Abstract
Background/Aims
Variceal rupture is one of the main causes of mortality in cirrhotic patients. However, there are limited data on the long-term outcomes of variceal bleeding.
Methods
We investigated the incidence and mortality of variceal bleeding at three endoscopic centers in Gangwon province during 3 periods (August 1996 to July 1997, August 2001 to July 2002, and August 2006 to July 2007).
Results
A total of 1,704 upper gastrointestinal (GI) bleedings occurred during the study periods. Peptic ulcers were found in 825 patients (48.5%), and variceal ruptures were found in 607 patients (35.6%). The variceal bleeding rate did not decrease in each period (26.0% vs 43.7% vs 33.9%, respectively). In the variceal bleeding group, the 6-week mortality rate steadily and significantly decreased (15.5% vs 10.8% vs 6.4%, respectively, p=0.027). In addition, the mortality rate was significantly higher in the variceal bleeding group than in the non-variceal bleeding group (10.4% vs 2.0%, p<0.001; odds ratio, 5.659; 95% confidence interval, 3.445 to 9.295).
Conclusions
Variceal bleeding was still the major cause of upper GI bleedings and did not decrease in prevalence over the 10-year period in Gangwon province, South Korea. However, the mortality rate of variceal bleeding decreased significantly.
Keywords: Gastrointestinal hemorrhage, Liver cirrhosis, Esophageal and gastric varices, Mortality
INTRODUCTION
Variceal rupture is one of the important causes of upper gastrointestinal (GI) bleedings, and it is a major cause of death and complications in patients with liver cirrhosis.1 Variceal bleeding is known to stop spontaneously in 40% to 50% of patients, but even so, the consensus indicated that the early mortality rate of the first variceal bleeding is over 30% in cirrhotic patients.2 In addition, any deaths occurring within 6 weeks of admission due to variceal bleeding is considered as bleeding-related mortality, because the risk of rebleeding and mortality is the highest during the first 6 weeks.3 The risk of rebleeding decreased to similar level of the previous bleeding risk,1 and about 30% of variceal bleeding patients are known to have died within 12 months after bleeding.4
Though many treatment modalities for variceal bleedings are available in clinical practice and improved outcomes in variceal bleeding in cirrhotic patients have been reported over decades,4-6 little is known about the recent changes in clinical outcomes and characteristics of cirrhotic patients with variceal bleeding in South Korea.
The aim of our study was to investigate the clinical outcomes and characteristics of patients with variceal bleeding during the past 10 years in Gangwon province, South Korea.
MATERIALS AND METHODS
1. Study population
We retrospectively reviewed the medical records of all patients diagnosed with upper GI bleeding who had visited the emergency room during the three periods (August 1996 to July 1997, August 2001 to July 2002, and August 2006 to July 2007) in three endoscopic centers where emergent therapeutic endoscopy was available, in Gangwon province, South Korea. We used the International Classification of Disease coding in each hospital databases when we identified the patients.
Upper GI bleeding is diagnosed with emergent esophagogastroduodenoscopy as well as by utilizing clinical findings, such as hematemesis and/or melena, bloody aspirates via nasogastric tube by saline irrigation, hypotension, tachycardia, and decreased hemoglobin level, etc. Diagnosis of variceal bleeding is made using endoscopic findings, such as active bleeding from varix, bleeding stigmata on varix such as adherent clot, and presence of large varices with blood in the stomach without other definite recognizable source of bleeding.
All upper GI bleedings were controlled with therapeutic endoscopy by expert endoscopists. When emergent endoscopic bleeding control was not available in patients with liver cirrhosis, adequate additional endoscopic hemostasis was performed until the patient reached hemodynamic stabilization.
The exclusion criteria was as follows: patients under 16 years of age, history of coagulopathy except cirrhosis, traumatic injury related bleeding, malignancy related bleeding, lower GI tract bleeding or small bowel bleeding.
Diagnosis of liver cirrhosis was made by histology, presence of varices by endoscopic examination, or combination of biochemical and clinical findings of portal hypertension as well as abdominal ultrasonographic or computed tomography findings.
2. Assessment
In the study periods, baseline characteristics (sex, age, past medical history, smoking and alcohol drinking history, medication history, and etiology of liver cirrhosis, etc.), clinical variables (number of episodes of GI bleeding, etiology of bleeding, incidence of variceal bleeding, and methods of hemostasis, etc.), and clinical outcomes (hospital stay, in-hospital mortality, etc.) of patients were investigated and compared between the study periods.
Also, subgroup analysis for patients with variceal bleeding was performed. We investigated the number of episodes and etiology of bleeding, and severity of cirrhosis (Child-Pugh class). Characteristics of non-survivor and survivor groups were compared.
We defined the bleeding-related mortality as any death occurring within 6 weeks of admission due to variceal bleeding.
3. Statistical analysis
Continuous variables are presented as mean±standard deviation, and categorical variables as frequency and percentage. Student's t-test, one-way ANOVA, and Pearson's chi-square test were performed to compare each group, and logistic regression analysis was also performed to determine the factor for predicting mortality. SPSS software version 11.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and p<0.05 was considered as statistically significant.
This study was approved by the Institutional Review Board of all participating hospitals.
RESULTS
1. Baseline characteristics
During the 3 study periods, a total of 1,704 upper GI bleedings had occurred. The mean age was 54.1±14.7 years (range, 16 to 97) with 1,445 being males (84.8%) and 259 being females (15.2%).
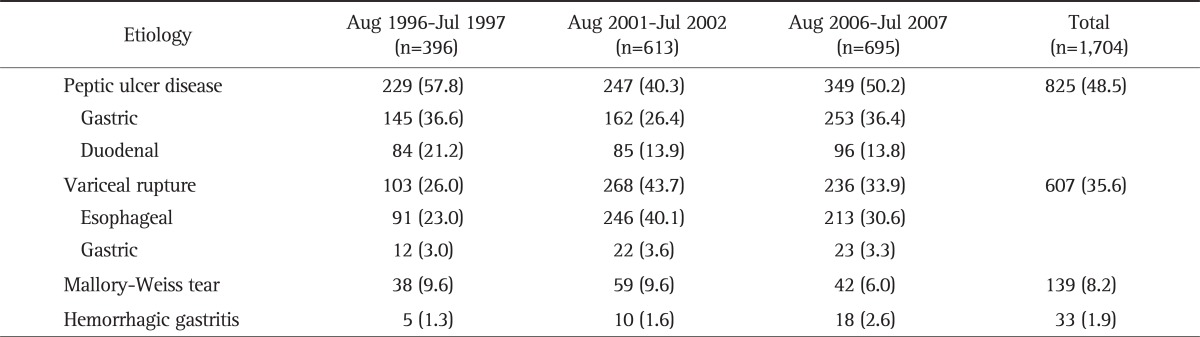
The most common etiology of upper GI bleedings was peptic ulcer (n=825, 48.5%), followed by variceal rupture (n=607, 35.6%), Mallory-Weiss tear (n=139, 8.2%), hemorrhagic gastritis (n=33, 1.9%), and others (Table 1).
Table 1.
Etiology of Upper Gastrointestinal Bleeding by Study Period
Data are presented as number (%).
The total number of cirrhotic patients was 732 (43.0%), and their etiologies were alcohol in 486 (66.4%), hepatitis B virus in 194 (26.5%), hepatitis C virus in 22 (3.0%), autoimmune in 3 (0.4%), and unknown in 27 (3.7%) patients.
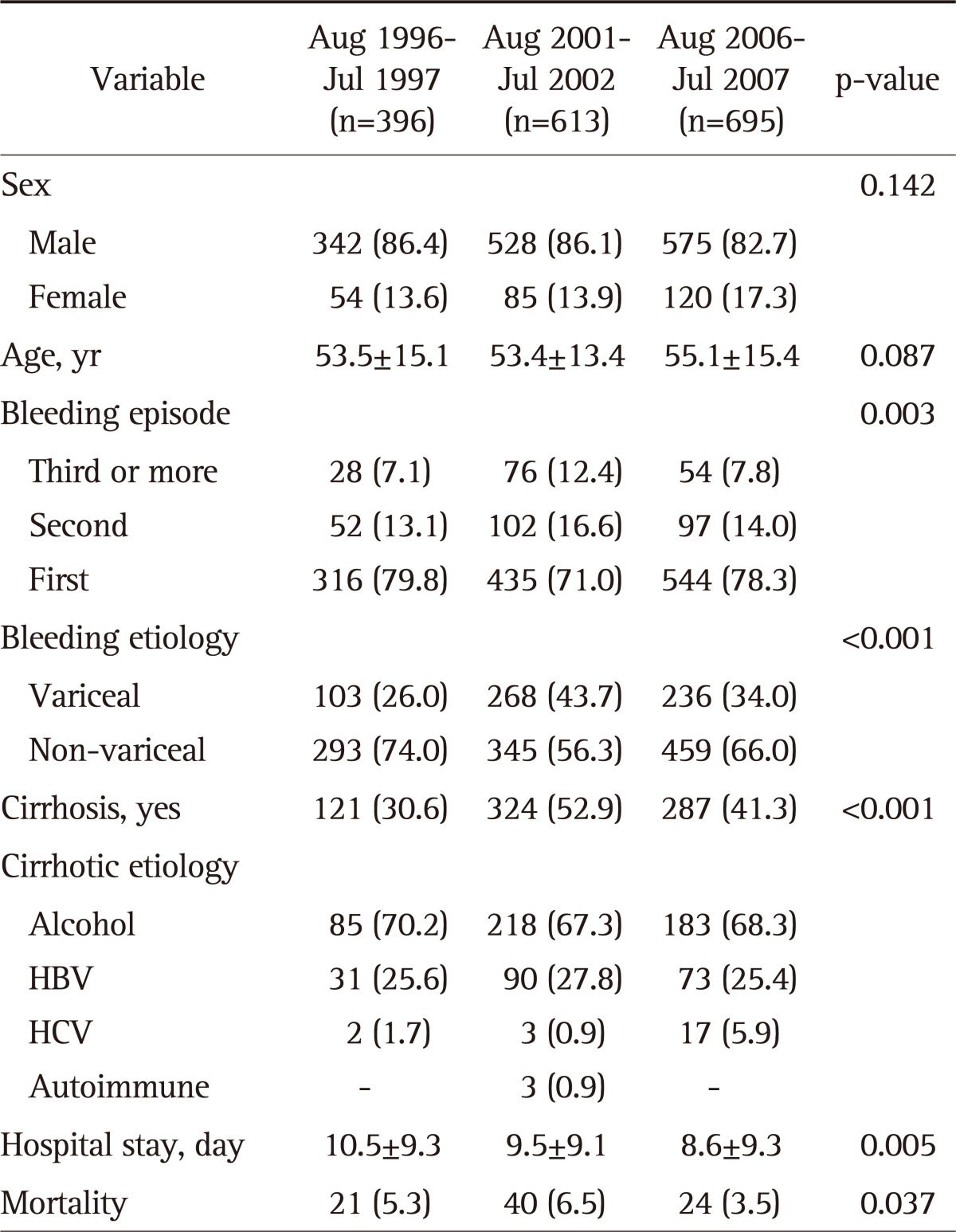
Characteristics of patients with upper GI bleeding by each period are shown in Table 2.
Table 2.
Characteristics of the Patients with Upper Gastrointestinal Bleeding by Study Period
Data are presented as mean±SD or number (%).
GI, gastrointestinal; HBV, hepatitis B virus; HCV, hepatitis C virus.
2. Clinical outcomes
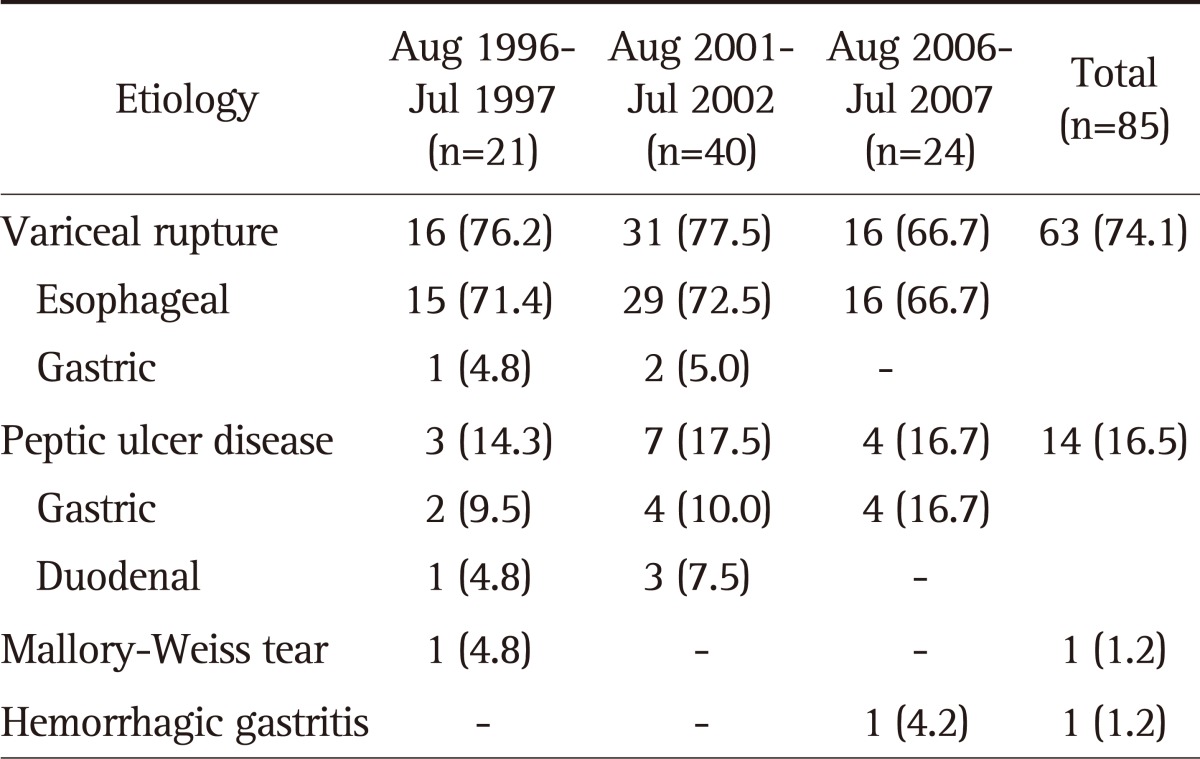
Total mortality rate of upper GI bleedings was 5.0% (n=85) during the study periods. Variceal rupture was the leading cause of mortality (n=63, 74.1%), followed by peptic ulcer (n=14, 16.5%), and others (n=2, 2.2%) (Table 3). Two other etiologies of mortality were Mallory-Weiss tear and hemorrhagic gastritis.
Table 3.
Etiology of Upper Gastrointestinal Bleeding in the Mortality Group
Data are presented as number (%).
The mean days of hospitalization decreased significantly over the periods (10.5±9.3 days vs 9.5±9.1 days vs 8.6±9.3 days, respectively, p=0.005), but in-hospital mortality rate did not decrease (5.3% vs 6.5% vs 3.5%, respectively, p=0.037). In a total of 1,704 patients, the mortality rate was significantly higher in the variceal bleeding group compared to that of the non-variceal bleeding group (10.4% vs 2.0%, p<0.001; odds ratio [OR], 5.66; 95% confidence interval [CI], 3.445 to 9.295).
3. Subgroup analysis for variceal bleeding
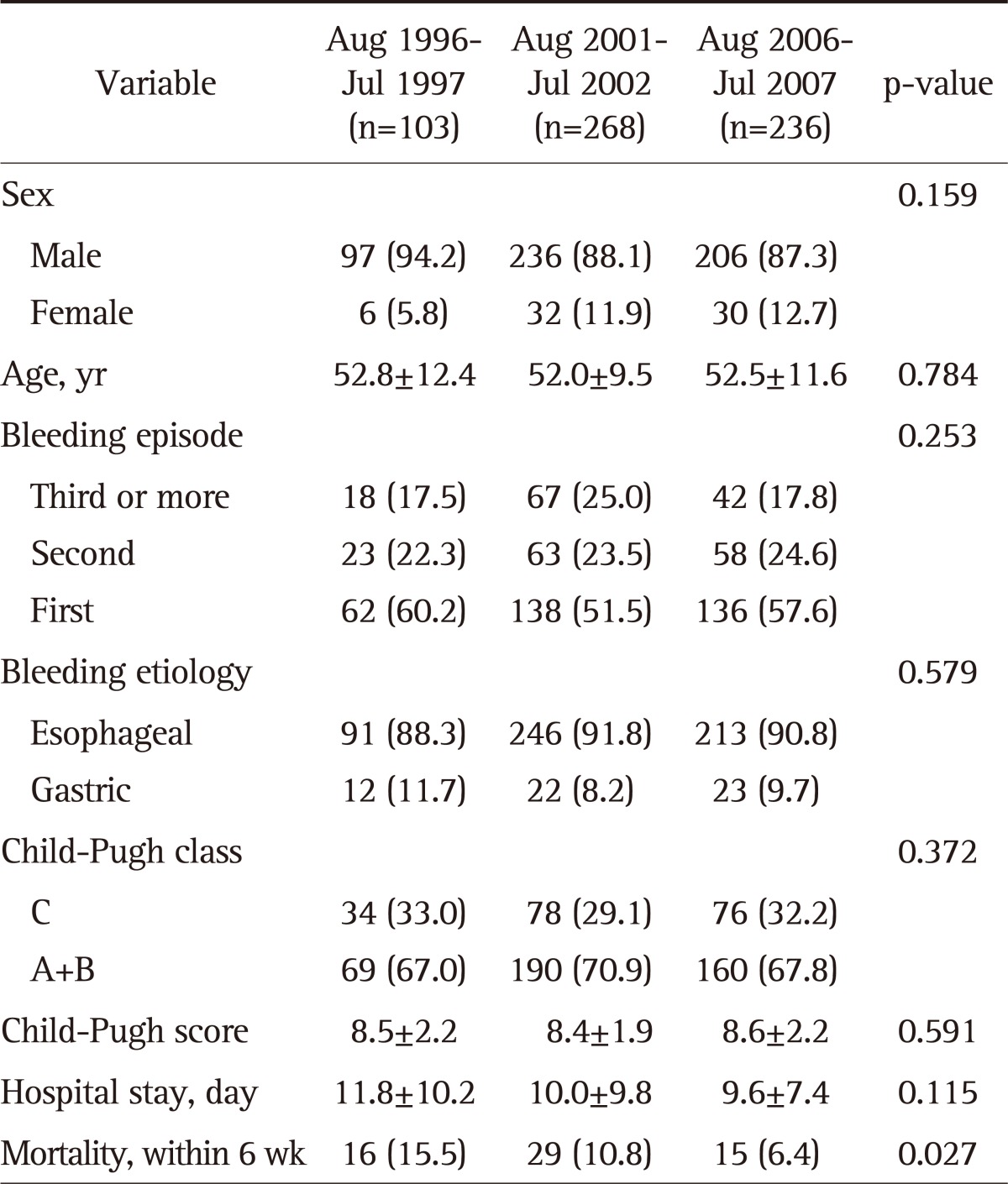
Characteristics of patients with variceal bleeding by each period are shown in Table 4.
Table 4.
Characteristics of the Patients with Variceal Bleeding by Study Period
Data are presented as mean±SD or number (%).
In a total of 607 variceal bleeding patients, the mean age was 52.3±10.9 years (range, 16 to 91), 539 patients were male (88.8%). Also, there were 550 (90.6%) esophageal and 57 (9.4%) gastric variceal bleedings. The variation of variceal bleeding rate by month was not statistically different among the three periods (data not shown). Also, there were no differences between the periods in sex, age, number of bleeding episodes, the rate of ruptured variceal type (esophageal varix, 88.3% vs 91.8% vs 90.3%, respectively, p=0.579), and the severity of cirrhosis (Child-Pugh class C, 33.0% vs 29.1% vs 32.2%, respectively, p=0.372).
Mortality rate of 6-week period of 607 variceal bleedings was 9.9% (n=60). The mean days of hospitalization decreased over the periods, yet showing no statistical significance (11.8±10.2 days vs 10.0±9.8 days vs 9.6±7.4 days, respectively, p=0.115). However, mortality rate decreased significantly (15.5% vs 10.8% vs 6.4%, respectively, p=0.027).
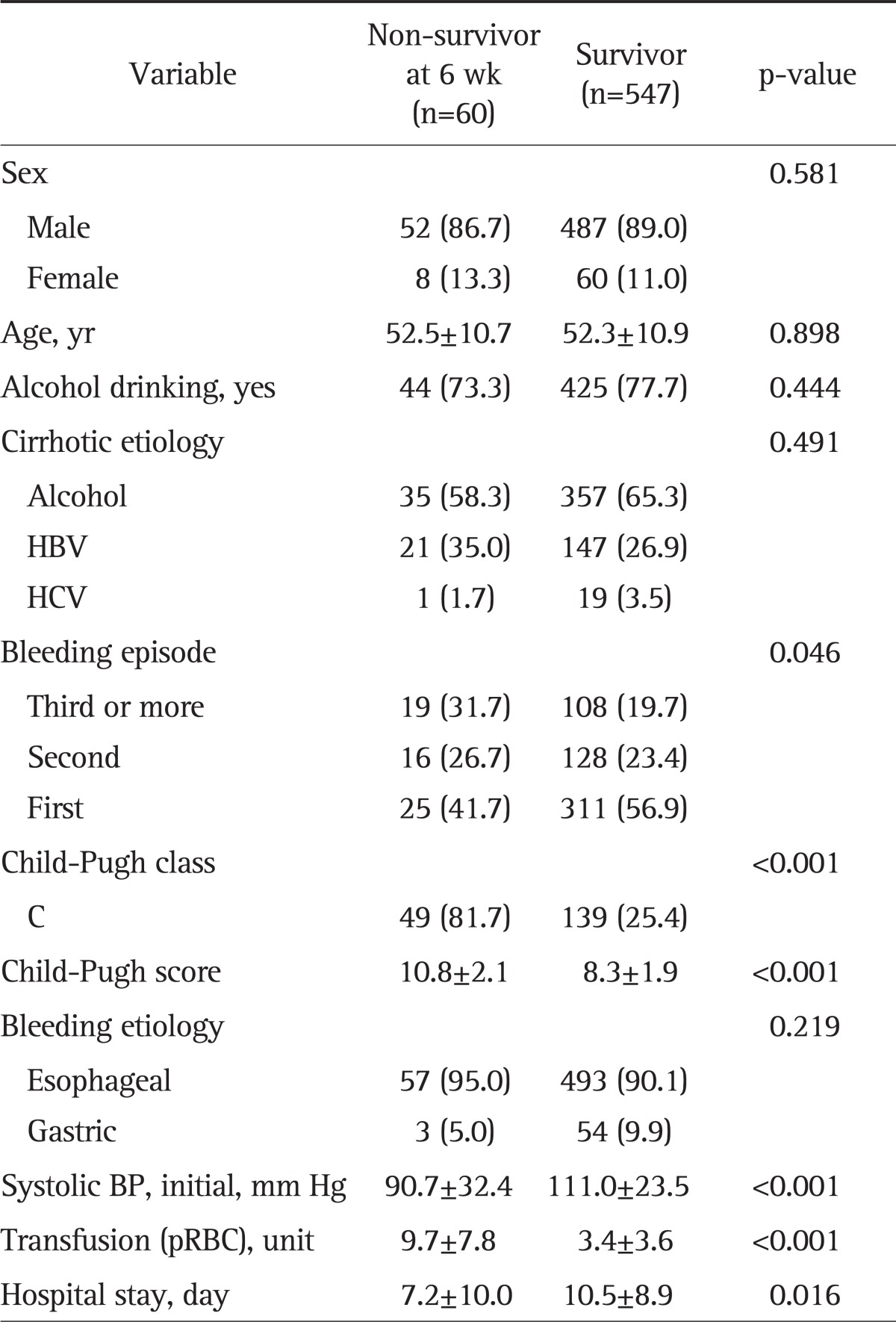
When the non-survivor group was compared to the survivor group, there were no differences in sex, age, alcohol drinking history, etiology of liver cirrhosis, and location of the variceal rupture (esophageal vs gastric, p=0.219) in each group. However, there were significant differences depending on the degree of advancement of liver cirrhosis (Child-Pugh class C, 81.7% vs 25.4%, p<0.001), as well as number of bleeding episodes, 3 or more (31.7% vs 19.7%, p=0.046) (Table 5).
Table 5.
Comparison of the Clinical Characteristics of the Variceal Bleeding Group by Survival
Data are presented as mean±SD or number (%).
HBV, hepatitis B virus; HCV, hepatitis C virus; BP, blood pressure; pRBC, packed red blood cell.
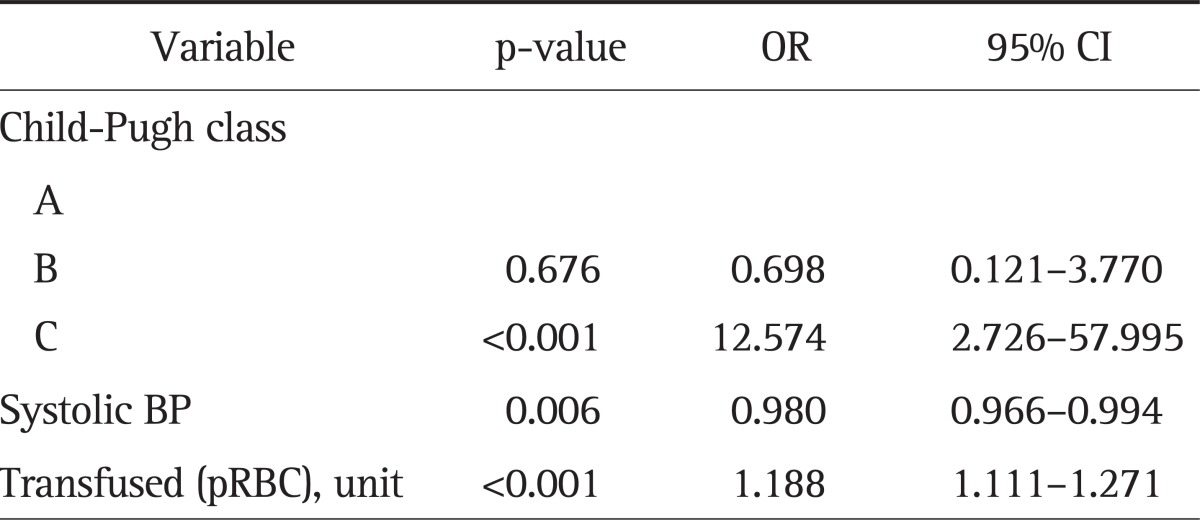
In multivariate analysis, the prognostic factors for predicting mortality in patients with variceal bleedings were Child-Pugh class C versus A (OR, 12.574; 95% CI, 2.726 to 57.995), initial systolic blood pressure (OR, 0.980; 95% CI, 0.966 to 0.994), total number of transfused red blood cell units (OR, 1.188; 95% CI, 1.111 to 1.271) (Table 6).
Table 6.
Multivariate Analysis to Predict Mortality within 6 Weeks in the Variceal Bleeding Group
OR, odds ratio; CI, confidence interval; BP, blood pressure; pRBC, packed red blood cell.
DISCUSSION
To date, many studies from other countries have reported decreasing incidence and mortality rates of variceal bleeding5-7 by progress of treatment modalities.8 However, in South Korea, no long-term community-based epidemiologic data was available until up to recently.
Higher mortality and morbidity rate of variceal bleedings due to liver cirrhosis causes a large socioeconomic burden to the community. Therefore, community-based and nationwide epidemiologic data are essential for a better management of cirrhotic patients. Moreover, there have been several reports about a gradual increase in socioeconomic costs due to continued increase of alcohol consumption, which is an important cause of cirrhosis in South Korea.9,10
With these points in mind, we investigated long-term changes in clinical outcomes of variceal bleedings in the Gangwon province. We also analyzed the changes in etiology of liver cirrhosis, with special focus on alcohol consumption to provide a baseline resource for further nationwide studies.
During the study periods, peptic ulcer was the leading etiology in all of the upper GI bleeding patients, followed by variceal rupture, Mallory-Weiss tear, hemorrhagic gastritis, and others. The etiology of upper GI bleedings did not change over the study periods.
But in the mortality group of upper GI bleeding (n=85), the leading etiologies were variceal ruptures (n=63, 74.1%), followed by peptic ulcers (n=14, 16.5%), and others. This results are consistent with the results of several previous retrospective studies.11,12 In addition, total mortality rate of upper GI bleeding did not decrease over the study period (5.1% vs 6.6% vs 4.3%, respectively).
When compared to the non-variceal bleeding group, possible explanations for the higher mortality rate in the variceal bleeding group are massive bleeding tendency despite the chance of spontaneous hemostasis: decreased remaining hepatic function which can result in hepatic failure: and other risk factors, such as combined infections, hepatorenal syndrome, hepatic encephalopathy, etc.
One recent prospective, multi-center study in South Korea showed that the 6 week mortality rate of variceal bleeding in patients with liver cirrhosis was 26.0%, which was about two times higher than that of non-varceal bleedings,13 but long term changes in mortality rates were not investigated. Our data also reconfirmed that the mortality rate in patients with upper GI bleedings was significantly higher in the variceal bleeding group compared to that of the non-variceal bleeding group (10.4% vs 2.0%, p<0.001; OR, 5.659; 95% CI, 3.445 to 9.295).
The significant decrease in mortality in other studies is probably due to the improvement in treatment modalities such as endoscopic treatment, transjugular intrahepatic portosystemic shunt, and pharmacologic treatment including the use of prophylactic antibiotics and vasoactive drugs.2,14,15 This is also applicable to our study.
Actually, in the variceal bleeding subgroup analysis, 6 week mortality rate had gradually decreased over the study period significantly (15.5% vs 10.8% vs 6.4%, respectively, p=0.027), in spite of its incidence not decreasing.
Another remarkable finding of this study is the change of etiology in liver cirrhosis. Until now, chronic viral hepatitis B was known to be the most common cause of liver cirrhosis in South Korea.16 However, several recent reports showed that alcohol should be regarded as an important cause due to its incidence gradually increasing as the etiology of liver cirrhosis.17,18 This etiologic change may have influence on the results in the change of incidence and mortality rate of variceal bleeding in our study.
Our study also revealed that alcohol is the leading cause of liver cirrhosis in the Gangwon province, South Korea. Previously, we had already reported that more than 60% of all liver cirrhotic patients admitted to the tertiary centers in the Gangwon province had alcohol etiology in 2009.19
Regarding the selection bias of excluding cirrhotic patients managed by outpatient clinics, we can postulate that this is due to geographical variations. There is higher incidence of alcohol related liver diseases, including liver cirrhosis, in our province, as reported in a recent nationwide report.20
However, we should interpret our results with caution. That is, our result should not be generalized to the nationwide statistics yet because of the limitation below. First, there is selection bias because we only included admitted patients, not outpatients, and also because patients with severe forms of alcoholic hepatitis who are indistinguishable from patients with cirrhosis could have been included in our study population. Second, the combined etiology of liver cirrhosis was not clearly investigated due to missing or incomplete old data. Third, there were incomplete data regarding endoscopic findings (variceal grade, location, etc.), rebleeding rate, and changes in treatment modality.
In spite of such limitations, our study has useful clinical implications, because this is the first long term, population-based cohort study in South Korea, and provides the baseline epidemiologic data and resources on changes in clinical outcomes of patients with variceal bleeding, as well as illustrating the importance of alcohol as the etiology of liver cirrhosis.
In conclusion, the result of our study demonstrates that variceal bleeding is still one of the major causes of upper GI bleedings. However, the mortality rate of variceal bleeding has gradually decreased significantly over the past decade in the Gangwon province, South Korea, in spite of incidence of variceal bleeding not decreasing. And a nationwide, large scale prospective study is needed for further assessment.
ACKNOWLEDGEMENTS
This work was supported by a fund from Gangwon branch of the Korean Association for the Study of the Liver, and also by a grant from the Korea Ministry of Health and Welfare, Republic of Korea (No. A102065).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–809. [PubMed] [Google Scholar]
- 2.D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 3.de Dombal FT, Clarke JR, Clamp SE, Malizia G, Kotwal MR, Morgan AG. Prognostic factors in upper G.I. bleeding. Endoscopy. 1986;18(Suppl 2):6–10. doi: 10.1055/s-2007-1018418. [DOI] [PubMed] [Google Scholar]
- 4.Burroughs AK. The natural history of varices. J Hepatol. 1993;17(Suppl 2):S10–S13. doi: 10.1016/s0168-8278(05)80448-9. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol. 2000;95:3566–3573. doi: 10.1111/j.1572-0241.2000.03376.x. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 7.Di Fiore F, Lecleire S, Merle V, et al. Changes in characteristics and outcome of acute upper gastrointestinal haemorrhage: a comparison of epidemiology and practices between 1996 and 2000 in a multicentre French study. Eur J Gastroenterol Hepatol. 2005;17:641–647. doi: 10.1097/00042737-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 9.Chung WJ, Chun HJ, Lee SM. Socioeconomic costs of alcohol drinking in Korea. J Prev Med Public Health. 2006;39:21–29. [PubMed] [Google Scholar]
- 10.Lee JK, Kim YI, Yoon SJ, et al. Estimating the burden of diseases due to high alcohol consumption in Korea. J Prev Med Public Health. 2005;38:175–181. [PubMed] [Google Scholar]
- 11.van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–1499. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 12.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945–953. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Um SH, Seo YS, Kim YH, et al. Clinical features of upper gastrointestinal bleeding in patients with cirrhosis. Korean J Hepatol. 2006;12(Suppl 3):S50. [PubMed] [Google Scholar]
- 14.Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico G, Pietrosi G, Tarantino I, Pagliaro L. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124:1277–1291. doi: 10.1016/s0016-5085(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim CY, Kim JW, Lee HS, Yoon YB, Song IS. Natural history and survival rate of chronic liver diseases in Korea: 20 years prospective analysis. Korean J Med. 1994;46:168–181. [Google Scholar]
- 17.Han YS, Kim BH, Baek IY, et al. The change of the etiology, complications and cause of death of the liver cirrhosis in 1990s. Korean J Hepatol. 2000;6:328–339. [Google Scholar]
- 18.Kim YS, Um SH, Ryu HS, et al. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci. 2003;18:833–841. doi: 10.3346/jkms.2003.18.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YD, Choi DH, Seok KT, et al. Changing epidemiology of chronic liver disease in Gangwon province: importance of alcoholic liver disease in Korea. Korean J Hepatol. 2009;15(Suppl 3):S83. [Google Scholar]
- 20.Korea Centers for Disease Control and Prevention. 2005 Health behavior and chronic disease statistics. Seoul: Korea Centers for Disease Control and Prevention; 2006. [Google Scholar]