Abstract
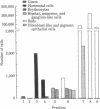
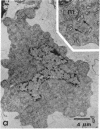
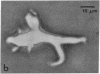
Horizontal cells of the carp retina were separated from other retinal cell types by using enzymatic dissociation and velocity sedimentation at unit gravity. Fractions containing horizontal cells were tested for their ability to accumulate cyclic AMP in the presence of various putative neurotransmitters. Micromolar concentrations of dopamine, when added in the presence of 3-isobutyl-1-methylxanthine, stimulated cyclic AMP accumulation in these isolated cells. The dopamine-dependent accumulation of cyclic AMP in intact isolated horizontal cells was blocked by nanomolar concentrations of dopamine antagonists such as haloperidol, (+)-butaclamol, and fluphenazine. The results indicate that there is a postsynaptic dopamine receptor on carp horizontal cells that is associated with adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Gorman R. E., Miller W. H. Adenyl cyclase as a link between photon capture and changes in membrane permeability of frog photoreceptors. Proc Natl Acad Sci U S A. 1971 Mar;68(3):561–562. doi: 10.1073/pnas.68.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries G. W., Cohen A. I., Lowry O. H., Ferrendelli J. A. Cyclic nucleotides in the cone-dominant ground squirrel retina. Exp Eye Res. 1979 Sep;29(3):315–321. doi: 10.1016/0014-4835(79)90010-1. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Watling K. J. Dopaminergic mechanisms in the teleost retina. II. Factors affecting the accumulation of cyclic AMP in pieces of intact carp retina. J Neurochem. 1981 Feb;36(2):569–579. doi: 10.1111/j.1471-4159.1981.tb01629.x. [DOI] [PubMed] [Google Scholar]
- El-Refai M. F., Exton J. H. Effects of trypsin on binding of [3H]epinephrine and [3H]-dihydroergocryptine to rat liver plasma membranes. Evidence for interconversion of binding sites. J Biol Chem. 1980 Jun 25;255(12):5853–5858. [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Hedden W. L., Jr, Dowling J. E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Dopamine receptors in the brain. Science. 1975 Jun 13;188(4193):1084–1089. doi: 10.1126/science.2976. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Leysen J. E. Unitary dopaminergic receptor composed of cooperatively linked agonist and antagonist sub-unit binding sites. Commun Psychopharmacol. 1979;3(6):397–410. [PubMed] [Google Scholar]
- Orr H. T., Lowry O. H., Cohen A. I., Ferrendelli J. A. Distribution of 3':5'-cyclic AMP and 3':5'-cyclic GMP in rabbit retina in vivo: selective effects of dark and light adaptation and ischemia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4442–4445. doi: 10.1073/pnas.73.12.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redburn D. A., Clement-Cormier Y., Lam D. M. Dopamine receptors in the goldfish retina: 3H-spiroperidol and 3H-domperidone binding; and dopamine-stimulated adenylate cyclase activity. Life Sci. 1980 Jul 7;27(1):23–31. doi: 10.1016/0024-3205(80)90015-6. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. Biochemical studies of isolated glial (Müller) cells from the turtle retina. J Cell Biol. 1978 Sep;78(3):675–684. doi: 10.1083/jcb.78.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. Endogenous levels of neurotransmitter candidates in photoreceptor cells of the turtle retina. J Neurochem. 1979 Feb;32(2):455–461. doi: 10.1111/j.1471-4159.1979.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Stell W. K., Lightfoot D. O. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975 Feb 15;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Watling K. J., Dowling J. E. Dopaminergic mechanisms in the teleost retina. I. Dopamine-sensitive adenylate cyclase in homogenates of carp retina; effects of agonists, antagonists, and ergots. J Neurochem. 1981 Feb;36(2):559–568. doi: 10.1111/j.1471-4159.1981.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Watling K. J., Dowling J. E., Iversen L. L. Dopamine receptors in the retina may all be linked to adenylate cyclase. Nature. 1979 Oct 18;281(5732):578–580. doi: 10.1038/281578a0. [DOI] [PubMed] [Google Scholar]