Abstract
In the pathogenesis of pancreatitis, oxidative stress is involved in the activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway and cytokine expression. High serum levels of cholecystokinin (CCK) have been reported in patients with acute pancreatitis, and treatment with cerulein, a CCK analogue, induces acute pancreatitis in a rodent model. Recent studies have shown that cerulein-activated nicotinamide adenine dinucleotide phosphate oxidase elicits reactive oxygen species, which trigger the phosphorylation of the JAK1, STAT1, and STAT3 proteins and induce the production of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1β, and IL-6, in pancreatic acinar cells. The JAK/STAT pathway also stimulates cell proliferation and malignant transformation and inhibits apoptosis in the pancreas. This review discusses the possible role of the JAK/STAT pathway in the pathogenesis of pancreatitis and pancreatic cancer in response to oxidative stress.
Keywords: JAK/STAT, Pancreatitis, Pancreatic cancer, Oxidative stress
INTRODUCTION
Pancreatic oxidative stress occurs during an early stage of pancreatitis.1-3 Scavenger therapy for reactive oxygen species (ROS) showed some success in experimental pancreatitis models, including cerulein pancreatitis.4,5 In human acute pancreatitis, the increased levels of lipid peroxide in the bile or pancreatic tissue and subnormal levels of antioxidant vitamins in the blood were reported.6 Cerulein is an analogue of cholecystokinin (CCK) and causes the maximum pancreatic secretion of amylase and lipase,7,8 cytoplasmic vacuolization, death of acinar cells, edema formation, and infiltration of inflammatory cells into the pancreas,9,10 which are observed in human pancreatitis. The mechanism of action of cerulein is suggested to involve production of large amounts of ROS, activation of oxidant-sensitive transcription factor nuclear factor-κB (NF-κB) and induction of cytokine expression in pancreas.11,12 Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase components have been detected in pancreatic acinar AR42J cells.13 AR42J cells are derived from a transplantable tumor of a rat exocrine pancreas and are tumorigenic in nude mice and maintain many characteristics of normal pancreatic acinar cells.14 Cerulein-induced NF-κB activation and interleukin (IL)-6 expression were inhibited by treatment with the antioxidant rebamipide, diphenylene iodonium (nonspecific inhibitor for NADPH oxidase), or by transfection with antisense oligodeoxynucleotides for NADPH oxidase subunits p22phox and p47phox in pancreatic acinar AR42J cells. Therefore, NADPH oxidase has been considered as a main source of ROS during development of pancreatitis induced by cerulein. Both NF-κB and Janus kinase/signal transducer and activator of transcription (JAK/STAT) are activated in response to ROS in pancreas.11,15 Although there are reports indicating the involvement of NF-κB in the pathogenesis of pancreatitis and pancreatic cancer, only limited data are available on the role of JAK/STAT in the pathogenesis of these diseases. It has been reported that inhibition of JAK1/STAT1 improves the severity of cerulein-stimulated pancreatic injury by inhibiting NF-κB activation, indicating that activation of JAK1/STAT1 is an early event in pancreatic injury.16 Since JAK/STAT mediates cell proliferation and malignant transformation17 as well as inflammatory signaling18,19 in pancreas, we focus our discussion on the possible role of JAK/STAT in the pathogenesis of pancreatitis and pancreatic cancer in this review.
JAK/STAT IN PANCREAS
The JAK family kinases consist of four members JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) in mammals, whereas there are seven STAT family members such as STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. Cytokine receptors constitutively associate with JAKs, which allows activation of the signaling cascade. The binding of a cytokine to its receptor induces conformational changes of the receptor, which subsequently results in the activation of the associated JAKs. Activated JAKs lead to phosphorylation of one or more of seven STATs. Phosphorylation of STATs leads to their dimerization and translocation into the nucleus, in which they bind specific DNA sequences to activate gene transcription. STAT1, STAT2, STAT3, STAT5, and STAT6 are widely distributed in various tissues, whereas STAT4 is restricted mainly to thymus, spleen, testis, myeloid cells, and monocytes.20 In pancreatic tissue and acinar cells, JAK1, JAK2, TYK2, STAT1, STAT2, STAT3, STAT5, and STAT6 are expressed. However, expression of STAT4 is detected in pancreatic tissue but not in isolated pancreatic acinar cells.21 In pancreatic acinar AR42J cells, tumor necrosis factor-α (TNF-α) stimulates the activation of STAT1, STAT3, and STAT422 and STAT3 and suppressor of cytokine signaling 3 (SOCS3) which are all signaling pathways involved in tissue inflammation.23 TNF-α and TNF-α receptors (TNFR1 and TNFR2) have been shown to be expressed in normal rat pancreatic acinar cells and cerulein upregulated the expression of TNF-α receptors24 and activated JAK2/STAT3 and IL-1β in pancreatic acinar cells.25 Together these studies suggest that STAT3 activation by cerulein is mediated by TNF-α signaling in pancreas (Fig. 1).
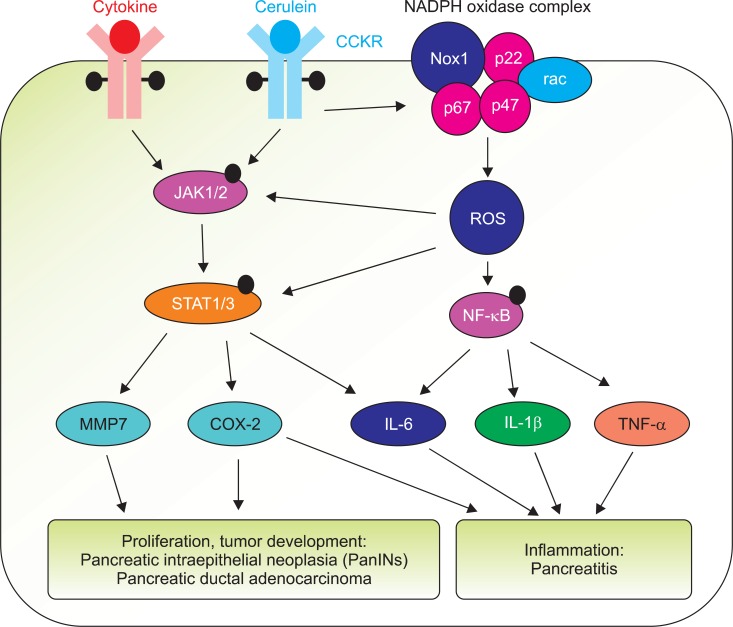
Fig. 1.
Possible role of Janus kinase/signal transducers and activators of transcription (JAK/STAT) in the pathogenesis of pancreatitis and pancreatic cancer development. Cerulein/cholecystokinin receptor (CCKR) or cytokine (including tumor necrosis factor-α [TNF-α])/cytokine receptor activates JAK(1/2)/STAT(1/3) which induces the expression of inflammatory cytokines (interleukin [IL]-6, IL-1β, TNF-α) and cancer-related genes (metalloproteinase 7 [MMP7], cyclooxygenase [COX]-2) in pancreas. COX-2 mediates the development of pancreatitis. Cerulein/CCKR may activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (Nox1, p67, p22, p47, a small G protein rac) and produce reactive oxygen species (ROS) which in turn activates JAK/STAT as well as nuclear factor-κB (NF-κB), and induction of inflammatory genes and COX-2 in pancreas. Prolonged induction of MMP7 and COX-2 may lead to the development of pancreatic intraepithelial neoplasia (PanINs) and pancreatic ductal adenocarcinoma. Black dots on cytokine receptor, CCKR, JAK, STAT, and NF-κB indicate phosphorylated sites of each protein.
CCK AND JAK/STAT IN PANCREAS
Although various hormones such as growth hormone and prolactin have been reported to activate the JAK/STAT pathway,26 there have been little studies on the involvement of JAK/STAT on CCK signaling. A CCK analogue cerulein triggers phosphorylation of JAK1, STAT1, and STAT3 proteins and induce inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in pancreas.27 CCK receptors are a group of G-protein coupled receptors (GPCRs) which bind two subtypes of CCK, CCK1, and CCK2. CCK2 receptor (CCK2R) upon binding of CCK activated the JAK2/STAT3 pathway in pancreatic acini in transgenic mice expressing CCK2R.17 The mechanism of JAK2 activation involves Gq proteins and requires the NPXXY motif, located at the end of the seventh transmembrane domain of CCK2R. This motif, which is critical for Gq-dependent signaling pathways, is also required for STAT3 activation by CCK2R. Thus, JAK/STAT signaling is involved in the proliferative effect of CCK2R, which contributes to the increase in the growth of pancreas and pancreatic tumor development.
OXIDATIVE STRESS AND JAK/STAT IN PANCREAS
The STAT family of transcription factors is known to be activated by various cytokines and growth factors and may be activated by oxidative stress. In epithelial cells and fibroblasts, activation of STAT3 and STAT1 in response to H2O2 occurs within minutes.15 ROS act as upstream signaling molecules for JAK2 activation in smooth muscle cells.28 The mechanism of H2O2 activation of STAT proteins involves activation of the JAK2 and TYK2 kinases. STATs are phosphorylated in response to H2O2 subsequent to the phosphorylation of JAK2 and TYK2 kinases.15 The possible mechanism for activation of JAK2 by H2O2 is inactivation of protein tyrosine phosphatases (PTPs). Using PTP inhibitors vanadate and pervanadate results in STAT activation.29,30 In human pancreatic cancer MIA PaCa-2 and PANC-1 cells, NADPH oxidase 4 (NOX4), which produces ROS, mediates the sustained phosphorylation of JAK2.31 NOX4 inhibits PTP, leading to enhanced and sustained phosphorylation of JAK2 and suppresses apoptosis of pancreatic cancer cells. These studies demonstrate that ROS mediate activation of JAK/STAT and inhibit apoptotic cell death of pancreatic cancer cells.
JAK/STAT IN PANCREATITIS
We previously reported that activation of JAK2/STAT3 results in edematous and inflammatory changes in the pancreas and increased serum levels of IL-1β in rats with experimental pancreatitis.19 The maximal lung injury after cerulein-induced acute pancreatitis occurs in STAT4- or STAT6-deficient mice.32 In pancreatic acinar cells stimulated with TNF-α, the activation of JAK2/STAT3 mediated the development of pancreatic injury.22 Inhibition of JAK/STAT as well as NF-κB improved the severity of pancreatitis.27 Since ROS mediates induction of inflammatory cytokines and NADPH oxidase produces ROS in pancreas, inhibition of NADPH oxidase may be beneficial for the prevention and the treatment of pancreatitis by suppressing JAK2/STAT3 and mitogen activated protein kinases (MAPKs) and expression of TGF-β1 in pancreatic acinar cells.25 Lipopolysaccaride (LPS), TNF-α, and cerulein are potent mediators of STAT3 and SOCS3 expression as well as the induction of IL-6 and IL-1β in pancreas.23,33 Targeting MAPKs, NF-κB, and STAT3 pathways by dexamethasone treatment downregulated the expression of chemokines during the course of acute pancreatitis, resulting in decreased severity of the disease in the rats with bile duct ligation.34 Administration of cerulein and LPS induced an increase in serum amylase and IL-6 levels, severe acute pancreatitis, pancreatitis-associated lung injury, and phosphorylation of STAT3 in the pancreas. Blocking IL-6 suppresses STAT3 activation in the pancreas and consequently attenuated the severity of pancreatitis by promoting pancreatic acinar cell apoptosis.35 Pancreatitis-associated ascitic fluid (PAAF) which is known to contribute to the progression of acute pancreatitis activated the expression of monocyte chemoattractant protein-1 (MCP-1) and induced the phosphorylation of p38-MAPK, degradation of IκBα, and increases in nuclear level of p65, and STAT3 activity in rat pancreatic acinar cells.36 It was suggested from this study that acinar cells are activated by PAAF to produce MCP-1, mediated mainly by the activation of NF-κB and STAT3 pathways. In early acute pancreatitis in the rat, oxidant-mediated activation of MAPK, NF-κB, and STAT3 triggers the expression of chemokines in acini but not in non-acinar cells in the pancreas.37 Cyclooxygenase-2 (COX-2)-derived bioactive lipids including prostaglandin E2 are potent inflammatory mediators that promote tumor growth and metastasis by stimulating cell proliferation, invasion and angiogenesis.38 In COX-2 deficient mice, deletion of COX-2 gene prevented the histologic and inflammatory changes associated with pancreatitis compared with the wild-type littermates, indicating that COX-2 is an important regulator of the severity of acute pancreatitis.38 In the rats treated with a JAK/STAT inhibitor, AG490, the expression of COX-2, IL-1β, and IL-6 induced by cerulein was inhibited by the suppression of STAT activation.27 Therefore, STAT may play an important regulatory role in the expression of inflammatory cytokine IL-6 and COX-2 in cerulein-treated pancreatic acini.
CCK AND JAK/STAT IN PANCREATIC CANCER
JAK2/STAT3 pathway is activated by the CCK2R in pancreatic tumor cells and contributes to cell proliferation.17 Targeted expression of CCK2R, a GPCR, in mouse pancreatic acinar tissue led to the activation of JAK2 and STAT3.16 The activation of JAK2/STAT3 increased growth of the pancreas and resulted in the development of preneoplastic lesions, which is similar to those found in human pancreatic cancers. Deregulation of JAK2/STAT3 pathway by CCK2R represents an early step in pancreatic carcinogenesis, contributing to cell proliferation and pancreatic tumor development.17 Recent studies indicate that STAT3 controls the development of the earliest premalignant pancreatic lesions, acinar-to-ductal metaplasia and pancreatic intraepithelial neoplasia (PanIN).39 STAT3 directly regulates vascular endothelial growth factor expression and hence angiogenesis, growth, and metastasis of human pancreatic cancer FG and PANC-1 cells.40 On malignant transformation, activated STAT3 promotes cellular proliferation by acceleration of G1/S-phase progression and thereby contributes to the malignant phenotype of human pancreatic cancer CAPAN-1 cells.41 The deregulation of JAK2/STAT3 pathway by CCK2R represents an early step contributing to cell proliferation and pancreatic tumor development.17
The transcription factor pancreatic and duodenal homeobox factor 1 (PDX-1) is expressed in pancreatic progenitor cells. In exocrine pancreas, PDX-1 is down-regulated during late development, while re-up-regulation of PDX-1 has been reported in pancreatic cancer and pancreatitis. The pancreas of PDX-1 expressing transgenic mouse was markedly small with the replacement of acinar cells by duct-like structures (acinar cell-ductal metaplasia), accompanied by activated STAT3. Genetic ablation of STAT3 in the transgenic pancreas profoundly suppressed the metaplastic phenotype. These results provide a mechanism of pancreatic metaplasia by which persistent PDX-1 expression induces acinar-to-ductal transition through STAT3 activation.42 Inactivation of IL-6 transsignaling or STAT3 inhibits PanIN progression and reduces the development of pancreatic ductal adenocarcinoma (PDAC). Aberrant activation of STAT3 through homozygous deletion of SOCS3 in the pancreas accelerates PanIN progression and PDAC development.43 Thus, inflammatory mediator STAT3 is a critical component of spontaneous and pancreatitis-accelerated PDAC precursor formation and contributes to cell proliferation, metaplasia-associated inflammation, and matrix metalloproteinase 7 (MMP7) expression during neoplastic development. It was also shown that STAT3 signaling enforces MMP7 expression in PDAC cells and that MMP7 deletion limits tumor size and metastasis in mice. These studies suggest that STAT3 and MMP7 are important mediators for PDAC initiation and progression.44 In cultured human pancreatic cancer Su 86.86 cells, COX-2 was induced by treatment with tumor-promoting phorbol esters45 and in COX-2-positive pancreatic cancer BxPC-3 cells, COX-2 inhibitor reduced angiogenesis and growth.46 Recently, a novel pathway in which COX-2 activates STAT3 by inducing IL-6 expression has been suggested in non-small cell lung cancer cells.47 Together, these studies provide a rationale for the development of STAT3, IL-6, COX-2, and MMP7 targeted therapy for the treatment of pancreatic cancer.
CONCLUSIONS
ROS are critical mediator in inflammatory process in initiation and development of pancreatitis. In addition to NF-κB, ROS activates JAK/STAT pathway, which regulates inflammatory gene expression, cell proliferation, and transformation in pancreas. Thus, the activation of NF-κB and JAK/STAT seems to be the molecular mechanisms underlying the pathogenesis of pancreatitis and pancreatic cancer. Inhibition of either JAK/STAT or NF-κB may alleviate inflammation and carcinogenesis of pancreatic tissues. Therefore, JAK/STAT may serve as the potential therapeutic targets in the development of new drugs in the treatment of pancreatitis and/or pancreatic cancer.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) funded by Ministry of Education, Science and Technology (2011-0001177). Hyeyoung Kim is grateful to the Brain Korea 21 Project, Yonsei University College of Human Ecology.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Schoenberg MH, Büchler M, Gaspar M, et al. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough DB, Boyle B, Joyce WP, et al. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br J Surg. 1990;77:1256–1259. doi: 10.1002/bjs.1800771119. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Seo JH, Lim JW, Kim H. Membrane proteome analysis of cerulein-stimulated pancreatic acincar cells: implication of acute pancreatitis. Gut Liver. 2010;4:84–93. doi: 10.5009/gnl.2010.4.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisner J, Green D, Ferrell L, Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988;29:1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H. Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann NY Acad Sci. 2011;1229:99–102. doi: 10.1111/j.1749-6632.2011.06107.x. [DOI] [PubMed] [Google Scholar]
- 6.Scott P, Bruce C, Schofield D, Shiel N, Braganza JM, McCloy RF. Vitamin C status in patients with acute pancreatitis. Br J Surg. 1993;80:750–754. doi: 10.1002/bjs.1800800632. [DOI] [PubMed] [Google Scholar]
- 7.Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989;10:418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989;257(2 Pt 1):G202–G209. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- 9.Gorelick FS, Adler G, Kerin HF. Cerulein-induced pancreatitis. In: Go VW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The pancreas: biology, pathobiology, and disease. 2nd ed. New York: Raven; 1993. pp. 501–526. [Google Scholar]
- 10.Willemer S, Elsässer HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24(Suppl 1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- 11.Yu JH, Lim JW, Namkung W, Kim H, Kim KH. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab Invest. 2002;82:1359–1368. doi: 10.1097/01.lab.0000032377.09626.c7. [DOI] [PubMed] [Google Scholar]
- 12.Kim H. Cerulein pancreatitis: oxidative stress, inflammation, and apoptosis. Gut Liver. 2008;2:74–80. doi: 10.5009/gnl.2008.2.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu JH, Lim JW, Kim H, Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2005;37:1458–1469. doi: 10.1016/j.biocel.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Jessow NW, Hay RJ. Characteristics of two rat pancreatic exocrine cell lines derived from transplantable tumors. In Vitro. 1980;16:212. [Google Scholar]
- 15.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275(6 Pt 1):C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Huang L, Zhang Y, Qiao M, Yao W, Yuan Y. The antagonist of the JAK-1/STAT-1 signaling pathway improves the severity of cerulein-stimulated pancreatic injury via inhibition of NF-kappaB activity. Int J Mol Med. 2011;27:731–738. doi: 10.3892/ijmm.2011.632. [DOI] [PubMed] [Google Scholar]
- 17.Ferrand A, Kowalski-Chauvel A, Bertrand C, et al. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 18.Ihle JN, Witthuhn BA, Quelle FW, et al. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 19.Yu JH, Kim KH, Kim H. Suppression of IL-1beta expression by the Jak 2 inhibitor AG490 in cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol. 2006;72:1555–1562. doi: 10.1016/j.bcp.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Jatiani SS, Baker SJ, Silverman LR, Reddy EP. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallmeier E, Schäfer C, Moubarak P, et al. JAK and STAT proteins are expressed and activated by IFN-gamma in rat pancreatic acinar cells. J Cell Physiol. 2005;203:209–216. doi: 10.1002/jcp.20216. [DOI] [PubMed] [Google Scholar]
- 22.Robinson K, Vona-Davis L, Riggs D, Jackson B, McFadden D. Peptide YY attenuates STAT1 and STAT3 activation induced by TNF-alpha in acinar cell line AR42J. J Am Coll Surg. 2006;202:788–796. doi: 10.1016/j.jamcollsurg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Vona-Davis LC, Frankenberry KA, Waheed U, Peterson E, McFadden DW. Expression of STAT3 and SOCS3 in pancreatic acinar cells. J Surg Res. 2005;127:14–20. doi: 10.1016/j.jss.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju KD, Lim JW, Kim KH, Kim H. Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-beta1 in the pathophysiology of acute pancreatitis. Inflamm Res. 2011;60:791–800. doi: 10.1007/s00011-011-0335-4. [DOI] [PubMed] [Google Scholar]
- 26.Schindler C, Strehlow I. Cytokines and STAT signaling. Adv Pharmacol. 2000;47:113–174. doi: 10.1016/s1054-3589(08)60111-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen P, Huang L, Zhang Y, Qiao M, Yao W, Yuan Y. The antagonist of the JAK-1/STAT-1 signaling pathway improves the severity of cerulein-stimulated pancreatic injury via inhibition of NF-κB activity. Int J Mol Med. 2011;27:731–738. doi: 10.3892/ijmm.2011.632. [DOI] [PubMed] [Google Scholar]
- 28.Frank GD, Mifune M, Inagami T, et al. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003;23:1581–1589. doi: 10.1128/MCB.23.5.1581-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David M, Romero G, Zhang ZY, Dixon JE, Larner AC. In vitro activation of the transcription factor ISGF3 by interferon alpha involves a membrane-associated tyrosine phosphatase and tyrosine kinase. J Biol Chem. 1993;268:6593–6599. [PubMed] [Google Scholar]
- 30.Haque SJ, Flati V, Deb A, Williams BR. Roles of protein-tyrosine phosphatases in Stat1 alpha-mediated cell signaling. J Biol Chem. 1995;270:25709–25714. doi: 10.1074/jbc.270.43.25709. [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Edderkaoui M, Truong P, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Simovic MO, Ballard BR, Gray KD, Stain SC. The STAT4 and STAT6 pathways in pancreatitis-associated lung injury. J Surg Res. 2007;137:10–15. doi: 10.1016/j.jss.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 2008;40:677–688. doi: 10.1016/j.biocel.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Yubero S, Ramudo L, Manso MA, De Dios I. Mechanisms of dexamethasone-mediated chemokine down-regulation in mild and severe acute pancreatitis. Biochim Biophys Acta. 2009;1792:1205–1211. doi: 10.1016/j.bbadis.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Chao KC, Chao KF, Chuang CC, Liu SH. Blockade of interleukin 6 accelerates acinar cell apoptosis and attenuates experimental acute pancreatitis in vivo. Br J Surg. 2006;93:332–338. doi: 10.1002/bjs.5251. [DOI] [PubMed] [Google Scholar]
- 36.Ramudo L, Yubero S, Manso MA, Vicente S, De Dios I. Signal transduction of MCP-1 expression induced by pancreatitis-associated ascitic fluid in pancreatic acinar cells. J Cell Mol Med. 2009;13:1314–1320. doi: 10.1111/j.1582-4934.2008.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yubero S, Ramudo L, Manso MA, De Dios I. The role of redox status on chemokine expression in acute pancreatitis. Biochim Biophys Acta. 2009;1792:148–154. doi: 10.1016/j.bbadis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Ethridge RT, Chung DH, Slogoff M, et al. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322. doi: 10.1053/gast.2002.35951. [DOI] [PubMed] [Google Scholar]
- 39.Corcoran RB, Contino G, Deshpande V, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 41.Scholz A, Heinze S, Detjen KM, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 42.Miyatsuka T, Kaneto H, Shiraiwa T, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda A, Wang SC, Morris JP, 4th, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 46.Eibl G, Takata Y, Boros LG, et al. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Res. 2005;65:982–990. [PubMed] [Google Scholar]
- 47.Dalwadi H, Krysan K, Heuze-Vourc'h N, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11:7674–7682. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]