Abstract
Background/Aims
This study assessed the efficacy of a rifaximin plus levofloxacin-based rescue regimen in patients that had failed both triple and quadruple standard regimens for the eradication of Helicobacter pylori.
Methods
We treated patients for H. pylori between August 2009 and April 2011. The triple regimen consisted of combined treatment with amoxicillin, clarithromycin, and pantoprazole for 1 week. For failed cases, a quadruple regimen of tetracycline, metronidazole, bismuth dicitrate, and lansoprazole for 1 week was administered. The rescue regimen for persistently refractory cases was rifaximin 200 mg t.i.d., levofloxacin 500 mg q.d., and lansoprazole 15 mg b.i.d. for 1 week.
Results
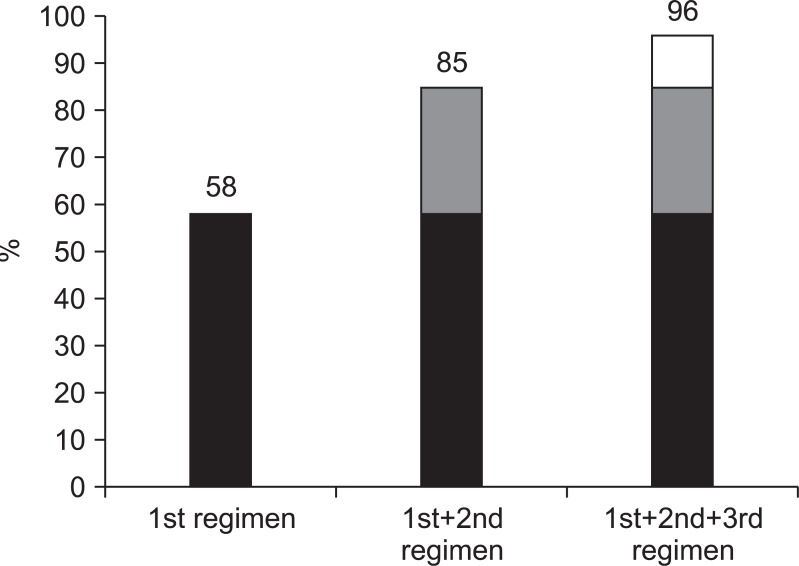
In total, 482 patients were enrolled in this study. The eradication rates associated with the first and second regimens were 58% and 60%, respectively. Forty-seven out of 58 patients who failed with the second-line regimen received rifaximin plus levofloxacin-based third-line therapy. The eradication rate for the third regimen was 65%. The cumulative eradication rates were 58%, 85%, and 96% for each regimen, respectively.
Conclusions
A rifaximin plus levofloxacin-based regimen could be an alternative rescue therapy in patients with resistance to both triple and quadruple regimens for the eradication of H. pylori.
Keywords: Helicobacter pylori, Eradication, Rifaximin
INTRODUCTION
Chronic Helicobacter pylori infection causes distal stomach-predominant atrophic gastritis. It eventually causes many disorders such as peptic ulcer diseases, gastric cancer, and mucosa-associated lymphoid tissue lymphoma.
The survival capability of this organism within the stomach makes it difficult to eradicate and requires multi-drug regimens consisting of two antibiotics and a strong acid suppressant.1 Several guidelines suggest the use of 7-day triple therapy, comprising a proton pump inhibitor (PPI), clarithromycin and amoxicillin, as the first line therapy, whereas the 7-day quadruple therapy includes bismuth salts, and is indicated for eradication in patients who failed first line therapy.2-6
Nowadays, the prevalence of H. pylori resistance to antibiotics is increasing, which is the major cause of treatment failure.7 Standard triple therapy had failed to eradicate H. pylori in up to 25% of patients.2,8 Concomitant and sequential therapy have been attempted to overcome the treatment failure.9,10
New antibiotics have also been applied to increase the eradication rate. Rifaximin is a poorly absorbed rifamycin derivative with a broad spectrum of antibacterial activity covering gram-positive and gram-negative organisms, both aerobes and anaerobes, including H. pylori.11-14 Levofloxacin, a synthetic antibiotic of the fluoroquinolone drug class, is also reported to be as effective for H. pylori eradication.15,16
However, there is no consensus about treatment in patients who have failed to be eradicated of H. pylori infection with both first line triple therapy and second line quadruple therapy.
The aim of this study was to assess the efficacy of a rifaximin plus levofloxacin based regimen for the treatment of two consecutive failure cases with first and second eradication regimens.
MATERIALS AND METHODS
1. Patients
We reviewed medical records of patients in need for H. pylori eradication because of peptic ulcer disease, early gastric cancer, family history of gastric cancer, and chronic atrophic gastritis with intestinal metaplasia at CHA Bundang Medical Center between August 2009 and April 2011. All patients underwent an esophagogastroduodenoscopy. H. pylori infection was confirmed by rapid urease test or histology. Patients who had history of previous eradication therapy were excluded. The Institutional Review Board of CHA Bundang Medical Center permitted this study. A total of 482 patients were finally enrolled.
2. Treatment strategy
First-line H. pylori eradication regimen was clarithromycin 500 mg b.i.d., amoxicillin 1 g b.i.d., and pantoprazole 20 mg b.i.d. for 7 days. Second-line regimen for patients who had failed after the first triple regimen was metronidazole 500 mg t.i.d., tripotassium bismuth dicitrate 300 mg (Bi2O3 120 mg) b.i.d., tetracycline 500 mg t.i.d., and lansoprazole 15 mg b.i.d. for 7 days. Patients who had failed with second-line quadruple regimen were treated for 7 days with a third-line regimen of rifaximin 200 mg t.i.d., levofloxacin 500 mg q.d., and pantoprazole 20 mg b.i.d. Eradication of H. pylori was determined by the urea breath test (UBT) performed 4 weeks after treatment.
3. UBT
The UBT is based on the ability of H. pylori to convert urea to ammonia and carbon dioxide. A 100 mg tablet of 13C-urea was ingested and 13CO2 was measured in the expiration breath after 20 minutes. A delta 13C-UBT over baseline value higher than 2% was considered positive as active H. pylori infection. Patients were asked to avoid acid-lowering medicine or antibiotics for 1 week before UBT.
4. Adverse effects
During the follow-up visits, presence of drug-related adverse effects such as nausea, vomiting, epigastric discomfort, diarrhea, constipation, bitter taste, and skin rash were recorded.
5. Analysis
Patients who did not perform the urease breath test were grouped as follow-up loss. Any patient who called off further treatment in the group of eradication failure was excluded as dropouts. Per-protocol analysis was used to evaluate the effect of regimens.
RESULTS
1. H. pylori eradication rates
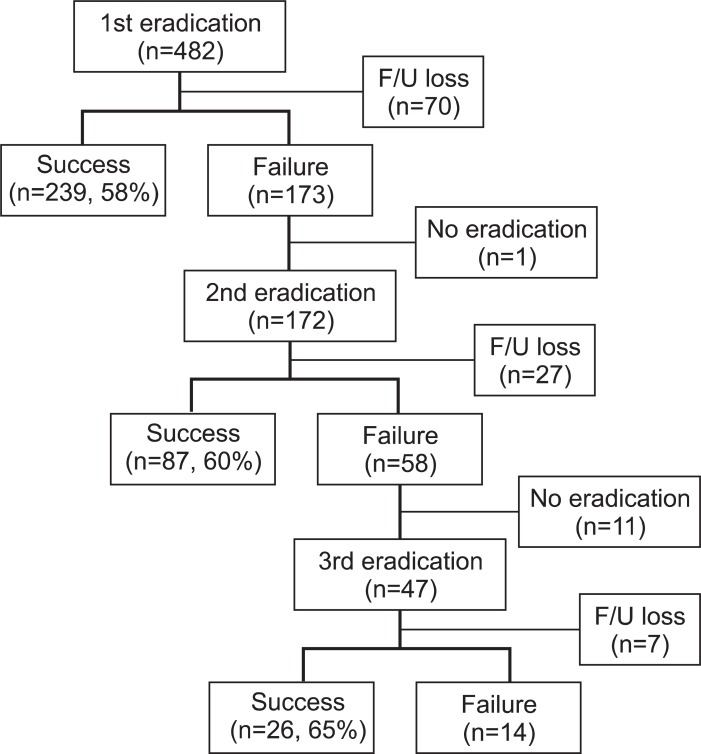
A total of 482 patients (mean age, 53±13 years; male:female=2:1) received 7-day triple therapy for H. pylori eradication (Fig. 1). Two hundred and thirty-nine (58%) patients succeeded in having H. pylori infection eradicated but 173 patients failed. Patients who did not receive UBT after treatments were categorized as follow-up loss groups, and 70 patients did not perform UBT after triple therapy.
Fig. 1.
Enrollment and follow-up (F/U).
One patient did not want second line treatment due to side effects such as nausea and diarrhea. Therefore, 172 patients who failed in triple therapy received the second quadruple therapy. Eighty-seven patients (60%) were eradicated of H. pylori but 58 patients failed. Twenty-seven patients were lost in follow-up.
Forty-seven out of the 58 patients received third eradication medicine. Twenty-six (65%) patients achieved success with eradication of H. pylori and 14 patients failed to eradicate. Cumulative eradication rates of three consecutive regimens were 58%, 85%, and 96%, respectively (Fig. 2).
Fig. 2.
Cumulative eradication rates of Helicobacter pylori (per protocol).
2. Adverse effects
Six patients (15%) reported side effects in third-line treatment: five patients complained of mild epigastric discomfort and one patient reported transient dizziness.
3. Compliance to treatment
Seven of 47 patients who agreed to third line treatment were lost for follow-up UBT, but any drop-out was not reported due to drug intolerance.
DISCUSSION
The prevalence of H. pylori infection in Korea is considerably high in adults (59.6%).17 Patients who need to eradicate H. pylori have increased due to the national screening program for early detection of gastric cancer. However, the success rate of eradication is continuously decreasing, which is of great concern.17 Several factors have been suggested as the reasons behind this failure. Poor compliance and resistance to antibiotics are generally considered to be the main causes.
Resistance of H. pylori to metronidazole and clarithromycin is a growing concern. According to a single center study in Korea, the rate of resistance to clarithromycin increased from 16.7% to 38.5% between the year 2007 and 2009 compared with the period between 2003 and 2005.18 The resistance rate to metronidazole in Korea was 27.1% to 27.5% between 2003 and 2010.19,20 As resistance to these antibiotics has recently been increasing, the eradication rate has been decreasing even as low as 61% to 76% in primary care settings in Italy21 and even 47.4% in Turkey.22 Therefore, more patients are still H. pylori positive even after standard triple and quadruple eradication therapy, but we do not have any recommended guideline for third line therapy.
Rifamycin deriatives (like rifampicin, rifabutin, and rifaximin) display antibacterial activity against H. pylori.11,13 Rifabutin is being used increasingly in some rescue therapies after failed first or second eradication therapy. Some studies using rifabutin based regimens reported 60% to 79% eradication rates.23,24 There are no reported resistant strains of H. pylori against rifabutin, which is why it may be effective as a rescue therapy for patients who did not respond to the existing eradication therapy.12 However, popular use of rifabutin is able to induce serious side effects such as bone marrow suppression and the development of rifampicin-resistant tuberculosis in areas where prevalence of tuberculosis is high.
Rifaximin is one of the new alternative antibiotics. It is a poorly absorbed drug and almost devoid of adverse effects. Bioavailability within the gastrointestinal tract is fairly high and capable of inhibiting the growth of H. pylori with intermediate minimum inhibitory concentration (MIC) value, which is between that of amoxicillin and colloidal bismuth subcitrate.25 MIC value of rifaximin is affected even by lowering the pH from 7.2 to 6, because of its low absorption. A subsequent study showed lack of antagonism towards metronidazole and omeprazole.26 Eradication rates of H. pylori with rifaximin plus clarithromycin and rifaximin plus metronidazole were 73% and 60%, respectively in a single-blind randomized study.27
Levofloxacin was recently used to treat H. pylori infection both for first- and second-line treatments. Studies using levofloxacin as an alternative to clarithromycin as a triple regimen reported eradication rates of 90% to 92%, which were better than standard quadruple regimens as a second-line option for H. pylori eradication.28-30 However, Korean studies reported much worse eradication results of 69.8% and 53.3% with levofloxacin based first or second line treatment.31,32 Recent studies showed the possibility of rifaximin plus levofloxacin combination treatment as an alternative regimen of H. pylori eradication therapy.33,34
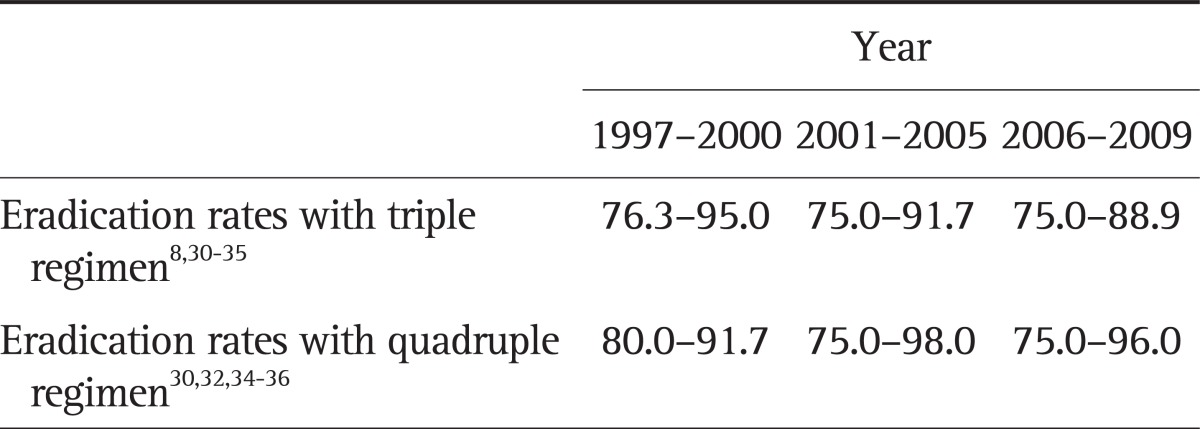
The result of first line triple therapy performed in this study was lower than the expected score, being only 58%. This may be due to either higher regional antibiotic resistance rate of H. pylori or poor compliance. Based on published eradication rates of H. pylori in Korea from 1997 to 2009, the success rates of eradication showed a tendency to decrease (Table 1).8,35-41 Eradication rates varied according to the places the study was performed, such as city versus country. The eradication rate of quadruple therapy in this study was 60%. It was also lower than that of other studies.35,37,39-42 The causes of this high failure rate might be due to reduced dose of tetracycline, higher regional rate of antibiotic resistance or short-term treatment period of 1 week.
Table 1.
Changes in the Eradication Rates of Helicobacter pylori with Each Standard Regimen in Korea
Data are presented as percentage.
The Maastricht III Consensus Report recommended rescue treatment should be based on antimicrobial susceptibility.43 Performing culture has many limitations in practice and we investigated new antibiotics combination as a rescue regimen. In considering multi-drug resistant H. pylori shown by consecutive eradication failures, the eradication rate with a rifaximin plus levofloxacin combination regimen of 65% was not so low, and the cumulative cure rate reaching 96% was a worthy result.
As a rescue treatment after failure of standard first and second-line therapy, repeated quadruple therapy for 2 weeks was studied.44 Eradication rate was 66.7% at intention-to-treatment and 75% at per-protocol analysis. Of the 45 retreated patients, two (4.4%) patients complied poorly with medication. Compared with this study, present study regimen was just 1-week medication and compliance was excellent without severe side effects.
The present study has some limitations besides lack of microbial susceptibility test. We had only one treatment arm without a comparable regimen such as one antibiotic plus PPI regimen and no data with rifaximin plus levofloxacin treatment in H. pylori positive naïve patients as a first line regimen.
In summary, results of the present investigation demonstrated an acceptable efficacy and compliance of rifaximin plus levofloxacin based triple eradication regimen in patients who had failed in standard first and second line treatment. Therefore, this combination regimen could be an alternative rescue therapy for the eradication of H. pylori.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Malfertheiner P, Mégraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 2.Scarpignato C. Towards the ideal regimen for Helicobacter pylori eradication: the search continues. Dig Liver Dis. 2004;36:243–247. doi: 10.1016/j.dld.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Romano M, Cuomo A. Eradication of Helicobacter pylori: a clinical update. MedGenMed. 2004;6:19. [PMC free article] [PubMed] [Google Scholar]
- 4.McLoughlin RM, O'Morain CA, O'Connor HJ. Eradication of Helicobacter pylori: recent advances in treatment. Fundam Clin Pharmacol. 2005;19:421–427. doi: 10.1111/j.1472-8206.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 5.Candelli M, Nista EC, Carloni E, et al. Treatment of H. pylori infection: a review. Curr Med Chem. 2005;12:375–384. doi: 10.2174/0929867053363027. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–1855. doi: 10.1111/j.1572-0241.2004.40014.x. [DOI] [PubMed] [Google Scholar]
- 7.Pilotto A, Rassu M, Leandro G, Franceschi M, Di Mario F GISU. Interdisciplinary Group for the Study of Ulcer. Prevalence of Helicobacter pylori resistance to antibiotics in Northeast Italy: a multicentre study. Dig Liver Dis. 2000;32:763–768. doi: 10.1016/s1590-8658(00)80352-7. [DOI] [PubMed] [Google Scholar]
- 8.Cho DK, Park SY, Kee WJ, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol. 2010;55:368–375. doi: 10.4166/kjg.2010.55.6.368. [DOI] [PubMed] [Google Scholar]
- 9.Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17:719–726. doi: 10.1046/j.1365-2036.2003.01461.x. [DOI] [PubMed] [Google Scholar]
- 10.Parente F, Cucino C, Bianchi Porro G. Treatment options for patients with Helicobacter pylori infection resistant to one or more eradication attempts. Dig Liver Dis. 2003;35:523–528. doi: 10.1016/s1590-8658(03)00268-8. [DOI] [PubMed] [Google Scholar]
- 11.Pilotto A, Franceschi M, Rassu M, Furlan F, Scagnelli M. In vitro activity of rifabutin against strains of Helicobacter pylori resistant to metronidazole and clarithromycin. Am J Gastroenterol. 2000;95:833–834. doi: 10.1111/j.1572-0241.2000.01900.x. [DOI] [PubMed] [Google Scholar]
- 12.Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1497–1499. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity: a review. Chemotherapy. 2005;51(Suppl 1):67–72. doi: 10.1159/000081991. [DOI] [PubMed] [Google Scholar]
- 14.Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201–211. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Zullo A, Hassan C, De Francesco V, et al. A third-line levofloxacin-based rescue therapy for Helicobacter pylori eradication. Dig Liver Dis. 2003;35:232–236. doi: 10.1016/s1590-8658(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 16.Gisbert JP, Gisbert JL, Marcos S, Moreno-Otero R, Pajares JM. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther. 2006;24:1469–1474. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 17.Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Jung SW. Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2011;58:67–73. doi: 10.4166/kjg.2011.58.2.67. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Kim N, Park HK, et al. Primary antibiotic resistance of Helicobacter pylori strains and eradication rate according to gastroduodenal disease in Korea. Korean J Gastroenterol. 2011;58:74–81. doi: 10.4166/kjg.2011.58.2.74. [DOI] [PubMed] [Google Scholar]
- 20.Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 21.Della Monica P, Lavagna A, Masoero G, Lombardo L, Crocellá L, Pera A. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther. 2002;16:1269–1275. doi: 10.1046/j.1365-2036.2002.01244.x. [DOI] [PubMed] [Google Scholar]
- 22.Sezikli M, Cetinkaya ZA, Güzelbulut F, et al. Efficacy of the combination of tetracycline, amoxicillin, and lansoprazole in the eradication of Helicobacter pylori in treatment-naive patients and in patients who are not responsive to clarithromycin-based regimens: a pilot study. Gut Liver. 2012;6:41–44. doi: 10.5009/gnl.2012.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gisbert JP, Calvet X, Bujanda L, Marcos S, Gisbert JL, Pajares JM. 'Rescue' therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter. 2003;8:90–94. doi: 10.1046/j.1523-5378.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Perri F, Festa V, Clemente R, Quitadamo M, Andriulli A. Rifabutin-based 'rescue therapy' for Helicobacter pylori infected patients after failure of standard regimens. Aliment Pharmacol Ther. 2000;14:311–316. doi: 10.1046/j.1365-2036.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 25.Mégraud F, Bouffant F, Camou Juncas C. In vitro activity of rifaximin against Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1994;13:184–186. doi: 10.1007/BF01982198. [DOI] [PubMed] [Google Scholar]
- 26.Holton J, Vaira D, Menegatti M, Barbara L. The susceptibility of Helicobacter pylori to the rifamycin, rifaximin. J Antimicrob Chemother. 1995;35:545–549. doi: 10.1093/jac/35.4.545. [DOI] [PubMed] [Google Scholar]
- 27.Pretolani S, Bonvicini F, Brocci E, et al. Effect of rifaximin, a new non-absorbed antibiotic, in the treatment of Helicobacter pylori infection. Acta Gastroenterol Belg. 1993;56:144A. [Google Scholar]
- 28.Cammarota G, Cianci R, Cannizzaro O, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1339–1343. doi: 10.1046/j.1365-2036.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Caro S, Zocco MA, Cremonini F, et al. Levofloxacin based regimens for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 2002;14:1309–1312. doi: 10.1097/00042737-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Nista EC, Candelli M, Cremonini F, et al. Levofloxacin-based triple therapy vs. quadruple therapy in second-line Helicobacter pylori treatment: a randomized trial. Aliment Pharmacol Ther. 2003;18:627–633. doi: 10.1046/j.1365-2036.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Hong SP, Kwon CI, et al. The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2006;48:19–24. [PubMed] [Google Scholar]
- 32.Jung HS, Shim KN, Baik SJ, et al. Efficacy of levofloxacin-based triple therapy as second-line Helicobacter pylori eradication. Korean J Gastroenterol. 2008;51:285–290. [PubMed] [Google Scholar]
- 33.Gasbarrini A, Gasbarrini G, Pelosini I, Scarpignato C. Eradication of Helicobacter pylori: are rifaximin-based regimens effective? Digestion. 2006;73(Suppl 1):129–135. doi: 10.1159/000089788. [DOI] [PubMed] [Google Scholar]
- 34.Gasbarrini A, Lauritano EC, Nista EC, et al. Rifaximin-based regimens for eradication of Helicobacter pylori: a pilot study. Dig Dis. 2006;24:195–200. doi: 10.1159/000090330. [DOI] [PubMed] [Google Scholar]
- 35.Song JG, Lee SW, Park JY, et al. Trend in the eradication rates of Helicobacter pylori infection in the last 11 years. Korean J Med. 2009;76:303–310. [Google Scholar]
- 36.Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006;48:156–161. [PubMed] [Google Scholar]
- 37.Chung JW, Lee GH, Han JH, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011;58:246–250. [PubMed] [Google Scholar]
- 38.Chung WC, Lee KM, Paik CN, et al. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009;53:221–227. [PubMed] [Google Scholar]
- 39.Na HS, Hong SJ, Yoon HJ, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and reinfection rate after successful eradication. Korean J Gastroenterol. 2007;50:170–175. [PubMed] [Google Scholar]
- 40.Cho HJ, Bae RC, Lee SH, et al. The trend in the eradication rates of first- and second-line therapy for Helicobacter pylori infection in Daegu and Kyungpook provinces: a single center experience for the most recent 9 years. Korean J Med. 2009;76:186–192. [Google Scholar]
- 41.Oh JH, Kim TH, Cheung DY, et al. Eradication rates of bismuth-based quadruple therapy as a second-line treatment for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2009;39:131–135. [Google Scholar]
- 42.Cho EJ, Lee DH, Chun JY, et al. Recent trends in the eradication rates of second-line quadruple 14 therapy for Helicobacter pylori and the clinical factors that potentially affect the treatment outcome. Korean J Gastrointest Endosc. 2009;38:14–19. [Google Scholar]
- 43.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SK, Lee SW, Park JY, et al. Effectiveness and safety of repeated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy: repeated quadruple therapy as a third-line therapy. Helicobacter. 2011;16:410–414. doi: 10.1111/j.1523-5378.2011.00870.x. [DOI] [PubMed] [Google Scholar]