Abstract
Chronic mucosal inflammation and tissue damage predisposes patients to the development of colorectal cancer (CRC)1. This association could be explained by the hypothesis that the same factors and pathways important for wound healing also promote tumorigenesis. A sensor of tissue damage should induce these factors to promote tissue repair and regulate their action to prevent development of cancer. IL-22, a cytokine of the IL-10 superfamily, plays an important role for colonic epithelial cell repair, and is increased in the blood and intestine of IBD patients2, 3. This cytokine can be neutralized by the soluble IL-22 receptor, known as the IL-22 binding protein (IL-22BP, IL-22RA2), however the significance of endogenous IL-22BP in vivo and the pathways that regulate this receptor are unknown4, 5. We describe herein that IL-22BP plays a crucial role in controlling tumorigenesis and epithelial cell proliferation in the colon. IL-22BP is highly expressed by dendritic cells (DC) in the colon in steady state conditions. Sensing of intestinal tissue damage via the NLRP3 or NLRP6 inflammasomes led to an IL-18-dependent down regulation of IL-22BP, thereby increasing the ratio of IL-22/IL-22BP. IL-22, which is induced during intestinal tissue damage, exerted protective properties during the peak of damage, but promoted tumor development if uncontrolled during the recovery phase.
Thus the IL-22-IL-22BP axis critically regulates intestinal tissue repair and tumorigenesis in the colon.
IL-22 is produced by innate lymphoid cells (ILCs),TH17 cells, and TH22 cells, particularly at mucosal surfaces5–7. The membrane bound IL-22 receptor 1 (IL-22R1, IL-22RA1) is absent on immune cells, but expressed within tissues, such as the epithelial cells of the gastrointestinal tract and skin4. IL-22 has an important function in the promotion of antimicrobial immunity via induction of antimicrobial peptides, and in tissue repair via induction of epithelial cell proliferation and survival5, 8, 9. However, IL-22 can also promote pathological inflammatory responses in the skin10 or intestine11 in mouse models, and its concentration is increased in a variety of human diseases including psoriasis, rheumatoid arthritis, infections, and IBD5. In line with the pleiotropic role(s) of IL-22, it is known that this cytokine signals via STAT3, which is important for wound healing, but also for tumor development1. However the role of IL-22 during tumor development needs to be clarified, since both inhibitory and promoting effects have been reported (for review see4).
IL-22BP is a soluble IL-22 receptor, which lacks a trans-membrane and intracellular domain. IL-22BP specifically binds to IL-22 but not other IL-10 family members, and prevents the binding of IL-22 to membrane bound IL-22R112–15. The binding of IL-22 to IL-22BP is of 20- to 1000-fold higher affinity compared to its binding to the membrane bound IL-22R13, 16. It is also known that IL-22BP expression is down-regulated in the intestine during tissue damage. However the cellular source of IL-22BP is unclear. Moreover, the mechanism regulating IL-22BP expression and the significance of endogenous IL-22BP in vivo are unknown4, 5.
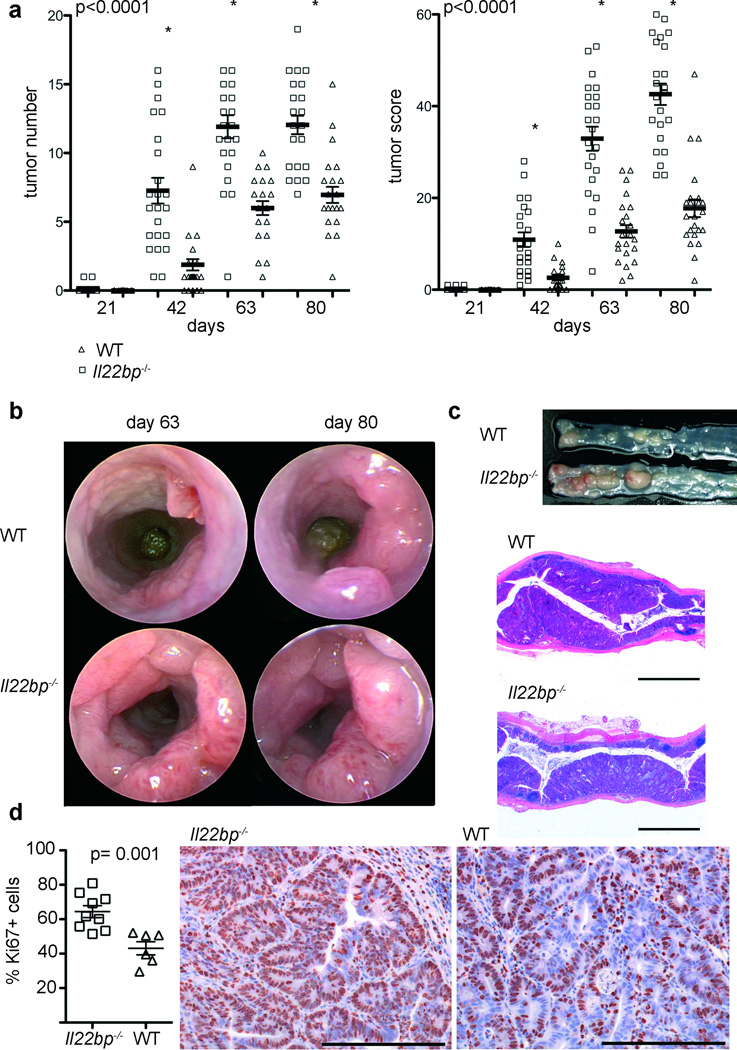
IL-22BP is highly expressed in the colon (Supplemental Figure1) and IL-22 has been suggested to play a role in tumor development. We therefore generated Il22bp−/− mice (Supplemental Figure 2) and used a colitis-associated colon cancer model, which resembles the pathology of human colitis-associated neoplasia17,18 (Supplemental Figure 3), in order to analyze the role of IL-22BP during tumorigenesis in the colon. Interestingly, tumor development was strongly accelerated and the number and size of the tumors were increased in Il22bp−/− compared to wild type control mice (Figure 1A–C). Tumor-morphology was similar between Il22bp−/− and wild type control mice (Figure 1C). Thus, IL-22BP deficiency leads to accelerated and increased tumorigenesis in a colitis-associated colon cancer model.
Figure 1. Increased tumorigenesis in Il22bp−/− mice in a colitis-associated colon cancer model.
A: Time course of the tumor number and tumor score. Representative endoscopic view of the mouse colon at the indicated time points (B), macroscopic view and histology on day 80 (bars= 2000µm) (C). Results are cumulative from two independent experiments. D: Frequency of Ki67+ cells in tumors from wild type and Il22bp−/− mice and representative histology are shown (bars= 200µm). Ki67-expression was analyzed in a total number of 48 KO and 28 WT tumors. Each dot represents one mouse; bars= mean +/− sem.
Inflammation is one of the major drivers of tumor development in the colitis associated colon cancer model1, 18. IL-22 has been reported to have both protective and pathogenic properties during colitis9, 11. However wild type and Il22bp−/− mice showed no difference in colitis disease severity in the acute DSS-induced colitis model (Supplemental Figure 4A–G). In line with a previous report3 we found that Il22bp was down-regulated during acute DSS-colitis, and was not detectable on day 10 of the experiment (Supplemental Figure 4G), which could explain the lack of phenotype in Il22bp−/− mice. We then examined a model of chronic DSS-induced colitis in which DSS is administered for 5 days followed by 16 days of a water regimen for four separate cycles, similar to the colitis associated colon cancer model. No difference in disease severity was observed between wild type and Il22bp−/− in this chronic colitis model (Supplemental Figure 5).
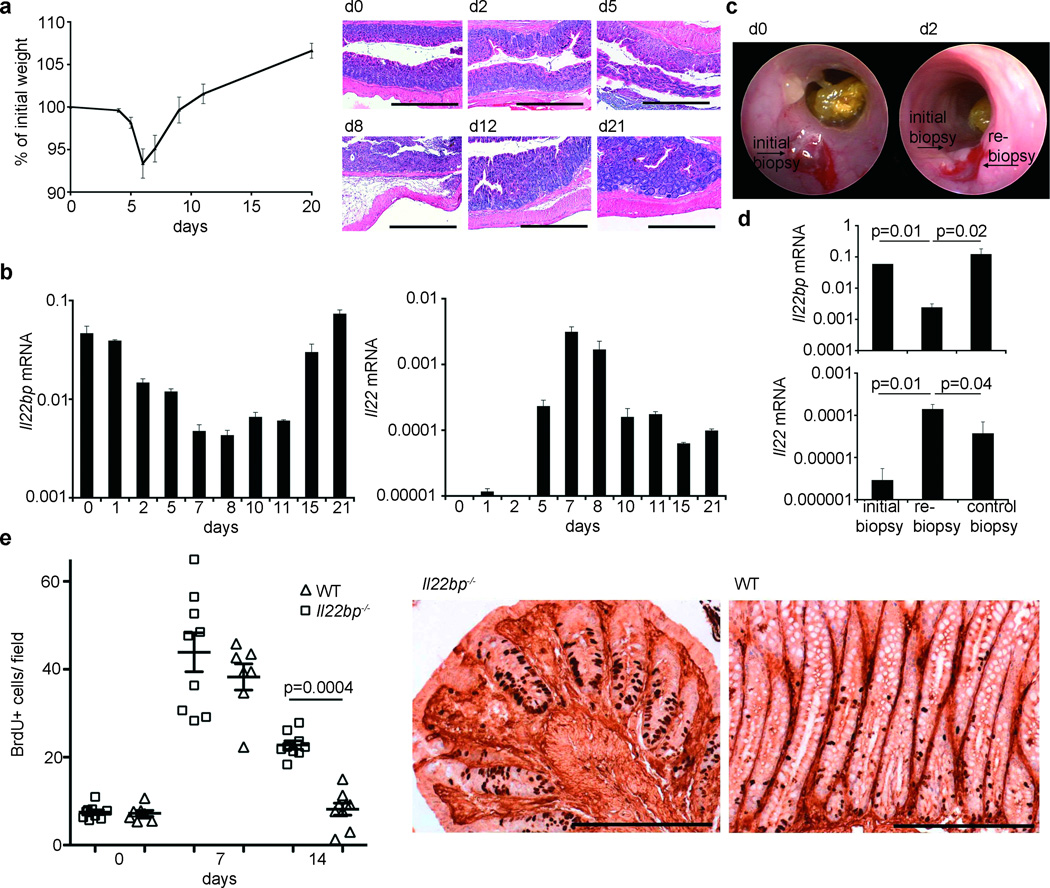
IL-22 is well known to promote epithelial cell proliferation4, 8. Therefore another hypothesis for the increased experimental colon tumorigenesis in Il22bp−/− mice is that the effect of IL-22BP is to inhibit IL-22 induced epithelial cell proliferation. However the significance of endogenous IL-22BP for the control of IL-22 is currently unclear4, 5. Furthermore both IL-22 and IL-22BP expression are modulated during intestinal tissue damage3, 19. We examined the expression of IL-22 and IL-22BP during one cycle of DSS administration for 5 days followed by 16 days of water. Mice treated with DSS lost weight and developed histological signs of colitis (Figure 2 A). The peak of disease occurred between day 7 and 8 (Figure 2 A). Il22bp was expressed under steady state conditions. The lowest expression of Il22bp correlated with the peak of disease. However Il22bp was expressed once again during the recovery phase (Figure 2 A+B). Consistent with previous studies 3, 9, we found increased Il22 levels in the colon with maximal expression between day 7 and 8 (Figure 2B). Accordingly IL-22 serum levels were increased with the same pattern (data not shown). We next validated the inverse expression pattern of IL-22 and IL-22BP in another model of intestinal damage. Following wounding of the colon using an endoscopic biopsy forceps8 (Figure 2C), Il22bp was expressed in the initial biopsy (Figure 2D). However, two days later Il22bp expression was reduced in a re-biopsy close to the wound, but not in a control biopsy taken in a distance of 0.5 cm from the initial biopsy (Figure 2 D). Thus IL-22BP is down regulated upon damage of the epithelial barrier in the colon, but induced once again during the recovery, and IL-22 shows an inverse expression pattern to IL-22BP.
Figure 2. Inverse Expression of Il22bp and Il22 during chemical and mechanical intestinal tissue damage.
A: Weight loss during DSS-colitis (2.5% for 5 days; mean +/− sem; n=11), and histology (bars= 500µm). B: Il22bp and Il22 mRNA (normalized to Hprt) in total colon (mean +/− sem of triplicates). C+D: Il22bp and Il22 mRNA after mechanical wounding (mean +/− sem; n=4). E: Number of BrdU-positive cells per crypt during DSS-colitis and BrdU immunohistochemistry on day 14 (bars= 200µm). Each dot represents one animal. Lines indicate mean +/− sem. Results are representative of at least 3 experiments.
Based on these results we tested if IL-22BP has a significant impact on epithelial cell proliferation during DSS-induced intestinal tissue damage. There was no difference in epithelial cell proliferation between wild type and Il22bp−/− mice on day 0 or 7 of the experiment. However while in wild type mice epithelial cell proliferation on day 14 was comparable to steady state conditions, Il22bp−/− mice continued to demonstrate elevated epithelial cell proliferation (Figure 2E, Supplemental Figure 6). At this time point both Il22 and Il22bp were expressed in the colon (Figure 2B). In line with these data, tumor cells of Il22bp−/− mice proliferated more compared to wild type (Figure 1 D). Thus endogenous IL-22BP is required to terminate the IL-22 induced regenerative program.
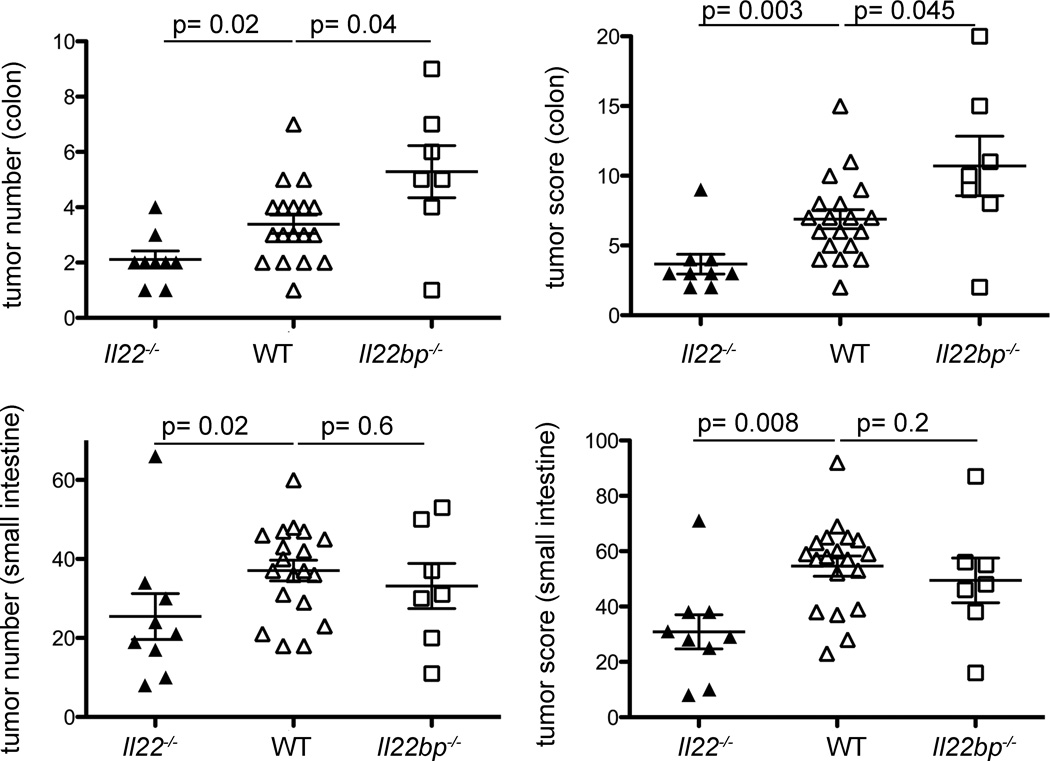
We generated Il22bp−/−Il22−/− double KO (dKO) mice in order to establish that the effect of IL-22BP is dependent on the presence of IL-22. Il22bp−/−Il22−/− mice showed a similar tumor number and score compared to Il22−/− mice, which was lower than the score observed in Il22bp−/− mice. These data confirm that the effect of IL-22BP is dependent on the presence of IL-22 (Supplemental Figure 7). Unexpectedly Il22−/− mice developed a higher tumor load compared to wild type mice (Supplemental Figure 7), which appears to be in contrast to the increased tumor load in Il22bp−/− mice. It is known, however, that Il22−/− mice exhibit an increased disease severity in the DSS-colitis model9. We therefore proposed the hypothesis that IL-22 has a dual function during colitis associated colon cancer: Deficiency of IL-22 might lead to delayed colonic repair and increased intestinal inflammation thereby promoting tumor development. However the increased availability of IL-22 in Il22bp−/− mice during the recovery phase caused prolonged epithelial proliferation, thereby also promoting the development of intestinal tumors. In support of this hypothesis, we confirmed that Il22−/− mice have increased disease and inflammation with marked IL-6 production after DSS administration (Supplemental Figure 8). To further validate our hypothesis we performed two sets of experiments. First we showed, by the administration of neutralizing IL-22 antibody, that IL-22 is protective during the peak of disease, but detrimental during the recovery phase (Supplemental Figure 9). Secondly we used a model of spontaneous tumorigenesis, in which a genetic mutation and not inflammation induces tumor development in the colon. We crossed Il22−/− and Il22bp−/− with Apcmin/+ mice. Min (Multiple intestinal neoplasia) mice carry a dominant mutation in the adenomatous polyposis coli (Apc) gene and develop multiple adenomas throughout their intestinal tract, mainly in the small intestine20. This mouse model resembles the human disease known as familial adenomatous polyposis (FAP), which is also caused by mutations in the APC gene. Humans carrying this mutation develop polyps mainly in the colon and malignant transformation into colon cancer occurs, if untreated, in almost 100% of the cases.
Il22bp−/−Apcmin/+ developed an increased number and size of tumors while Il22−/−Apcmin/+ developed fewer tumors in the colon compared to Apcmin/+. In contrast to the human disease, in the mouse model most tumors are seen in the small intestine. The tumor number and load was also significantly lower in the small intestine of Il22−/−Apcmin/+ compared to wild type mice, although Il22bp−/−Apcmin/+ showed similar tumor development in the small intestine compared to wild type mice (Figure 3). This lack of effect of the Il22bp genotype in the small intestine is in line with the expression of Il22bp, which is high in the colon and low in the small intestine (Supplemental Figure 1). Taken together these data support our hypothesis that the increased tumor burden in Il22−/− mice in the colitis associated colon cancer model is due to the increased susceptibility to DSS-induced colitis. In summary, IL-22 and IL-22BP are regulated during intestinal tissue damage. IL-22 has both protective and detrimental effects during intestinal tissue damage and therefore needs to be controlled via IL-22BP.
Figure 3. IL-22BP controls tumorigenesis in APCmin/+ mice.
Il22bp−/− and Il22−/− mice were crossed with Apcmin/+ mice. 6 months old mice were analyzed. Tumor number and size were measured using a dissecting microscope (size 1: < 2mm, size 2: 2–5 mm, size 3: > 5mm). Each dot present one mouse, lines indicate mean +/− sem. All mice displayed are Apcmin/+. No tumors were observed in Apc+/+ mice regardless of the Il22 and Il22bp genotype.
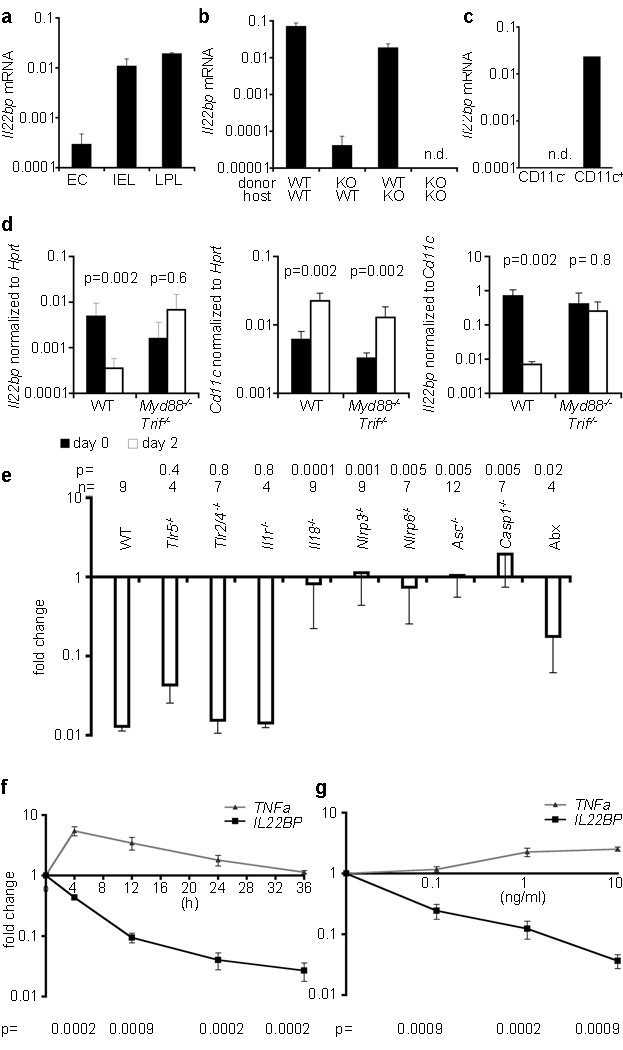
The cellular source of IL-22 and the mechanism regulating IL-22 expression have been studied5, 21. In contrast the mechanism regulating IL-22BP expression is unknown4, 5. IL-22BP expression has been reported in different hematopoietic cells and also in intestinal epithelial cells4, 14. However these studies contradict each other. Interestingly, we observed Il22bp expression in hematopoietic cells and preparations of epithelial cells isolated from the colon (Figure 4A). One caveat, however, was that the purity of the epithelial cells was about 98% and the Il22bp expression was about 50 times lower than in the hematopoietic cells. We used bone marrow chimeras to show that the vast majority of IL-22BP expression in the colon was due to the hematopoietic compartment both in steady state condition (Figure 4B) and in the colitis-associated colon cancer model (Supplemental Figure 10). We next used a stepwise approach to identify the hematopoietic source of IL-22BP in the colon. Il22bp was expressed in TCRbeta− cells, but not in TCRbeta+ cells (data not shown). Within the TCRbeta− cells MHCII+CD11c+ cells were the main source of Il22bp (Figure 4C, Supplemental Figure 10).
Figure 4. IL-18 regulates Il22bp expression by CD11c+ cells.
A–D: Il22bp expression in the colon. A: EC: epithelial cells, IEL, LPL: intraepithelial and lamina propria lymphocytes; mean of triplicates. B: Analysis of bone marrow chimeras (n=8). C: Isolation of CD11c+ cells; mean of duplicates. D: During wounding of WT and Myd88−/−Trif−/− mice (n=3). E: Il22bp expression (re-biopsy relative to initial biopsy; Abx: antibiotic treated WT mice). F+G: IL-22BP expression in cultured human monocyte-derived DC (n=8). (F:10ng/ml IL-18, variable time; G:24h, variable IL-18 concentration). Results are representative of two independent experiments (A–D, F, G), cumulative from 7 experiments (E). Mean+/−sem.
Next we aimed to identify the trigger and the pathway regulating IL-22BP expression. As shown in Figure 2 we found that Il22bp is down regulated at day two after DSS administration prior to the development of histological signs of disease. However it is known that DSS induces the loss of tight junctions within two days, and that the epithelial barrier is therefore permeable, allowing penetrance of bacteria and bacterial products22. Interestingly depletion of the bacterial flora by administration of antibiotics in the DSS-colitis model impaired the down regulation of IL-22BP (data not shown, Figure 4E). We therefore tested, whether the down regulation of IL-22BP is dependent on Myd88/Trif signaling. To that end we used the endoscopic wounding model as described in Figure 2. Strikingly Il22bp was not down regulated in Myd88−/−Trif−/− dKO mice after wounding of the colon. Moreover, expression appeared to be elevated in the wounded dKO mice compared to the unwounded tissue of these mice. As expected, Cd11c was also upregulated in the wounded compared to the unwounded tissue. We therefore normalized Il22bp expression to Cd11c, which showed that the Il22bp expression remained stable in Myd88−/−Trif−/− dKO mice, but was drastically reduced in wild type mice after wounding (Figure 4D). Finally, we could demonstrate that the down regulation of Il22bp was independent of the TLR2, TLR4, TLR5 or IL-1 pathways, but dependent on IL-18 (Figure 4E).
Inflammasomes are cytoplasmic multiprotein complexes, which function as sensors of endogenous or exogenous stress. Inflammasomes typically assemble with the adaptor protein ASC (apoptosis-associated speck-like protein) into a multiprotein complex that leads to caspase1 activation and subsequent cleavage of pro-IL-18 (or pro-IL-1) 23. Accordingly the down regulation of Il22bp was blocked in Caspase1−/− and Asc−/− mice, and we found furthermore that the NLRP3 and NLRP6 inflammasomes but not NLRC4 were important for this process (Figure 4E, data not shown). Inflammasomes can be activated by host-derived factors which are released upon tissue damage, such as ATP, uric acid, and hyaluronan, and also by microbial ligands 23. Using the antibiotic treatment that we validated previously24, we found that the down regulation of Il22bp was partially dependent on the intestinal microbiota (Figure 4E). We recently reported that Nlrp6−/− mice have an altered micro-flora24. We therefore co-housed wild type mice with Nlrp6−/− mice, conditions under which the dysbiotic flora are transferred to co-housed recipients. But the down regulation of Il22bp upon wounding of the wild type intestine was not impaired (fold reduction of Il22bp: WT 0.0123 +/− 0.002, WT (co-housed) 0.0118 +/− 0.0003, Nlrp6−/− (co-housed) 0.405 +/− 0.152). These data show that, although the down regulation of Il22bp is partially dependent on the micro-flora(Fig 4E), it is not affected by the transmissible flora of inflammasome deficient mice.
It is known that IL-18 is up-regulated very early after DSS-induced intestinal tissue damage25, 26. We therefore tested the role of IL-18 in the modulation of IL-22BP in this model. We observed that IL-18 is crucial for the complete down regulation of Il22bp also upon DSS-induced tissue damage (Supplemental Figure 11). While loss of IL-18 strongly reduced the level of down regulation of Il22bp, there was some residual down regulation of Il22bp even in the absence of IL-18, suggesting that other factors may also contribute to the regulation of Il22bp.
We next aimed to further support and extent our murine in vivo data to human biology. We first validated that human DC express IL-22BP, and then we established, in vitro, that IL-18 is able to down regulate IL-22BP in human (Figure 4F + G) and also mouse DC (data not shown) in a time- and dose-dependent manner. Taken together these data show that IL-18 down regulates Il22bp expression by CD11c+ cells upon intestinal wounding.
Recent publications have shown that Il18−/− mice have increased inflammation and tumor development in the colitis associated colon cancer model 26, 27. This is in line with our finding that IL-18 down regulates IL-22BP, and that Il22−/− mice have increased inflammation and tumorigenesis in the colitis associated colon cancer model. However our data do not exclude other important functions of IL-18 besides the regulation of IL-22BP.
Chronic mucosal inflammation and tissue damage as seen in IBD predisposes patients to the development of colorectal cancer, one of the most frequent fatal cancers in the world. This association could be explained by the hypothesis that the same factors and pathways that are important for wound healing, also promote tumorigenesis. Our data indicate that IL-22 is such a factor. Several studies have linked the microbial status with colon tumorigenesis28–30. These data are in line with our results that sensing of microbial ligands and intestinal tissue damage by the NLRP3 and NLRP6 inflammasomes regulates IL-22BP expression by CD11c+ cells via caspase 1-mediated IL-18 activation. This regulation of IL-22BP is crucial to control the effects of IL-22 during intestinal tissue damage and tumorigenesis (Supplemental Figure 12). Therefore we propose a link between the micro-flora, the epithelium, and the immune system regulating the balance between tissue regeneration and tumor development in the intestine. The regulation of IL-22BP via the inflammasome provides an unanticipated mechanism, controlling IL-22 and thereby the development of colon cancer.
Methods summary
Tumor induction
Co-housed mice were injected intraperitoneally with AOM (Sigma) at a dose of 7.5mg/kg body weight. After 5 days, mice were fed 2.5% DSS (MP biomedicals, M.W. =36,000–50,000 Da) in the drinking water for 5 days, followed by 16 days of regular water. This cycle was repeated twice.
Endoscopic Procedures
Colonoscopy was performed in a blinded fashion for colitis and tumor monitoring using the Coloview system (Karl Storz, Germany). Colitis scoring was based on granularity of mucosal surface, stool consistence, vascular pattern, translucency of the colon and fibrin visible (0–3 points for each). Tumor sizes were graded from 1 to 5. The total tumor score per mouse was calculated as sum of all tumor sizes. In some experiments the colon was wounded using the endoscopy and a biopsy forceps. A re-biopsy was taken two days after the wounding close to the initial biopsy. A control biopsy was taken in a distance of about 0.5 cm.
Statistical Analysis
For comparison of groups, the non-parametric two-sided Mann Whitney test or Anova and post-hoc analysis was applied. The significance level α was set to 0.05.
Supplementary Material
Acknowledgements
The authors would like to thank F. Manzo for expert administrative assistance, E. Eynon and J. Alderman for managing the mouse program. We also thank T. Taylor and G. Tokmoulina for expert help with the FACS sorting. R.A.F. is an Investigator of the Howard Hughes Medical Institute. S.H. was supported by a post-doctoral fellowship from the Crohn’s and Colitis Foundation of America, the ‘Stiftung experimentelle Biomedizin’ and the Ernst Jung Foundation. N.G. was supported by an EMBO post-doctoral fellowship. L.A.Z. was supported by a post-doctoral fellowship from the American Cancer Society. A.J.M., D.M.V. and G.D.Y. were employees of Regeneron Pharmaceuticals at the time this work was performed. W.O. is an employee of Genentech Inc.. This work was supported by R01DK077905, DK-P30-34989, and U19-AI082713 (to C.A. and R.A.F.) and by the DFG, SFB841 (to S.H.).
Footnotes
Author contribution
R.A.F., S.H., and N.G. designed the experiments, analyzed the data, and wrote the manuscript. L.A.Z. performed CAC experiments with Il22−/− single KO mice, and provided Il22−/−mice. F.J.H. assisted during the mouse endoscopy. W.O.C. made key suggestions for experiments and edited the manuscript. A.J.M., D.M.V., and G.D.Y. generated Il22bp−/−mice and are employees of Regeneron Pharmaceuticals Inc.. C.J.B. performed the histopathological analyses. W.O. provided IL-22 antibody and is an employee of Genentech. L.B. performed immuno histochemistry. B.H. provided mice for colitis-associated cancer experiments. W.Z., J.H.C, C.A., M.H. did the experiments using human material. S.H. and N.G. performed all other experiments.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand S, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 3.Wolk K, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 4.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL 22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 6.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 7.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 8.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 11.Kamanaka M, et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei CC, Ho TW, Liang WG, Chen GY, Chang MS. Cloning and characterization of mouse IL-22 binding protein. Genes Immun. 2003;4:204–211. doi: 10.1038/sj.gene.6363947. [DOI] [PubMed] [Google Scholar]
- 13.Kotenko SV, et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A. 2001;98:9511–9516. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–7095. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- 16.Jones BC, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure. 2008;16:1333–1344. doi: 10.1016/j.str.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 21.Kinnebrew MA, et al. Interleukin 23 Production by Intestinal CD103(+)CD11b(+) Dendritic Cells in Response to Bacterial Flagellin Enhances Mucosal Innate Immune Defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poritz LS, et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 23.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivakumar PV, et al. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 2010;32:300–302. doi: 10.1016/j.immuni.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 29.Uronis JM, et al. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. plos one. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.