Abstract
Problem
Although it is established that the levels of individual cytokines are altered by HIV-1 infection, the changes in cytokine interrelations that organize them into networks have been poorly studied. Here, we evaluated these networks in HIV-infected and -uninfected individuals in fluid compartments that are critical for HIV-1 pathogenesis and transmission, namely blood and semen.
Method of Study
In samples collected from therapy-naïve HIV-1-infected and HIV-1-uninfected individuals, we measured HIV-1-load, CD4-cell-count, and levels of 21 cytokines using a multiplex bead-assay.
Results
Cytokine networks in blood and semen were different for HIV-1-infected and -uninfected individuals. In both compartments of HIV-1-infected individuals, the cytokine networks were more interlocked than in controls: HIV -1 infection results in the establishment of new correlations and the strengthening of pre-existing correlations between different cytokines. In blood and semen of HIV-infected patients there were, respectively, 68 and 72 statistically significant correlations between cytokines, while in uninfected individuals there were 18 and 21 such correlations.
Conclusions
HIV-1 infection reorganizes the cytokine networks, establishing new strong correlations between various cytokines and thus imposing a high rigidity on the cytokine network. This rigidity may reflect the impairment of the ability of the immune system to respond to microbial challenges.
Introduction
The majority of HIV-1 transmission worldwide occurs via sexual intercourse with an infected partner, predominantly male. Therefore, semen of HIV-1-infected men represents not only the main vehicle for HIV-1 transmission, but also constitutes an immunological milieu in which the viral variants that transmit infection reside and are selected 1. Cytokines are considered to be the main soluble components that determine such a milieu.
Often, it is assumed that HIV-1 infection is associated with the disruption of the cytokine network and that such a disruption contributes to the immunodeficiency2, 3. Here, we measured the concentrations of individual seminal cytokines and also evaluated their networks by measuring correlations between their concentrations in semen as well as in blood of HIV-1-infected and -uninfected men. We found that HIV-1 decreases the degree of freedom of the cytokine network by imposing more correlations between changes of individual cytokines. A similar effect was noticed in blood. We speculate that to respond successfully to various microbial challenges the network of cytokines should be flexible and that the increase due to HIV-1 in the cytokines’ correlations in this network may be one of the important factors determining HIV-1-mediated immunodeficiency.
Materials and Methods
Blood was collected by the All India Institute of Medical Sciences (AIIMS) from 66 HIV-infected therapy-naïve males who were enrolled in this study between September 2008 and November 2009. Forty seven of the experimental subjects also provided semen samples. HIV-1 infection was detected with ELISA and then confirmed with Western blot and HIV-1 blood plasma qualitative PCR. The presence of genital ulcers, genital discharge, or VDRL (Venereal Disease Research Laboratory test) reactivity was an exclusion criterion for enrollment. Also, blood was collected from 33 HIV-1-uninfected healthy volunteers referred to the AIIMS fertility clinic. Twenty four of these volunteers also provided semen samples.
Collection and analysis of blood and semen were performed in conformity with a protocol approved by the Institutional Review Boards of both NIH and AIIMS. Plasma samples were separated from blood by centrifugation. Semen samples were obtained by masturbation into a sterile container and were centrifuged at 800 g for 10 min within 1 h of ejaculation. The resulting seminal plasma (supernatant) was separated from the centrifugation pellet, aliquoted, and stored at −80°C until further use. We measured the levels of 21 cytokines (indicated in Figure 1) using a multiplex bead array assay (Luminex, Austin, TX). The assay was performed as previously described 4. Because of the nonspecific spontaneous degranulation of CCL5 (RANTES) from platelets upon venipuncture, the measured levels of this cytokine were not included in the analysis of the blood plasma. Values that were below the lower limit of detection were reported as the midpoint between zero and the lower level of detection. Nucleic acids were extracted with the EasyMag 2.0 instrument (Biomerieux, Durham, NC), and a SYBR green real-time PCR assay was used to quantify HIV-1 load. We conducted the amplifications using a set of primers (MH531: 5′-TGTGTGCCCGTCTGTTGTGT-3′; MH532: GAGTCCTGCGTCGAGAGAGC) amplifying 100 base pairs of a highly conserved region of HIV-1gag as described earlier5.
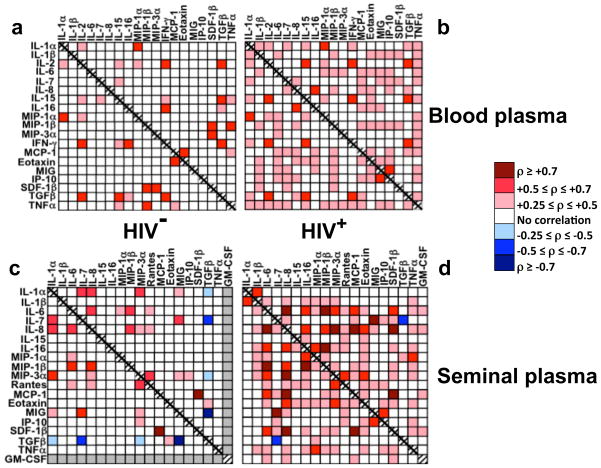
Figure 1. HIV-1 infection affects the cytokine networks in blood and seminal plasma.
Correlations between cytokine concentrations in HIV-1-uninfected (a, c) and HIV-1-infected (b, d) individuals in blood (a, b) and seminal (c, d) plasmas. The levels of the following cytokines were measured: IL-1α, IL-1β, IL-2, IL-6, IL-7, CXCL8 (IL-8), IL-15, IL-16, CCL3 (MIP-1α), CCL4 (MIP-1β), CCL20 (MIP-3α), CCL5 (RANTES), CCL2 (MCP-1), CCL11(eotaxin-1), IFN-γ, CXCL9 (MIG), CXCL10 (IP-10), CXCL12b (SDF-1β), GM-CSF, TNF–α, and TGF-β. Neither IL-2 nor IFN-γ was found in seminal plasma. GM-CSF was not detected in any of the seminal plasma samples from HIV-1-uninfected individuals; therefore, correlations of the concentration of GM-CSF with that of other cytokines were not evaluated. Similarly, as GM-CSF was not detected in blood plasma samples from either HIV-1-infected or -uninfected individuals, correlations of its concentration with those of other cytokines in blood plasma were not evaluated. Because of the nonspecific spontaneous degranulation of CCL5 from platelets upon venipuncture, the measured levels of this cytokine were not included in the analysis of the blood plasma. Statistically significant correlations are presented as a heat map: different shades of red represent positive correlations of various strengths and different shades of blue represent negative correlations of various strengths. White cells in the matrix denote the absence of a statistically significant correlation between cytokines. Note that HIV-1 infection resulted in a dramatic increase of the number of correlations between different cytokines, thus making the cytokine network more interconnected.
We compared continuous variables between groups using the Mann-Whitney U test and assessed the linear association of continuous variables using the non-parametric Spearman’s rank correlation coefficient (ρ), as this is an established method that is less susceptible to uncensored data bias. McNemar’s chi-squared test was used to determine whether the number of correlations differed by HIV-1 status. All tests were 2-tailed, and a p value of 0.05 or lower was considered significant. Multivariate MANOVA analysis of the subsets of cytokines in blood and semen of HIV-1-infected and -uninfected men that resulted in a measured value in all tested samples was performed to exclude a possible effect on the correlations by the cytokines that were below the lower limit of detection.
Results
HIV-1 RNA median blood and semen plasma loads in HIV-1-infected individuals were 4.58 log10 copies/mL (inter-quartile range [IQR]: 2.0–5.2) and 4.05 log10 copies/mL (IQR: 3.2–5.1), respectively. The median CD4 T-cell count was 281 cells/mm3 (IQR: 185–436). In agreement with earlier reports, we found that the levels of individual cytokines were different in blood and seminal plasmas, confirming that these compartments are immunologically distinct 6, 7. Moreover, cytokine production was altered in blood and semen of HIV-1-infected compared with HIV-1-uninfected individuals, with a significant change in the production of 9 and 17 cytokines in blood and seminal plasmas, respectively: In blood plasma there was a significant decrease in the production of CCL4 and CXCL12b (SDF-1β) and an increase in the production of IL-16, CCL20 (MIP-3α), CCL2 (MCP-1), CCL11(eotaxin-1), CXCL9 (MIG), CXCL10 (IP-10), and TNF-α, while in seminal plasma the production of IL-1α, IL-1β, IL-6, IL-7, CXCL8 (IL-8), IL-16, CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL20, CCL2, CCL11, CXCL9, CXCL10, CXCL12b (SDF-1β), TGF-β, and GM-CSF was significantly upregulated (p<0.05). On the basis of their functional roles, we classified the measured cytokines as (i) mediators of innate immunity and inflammation (IL-1α, IL-1β, IL-6, CXCL8, CXCL9, CXCL10, TNF-α), (ii) mediator of hematopoiesis (GM-CSF), (iii) mediators of chemotaxis (CCL3, CCL4, CCL5, CCL20, CCL2, CCL11, CXCL12b), (iv) anti-inflammatory cytokine (TGF-β), and (v) mediators of activation, proliferation, and differentiation of lymphocytes (IL-2, IL-7, IL-16, CCL3, CCL4, CCL5, CCL20). In consideration of their pleotropic effects, CCL3, CCL4, CCL5, and CCL20 were included in two different functional groups, both as mediators of chemotaxis and as mediators of activation, proliferation, and differentiation of lymphocytes.
This classification revealed that chronic HIV-1 infection has an opposite effect on different mediators of chemotaxis in blood plasma: CCL4 and CXCL12b were downregulated while CCL2 and CCL20 were upregulated compared with HIV-1-uninfected individuals. Furthermore, in blood plasma of HIV-1-infected individuals we observed an increased production of two mediators of lymphocyte activation, proliferation, and differentiation (IL-16 and CCL20), together with an increased production of three mediators of innate immunity and inflammation (CXCL9, CXCL10, and TNF-α).
In seminal plasma, with the exceptions of the mediator of innate immunity and inflammation, TNF-α, and the mediator of activation, proliferation, and differentiation of lymphocytes, IL-2 (not detected in any of the samples), all the measured cytokines belonging to the five functional groups were upregulated in HIV-1-infected individuals comparted with HIV-1-uninfected individuals. The upregulated cytokines comprised six mediators of innate immunity and inflammation (IL-1α, IL-1β, IL-6, CXCL8, CXCL9, and CXCL10), seven mediators of chemotaxis (CCL3, CCL4, CCL5, CCL20, CCL2, CCL11, and CXCL12b), the anti-inflammatory cytokine TGF-β, the mediator of hematopoiesis GM-CSF, and six mediators of activation, proliferation, and differentiation of lymphocytes (IL-7, IL-16, CCL3, CCL4, CCL5, and CCL20).
Next, we performed a correlation analyses to reveal possible links between the concentrations of cytokines, HIV-1 viral load, and CD4 cell counts. In agreement with multiple studies published earlier 8, we found a significant negative correlation between the HIV-1 plasma load and the CD4 cell count (ρ = −0.49; p = 0.0001) and a positive correlation between the HIV-1 loads in blood and seminal plasma (ρ = 0.64; p = 0.0001). Also, we found that the CD4 cell count negatively correlates with the blood plasma concentrations of IL-7, CXCL9, CXCL10, and TNF-α (ρ = −0.26, r = −0.34, ρ= −0.51, ρ= −0.26, respectively; p ≤ 0.04). A significant negative correlation was also revealed between the CD4 T-cell count and the seminal plasma levels of CCL2 (ρ=−0.39; p = 0.006). In regard to the HIV-1 viral loads, we found that the seminal HIV-1 RNA levels correlated positively with the seminal concentrations of IL-6, IL-16, CCL2, CCL11, and CXCL12b (respectively ρ = 0.31, ρ = 0.30, ρ = 0.32, ρ = 0.35, ρ = 0.34; p≤ 0.05), while the blood plasma HIV-1 RNA levels correlated positively with the blood plasma concentrations of IL-7, CXCL9, and CXCL10 (ρ = 0.29, ρ = 0.28, ρ = 0.38; p≤ 0.02) and negatively with that of IFN-γ (r = −0.32; p = 0.008).
Although important, the changes in the levels of individual cytokines and their correlation with HIV-1 loads and CD4 cell count give no information as to the global pattern of cytokines’ interactions that organizes them in a network. To quantify the latter, we determined whether individual cytokines change in a coordinated way by measuring the correlations between the production of each cytokine and that of each of the other cytokines. We deduced the organization of the cytokine network from (i) the ρ values, which indicate the signs and strengths of correlations between individual cytokines, and (ii) the total number of statistically significant correlations, which reflects the global pattern of cytokine correlations. Figure 1 shows the results of our analysis as a “heat map” in which the signs (positive or negative) and the strengths [weak (0.3 ≤ρ≤ 0.5), strong (0.5 ≤ρ≤ 0.7), or very strong ( ≥ 0.7)] of the statistically significant correlations are presented.
Both in blood and in seminal plasmas, HIV-1 infection resulted in a significant modification of the interconnections between cytokines belonging to functionally distinct classes: the midpoints of the cumulative distribution functions of all the correlation coefficients were significantly different in HIV-infected and -uninfected individuals (p < 0.05). Furthermore, in the blood and seminal plasmas of HIV-uninfected individuals there were, respectively, 18 and 21 statistically significant correlations between the levels of individual cytokines. In contrast, in HIV-infected patients there were, respectively, 68 and 72 such correlations (p < 0.01). Thus, in HIV-1-infected individuals the cytokine networks in blood and semen become more interlocked: in blood and seminal plasmas of these individuals, respectively 54 and 57 new correlations were established, and 3 and 4 pre-existing correlations increased their strengths. In contrast only 10 correlations, were lost in both blood and seminal plasmas in the course of HIV-1 infection, 3 and 0 decreased their strength in blood and seminal plasma respectively, and 8 and 7 did not change their strength (Fig. 1).
For example, for CCL2 only a correlation with CXCL12b was found in semen of HIV-1-uninfected individuals, while seven new statistically significant correlations were established for this cytokine in the course of HIV-1 infection, including a new strong one with CXCL8. In another example, a weak correlation of CXCL8 with CCL20 (two cytokines both reported to determine HIV-1 transmission 9) in semen of HIV-1-uninfected individuals (ρ= 0.43) became a very strong one upon HIV-1 infection (ρ = 0.74). MANOVA multivariate analysis confirmed that the measured correlations did not result from random error (p<0.01).
Discussion
Cytokines constitute a complex and redundant system of immunomodulators that are changed upon infection by various pathogens as a part of immune defense mechanisms, including a generalized nonspecific immune activation in case of HIV-1 infection. Unfortunately, in this case all these immune reactions fail to protect the infected host that develops immunodeficiency.
Cytokine concentrations correlate with other important hallmarks of HIV disease: We found correlations between the concentrations of various seminal cytokines and viral load/CD4 T-cell counts in infected men. In particular, CD4 T-cell count negatively correlates with the blood plasma concentrations of IL-7, CXCL9, CXCL10, and TNF--α and with the seminal plasma levels of CCL2. In contrast, blood plasma HIV-1 RNA levels correlate positively with the blood plasma concentrations of IL-7, CXCL9, and CXCL10, while seminal HIV-1 RNA levels correlated positively with the seminal concentrations of IL-6, IL-16, CCL2, CCL11, and CXCL12b.
Some of these correlations have not been revealed before; with regard to those that have been reported, our data are in agreement with previous reports. In particular, this applies to the correlations between blood plasma IL-7, CD4 T-cell count, and blood plasma HIV-1 RNA10, 11.
HIV-1 infection dramatically affects correlations between the cytokines themselves: We found that HIV-1 infection results in changes in the levels of the key cytokines. In particular, in seminal plasma 17 of the 21 measured cytokines were upregulated (IL-1α, IL-1β, IL-6, IL-7, CXCL8, IL-16, CCL3, CCL4, CCL5, CCL20, CCL2, CCL11, CXCL9, CXCL10, CXCL12b, TGF-β, and GM-CSF), including mediators of innate immunity and inflammation (IL-1α, IL-1β, IL-6, CXCL9, CXCL10), mediators of hematopoiesis (GM-CSF), mediators of chemotaxis (CCL3, CCL4, CCL5, CCL20, CCL2, CCL11, CXCL12b), an anti-inflammatory cytokine (TGF-β,), and mediators in activation, proliferation, and differentiation of lymphocytes (IL-7, IL-16, CCL3, CCL4, CCL5, CCL20). These changes were different in semen and blood, emphasizing once more that these two body compartments are immunologically different12, 13.
It is widely accepted that cytokines constitute a regulated network with coordinated changes in the levels of individual compounds. Here, we attempted to evaluate the networking of cytokines, quantitatively providing a new insight into how the cytokine network is organized and is altered in the course of HIV-1 infection. This new analysis allowed us to reveal the cross-talk between different cytokines independently of their absolute levels. For example in semen, the upregulated production of CCL5 positively correlated with the production of CCL20, which is a critical chemokine that facilitates HIV-1 infection in the female genital tract 9. However, we showed that, in the course of HIV-1 infection, CCL20 strongly positively correlates with CXCL8, which in its turn upregulates IL-6, IL-16, CCL4, CCL20, CCL2, CCL11, and CXCL12b. Thus, it appears that CCL5 production correlates with that of several pro-inflammatory cytokines and produces a positive feedback on CCL20 production. On the other hand, the production of an immunosuppressive cytokine like TGF-β negatively correlated with that of IL-1α, IL-7, CCL20, and CXCL9 in HIV-1-negative individuals, while after HIV-1 infection only the negative correlation with IL-7 is maintained and the relations with IL-1α, CXCL9, and CCL20 are lost. Although the functions of many of individual cytokines are poorly understood, it appears from our results that in the course of HIV-1 infection more positive correlations among pro-inflammatory cytokines are built, while negative correlations of immunosuppressive cytokines are lost. This may be reflected in the general immunoactivation observed in HIV-1-infected individuals.
Although the number of enrolled individuals prevented us from performing a fine subgroup analysis, the evidence of significant correlations between CD4 cell count, HIV-1 viremia in blood and in seminal plasma, and the amounts of some cytokines suggests that both CD4 cell count and HIV-1 load may determine the complex pattern and the strength of the correlation between cytokines. In its turn the complexity of this pattern is the reflection of a probably even more complex network of immune and non-immune cells responding to various microbial challenges. Therefore, although, our enrollment protocol excludes patients with symptomatic genital ulcers or genital discharge, it is conceivable that besides HIV-1 some other copathogens residing and/or replicating in the genital tract of HIV-1 infected individuals causing only subclinical inflammation contribute to the modulations of the inter-cellular network and thus of the cytokine network.
In summary, our data show that in two immunologically separate compartments, semen and blood, which respectively are crucial for HIV-1 transmission and mirror its pathogenesis14, 15, this virus induces not only quantitative changes in the levels of individual cytokines but also triggers dramatic qualitative changes in the cytokine network by imposing new and stronger correlations between its elements. These correlations may result from a robust and persistent immune response to HIV-1 infection, which triggers general immunoactivation. Whichever the mechanisms responsible for the increase in strength and number of correlations are, they result in a cytokine network that appears more “orderly”.
How can this new order affect the immune system? We speculate that for efficient functioning, all complex multi-element systems have to be flexible and adaptable, allowing their elements to operate relatively independently. The rigidity of a system of strongly interdependent elements may result in diminished capacity to meet complex external challenges. In our case, HIV-1-triggered rigidity of the cytokine network may reflect the fact that the immune system is ‘globally’ engaged and therefore less adaptable to fight other infections or neoplasms that are frequently associated with HIV-1 disease and are fatal for AIDS patients. However, this speculation needs experimental testing 16.
Multiple publications, including ours 4, have demonstrated that in the course of HIV-infection various cytokines are up- or downregulated in blood. Recently, it was found that the same is true for semen 6, 17. These data led to a common notion that in HIV-1-infected individuals the cytokine network deviates from normality and is “disrupted”. Here, we more precisely characterize this “disruption” and introduce a quantitative measure of the network deviation from normality: changes in the number of correlations between various cytokines irrespective of their absolute amounts. With this analysis we have revealed that, in addition to profoundly altering the absolute amounts of different cytokines, HIV-1 infection imposes a qualitatively new order on the cytokine network and its underlying cellular networks, which may contribute to the immunodeficiency. Since for many cytokines their multiple functions are not deciphered yet, we do not know the exact functional meaning of various cytokine correlations and whether they have direct detrimental effects on the immune response by imposing this new order on the cytokine network HIV-1 manages to overcome the immune response, or whether this new order reflects a failed attempt of the activated immune system to fight HIV. In any case, possible triggering of a similar effect by other pathogens might explain why many viral infections (e.g., influenza) make humans more susceptible to other infections (e.g., bacterial pneumonia) that often complicate the course of the primary disease 18.
Whatever the as-yet little-known functional effects and causes of the modulation of the cytokine network are, our study shows that many positive correlations are built especially among pro-inflammatory cytokines in both blood and semen of HIV-1-infected individuals, apparently creating a pro-inflammatory milieu. This milieu may facilitate HIV-1 replication by recruiting and activating HIV-1 target cells and thus ultimately facilitating HIV-1 transmission in the course of sexual intercourse. Also, co-transferred seminal cytokines may affect HIV acquisition by the genital mucosa and HIV-1 dissemination through the receiver’s secondary lymphoid organs.
Future studies designed to identify the molecular mechanisms of HIV-1-mediated increase in cytokine correlations, as well as multivariate analysis on cytokine production in larger cohorts of HIV-1-infected patients, may reveal critical common factors associated with the regulation of the cytokine network in the course of HIV-1 infection and indicate novel targets for antiviral strategies.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health and the NIH Intramural-to-India (I-to-I) Program.
Contributor Information
Andrea LISCO, Email: andrea.lisco2@gmail.com.
Andrea INTROINI, Email: introinia@mail.nih.gov.
Arshi MUNAWWAR, Email: arshimunawwar@yahoo.co.in.
Christope VANPOUILLE, Email: vanpouic@mail.nih.gov.
Jean-Charles GRIVEL, Email: grivelj@mail.nih.gov.
Paul BLANK, Email: blankp@mail.nih.gov.
Sarman SINGH, Email: sarman_singh@yahoo.com.
Leonid MARGOLIS, Email: margolil@mail.nih.gov.
References
- 1.Bronson R. Biology of the male reproductive tract: its cellular and morphological considerations. Am J Reprod Immunol. 2007;65:212–219. doi: 10.1111/j.1600-0897.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 2.Alfano M, Poli G. The cytokine network in HIV infection. Curr Mol Med. 2002;2:677–689. doi: 10.2174/1566524023361925. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 4.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ptak RG, Gallay PA, Jochmans D, Halestrap AP, Ruegg UT, Pallansch LA, Bobardt MD, de Bethune MP, Neyts J, De Clercq E, Dumont JM, Scalfaro P, Besseghir K, Wenger RM, Rosenwirth B. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob Agents Chemother. 2008;52:1302–1317. doi: 10.1128/AAC.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, Tang Y, Hobbs M, Hoffman I, Kazembe P, Jones CD, Borrow P, Fiscus S, Cohen MS, Swanstrom R. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 8.Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, Herndier BG, Andersson J, McCune JM. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 11.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, Asmuth DM, Tenorio AR, Altman JD, Fox L, Moir S, Malaspina A, Morre M, Buffet R, Silvestri G, Lederman MM. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheth PM, Danesh A, Sheung A, Rebbapragada A, Shahabi K, Kovacs C, Halpenny R, Tilley D, Mazzulli T, MacDonald K, Kelvin D, Kaul R. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–48. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 13.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, Grivel JC, Singh S, Margolis L. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 15.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Puronen CE, Sereti I. Immunopathogenesis of asymptomatic chronic HIV Infection: the calm before the storm. Curr Opin HIV AIDS. 2009;4:206–214. doi: 10.1097/COH.0b013e328329c68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berlier W, Bourlet T, Levy R, Lucht F, Pozzetto B, Delezay O. Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol. 2006;36:204–207. doi: 10.1016/j.jcv.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011;203:880–888. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]