Abstract
Background
Deficient behavioral regulation may be a risk factor for substance use disorders in adolescents. Abnormalities in brain regions critical to cognitive control have been linked to more intense and problematic future substance use (e.g., (Durazzo, Gazdzinski, Mon, & Meyerhoff, 2010; Falk, Berkman, Whalen, & Lieberman, 2011; Paulus, Tapert, & Schuckit, 2005). The goal of this study was to examine the degree to which brain response to an inhibition task measured in mid-adolescence can predict substance use 18 months later.
Method
Adolescents aged 16–19 (N=80) performed a go/no-go response inhibition task during fMRI at project baseline, and were followed 18 months later with a detailed interview on substance use and dependence symptoms. Participants were 39 high frequency users and 41 demographically similar low frequency users (458 versus 2 average lifetime drug use occasions at baseline, respectively).
Results
Across all subjects, no-go trials produced significant increases in neural response in the ventromedial prefrontal cortex and a region including the left angular and supramarginal gyri (p(FWE)<.01, cluster threshold ≥30 voxels). Less ventromedial prefrontal activation but more left angular gyrus activation predicted higher levels of substance use and dependence symptoms in the following 18 months, particularly for those who were high frequency users in mid-adolescence (p<.05).
Conclusions
These findings are consistent with studies showing that impairments in cognitive control have strong associations with substance use. We found a predictive relationship between atypical activation patterns at baseline and substance use behavior 18 months later, particularly among adolescents with histories of previous heavy use.
Keywords: predictors of substance use, fMRI, executive functioning, adolescence
1. Introduction
Experimentation with drugs and alcohol is quite common during adolescence. Spanning the years from 8th grade to 12th grade, the proportion of youth reporting recent use of an illicit drug increases from 10% to 24% (Johnston, 2010). Prevalence of recent drinking to intoxication similarly rises from 5% to 27% across the same time frame (Johnston, 2010). Among young adults ages 18–25, over 15% meet criteria for alcohol or drug abuse or dependence (Substance Abuse and Mental Health Services Administration (SAMHSA), 2010). Given the detrimental functional and social consequences of substance use disorders, identifying which youth are particularly susceptible to dependence is of great clinical significance. Research that isolates predictors of future substance use problems can refine the etiology of addictive behaviors and point to potential targets and strategies for early intervention.
A number of studies have used longitudinal and cross-sectional methods to identify risk factors for developing substance use disorders in mid to late adolescence. Psychiatric disorders (e.g., conduct disorder, attention deficit/hyperactivity disorder) (Boyle et al., 1992; Elkins, McGue, & Iacono, 2007), personality factors (e.g., novelty seeking, harm avoidance) (Mâsse & Tremblay, 1997), genetic characteristics (e.g., hypodopaminergic genotypes) (Conner, Hellemann, Ritchie, & Noble, 2010), and cognitive ability (e.g., executive functioning) (Nigg et al., 2006; Tarter et al., 2003) have all been implicated as predictors for alcohol and illicit drug use in adolescence. However, research focusing on brain-behavior relationships to elucidate the neurocognitive correlates of substance use risk is limited.
Recently, some researchers have used neuroimaging (Durazzo et al., 2010), and functional magnetic resonance imaging (fMRI) in particular, to help clarify the neural precursors of future substance-related behavior in adults. For example, Falk and colleagues (2011) found that medial prefrontal blood oxygen level dependent (BOLD) response during exposure to videos on smoking abstinence predicted subsequent reduction in cigarette use. Among cocaine users, greater fMRI response to a Stroop task in the ventromedial prefrontal cortex (vmPFC) predicted longer durations of abstinence (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008) and, during a drug cue reactivity task, correlated with post-treatment cocaine urine toxicology (Kosten et al., 2006). Dampened neural response in right middle frontal regions during a decision-making task administered to methamphetamine users who were undergoing drug treatment predicted a higher probability of post-treatment relapse (Paulus, Tapert, & Schuckit, 2005).
Response inhibition, which is an executive functioning faculty of cognitive control, has shown strong associations with substance use disorders (Hester & Garavan, 2004; Lawrence, Luty, Bogdan, Sahakian, & Clark, 2009; Verdejo-García, Bechara, Recknor, & Perez-Garcia, 2006). In addition, increasing evidence suggests that poor performance on measures of response inhibition and impulsivity indicate a vulnerability to substance use initiation and escalation (for a review, see (Verdejo-García, Lawrence, & Clark, 2008)). We recently examined differential BOLD responses to a go/no-go task in substance-naïve adolescents (ages 12–14); comparing 21 youth who initiated substance use in the subsequent three years to 17 youth who remained non to minimal users throughout adolescence (Norman et al., 2011). Those who went on to use substances had shown significantly less activation at baseline in 12 brain regions including and extending beyond typically observed inhibitory circuitry (Aron, 2007), particularly prefrontal regions.
The purpose of this study was to investigate whether certain characteristics of brain activation during response inhibition could predict future substance use in adolescents with a range of baseline use patterns. We employed a go/no-go task administered during fMRI to high and low frequency users, then longitudinally assessed the adolescents’ alcohol and drug use for the subsequent 18 months. Given the importance of executive cognitive control in identifying vulnerability to substance use, we hypothesized that less prefrontal cortex activation during an inhibition task would predict more substance use and problems above and beyond substance use history up until the time of the imaging session.
2. Method
2.1 Participants
Participants were 39 adolescents with a history of high frequency substance use (HF group) and 41 demographically matched peers with low frequency substance use histories (LF group). Both groups were recruited from the same public schools in the San Diego metropolitan area (Bava, Frank et al., 2009; Bava, Jacobus, Mahmood, Yang, & Tapert, 2009; Medina et al., 2007; Schweinsburg et al., 2008; S. F. Tapert et al., 2007). In accordance with the University of California San Diego Human Research Protections Program, informed consent and assent were obtained from each parent and participating adolescent, respectively. Detailed interviews were used to confirm participant eligibility, obtain demographic information, assess psychosocial functioning, and establish substance use history. Individuals were excluded if they had a history of prenatal alcohol or drug exposure; complications at birth; history of traumatic brain injury; neurological condition; serious medical illness; learning disorder; or psychiatric diagnosis other than alcohol or cannabis use disorder; left handedness; or fMRI contraindication (e.g., claustrophobia, sensory limitations, or ferrous metal in the body).
LF users had less than 15 total lifetime use occasions of any illicit drug (including marijuana, other street drugs, and use of prescription or over-the-counter medications to get a high or buzz). HF users had over 180 total lifetime use occasions of any drug (the most common drug used was marijuana). Groups were statistically similar on demographic characteristics, premorbid intellectual functioning estimates, and state anxiety at the time of scanning (see Table 1). HF users reported more Beck Depression Inventory symptoms than LF users (3.1 vs. 1.6; p<.05), but all participants were well within normal limits on state anxiety and depressed mood at the time of scanning. By definition, HF users had significantly more total lifetime drinking, marijuana, and other drug use occasions than LF users (see Table 2). Only 4 of the LF users and 13 of the HF users reported smoking one or more cigarettes in the month prior to study participation. Three of the HF users who reported cigarette use exhibited symptoms of nicotine dependence; two were classified as having “very low dependence” and one as “low dependence” according to the Fagerström Test for Nicotine Dependence.
Table 1.
Participant characteristics at project baseline.
| Low Frequency Drug Users (n = 41) M (SD) or % |
High Frequency Drug Users (n =39) M (SD) or % |
|
|---|---|---|
| Age (range 16 to 19) | 17.6 (1.0) | 17.4 (0.9) |
| % Female | 27% | 31% |
| % Caucasian | 79% | 76% |
| % with familial substance use disorder | 29% | 36% |
| % reporting more than one cigarette smoked in the past month * | 10% | 33% |
| Average cigarettes smoked per month (for those reporting past smoking) | 11.8 (13.5) | 9.5 (8.3) |
| Parent annual salary ($ thousands) | 123.8 (67.5) | 151.6 (124.6) |
| WRAT-3 Reading standard score | 110.0 (6.7) | 107.3 (7.6) |
| WASI Vocabulary T-score | 59.0 (8.8) | 58.8 (7.5) |
| Grade point average | 3.5 (0.6) | 3.1 (0.8) |
| Beck Depression Inventory total * | 1.6 (2.3) | 3.1 (3.3) |
| Spielberger State Anxiety total | 25.7 (6.8) | 29.4 (7.4) |
Group difference p < .05
Table 2.
Participant substance use histories at project baseline, and over the 18-month follow-up.
| LF Users (n = 41) M (SD) or % [range] |
HF Users (n = 39) M (SD) or % [range] |
|
|---|---|---|
| Lifetime drug use occasions (baseline)** | 2.4 (3.8) [0 – 14] |
458.4 (335.8) [180 – 1867] |
| Lifetime drug dependence symptoms (baseline)** | 0.1 (0.5) [0 – 2] |
8.2 (4.7) [1 – 24] |
| Lifetime alcohol use occasions (baseline)** | 28.1 (38.1) [0 – 196] |
202.0 (151.8) [22 – 736] |
| Lifetime alcohol dependence symptoms (baseline)** | 0.9 (1.3) [0 – 4] |
3.6 (3.3) [0 – 12] |
| Drug use occasions (18-month follow-up)** | 54.5 (134.0) [0 – 554] |
313.2 (297.7) [0 – 1666] |
| Drug dependence symptoms (18-month follow-up)* | 1.4 (3.4) [0 – 15] |
6.1 (4.2) [0 – 17] |
| Alcohol use occasions (18-month follow-up)** | 90.1 (110.5) [0 – 474] |
247.2 (234.6) [0 – 1130] |
| Alcohol dependence symptoms (18-month follow-up)** | 1.4 (1.7) [0 – 8] |
3.0 (2.5) [0 – 8] |
Group difference p < .01
Group difference p < .05
2.2 Measures
Substance use measures
The Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) is a detailed interview used to obtain alcohol and other drug use history. At baseline, participants are asked about lifetime and past 3-month use of alcohol, marijuana, nicotine, and eight other classes of illicit drugs. In addition, the measure tallies DSM-IV abuse and dependence criteria, hangover/withdrawal symptoms, and negative consequences associated with substance use. At follow-up, the same measure is administered, but replacing the lifetime questions with past-18-month items. A brief version was administered via phone at 6- and 12-months after the baseline scan. For participants who reported cigarette use, the Fagerström Test for Nicotine Dependence (FTND; Fagerström, Heatherton, & Kozlowski, 1990) was administered. Scores on the FTND categorizes respondents as “very low,” “low,” “medium,” “high,” or “very high” in nicotine dependence.
Neuropsychological and Emotional Measures
At the baseline appointment, all participants were administered a battery of neuropsychological tests including the Wide Range Achievement Test, 3rd edition (WRAT-3; Wilkinson, 1993) and Vocabulary subtest of the Wechsler Adult Scale of Intelligence (WASI; Wechsler, 1999). Participants completed selfreport questionnaires to assess mood symptoms, including the Beck Depression Inventory, 2nd Edition (Beck, Steer, & Brown, 1996) and the Spielberger State–Trait Anxiety Inventory (STAI; Spielberger & Gorsuch, 1970).
Go/no-go Response Inhibition Task
The go/no-go task is a computerized measure of cognitive control that requires participants to inhibit a button press when a visual target is presented (Norman et al., 2011; Schweinsburg et al., 2004; S.F. Tapert et al., 2007). In the fMRI environment, participants viewed a serial presentation of blue shapes on a computer screen, all according to the same fixed stimulus sequence: 18 blocks of 10 trials each with baseline rest periods of 0–40 seconds between blocks. The stimuli consisted of large circles (n=64), small circles (n=16), large squares (n=43), and small squares (n=57). Each stimulus appeared for 200 milliseconds (msec), with an inter-trial interval of 1300 msec. Subjects were instructed to press a button each time the large circle, small circle, or large square appeared (go stimuli), and not to press the button when the small square was shown (no-go stimulus). The task was constructed using an event-related design with interspersed baseline rest periods (visual fixation), for a total run time of 6 minutes, 24 seconds. During the fixation trials (jittered to varying durations), subjects were instructed to passively look at a plus sign that appeared in the center of their visual field.
2.3 Procedures
All participants were asked to undergo a monitored period of abstinence from substance use. To ensure cessation and abstinence, collection of urine and breath samples by a trained research assistant took place every 3–4 days for a period of 4 weeks. Anyone with a positive toxicology result was offered a chance to restart the abstinence period, or discontinue the study. At the time of the baseline scan, all 80 participants described here had completed at least 20 days of monitored abstinence.
Participants performed the go/no-go task while inside a 3-Tesla CXK4 short bore Excite-2 MR system (General Electric, Milwaukee, WI). Participants were placed comfortably on the scanner table and the head was stabilized within the 8-channel phase-array head coil using foam cushions (NoMoCo Pillow, La Jolla, CA). Scan sessions involved a 10-second scout scan to assure good head placement and slice selection covering the whole brain, and a sagittallyacquired high-resolution 3d T1-weighted anatomical MRI (FOV 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°, 7:26 minutes).
For fMRI, stimuli were presented via projector onto a large screen that participants could see using a small mirror placed above their face while lying in the bore of the scanner. Participants were provided with a non-ferrous button box that they used to respond to the task. BOLD signal data were acquired in the axial plane using a T2*-weighted axially acquired echoplanar imaging sequence (echo time =30 ms, flip angle 90°, ramped bandwidth 250 KHz, field of view = 240 mm, 64 × 64 matrix, 3.75 × 3.75 mm in-plane resolution, 32 5-mm slices covering the whole brain, 128 repetitions, repetition time = 3000 ms). The acquisition time equaled the task duration of 6 minutes and 24 seconds. Field maps using 2 different echo times to assess field inhomogeneities and signal distortions under the same parameters as echo-planar images were applied to all fMRI acquisitions to minimize warping and signal dropouts. A change in the imaging parameters (to repetition time = 1500 ms and 256 repetitions, and 25 5-mm slices covering the whole brain) occurred during the course of data collection. Exactly half of the participants (40 out of 80 individuals; 19 LF and 21 HF users) had functional brain images that were acquired with the newer parameters. There were no procedural changes in presentation of the task.
2.4 Data Analysis
Processing and analysis of functional MRI (fMRI) images was conducted using AFNI. Using an automated algorithm, each image (repetition) was analyzed for artifact and signal abnormalities and the time-series data were corrected for motion by registering each acquisition to the maximally stable base volume (Cox & Jesmanowicz, 1999). Next, scans for each participant that were identified by two independent raters as exhibiting residual visible head motion were excluded from the time series (all data sets retained >85% of repetitions). The fMRI dataset for each participant was deconvolved with a reference function coding trials requiring response inhibition (no-go trials) or response selection (go trials), resulting in a fit coefficient in each voxel for each participant representing their neural response unique to the response inhibition portions of the task. Deconvolution accounted for hemodynamic response and covaried for motion and linear trends (Bandettini, Jesmanowicz, Wong, & Hyde, 1993). Analyses focused on BOLD response contrast for no-go relative to go trials. There were no significant differences in task performance or fit coefficients as a function of protocol change (repetition time = 3000 ms versus repetition time = 1500 ms). However, to account for any influence that the change in fMRI protocol could theoretically present, a variable designating participants as pre- or post- protocol change was used as a covariate in all analyses. Each anatomical image was transformed into standard coordinates (Talairach & Tournoux, 1988; Lancaster et al., 2000), and functional data were resampled into 3 × 3 × 3 mm voxels and a Gaussian filter spatially smoothed the images (5 mm full width half maximum) to account for neuroanatomical variability. A single sample t-test (N=80) determined regions where adolescents on average showed significant (i.e., different from zero) BOLD response contrast for no-go relative to go trials (voxel-level threshold p<.01). To reduce the possibility of false positives, we utilized the AlphaSim program within AFNI to employ a family-wise error correction that generated a cluster threshold of 30 voxels, p(FWE)<.01.
The predictive effects of neural response to the go/no-go task at baseline, and its interaction with baseline use group status, on subsequent substance use were tested in hierarchal multiple regressions. To determine whether substance use history may influence the relationship between fMRI activation and alcohol/substance use 18-months later, an interaction term was computed by multiplying the substance use group code (1 = LF user, 2 = HF user) by the centered BOLD response fit coefficient. To control for any differences attributable to the aforementioned differences in fMRI scan protocol, a grouping variable (identifying individuals’ fMRI acquisition as pre- and post-scanner upgrade) was used as a covariate in all regression analyses. Separate regressions were run for each of four different outcomes characterizing substance use in the last 18 months: total drug use, total drug dependence symptoms, total alcohol use, and total alcohol dependence symptoms. Variables were entered into hierarchical regression equations by blocks as follows: (1) Covariates: fMRI protocol code and baseline substance use variable (lifetime drug use, life time drug dependence symptoms, lifetime alcohol use, or lifetime alcohol dependence symptoms); (2) use group status (LF user vs. HF user) and BOLD response fit coefficients; and (3) interaction terms (i.e., cross-product).
3. Results
Behavioral performance on the go/no-go task as measured by hits (correct go responses), misses (failures to respond), false alarms (failures to inhibit), and a discriminability index (Green, 1966) did not significantly differ between LF and HF users (ps ≥ .43).
For all participants on average (N= 80), no-go trials relative to go trials showed significant positive BOLD response contrast (p(FWE)<.01, cluster threshold ≥30 voxels) in: (1) a cluster centered on the vmPFC (680 voxels) that included a portion of the anterior cingulate gyrus, and (2) a cluster including the angular gyrus, portions of the left posterior superior temporal gyrus, and the supramarginal gyrus (308 voxels). Hierarchical multiple regressions estimated how well baseline user group status and baseline no-go relative to go BOLD response contrast in these two clusters predicted subsequent substance involvement 18 months later. Dependent variables, taken from the CDDR at 18-month follow-up, were: follow-up drug use occasions, follow-up drug dependence symptoms, follow-up alcohol use occasions, and follow-up alcohol dependence symptoms. The corresponding value from each of these variables at the initial baseline session was used as a covariate.
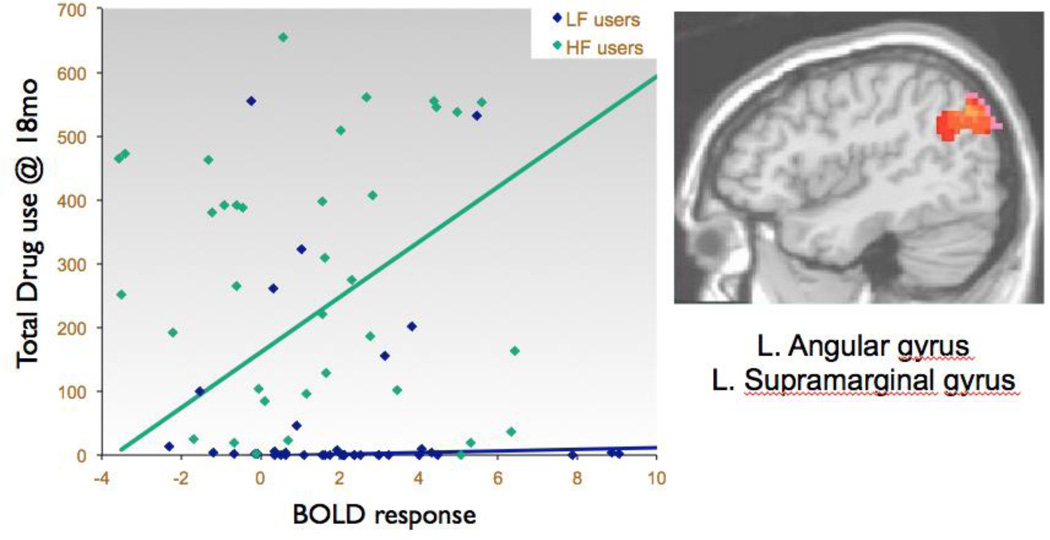
After accounting for the effects of the covariates entered into the first step the regression, the model predicting drug use occasions over the 18-month follow-up was significant in the second step (F(5,74) = 8.11, p<.01, R2Δ=10%). Specifically, user group status (β= .31, p<.05) and baseline no-go BOLD response contrast in the left angular gyrus region (β= .30, p<.05) predicted variability in 18-month follow-up drug use, above and beyond baseline drug use and other covariates. An interaction term (angular gyrus activity × LF/HF group) in the third step of the regression (β= .79, p = .058) trended toward significance, indicating that the relationship between increased angular gyrus activity and greater total drug use at 18 months differed between LF and HF users (see Table 3 and Figure 1). To probe this interaction, the same regression analyses were run separately for LF users and HF users (see Table 3). For HF users, more baseline activation in the angular gyrus predicted a trend for more drug use at 18-month follow-up (F(4,34) = 2.46, p=.06, R2Δ = 18%; β= .48, p<.05), while LF did not show a relationship between baseline activation and substance use outcome.
Table 3.
Hierarchical regressions predicting drug use at 18-month follow-up.
| Full Sample (N=80) |
Low-Frequency Users (n=41) |
High-Frequency Users (n=39) |
||||
|---|---|---|---|---|---|---|
| ΔR2 | β | ΔR2 | β | ΔR2 | β | |
| Step 1 | .25** | .28** | .08 | |||
| fMRI protocol | .09 | .20 | .11 | |||
| Lifetime drug use at baseline | .48** | .51** | .23 | |||
| Step 2 | .10** | .01 | .18† | |||
| Substance use group | .31* | |||||
| Medial prefrontal cortex activation | −.17 | −.13 | −.23 | |||
| Left angular gyrus activation | .30* | .17 | .48* | |||
| Step 3 | .04** | |||||
| Use group × medial prefrontal interaction | −.41 | |||||
| Use group × left angular gyrus interaction | .79† | |||||
p < .01
p < .05
p < .06
Figure 1.
Higher baseline left angular gyrus and supramarginal BOLD response contrast to inhibition trials predicts more drug use occasions in the subsequent 18 months, above and beyond baseline substance use levels (all participants: N=80, β=.30, p<.05; High-Frequency users: n=39; β=.48, p<.05).
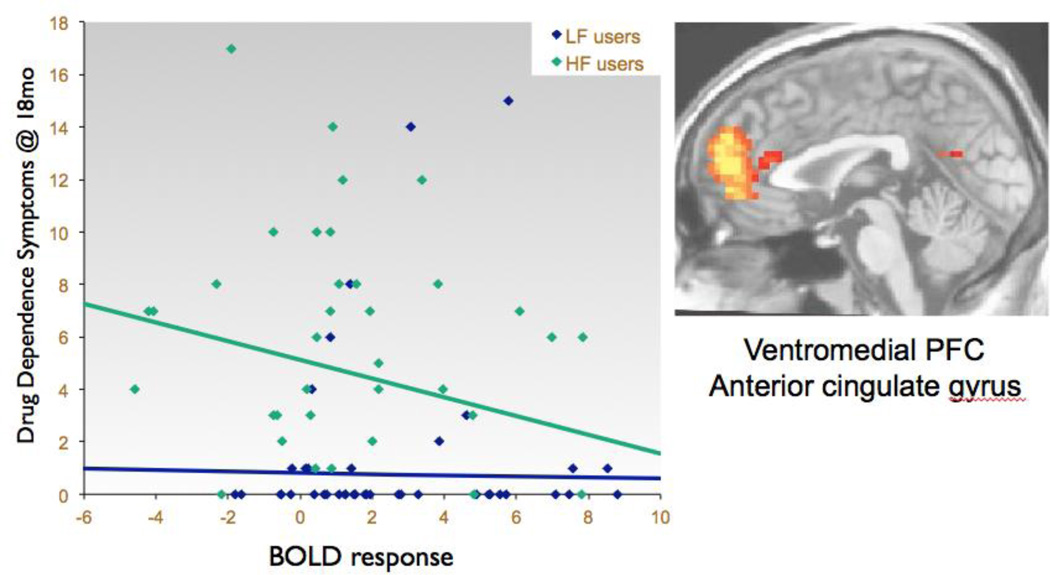
The model predicting drug dependence symptoms over the 18-month follow-up interval was significant in the second step (F(5,74) = 8.19, p<.01, R2Δ = 5%; see Table 4), with a trend for less no-go activation in the vmPFC to predict more drug dependence symptoms over the 18-month follow-up (β= −.22, p = .069; see Table 4 and Figure 2). Regression analyses run separately for each user group revealed that, for HF users, lower baseline no-go activation in the vmPFC predicted more follow-up drug dependence symptoms, above and beyond baseline drug dependence severity and other covariates (F(4,34) = 2.59, p=.05, R2Δ = 10%; β= −.39, p<.05; see Table 4).
Table 4.
Hierarchical regressions predicting drug dependence symptoms at 18-month follow up.
| Full Sample (N=80) |
Low-Frequency Users (n=41) |
High-Frequency Users (n=39) |
||||
|---|---|---|---|---|---|---|
| ΔR2 | β | ΔR2 | β | ΔR2 | β | |
| Step 1 | .30** | .01 | .13† | |||
| fMRI protocol | .02 | .04 | −.01 | |||
| Drug dependence symptoms at baseline | .54** | −.07** | .36* | |||
| Step 2 | .05** | .02 | .10† | |||
| Substance use group | .23 | |||||
| Medial prefrontal cortex activation | −.22‡ | −.02 | .39* | |||
| Left angular gyrus activation | .17 | .18 | .13 | |||
| Step 3 | .02** | |||||
| Use group × medial prefrontal interaction | −.49 | |||||
| Use group × left angular gyrus interaction | −.07 | |||||
p < .01
p < .05
p = .05
p = .07
Figure 2.
Lower baseline medial prefrontal cortex BOLD response contrast to inhibition trials predicts more drug dependence symptoms in the subsequent 18 months, above and beyond baseline symptom levels (all participants: N=80, β=−.22, p=.07; High-Frequency users: n=39; β=−.39, p<.05).
Follow-up alcohol use was not predicted by no-go activation in the vmPFC or angular gyrus, but the model predicting alcohol dependence symptoms over the 18-month follow-up was significant in the second step (F(5,74) = 5.79, p<.01, R2Δ = 6%; see Table 5). Specifically, lower baseline no-go activation in the vmPFC showed a trend for predicting more follow-up alcohol dependence symptoms, above and beyond baseline alcohol dependence and other covariates (β= −.24, p=.06). Separate regression analyses within each user group found that this relationship was driven primarily by the HF users (F(4,34) = 3.45, p<.05, R2Δ = 7%; β= −.31, p = .10; see Table 5). To account for the influence of age on the results, regression analyses were re-run with age included as a covariate. The variable did not contribute significantly to the model and did not change the overall main effects we observed in our findings.
Table 5.
Hierarchical regressions predicting alcohol dependence symptoms at 18-month follow.
| Full Sample (N=80) |
Low-Frequency Users (n=41) |
High-Frequency Users (n=39) |
||||
|---|---|---|---|---|---|---|
| ΔR2 | β | ΔR2 | β | ΔR2 | β | |
| Step 1 | .22** | .03 | .22** | |||
| fMRI protocol | .15 | −.01 | .27† | |||
| Alcohol dependence symptoms at baseline | .44** | .18 | .35* | |||
| Step 2 | .06** | .01 | .07* | |||
| Substance use group | .16 | |||||
| Medial prefrontal cortex activation | −.24† | −.08 | −.31‡ | |||
| Left angular gyrus activation | .11 | .17 | .07 | |||
| Step 3 | .02** | |||||
| Use group × medial prefrontal interaction | −.41 | |||||
| Use group × left angular gyrus interaction | −.14 | |||||
p < .01
p < .05
p = .06
p = .10
4. Discussion
Findings indicated that fMRI activation during a response inhibition task predicted variability in future alcohol and drug use above and beyond variability predicted by past substance use history. This predictive relationship between neural activity in mid-adolescence and substance use behavior in the subsequent 18 months was accounted for by the adolescents with histories of frequent substance use (the HF group) at the time of the imaging. Overall, these results indicated that the utilization of greater brain resources in response to the demands of a response inhibition task may help to identify youth at most risk for later drug and alcohol problems.
As expected, the go/no-go task elicited a cluster of activation in prefrontal regions, including the vmPFC and anterior cingulate cortices. These areas are part of the neural circuitry thought to support cognitive control, particularly response inhibition (Aron, 2007; Liddle, Kiehl, & Smith, 2001). Interestingly, participants also tended to exhibit a region of activation in response to the task centered on the left angular and supramarginal gyri, areas not typically associated with executive functioning. Those participants who were to progress to the highest levels of substance use were the most likely to utilize this unexpected region during inhibition versus non-inhibition trials. Given the involvement of left angular and supramarginal gyri in phonological short-term memory (Henson, Burgess, & Frith, 2000) and mathematical problemsolving (Grabner et al., 2009; van Harskamp, Rudge, & Cipolotti, 2002), it is possible that these participants sometimes employed a verbally-mediated calculation strategy to respond to the go/no-go task, instead of exerting executive control.
Importantly, we found that the observed neural response to the task (in vmPFC and left angular and supramarginal gyri) had a relationship with subsequent substance use. Among the high frequency (HF) substance users, greater reliance on neural circuitry that is atypical for inhibition tasks (left angular and supramarginal gyri) predicted more total drug use occasions during the 18-month follow-up period, suggesting a neurocognitive susceptibility to engage in future drug use. Conversely, greater engagement of the prefrontal/executive circuitry during response inhibition predicted better drug and alcohol outcomes, including for those who were heavy users at the time of the scan. Although unrelated to use occasions, stronger activation in the prefrontal cortex was related to fewer drug and alcohol dependence symptoms at follow-up. Unlike a purely quantitative measure of drug or alcohol use behavior (such as use occasions), a measure of dependence symptoms provides information of how problematic an individual’s drug or alcohol use is to the point of functional impairment, the threshold for which may differ from person to person. Thus, the observed relationship between deficient prefrontal activation during executive functioning and greater dependence symptoms in the future may suggest a neural inefficiency during cognitive control that corresponds to a greater likelihood of failing to selfregulate with regard to drug and alcohol use.
The current findings are consistent with recent cerebral perfusion data, indicating that decreased blood flow in frontal gray matter was associated with greater chance for resumption of drinking harmful amounts of alcohol after short-term abstinence in alcohol-dependent adults (Durazzo et al., 2010). Similarly, Paulus and colleagues (2005) found that after completion of an inpatient drug treatment program, individuals who later relapsed showed less activation in cortical areas important in decision-making, including the dorsolateral prefrontal cortex, compared to individuals who did not relapse. Finally, this study extends our previously reported finding that diminished overall brain fMRI response to inhibition trials on the go/no-go task was found in healthy adolescents who transitioned to heavy alcohol use compared to those who did not initiate heavy alcohol use (Norman et al., in press). Thus, there is accumulating evidence that neural circuitry supporting higher order cognition, particularly the frontal-executive regions underlying cognitive control, may be deficient in individuals who are susceptible to risky substance use behavior in the future.
Limitations to this study include that the sample had a bimodal distribution for substance involvement variables, and given the rigorous eligibility criteria, participants may reflect a high functioning subset of the adolescent substance using population. Furthermore, since this study did not image participants prior to the onset of substance use, it is difficult to ascertain whether the activation pattern that predicted future substance use behavior among the HF group was influenced by their heavy substance use, or whether it was a pre-existing trait.
This study can contribute to the development of approaches for detecting youths who may be susceptible to drug and alcohol, and targeted interventions for at-risk youth. Performance on a go/no-go task was recently found to be predictive of drinking behavior in college age students (Henges & Marczinski, in press). Thus, it may be worth exploring if training in response inhibition could improve this area of weakness for at-risk and substance-involved youth. There are subtle developmental changes in the frontal lobe that take place throughout adolescence (Giedd et al., 1999) and the delay in frontal lobe maturation compared to neural circuitry supporting reward processing may be what predisposes many adolescents to risk-taking behavior (Galvan et al., 2006). Future studies will examine whether fMRI has utility in predicting substance use behaviors for even longer follow up periods, such as early or even later adulthood, and to study what, if any, moderating effects result if samples include comorbid psychiatric disorders.
Highlights.
We examined whether fMRI activation could predict subsequent substance use behavior
Participants were adolescents with varying histories of substance use
Atypical activation during response inhibition predicted more drug and alcohol problems
Predictive relationship was strongest for youth with heavy histories of substance use
Acknowledgements
Role of Funding Sources
This research was supported by grants from the National Institute on Drug Abuse (R01 DA021182, PI: Tapert; P20 DA024194, PI: Mason; P20 DA027834, PI: Paulus) and the National Institute on Alcohol Abuse and Alcoholism (5T32 AA1352505, PI: Riley). NIDA and NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
The authors would like thank Dr. MJ Meloy and Mr. Anthony Scarlett for their expertise and research assistance; and the research participants and families who helped make this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of these findings were presented at the 2010 Research Society on Alcoholism (RSA) Annual Meeting and the 2011 Winter Conference on Brain Research.
Contributors
Authors Mahmood and Tapert designed the study and wrote the protocol. Authors Goldenberg and Migliorini conducted literature searches and provided summaries of previous research studies. Authors Thayer and Simmons conducted statistical analysis and summarized results. Author Mahmood wrote the first draft of the manuscript with specific areas of input from Author Goldenberg. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain and Cognition. 2009 doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- Boyle MH, Offord DR, Racine YA, Szatmari P, Fleming J, Links P. Predicting substance use in late adolescence: results from the Ontario Child Health Study follow-up. American Journal of Psychiatry. 1992;149(6):761–767. doi: 10.1176/ajp.149.6.761. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biological psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol and Drugs. 1998;4(59):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Conner BT, Hellemann GS, Ritchie TL, Noble EP. Genetic, personality, and environmental predictors of drug use in adolescents. Journal of Substance Abuse Treatment. 2010;38(2):178–190. doi: 10.1016/j.jsat.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, Meyerhoff DJ. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010;44(3):201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose, and Throat Journal. 1990;69:763–766. [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology. 2011;30(2):177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–862. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47(2):604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JM. Signal Detection Theory and Psychophysics. New York, NY: John Wiley and Sons Inc.; 1966. [Google Scholar]
- Henges AL, Marczinski CA. Impulsivity and alcohol consumption in young social drinkers. Addictive Behaviors. doi: 10.1016/j.addbeh.2011.09.013. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Burgess N, Frith C. Recoding, storage, rehearsal and grouping in verbal short-term memory: an fMRI study. Neuropsychologia. 2000;38(4):426–440. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use 1975–2009. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2010. [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brainmapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207(1):163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mâsse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry. 1997;54(1):62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13(05):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119(3):216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62(7):761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021(1):391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL. STAI manual for the State-trait anxiety inventory ("self-evaluation questionnaire") Consulting Psychologists Press; 1970. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. 2010 Retrieved from. [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Threedimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Vol. 194. Springer; 2007. pp. 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- van Harskamp NJ, Rudge P, Cipolotti L. Are multiplication facts implemented by the left supramarginal and angular gyri? Neuropsychologia. 2002;40(11):1786–1793. doi: 10.1016/s0028-3932(02)00036-2. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12(3):405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WASI manual. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test. Wilmington: Wide Range; 1993. [Google Scholar]