Abstract
Background/Aims
The rate of diagnosis of gastric adenoma has increased because esophagogastroduodenoscopy is being performed at an increasingly greater frequency. However, there are no treatment guidelines for low-grade dysplasia (LGD). To determine the appropriate treatment for LGD, we evaluated the risk factors associated with the categorical upgrade from LGD to high grade dysplasia (HGD)/early gastric cancer (EGC) and the risk factors for recurrence after endoscopic treatment.
Methods
We compared the complication rates, recurrence rates, and remnant lesions in 196 and 56 patients treated with endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR), respectively, by histologically confi rming low-grade gastric epithelial dysplasia.
Results
The en bloc resection rate was significantly lower in the EMR group (31.1%) compared with the ESD group (75.0%) (p<0.001). However, no significant difference was observed in the prevalence of remnant lesions or recurrence rate (p=0.911) of gastric adenoma. The progression of LGD to HGD or EGC caused an increase in the incidence of tumor lesions >1 cm with surface redness and depressions.
Conclusions
For the treatment of LGD, EMR resulted in a higher incidence of uncertain resection margins and a lower en bloc resection rate than ESD. However, there was no signifi cant difference in recurrence rate.
Keywords: Adenoma, Low-grade dysplasia, Endoscopic mucosal resection, Endoscopic submucosal dissection
INTRODUCTION
Because esophagogastroduodenoscopy is frequently performed during regular health checkups and for other various reasons, the diagnosis rate of gastric adenoma is increasing. In particular, a diagnosis of high-grade dysplasia (HGD, category 4 according to the Vienna classification) with forceps biopsy strongly suggests the presence of invasive carcinoma. HGD may be changed to invasive carcinoma following histological analysis after endoscopic resection. Therefore, HGD is considered an obvious precancerous lesion, requiring aggressive treatment such as surgery or endoscopic submucosal dissection (ESD).1-7
However, the risk of progression from low-grade dysplasia (LGD) to gastric cancer is relatively low (approximately 3% to 9%).8,9 Nonetheless, LGD can progress to invasive carcinoma4 or even advanced gastric cancer during follow-up.8 Recently, multiple studies have shown that specimens obtained by forceps biopsy are not representative of the entire lesion.
Therefore, endoscopic en bloc resection and complete resection are the preferred treatment methods for gastric adenoma. For this reason, ESD is favored by some clinicians as the preferred endoscopic treatment for gastric adenoma with LGD detected by histological examination.10,11 ESD has the advantage of en bloc resection and complete resection regardless of the lesion size. However, the procedure takes a long time, has a high risk of developing complications, and requires sophisticated skills.12
Therefore, this study evaluated the risk factors for the pathological change of LGD to HGD or early gastric cancer after endoscopic treatment. To determine whether ESD or endoscopic mucosal resection (EMR) is a more appropriate treatment for LGD, we compared the rates of complications and lesion recurrence as well as the presence of remnant lesions after performing the two methods.
MATERIALS AND METHODS
1. Patients
This study included patients who were diagnosed with LGD by histological examination with tissue forceps and who underwent EMR or ESD between July 2006 and July 2010 at the Chungnam National University Hospital. Retrospective analyses were initially performed on 260 patients who were followed up for one or more years after endoscopic treatment; eight patients who underwent gastrectomy were excluded because of differences in anatomy leading to different operation times and complications.
Ultimately, 252 patients were included in this study. EMR and ESD were performed in 77.8% and 22.2% of the subjects, respectively. Follow-up endoscopy was performed at 3, 6, and 12 months after procedure. The median of the follow-up period was 757 days. Because of the limitations of a retrospective study, there are no exact indications for EMR and ESD.
2. Evaluation of endoscopic features
Three endoscopists (H.Y. Jeong, J.K. Sung, and H.S. Moon) performed the study; each performed both diagnostic and therapeutic endoscopies. Endoscopic reports and photographs of the procedures were reviewed for morphological type, color, size, and location of the lesions. Independently, three endoscopists who performed endoscopy decide the cahratersitics of the lesions.
The surface gross type (i.e., pedunculated, elevated, flat, or depressed), color change (i.e., whitish, mixed, or red), ulceration, nodularity, and location of the lesions were also evaluated. During the first year, follow-up observations were performed at 3, 6, and 12 months after procedure. When endoscopic findings showed overgrowth, histological examination was performed to verify the recurrence of the lesion or presence of a remnant lesion. Thereafter, follow-up endoscopy was performed on a yearly basis.
3. EMR/ESD techniques
The subjects who were diagnosed with LGD (category 3 according to the Vienna classification) by endoscopic forceps biopsy and who underwent endoscopic resection such as EMR (i.e., EMR using a cap [EMR-C]13 or EMR with pre-cutting [EMR-P]14) or ESD15 were analyzed prospectively.
The three endoscopists performed all of the EMRs and ESDs, and three pathologists diagnosed most of the cases. Midazolam was administered intravenously for sedation, and cardiorespiratory functions were monitored. The EMR procedure was performed using a single-channel gastroduodenoscope (GIF H260; Olympus, Tokyo, Japan) or a double-channel gastroduodenoscope (GIF-ITQ 260M; Olympus).
Prior to the endoscopic resection, tumor shapes and margins were identified by spraying 0.1% indigo carmine on the lesions. After confirming the lesion, the border between the lesion and normal mucosa was electrosurgically marked approximately 1 to 2 mm away from the lesion. Resection was performed from outside of the marking to obtain secure margins.
Marking facilitated the identification of resection completeness and orientation of resected specimens.13,14 After completely marking the border, normal saline with dilute epinephrine (1:10,000) was injected into the submucosa near the tumor by using needle forceps until the tumor was completely elevated. For EMR, the lesion was elevated by retraction with grasping forceps that were passed through a polypectomy snare loop.14,15 After snaring, the lesion was resected. For ESD, an initial circumferential incision was performed around the lesion, and the lesion was then dissected using an electrosurgical knife (IT knife and/or a Hook knife) after submucosal injection.
An en bloc resection was defined as a resection in which the tumor was resected in a single piece. A complete resection was defined as a resection in which the resected tumor had tumor-free lateral and deep margins. In piecemeal resection complete resection was defined even if en bloc resection was not performed if the lesion was reconfigurable and the resection margin of the reconfigured lesions showed no dysplasia.
The following complications were assessed: immediate massive bleeding, which was defined as bleeding occurring within 24 hours after the endoscopic resection;14 and delayed bleeding, which was defined as gastrointestinal bleeding occurring later than 24 hours after the endoscopic resection.
Perforation was readily observed endoscopically or detected by the presence of free air on a plain radiograph taken after the procedure.14
During follow-up endoscopy, if a lesion with overgrowth was present after EMR or ESD, biopsy and histological diagnosis were performed; LGD or HGD (categories 3 and 4 in the Vienna classification, respectively) was defined as local recurrence.
Previous complete resection of the lesion was defined as local recurrence, and previous incomplete resection was defined as a remnant lesion.
4. Histological analysis
All of the specimens collected for histological examination were immediately fixed in neutral-buffered 10% formalin and embedded in paraffin. Histological sections were stained with hematoxylin and eosin and Wright-Giemsa stain, and examined by three experienced pathologists (S.K. Sang, D.Y. Kang, and C.S. Lee) who performed diagnoses according to the Vienna classification of gastrointestinal epithelial neoplasia.16
5. Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. One-way analysis of variance was used to compare continuous variables (i.e., age, size, and time). Pearson's chi-squared test was used for other parameters of nominal variables.
In order to determine the association between risk factors for change to HGD and invasive carcinoma (Vienna classification 4, 5), logistic regression analysis was performed.
RESULTS
1. Comparisons of endoscopic characteristics according to the Vienna classification
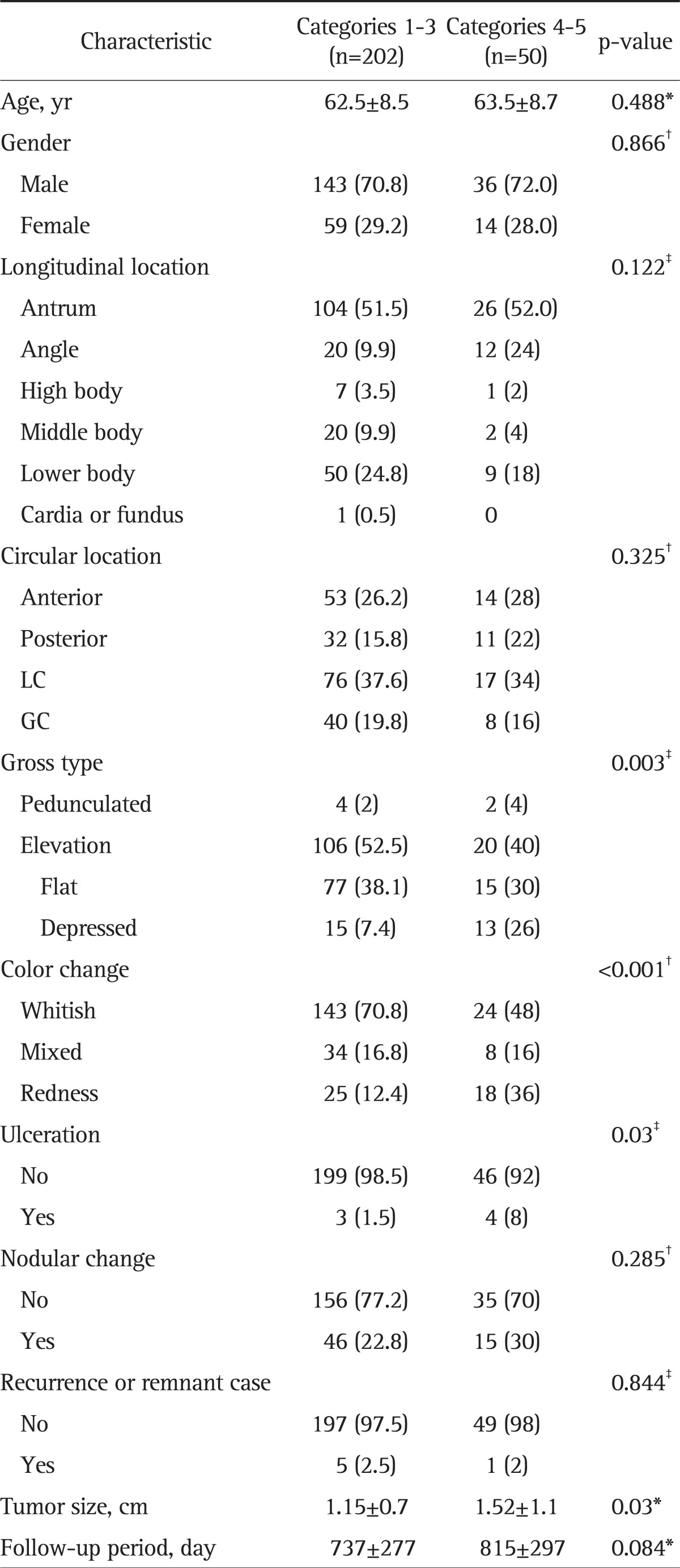
We compared the baseline characteristics and endoscopic findings between the patients with categories 1 to 3 (LGD or no tumor) and those with categories 4, 5 (HGD or invasive carcinoma) (Table 1). No differences were observed between the two groups with respect to age, gender, or lesion location. A significant number of patients had preoperative endoscopic findings including depressed-type lesions, ulceration, and redness, which are risk factors of upgraded histological outcomes. Moreover, a significant number of patients having large tumors also showed upgraded histological outcomes.
Table 1.
Baseline Characteristics and Endoscopic Findings of Lesions: Comparison between Category 1 to 3 and 4 to 5 Lesions according to the Vienna Classification
Data are presented as mean±SD or number (%).
LC, lesser curvature; GC, greater curvature.
*Unpaired t-test; †Pearson's chi-squared test; ‡Fisher's test.
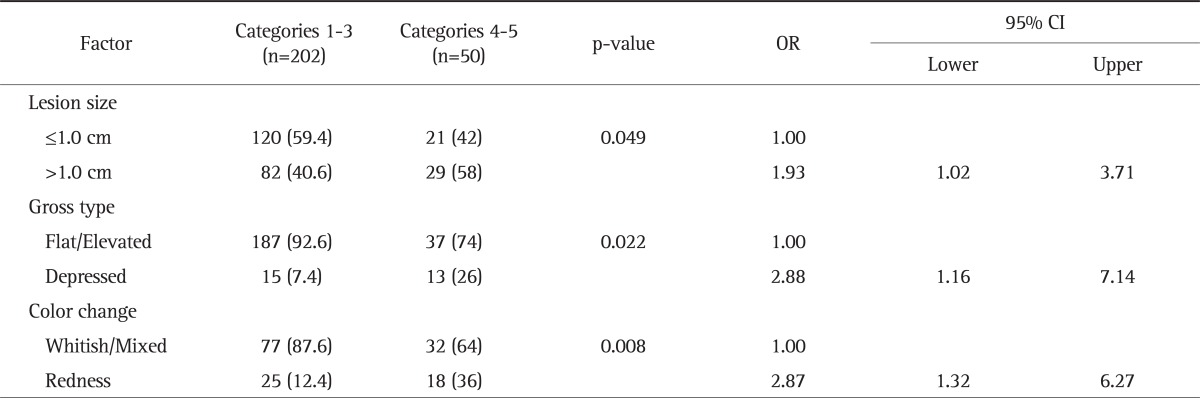
2. Logistic regression analysis of risk factors that resulted in category upgrades after histological analysis following the endoscopic procedures
The average odds ratio for patients showing a lesion size of >1 cm in the endoscopic findings and for whom the histological findings resulted in the upgrading of the category was 1.93 (95% confidence interval [CI] 1.02 to 3.71). The odds ratio for patients showing depressed-type lesions in the endoscopic findings and for whom the histological findings resulted in the upgrading of the category was 2.88 (95% CI, 1.16 to 7.14). The odds ratio for patients having a lesion accompanied by redness was 2.87 (95% CI, 1.32 to 6.27) (Table 2).
Table 2.
Logistic Regression Analysis of Risk Factors for High-Grade Dysplasia and Invasive Carcinoma (Vienna Classification 4 to 5) in Low-Grade Dysplastic Lesions Removed by Endoscopic Resection
Multivariate analysis. Data are presented as number (%).
OR, odds ratio; CI, confidence interval.
Other than the size of the lesion, endoscopic findings were associated with a high risk of histological upgrade. For endoscopic findings of depressed-type lesions or lesions accompanied by redness, the odds ratio was higher for the risk of histological upgrade.
3. Comparisons of endoscopic characteristics according to the endoscopic procedures performed
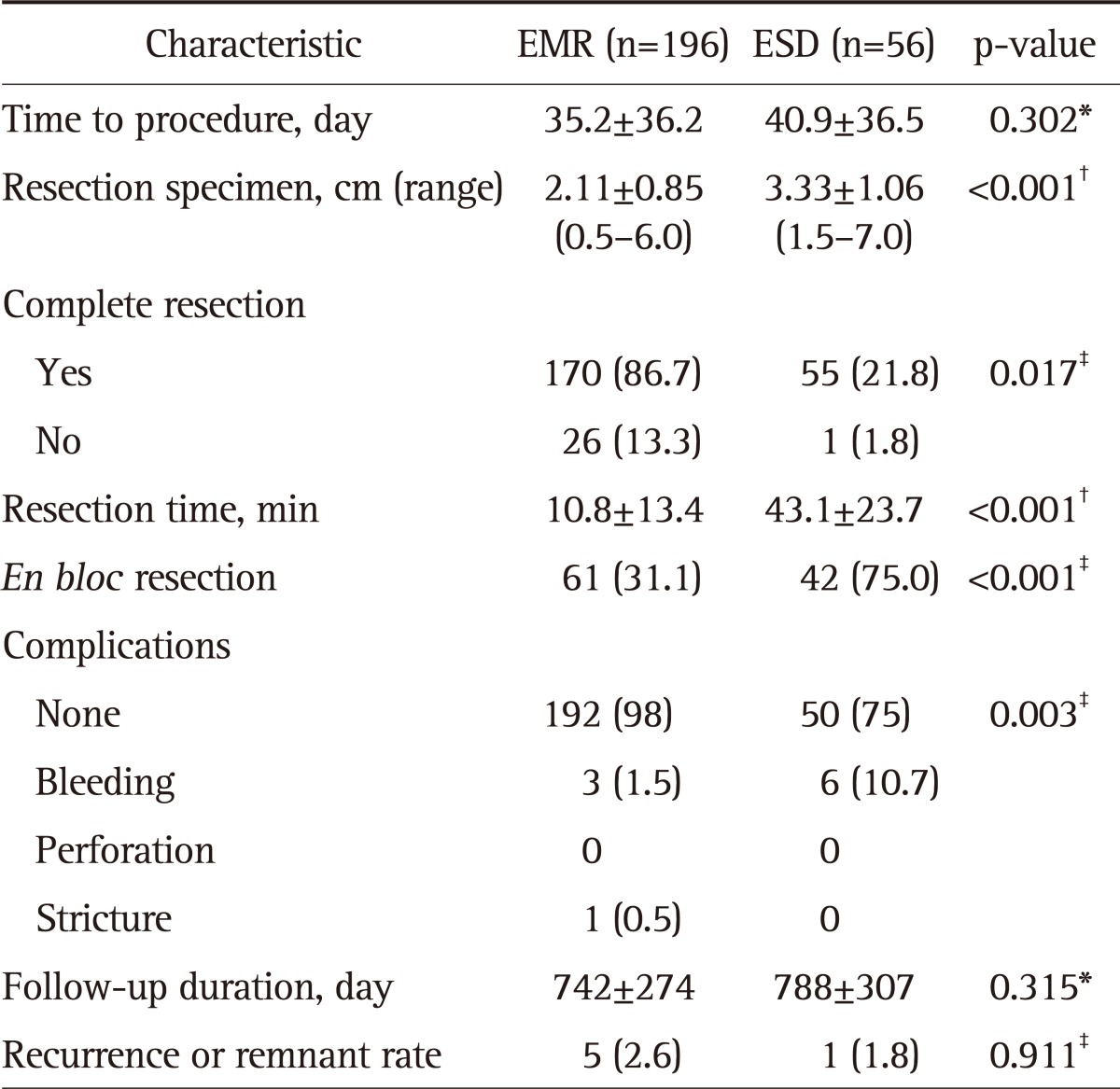
The baseline characteristics of LGD between the EMR and ESD groups were compared.
In the EMR and ESD groups, resection was performed approximately 1.0 and 1.6 cm from the lesions on average, respectively. The average resection size of the ESD group was larger (1.22 cm) than that of the EMR group (p<0.001).
After the endoscopic treatment, the complete resection rate after EMR (86.7%) was lower than that after ESD (98.2%, p=0.017); moreover, the en bloc resection rate after EMR (31.1%) was also significantly lower than that after ESD (75%, p<0.001). If frequency of snare-looping was greater, the complete resection rate was lower.
The operation time was defined as the time spent for resecting the lesion after marking. The average operation time for ESD was 43.1 minutes, which was significantly longer than that for EMR (10.8 minutes, p<0.001). ESD was associated with more complications, such as bleeding, that required postoperative endoscopic treatment.
However, these lesions were not obviously discernible, and were therefore analyzed together. No significant differences were observed between EMR (2.5%) and ESD (1.8%) with respect to the recurrence of lesions or the presence of remnant lesions (p=0.911) (Table 3).
Table 3.
Baseline Characteristics and Endoscopic Findings of Lesions: Comparison between EMR and ESD
Data are presented as mean±SD or number (%).
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
*Mann-Whitney U test; †Unpaired t-test; ‡Pearson's chi-squared test.
4. Clinical characteristics and endoscopic findings of cases with recurrent or remnant lesions
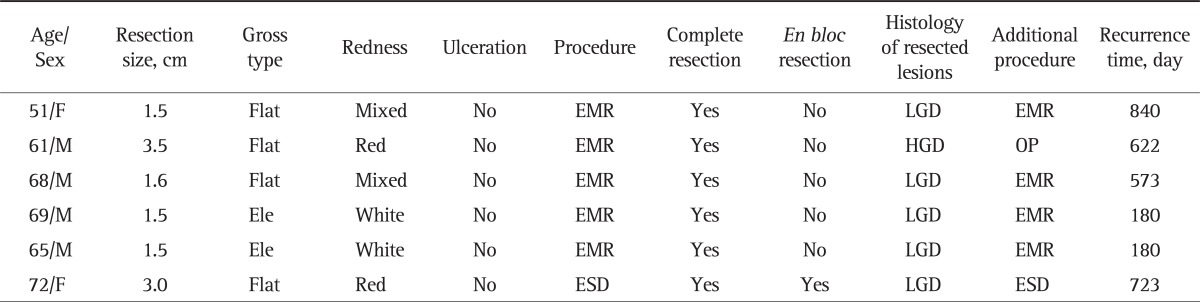
Table 4 summarizes the clinical and endoscopic findings of recurrent and remnant lesions (categories 3 and 4). Of the 252 patients who were diagnosed with LGD (category 3) by histological examination with tissue forceps and were followed-up for ≥1 year after endoscopic surgery, six patients showed recurrence of gastric adenoma or had remnant gastric adenoma (Table 4). Of these six patients, one was diagnosed with HGD (category 4) after EMR and underwent additional surgical treatment because of a positive resection margin. An additional endoscopic procedure (either EMR or ESD) was performed to remove recurrent or remnant adenomas in the remaining five patients; these patients are currently undergoing endoscopic follow-up.
Table 4.
Clinical Characteristics and Endoscopic Findings of Recurrent or Remnant Lesions
Flat, flat adenoma; Mix, mixed type; EMR, endoscopic mucosal resection; LGD, low-grade dysplasia; Red, redness; HGD, high-grade dysplasia; OP, operation; Ele, elevated adenoma; White, whitish; ESD, endoscopic submucosal dissection.
In the five cases of recurrence or remnant lesions after EMR, four to eight snare resections were usually required. Five EMR cases required complete resection, but this is an unusually large frequency of snare-looping compared to usual frequency of snare-looping required for EMR.
In the sixth ESD case, most of the ablation was performed using an IT knife, although the end of the resection was performed using snare resection because of the incomplete resection. This was thought to be associated with recurrence.
DISCUSSION
The increased use of esophagogastroduodenoscopy in recent times has resulted in an increase in the diagnosis of gastric adenoma and the subsequent endoscopic treatment of the disease.
HGD is a precancerous lesion. Therefore, resection of the lesion using an endoscopic or surgical method is strongly recommended. However, in the case of LGD, the change to invasive carcinoma and the process involved in this change are still under debate. Some researchers argue that if cases of LGD are unlikely to change into HGD, annual endoscopic surveillance with re-biopsy without resection is appropriate.17,18 Reports on the rate of gastric adenoma with LGD progressing into invasive carcinoma and its change time vary greatly.19-21 Nonetheless, it is possible (albeit unlikely) that LGD will progress into invasive carcinoma; therefore, follow-up after possible resection is considered desirable.
Thus, the present study investigated the risk factors involved in the change of LGD to HGD or invasive carcinoma by performing histological examination using tissue forceps, because the tissue by forceps biopsy do not represent the entire lesion. The retrospective data on the subjects who were diagnosed with LGD by endoscopic biopsy and underwent endoscopic treatment were analyzed.
Postprocedural histological analysis revealed that lesions >1 cm, with accompanying redness, or having depressed endoscopic findings have an increased risk of change to HGD or invasive carcinoma.
In addition, we investigated which endoscopic treatment method (i.e., EMR or ESD) is more appropriate for cases in which the risk of change to HGD or invasive carcinoma is not high. The rate of incomplete resection was higher in EMR (13.3%) than in ESD (p=0.017) among the different endoscopic treatment methods for LGD; moreover, the rate of en bloc resection was lower for EMR (31.1%) than for ESD (75%) (p<0.001). For ESD, lesions were dissected with an IT knife, and the en bloc resection rate was higher.
Meanwhile, the EMR procedure utilized an alligator and snare; en bloc resection cannot be performed in cases where the lesion does not get caught in the snare at least once. For this reason, the en bloc resection rate was lower in EMR than in ESD. Furthermore, for EMR, it is difficult to reconstruct the organization if the frequency of snare-looping is greater. Therefore, the en bloc resection rate was lower than that with ESD.
However, the two endoscopic techniques did not differ with respect to the rates of lesion recurrence or remnant lesions. The procedure time for ESD was longer (43.1 minutes) than that for EMR (10.8 minutes) (p<0.001). Moreover, ESD was associated with more complications (e.g., bleeding) than EMS (p=0.003).
Therefore, patients in whom the risk of LGD change to HGD or active carcinoma is low-such as patients with a lesion ≤l cm, with no redness, and a depressed appearance in endoscopic findings-should be treated with endoscopic resection using EMR. Although ESD is advantageous because it enables en bloc and complete resections regardless of the lesion size, the procedure takes a long time, has a high risk of complications, and requires sophisticated skills.
In contrast, even a less-experienced endoscopist can conduct EMR with a shorter operation time than that required for ESD.
In 6 recurrence and remnant cases, snare resection times were longer, and there was an increased frequency of recurrence or remnant lesions. Additional research is needed to clarify the relevance of the frequency of snare-looping with respect to recurrence or remnant lesions. Therefore, increasing the probability of en bloc resection by EMR may lead to lower recurrence rates.
The results of the present study show that cases of LGD in which the risk of change is low may be treated with EMR.
The limitations of this retrospective study are the differences in the baseline characteristics of the EMR and ESD groups. In addition, the average postoperative follow-up period (i.e., ≥1 year) is short. Furthermore, resection size can specifically affect complications during the procedure as well as the procedure time. However, additional research is required to clarify this.
Nonetheless, no appropriate endoscopic treatment for LGD has been established thus far. Therefore, this comparative analysis of EMR and ESD will be helpful for determining the optimal treatment method for LGD.
In conclusion, patients who are diagnosed with LGD by histological examination with tissue forceps and who have a low risk of change should be treated with endoscopic resection with EMR.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Simrén M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784–790. doi: 10.1136/gut.52.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farinati F, Rugge M, Di Mario F, Valiante F, Baffa R. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients. A prospective study. I.G.G.E.D.: Interdisciplinary Group on Gastric Epithelial Dysplasia. Endoscopy. 1993;25:261–264. doi: 10.1055/s-2007-1010310. [DOI] [PubMed] [Google Scholar]
- 3.Di Gregorio C, Morandi P, Fante R, De Gaetani C. Gastric dysplasia. A follow-up study. Am J Gastroenterol. 1993;88:1714–1719. [PubMed] [Google Scholar]
- 4.Rugge M, Farinati F, Baffa R, et al. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Gastroenterology. 1994;107:1288–1296. doi: 10.1016/0016-5085(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 5.Park DI, Rhee PL, Kim JE, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506. doi: 10.1055/s-2001-15089. [DOI] [PubMed] [Google Scholar]
- 6.Habu Y, Kawai K. The clinical significance of gastric dysplasia: the gastroenterologist's view. Endoscopy. 1993;25:296–297. doi: 10.1055/s-2007-1010319. [DOI] [PubMed] [Google Scholar]
- 7.Jung MK, Jeon SW, Park SY, et al. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 8.Rugge M, Cassaro M, Di Mario F, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–1116. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Min BH, Lee JH, Kim JJ, et al. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P) Dig Liver Dis. 2009;41:201–209. doi: 10.1016/j.dld.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 14.Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860–865. doi: 10.1016/j.gie.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S71–S73. doi: 10.1016/s1542-3565(05)00251-x. [DOI] [PubMed] [Google Scholar]
- 16.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SJ, Choi IJ, Kim CG, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011;43:465–471. doi: 10.1055/s-0030-1256236. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Jeon SW, Jung MK, et al. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–970. doi: 10.1097/MEG.0b013e3283013d58. [DOI] [PubMed] [Google Scholar]
- 19.Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31:977–983. doi: 10.1136/gut.31.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraga EP, Gardiol D, Costa J. Gastric dysplasia. A histological follow-up study. Am J Surg Pathol. 1987;11:788–796. [PubMed] [Google Scholar]
- 21.Kokkola A, Haapiainen R, Laxén F, et al. Risk of gastric carcinoma in patients with mucosal dysplasia associated with atrophic gastritis: a follow up study. J Clin Pathol. 1996;49:979–984. doi: 10.1136/jcp.49.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]