± 13 | 271 ± 20 | 200 ± 25 | 237 ± 15 | 260 ± 39 |
| Liver weight (g) | 6.4 ± 0.5 | 6.6 ± 0.3 | 7.0 ± 0.4 | 8.6 ± 0.7** | 23.1 ± 2.4** | 31.1 ± 2.6** |
| Liver weight/body weight ratio | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.04 ± 0.005** | 0.10 ± 0.008** | 0.12 ± 0.009** |
| Plasma measurements | ||||||
| Triglyceride (mg/dl) | 53.9 ± 20.2 | 41.4 ± 7.1 | 72.6 ± 18.0 | 71.4 ± 22.9 | 59.1 ± 11.7* | 366.2 ± 276.6* |
| Total cholesterol (mg/dl) | 29.7 ± 7.4 | 45.1 ± 7.6 | 47.1 ± 12.6 | 135.3 ± 57.0** | 112.6 ± 20.6** | 213.0 ± 116.8* |
| ALT (IU/l) | 16.7 ± 1.4 | 15.5 ± 1.4 | 23.3 ± 4.5 | 28.7 ± 3.8* | 35.5 ± 3.5** | 103.4 ± 59.1* |
| AST (IU/l) | 58.2 ± 12.3 | 101.6 ± 11.6 | 103.2 ± 25.9 | 68.8 ± 10.4 | 138.5 ± 35.6 | 245.5 ± 117.4* |
| Total adiponectin (μg/ml) | 5.4 ± 1.0 | 5.8 ± 0.6 | 6.1 ± 0.9 | 7.1 ± 0.8* | 4.7 ± 0.4* | 5.7 ± 1.1 |
| TNF-α (pg/ml) | 1.7 ± 0.3 | 2.4 ± 0.6 | 2.5 ± 1.6 | 4.7 ± 1.9* | 19.1 ± 7.8* | 13.7 ± 1.0* |
| Liver measurements: | ||||||
| Triglyceride (mg/g liver) | 13.4 ± 3.9 | 23.8 ± 18.2 | 17.2 ± 3.9 | 47.8 ± 5.7** | 31.8 ± 3.5 | 12.5 ± 4.3 |
| Total cholesterol (mg/g liver) | 2.3 ± 0.5 | 1.7 ± 0.5 | 1.6 ± 0.1 | 100.2 ± 6.6** | 170.0 ± 12.9** | 170.8 ± 24.6* |
Data are presented as the mean value ± standard deviation (SD) for each group
SP-diet, Stroke-prone control chow; HFC-diet, high-fat and high-cholesterol-containing diet; ALT alanine aminotransferase; AST, alanine aminotransferase; TNF-α, tumor necrosis factor-α
*p < 0.05, **p < 0.01; compared with the SP-diet group within each diet treatment period
Plasma and hepatic lipid levels and plasma aminotransferase activities
To evaluate whether there was a state of lipid accumulation and metabolism, we measured triglyceride and total cholesterol content in the plasma and liver tissue. The plasma triglyceride levels at 8 and 16 weeks in the HFC-diet group were greater than those in the SP-diet group during the entire treatment period. There was a considerable elevation at 16 weeks (a 5.1-fold change in the mean from the 2-week treatment period), although the inter-individual variance contributed largely at 16 weeks. Liver triglyceride content at 2 weeks was significantly greater in the HFC-diet group than in the SP-diet group, although it declined by 0.3-fold from 2 to 16 weeks.
Plasma total cholesterol concentration was significantly higher in the HFC-diet group than in the SP-diet group across the entire treatment period, with a 1.6-fold rise from 2 to 16 weeks. Hepatic total cholesterol content in the HFC-diet group was also significantly greater than that in the SP-diet group across the treatment period, exhibiting a 1.7-fold change from 2 to 16 weeks.
To evaluate a state of liver injury, we measured typical clinical markers of plasma ALT and AST values. For plasma ALT, mean values in the HFC-diet group were significantly different from those of the SP-diet group throughout the treatment period, with a 3.6-fold change from 2 to 16 weeks over time. Similarly, for plasma AST, a statistically significant difference was observed at 16 weeks with a 3.6-fold change from 2 to 16 weeks.
TNF-α, nuclear factor-κB, and oxidative stress responses
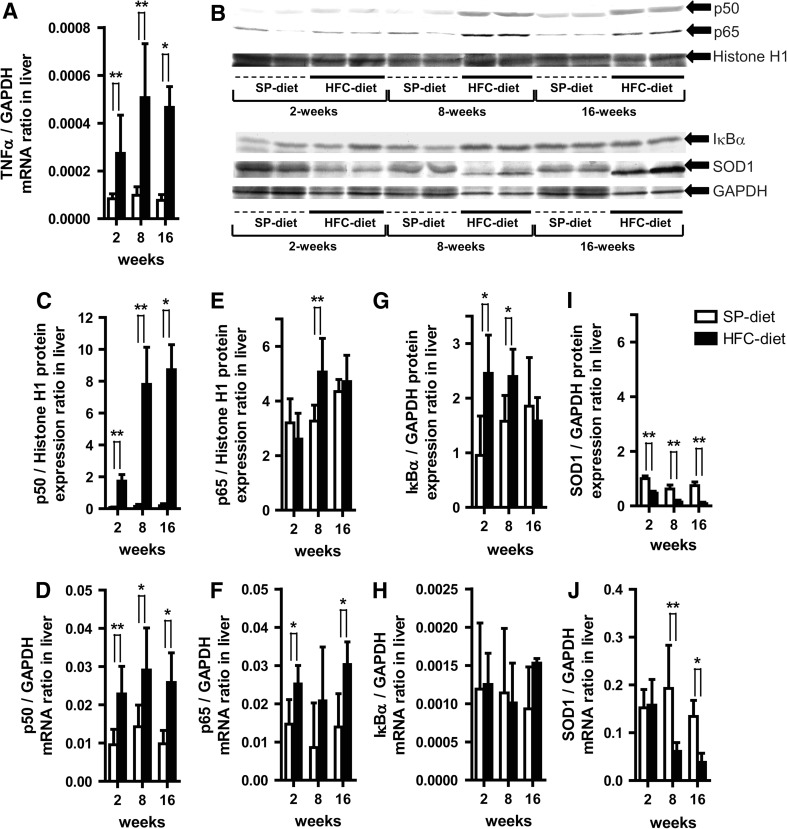
In order to evaluate response of inflammatory cytokine and association with total adiponectin, we measured TNF-α levels in the plasma and liver. Plasma TNF-α values in the HFC-diet group showed a convex time-course profile, being significantly greater than those in the SP-diet group and with a pronounced peak at 8 weeks, which was a 4.1-fold change from the mean value of the HFC-diet group at 2 weeks. Hepatic TNF-α protein expression did not show any statistically significant differences between the diet groups at each treatment period (data not shown) because of the possibility that it may be released into the blood circulation. In contrast, hepatic TNF-α mRNA expression in the HFC-diet group was significantly up-regulated at each treatment period (Fig. 1a) and exhibited an identical convex time-course profile to plasma TNF-α concentration in the HFC-diet group.
Fig. 1.
Effects of a high-fat and high-cholesterol-containing diet (HFC-diet) on hepatic tumor necrosis factor-α (TNF-α), nuclear factor κB (p50/p65), inhibitor of κBα (IκBα), Cu2+/Zn2+-superoxide dismutase (SOD1) protein and their mRNA expressions. a TNF-α mRNA expression ratio. b Western blot results of respective protein expressions in whole liver tissue homogenates or nuclear fractions. c, d Hepatic p50 protein (c) and mRNA expression (d) ratios. e, f Hepatic p65 protein (e) and mRNA expression (f) ratios. g, h Hepatic IκBα protein (g) and mRNA expression (h) ratios. i, j Hepatic SOD1 protein (i) and mRNA expression (j) ratios. Each histogram represents a mean ratio ± standard deviation (SD). *p < 0.05, **p < 0.01, compared with the stroke-prone control chow-fed diet (SP-diet) group within each diet–treatment period. GAPDH Glyceraldehyde-3-phosphate dehydrogenase
To evaluate nuclear factor κB (NF-κB) inflammatory signals in the liver, we analyzed the levels of p50, p65 and the inhibitor of κBα (IκBα) protein and their respective mRNA expression. Hepatic p50 protein and mRNA expression in the HFC-diet group were significantly up-regulated across the treatment period relative to those in the SP-diet group (Fig. 1b, c, d). Moreover, p65 protein expression in the HFC-diet group showed a significant 1.6-fold up-regulation at 8 weeks (Fig. 1e) while its mRNA expression was also significantly higher (1.7-, 2.4- and 2.2-fold at each treatment period) than that in the SP-diet group at 2 and 16 weeks (Fig. 1f). Furthermore, IκBα protein expression in the HFC-diet group was significantly up-regulated at 2 and 8 weeks and declined by 0.9-fold at 16 weeks (Fig. 1g). There was no significant difference in the expression of IκBα mRNA between HFC-diet and SP-diet groups (Fig. 1h).
To evaluate hepatic oxidative stress in relation to PPAR expression [17], we measured Cu2+/Zn2+-superoxide dismutase (SOD1) protein and mRNA expression. In the HFC-diet group, SOD1 protein was significantly down-regulated across the 2, 8, and 16 weeks of the study (Fig. 1i), while its mRNA expression was also significantly reduced from 8 weeks onwards over the 16-week period (Fig. 1j). This results suggested a decrease in anti-oxidative stress within the liver because SOD1 is known to catalyze the dismutation of superoxide radicals produced from the biological oxidation process and environmental stresses [17].
Plasma adiponectin and hepatic adiponectin receptors and 5′-adenosine monophosphate-activated protein kinase-alpha responses
The mechanistic association of adiponectin with liver diseases was evaluated. In the early phase of diet treatment (2 weeks), the mean total adiponectin concentration in plasma for the HFC-diet group was significantly different (1.3-fold higher) from that of the SP-diet group. Conversely, total adiponectin concentration at 8 weeks in the HFC-diet group was significantly reduced by 0.8-fold relative to that in the SP-diet group.
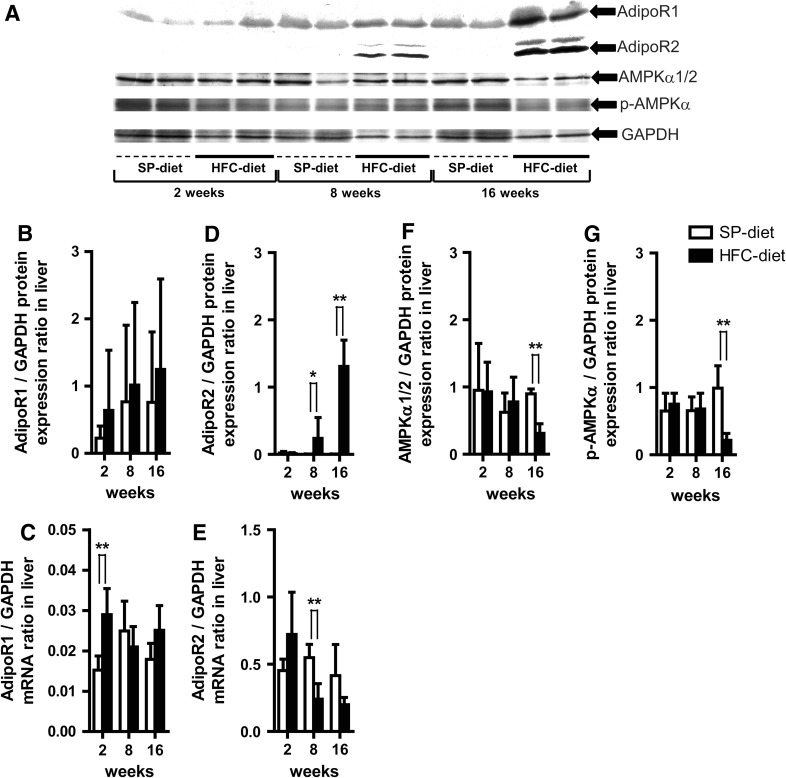
On the other hand, hepatic AdipoR1 protein expression detected in the HFC diet group was 2.8-, 1.3-, and 1.6-fold higher than that in the SP-diet group at each treatment period, with a 2.0-fold change from 2 to 16 weeks over the treatment period (Fig. 2a, b). Similarly, hepatic AdipoR1 mRNA expression in the HFC-diet group at 2 weeks was significantly higher than that in the SP-diet group (Fig. 2c).
Fig. 2.
Effects of HFC-diet treatment on hepatic adiponectin receptor 1 and 2 (AdipoR1, AdipoR2), 5′-adenosine monophosphate-activated protein kinase-α subunit ½ (AMPKα1/2) and phosphorylated AMPKα (p-AMPKα) protein levels and mRNA expressions. a Western blot results of respective protein expressions in whole liver tissue homogenates. b, c Hepatic AdipoR1 protein (b) and mRNA expression (c) ratios. d, e Hepatic AdipoR2 protein (d) and mRNA expression (e) ratios. f, g AMPKα1/2 (f) and p-AMPKα (g) protein expression ratios. Each histogram represents a mean ratio ± SD. *p < 0.05, **p < 0.01 compared with the SP-diet group within each diet–treatment period
AdipoR2 protein expression in the liver of the HFC-diet group showed a significant increase at 8 and 16 weeks (Fig. 2d), which was in contrast to hepatic AdipoR2 mRNA expression in the HFC-diet group, the expression of which was significantly lower than that in the SP-diet group at 8 weeks (Fig. 2e). Hepatic AdipoR2 mRNA expression in the HFC-diet group showed a 0.3-fold change from 2 to 16 weeks, which corresponded to the 0.8-fold decrease in plasma total adiponectin concentration over time.
We further analyzed 5′-adenosine monophosphate-activated protein kinase-alpha subunit 1/2 (AMPKα1/2) and phosphorylated AMPKα (p-AMPKα) in the liver to further evaluate their potential as a marker of energy metabolism associated with adiponectin and PPARα [20]. Hepatic protein expression of AMPKα1/2 and p-AMPKα in the HFC-diet group was significantly down-regulated at 16 weeks relative to that in the SP-diet group (Fig. 2f, g), indicating a downstream decrease of various energy metabolic reactions regulated by AMPKα [18–20].
PPARs and associated gene responses
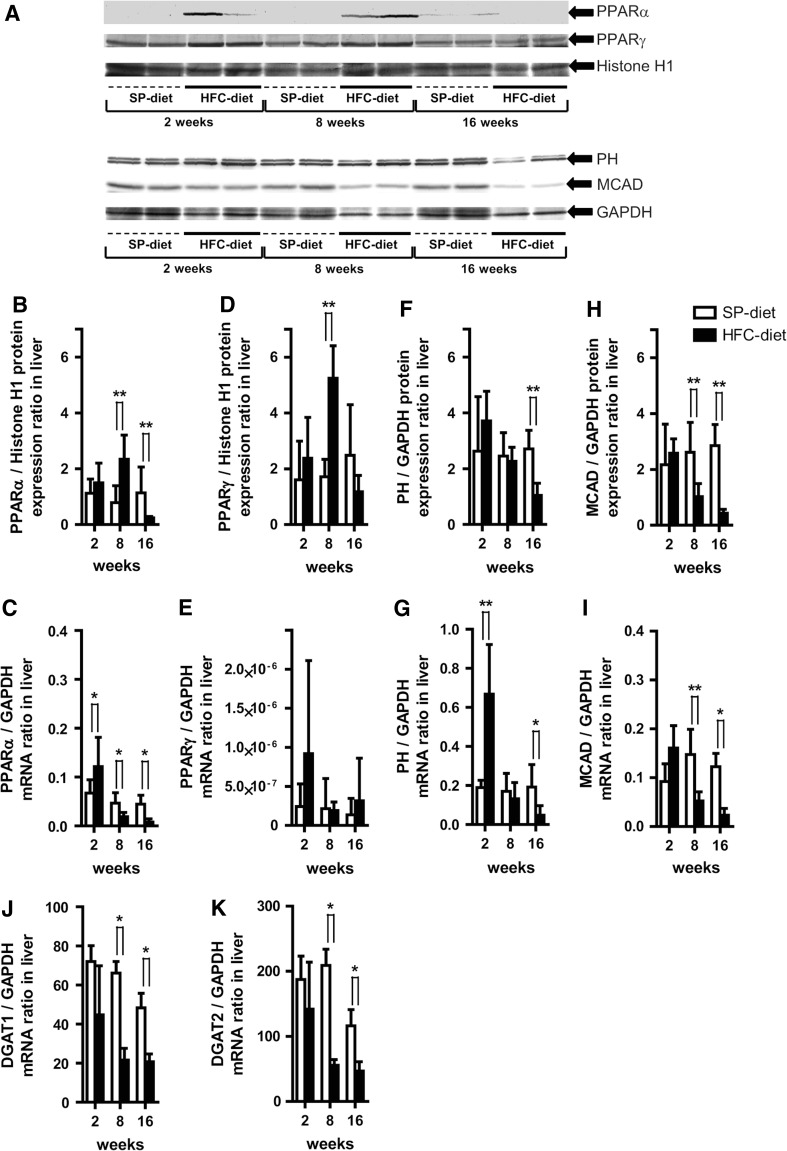
As a primary regulator of peroxisomal, mitochondrial, and microsomal fatty acid oxidation [21], hepatic PPARα protein expression in the HFC-diet group relative to that in the SP-diet group was induced by 1.3-fold at 2 weeks and significantly up-regulated at 8 weeks, but suppressed at 16 weeks (Fig. 3a, b). Hepatic PPARα mRNA expression in the HFC-diet group was significantly up-regulated at 2 weeks, followed by an earlier significant down-regulation at 8 and 16 weeks (Fig. 3c) relative to its own protein expression after 2 weeks, while the SP-diet group exhibited almost the same constant protein and mRNA expression across the treatment period. Similarly, as an indicator of hepatic lipid metabolism and fibrosis by stellate cells, peroxisome proliferator-activated receptor γ (PPARγ) protein expression in the HFC-diet group relative to that in the SP-diet group was induced by 1.5-fold at 2 weeks and significantly up-regulated at 8 weeks, whereas it was suppressed by 0.5-fold at 16 weeks (Fig. 3d). PPARγ mRNA expression in the HFC-diet group was induced by 3.8-fold at 2 weeks followed by a large reduction at 8 and 16 weeks (Fig. 3e).
Fig. 3.
Effects of HFC-diet treatment on hepatic peroxisome proliferator-activated receptor α and γ (PPARα, PPARγ), peroxisomal bifunctional protein (hydratase + 3-hydroxyacyl-CoA dehydrogenase) (PH), medium chain acyl-CoA dehydrogenase (MCAD), diacylglycerol acyltransferase 1 and 2 (DGAT1, DGAT2) protein and mRNA expressions. a Western blot results of respective protein expressions in whole liver tissue homogenates or nuclear fractions. b, c Hepatic PPARα protein (b) and mRNA expression (c) ratios. d, e Hepatic PPARγ protein (d) and mRNA expression (e) ratios. f–i Ratio of hepatic protein and mRNA expressions for PH (f, g) and MCAD (h, i). j, k Hepatic DGAT1 (j) and DGAT2 (k) mRNA expression ratio. Each histogram represents a mean ratio ± SD. *p < 0.05, **p < 0.01 compared with the SP-diet group within each diet–treatment period
To confirm changes in the down-stream target of PPARα transcription, we studied the protein expression of peroxisomal bifunctional protein (hydratase + 3-hydroxyacyl-CoA dehydrogenase) (PH). PH expression in the HFC-diet group was elevated by 1.4-fold at 2 weeks and declined significantly at 16 weeks (Fig. 3f), while its mRNA expression also increased significantly by 3.5-fold at 2 weeks, followed by a significantly large reduction at 16 weeks (Fig. 3g). Medium chain acyl-CoA dehydrogenase (MCAD) protein expression in the HFC-diet group was significantly suppressed from week 8 to week 16 (Fig. 3h), while mRNA expression was elevated 1.7-fold at 2 weeks, followed by a significant reduction at 8 and 16 weeks, when compared with the SP-diet group (Fig. 3i).
As a marker of triglyceride synthesis in liver, hepatic mRNA expression of diacylglycerol acyltransferase 1 (DGAT1) and 2 (DGAT2) was evaluated. In the HFC group, the expression of these molecules was significantly down-regulated at 8 and 16 weeks (Fig. 3j, k), suggesting an inhibition of triglyceride synthesis in the liver [22–24].
Fibrosis-associated gene responses
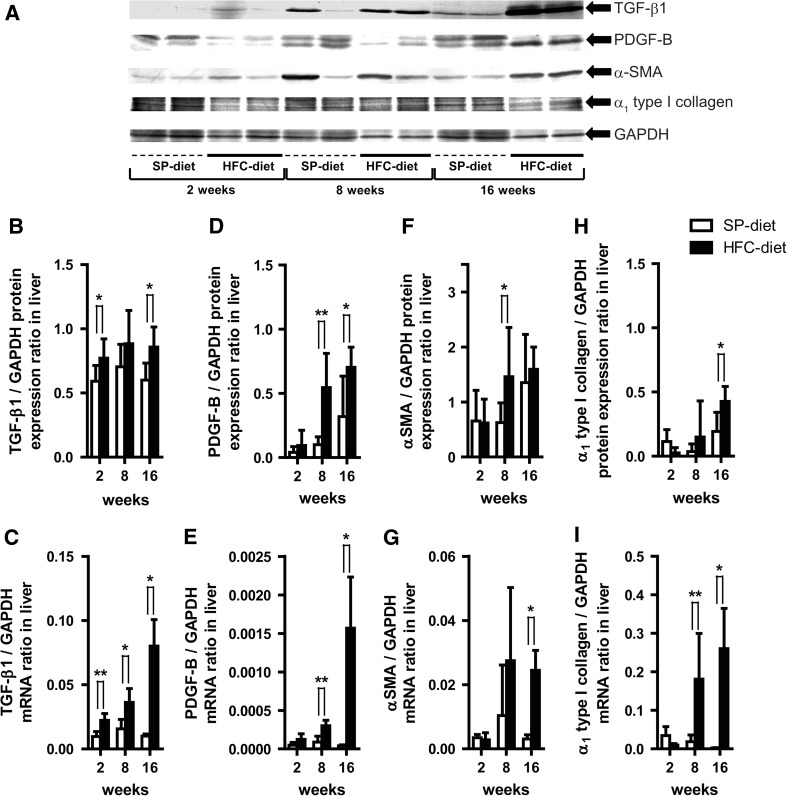
We then studied how well liver fibrogenesis (TGF-β1, PDGF-B, and α-SMA) and cellular matrix molecular markers (α1 type I collagen) were associated with our prior pathological observations [8]. With respect to TGF-β1, the expression of both hepatic proteins at 2 and 16 weeks and mRNA expression at each treatment period were significantly higher in the HFC-diet group than in the SP-diet group over time (Fig. 4a, b, c). PDGF-B protein and mRNA expression also showed a similar significant profile at 8 and 16 weeks, respectively, although those expressions at 2 weeks were 2.3- and 2.5-fold, respectively (Fig. 4d, e). A significant up-regulation of hepatic α-SMA protein at 8 weeks and mRNA expression at 16 weeks in the HFC-diet group became evident from the 8-week period up to 16 weeks, compared to the SP-diet group (Fig. 4f, g). Similar time-course profiles were observed for the significant increase in α1 type I collagen protein expression at 16 weeks, as well as its mRNA expression at 8 and 16 weeks in the liver for the HFC-diet group (Figs. 4h, i, 5).
Fig. 4.
Effects of HFC-diet treatment on hepatic transforming growth factor-β1 (TGF-β1), platelet-derived growth factor-B (PDGF-B), α-smooth muscle actin (α-SMA) and α1 type I collagen protein and mRNA expressions. a Western blot results of respective protein expressions in whole liver tissue homogenates. b, c Ratios of TGF-β1 protein (b) and mRNA (c) expressions. d, e Ratios of PDGF-B protein (d) and mRNA (e) expression. f, g Ratios of α-SMA protein (f) and mRNA (g) expression. h, i Ratio of α1 type I collagen protein (h) and mRNA (i) expression. Each histogram represents a mean ratio ± SD. *p < 0.05; **p < 0.01; compared with the SP-diet group within each diet-treatment period
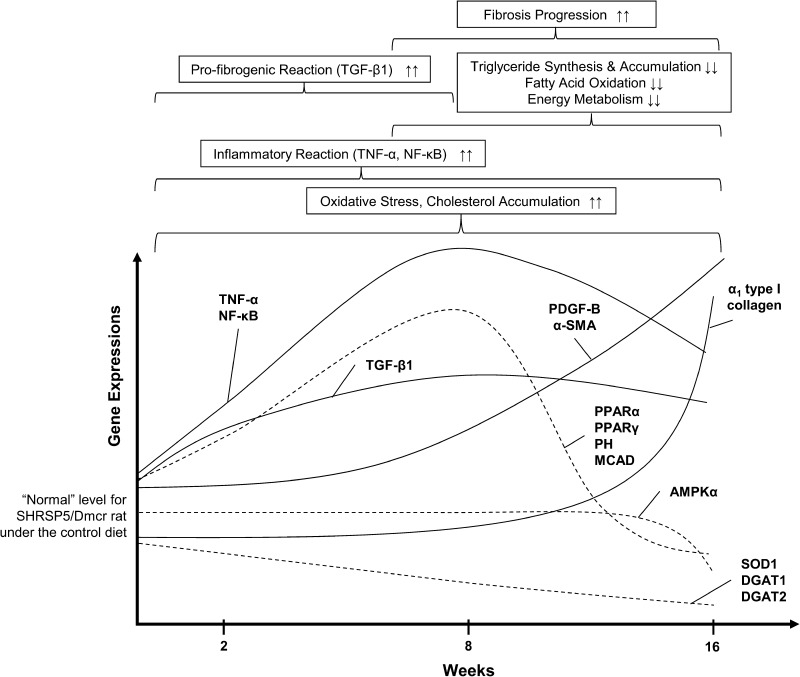
Fig. 5.
Schematic model for time-course changes in hepatic gene expressions of key factors during steatohepatitis and fibrosis progression in the stroke-prone, spontaneously hypertensive 5/Dmcr rat (SHRSP5/Dmcr) given the HFC-diet treatment. The current SHRSP5/Dmcr rat model appeared to show rather dynamic interplays and changes in the state of liver biochemical balances as shown by initial TNF-α and NF-κB hepatic inflammatory reactions in conjunction with pro-fibrogenic TGF-β1 responses that led to the progression of liver disease to extensive liver fibrosis, as indicated by the up-regulation of PDGF-B, α-SMA, and α1 type I collagen, the eventual down-regulation of proteins associated with PPARs and AMPKα, and down-regulation of DGAT1 and DGAT2 mRNA
Discussion
As we reported earlier [8], the present SHRSP5/Dmcr model under the HFC-diet treatment for 16 weeks demonstrated a transition from steatosis, inflammation with hepatocyte injury or ballooning, and a distinctive pattern of perivenular or pericellular liver fibrosis that resembled key features observed in NASH and liver fibrosis patients [1, 5, 25]. In contrast to the contemporary stepwise two- or multiple-hit hypothesis [26, 27], at the molecular level, we observed rather dynamic interplays and simultaneous changes in the state of liver biochemical balances as shown by TNF-α and p50/p65 hepatic inflammatory reactions in conjunction with pro-fibrogenic TGF-β1 responses that led to the progression of liver disease to extensive liver fibrosis, as indicated by the up-regulation of PDGF-B, α-SMA, and α1 type I collagen, the eventual down-regulation PPARα, PH, MCAD, AMPKα1/2, and p-AMPKα protein, and the down-regulation of DGAT1 and DGAT2 mRNA (Fig. 5).
Increased TNF-α gene expression and oxidative stress in the liver has been observed in NASH patients [1, 28, 29] and other rodent models [6, 30–32], with more advanced liver fibrosis accompanied by extensive hepatic TNF-α expression present in advanced NASH patients [1, 28]. We found that the HFC-diet treatment appeared to facilitate TNF-α-induced inflammatory reactions throughout the experimental period, with a pronounced peak at 8 weeks. The significant up-regulation of hepatic p50 and p65 protein in the HFC-diet group at 8 weeks implies liver inflammation induced by NF-κB, the p50/p65 heterodimer [33, 34]. This elevation in p50 and p65 protein might be due to the reduced anti-inflammatory functions of PPARα [21, 35–37], since the induction PPARα protein at 8 weeks did not activate its target genes of PH and MCAD during the same 8-week treatment period. One possible explanation for this elevation in p50 and p65 protein in the HFC-diet group may be the continuous inflammatory reactions of TNF-α, since NF-κB is located downstream of the TNF-α signal pathways and activated by TNF-α [21, 33]. Similarly, evident down-regulation of the SOD1 protein in the HFC-diet group across the treatment period was observed despite the induction of PPARα protein at 8 weeks, which regulates SOD1 for anti-oxidative effects in the liver [17, 21, 38]. This reduced abundance of SOD1 protein appeared to be driven primarily by TNF-α liver inflammation and partially by PPARα-induced fatty acid oxidation generating cytotoxic reactive molecules, thereby facilitating a decrease in the concentration of anti-oxidant enzymes in the liver [21, 32]. Moreover, a decline in plasma total adiponectin at 8 weeks inversely corresponded to the peak in plasma TNF-α, antagonizing anti-inflammatory effects of adiponectin [6, 39–41] and further supporting the presence of TNF-α-induced hepatic inflammation. Taken together with the increased plasma ALT and AST levels and aggravated liver pathological features [8], the hepatic inflammation and cell injury induced by TNF-α at 2 weeks were rather mild, but they did facilitate oxidative stress. In addition, TNF-α- and NF-κB-dependent inflammation and the superimposed oxidative stress became evident at 8 weeks, facilitating the progression of steatosis to steatohepatitis and extensive liver fibrosis after the 8-week treatment period in this SHRSP5/Dmcr model.
In terms of the fibrosis molecular markers, TGF-β1 in the HFC-diet group was clearly up-regulated at 2 weeks in the absence of any fibrosis features in the liver pathology [8]. While TGF-β1 is a cytokine known to facilitate pro-fibrogenic reactions and liver fibrosis via hepatic stellate cell (HSC) activation [25, 42, 43], this initial increase at 2 weeks might be triggered by the dietary cholate contained in the HFC-diet stimulating the expressions of the TGF-β1, α-SMA, and collagen genes [44], in parallel with hepatic Kupffer cell or HSC sensitization generating TGF-β1 in response to hepatic inflammation and cell injury [25, 42, 43]. However, α-SMA and α1 type I collagen were not induced at 2 weeks, thereby precluding a possibility of a dietary effect by cholate in the HFC-diet at 2 weeks. Thus, initial TGF-β1 activation at 2 weeks in association with TNF-α induction in the liver may be a good early marker of progressive liver fibrosis, at least in our current SHRSP5/Dmcr model. Following early TGF-β1 sensitization, the significant up-regulation of both PDGF-B and α-SMA protein expression in the HFC-diet group at 8 weeks, indicating the extensive activation of HSC in the HFC-diet group at 8 weeks [42, 43, 45], clearly corresponded to the initial appearance of liver fibrosis observed in our earlier pathology study at the same treatment period [8]. With diminishing hepatic anti-oxidant enzymes as well as increasing hepatic inflammation after 8 weeks, the significant up-regulation of hepatic α1 type I collagen protein together with the continuing effects of TGF-β1 and PDGF-B at 16 weeks suggest that the fundamental shifts in the extracellular matrix composition and collagen production for wound healing from liver cell injury [42] underlaid the progression to extensive liver fibrosis at the 16-week treatment period.
Circulating adiponectin is generally antagonized by TNF-α [6, 39–41], but we observed increased plasma total adiponectin and TNF-α in the HFC-diet group in the same 2-week treatment period that had been consistently observed in our earlier report [8]. This increased plasma total adiponectin at 2 weeks might possibly be related to PPARγ, since a mouse model without the liver-specific PPARγ-gene has been shown to have decreased serum adiponectin levels [46], indicating a role of hepatic PPARγ in adiponectin regulation. However, based on our results of plasma total adiponectin and TNF-α and on hepatic PPARγ at 8 weeks, the significant hepatic PPARγ protein induction did not lead to the observed elevation in plasma total adiponectin. Instead, the antagonizing effect of TNF-α against adiponectin appeared to be greater. Furthermore, together with the reduction of plasma total adiponectin in the HCF-diet group at 8 weeks, we expected a corresponding reduction in the hepatic AdipoR1/R2 and downstream AMPKα1/2 and PPARα pathways based on a recently proposed molecular signal transduction cascade considered to be important for energy metabolism and fatty acid oxidation in liver [20, 47]. However, our results, particularly those relating to hepatic protein expressions, did not support links with these proposed molecular signaling pathways. Instead, hepatic AdipoR2, which is predominantly expressed in the liver [20], showed a rather increased protein expression in the HFC-diet group that corresponds to the aggravation of liver fibrosis pathology at 8 and 16 weeks observed in our prior study [8]. Hence, further research in both liver and adipose tissues is needed to dissect the roles of adiponectin regulatory mechanisms in progressive liver fibrosis.
A net retention of lipids within hepatocytes, mostly in the form of triglycerides, is a prerequisite for the development of NASH and NAFLD in patients [2]. Our results show that hepatic triglyceride contents in the HFC-diet group initially increased and then declined after 8 weeks, whereas hepatic total cholesterol kept accumulating with increased liver weight and progressive liver pathological features [8]. We also observed a sharp rise in plasma triglyceride between 8 and 16 weeks in the HFC-diet group, which may be a reflection of hepatocyte necrosis and liver disease progression since increased levels of circulating blood triglyceride (hypertriglyceridemia) are a feature of human NASH patients [2, 29]. In addition, despite a collapse in homeostatic fatty acid oxidation and energy metabolism functions due to decreasing levels of PPARα, PH, MCAD, AMPKα1/2, and p-AMPKα proteins [20, 48, 49], hepatic triglyceride in the HFC-diet group did not accumulate at 16 weeks. One explanation might lay in observations made in an earlier study where the inhibition of triglyceride synthesis by DGAT2 inhibitor did not prevent fibrosis in the MCD-diet fed mouse [24]. These results suggest that triglyceride itself might not be hepatotoxic and that it may have a role in preventing progressive liver damage [24]. We actually observed clear down-regulation of DGAT1 and DGAT2 mRNA expression in the HFC-diet group at 8 and 16 weeks. Hence, from a perspective of lipid metabolism, it appears that the decline in hepatic triglyceride level and its synthesis might relate and aggravate the liver disease conditions so as to cause the extensive liver fibrosis in the current SHRSP5/Dmcr model.
Our SHRSP5/Dmcr model with non-obese features has some limitations when compared with a common metabolic abnormality observed in NAFLD or NASH patients, such as obesity [1, 2, 5], which are possibly due to an intrinsic trait of rats [5]. Results with our SHRSP5/Dmcr rats fed on the HFC-diet suggest that most lipids, especially total cholesterol, accumulated in liver with increased liver weight, since we did not find any extensive lipid accumulation in mesentery or visceral adipose tissue during the post-mortem examination. Moreover, unlike the fatty acid contents, our HFC-diet contained very high levels of cholesterol and relatively low levels of carbohydrates compared with the daily dietary intake of obese NASH patients [32, 50]. Hence, an investigation into the role of cholesterol in steatohepatitis and liver fibrosis in the SHRSP5/Dmcr rat model is necessary, and it is currently being studied by our co-workers (Naito et al., in preparation). Lastly, we lost two of five rats in the HFC-diet group undergoing the 16-week treatment period, possibly due to spontaneous stroke of SHRSP5/Dmcr rats [7]; therefore, the 14-week treatment period might be optimal for the current SHRSP5/Dmcr model [8]. Considering that there are non-obese, lean individuals who are affected by NASH and liver fibrosis [51–54] as well as patients with non-obese, non-diabetic NASH consuming high amounts of cholesterol and saturated fatty acid [50], our novel SHRSP5/Dmcr model may be suited for investigating the time-course of disease mechanisms for those lean, non-diabetic patients with steatohepatitis and associated liver fibrosis.
In conclusion, TNF-α and p50/p65 molecular signals appeared to be major factors for the HFC-diet-induced hepatic inflammation and oxidative stress facilitating the liver disease progression. While TGF-β1 up-regulation occurred before the appearance of any evident liver fibrosis and may, therefore, be a potential early biomarker of progressive liver fibrosis, the up-regulation of PDGF-B and α-SMA signified evident liver fibrosis at 8 weeks, followed by increased α1 type I collagen production and reduced triglyceride synthesis underlying extensive liver fibrosis at 16 weeks in our novel SHRSP5/Dmcr model.
Electronic supplementary material
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (B23390161) from the Japan Society for the Promotion of Science, and the Uehara Memorial Foundation in 2009. No additional external funding was received for this study. The organizations funding the study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None.
Abbreviations
- AdipoR1
Adiponectin receptor 1
- AdipoR2
Adiponectin receptor 2
- ALT
Alanine aminotransferase
- AMPKα1/2
5′-Adenosine monophosphate (AMP)-activated protein kinase α subunit ½
- AST
Aspartate aminotransferase
- DGAT1
Diacylglycerol acyltransferase 1
- DGAT2
Diacylglycerol acyltransferase 2
- ELISA
Enzyme-linked immunosorbent assay
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HFC-diet
High-fat and high-cholesterol-containing diet
- HSC
Hepatic stellate cells
- IκBα
Inhibitor of κBα
- MCAD
Medium chain acyl-CoA dehydrogenase
- MCD diet
Methionine and choline-deficient diet
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NF-κB
Nuclear factor-κB
- p-AMPKα
Phosphorylated AMPKα
- PDGF-B
Platelet-derived growth factor-B
- PH
Peroxisomal bifunctional protein (hydratase + 3-hydroxyacyl-CoA dehydrogenase)
- PPARα
Peroxisome proliferator-activated receptor α
- PPARγ
Peroxisome proliferator-activated receptor γ
- SHRSP5/Dmcr
Stroke-prone, spontaneously hypertensive 5/Dmcr rat
- α-SMA
α-Smooth muscle actin
- SOD1
Cu2+/Zn2+-superoxide dismutase
- SP-diet
Stroke-prone control chow
- TGF-β1
Transforming growth factor-β1
- TNF-α
Tumor necrosis factor-α
References
- 1.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46(Suppl 1):63–69. doi: 10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- 4.Powell EE, Jonsson JR, Clouston AD. Dangerous liaisons: the metabolic syndrome and nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:753–754. doi: 10.7326/0003-4819-143-10-200511150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 6.Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-α and –3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamori Y. Selection of arteriolipidosis-prone rats (ALR) Jpn Heart J. 1977;18:602–603. doi: 10.1536/ihj.18.602. [DOI] [PubMed] [Google Scholar]
- 8.Kitamori K, Naito H, Tamada H, Kobayashi M, Miyazawa D, Yasui Y, et al. Development of novel rat model for high-fat and high-cholesterol diet-induced steatohepatitis and severe fibrosis progression in SHRSP5/Dmcr. Environ Health Prev Med. doi:10.1007/s12199-011-0235-9 [DOI] [PMC free article] [PubMed]
- 9.Sansawa H, Takahashi M, Tsuchikura S, Endo H. Effect of chlorella and its fractions on blood pressure, cerebral stroke lesions, and life-span in stroke-prone spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2006;52:457–466. doi: 10.3177/jnsv.52.457. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M. Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, et al. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor α-independent pathway. J Occup Health. 2007;49:172–182. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- 12.Ramdhan DH, Kamijima M, Yamada N, Ito Y, Yanagiba Y, Nakamura D, et al. Molecular mechanism of trichloroethylene-induced hepatotoxicity mediated by CYP2E1. Toxicol Appl Pharmacol. 2008;231:300–307. doi: 10.1016/j.taap.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 14.Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, et al. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARα-null mice through attenuation of increases in oxidative stress. J Hepatol. 2009;50:1236–1246. doi: 10.1016/j.jhep.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 16.Bauer DF. Constructing confidence sets using rank statistics. J Am Stat Assoc. 1972;67:687–690. doi: 10.1080/01621459.1972.10481279. [DOI] [Google Scholar]
- 17.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/S0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 19.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 21.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor α target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J Biol Chem. 2005;280:21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- 23.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 28.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 29.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 30.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 31.Fu JH, Xie SR, Kong SJ, Wang Y, Wei W, Shan Y, et al. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp Clin Endocrinol Diabetes. 2009;117:354–360. doi: 10.1055/s-0028-1119406. [DOI] [PubMed] [Google Scholar]
- 32.Romestaing C, Piquet MA, Bedu E, Rouleau V, Dautresme M, Hourmand-Ollivier I, et al. Long term highly saturated fat diet does not induce NASH in Wistar rats. Nutr Metab (Lond) 2007;4:4. doi: 10.1186/1743-7075-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plumpe J, Malek NP, Bock CT, Rakemann T, Manns MP, Trautwein C. NF-κB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2000;278:G173–G183. doi: 10.1152/ajpgi.2000.278.1.G173. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty JB, Mann DA. NF-κB signalling: embracing complexity to achieve translation. J Hepatol. 2010;52:285–291. doi: 10.1016/j.jhep.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, et al. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 36.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 37.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, et al. Peroxisome proliferator-activated receptor α protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 39.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 40.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 41.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 42.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 43.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 44.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 45.Breitkopf K, Roeyen C, Sawitza I, Wickert L, Floege J, Gressner AM. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors α and β in activated rat hepatic stellate cells (HSC) Cytokine. 2005;31:349–357. doi: 10.1016/j.cyto.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 48.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, et al. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 2004;126:873–885. doi: 10.1053/j.gastro.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 50.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 51.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 52.Silverman JF, O’Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. [PubMed] [Google Scholar]
- 53.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26(Suppl 1):163–172. doi: 10.1111/j.1440-1746.2010.06548.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.