Abstract
Background/Aims
The management guidelines for cystic lesions of the pancreas (CLPs) are not yet well established. This study was performed to document the long-term clinical outcome of CLPs and provide guidelines for the management and surveillance of CLPs.
Methods
In this retrospective cohort study, an additional follow-up was performed in 112 patients with CLPs enrolled from 1998 to 2004 during a previous study.
Results
During follow-up for the median period of 72.3 months, the size of the CLPs increased in 18 patients (16.1%). Six of these patients experienced growth of their CLPs after 5 years of follow-up. Twenty-six patients underwent surgery during follow-up, and four malignant cysts were detected. The overall rate of malignant progression during follow-up was 3.6%. The presence of mural nodules or solid components was independently associated with the presence of malignant CLPs. Seven patients underwent surgery after 5 years of follow-up. The pathologic findings revealed malignancies in two patients. There was only one pancreas-related death during follow-up.
Conclusions
The majority of CLPs exhibit indolent behavior and are associated with a favorable prognosis. However, long-term surveillance for more than 5 years should be performed because of the potential for growth and malignant transformation in CLPs.
Keywords: Pancreatic cyst, Natural history, Prognosis
INTRODUCTION
Increasing numbers of cystic lesions of the pancreas (CLPs) are being incidentally recognized due to the improvements in high resolution abdominal imaging and an increasing frequency of the use of this tool.1,2 Some investigators3,4 have advocated aggressive surgical resection showing their data of the relatively high incidence of premalignant and malignant CLPs. However, data collected after surgical resection do not reflect the characteristics of cystic lesions incidentally found in the clinical setting.2 Other investigators2,5,6 advocate selective nonoperative management arguing the improvement of cross-sectional imaging and endoscopic ultrasonography (EUS), the high morbidity associated with pancreatic surgery, and a better understanding of the natural history of certain subgroups.
Despite the recent improvements of many diagnostic tools, current imaging modalities cannot differentiate benign from premalignant or malignant CLPs with sufficient accuracy.7 In addition, preoperative tissue diagnosis such as cyst aspiration via EUS does not provide a sufficient diagnostic yield.2 Therefore, knowledge about the long-term natural history of CLPs is essential for establishing the optimal management strategy. Although the knowledge about this natural history of CLPs has been gradually acquired from many studies,1-3,5,8-19 it is not fully understood yet because of insufficient number of patients and duration of follow-up in most of these studies. Therefore, in clinical practice, making an optimal management plan for CLPs is still challenging20 and the guidelines for the surveillance of these CLPs have not been well established to date.
In a previous study,2 the natural history of 182 patients with CLPs was reported (mean duration of follow-up, 35.4±22.9 months) and, afterward, extended follow-up of this cohort was performed at our institution. The current study was performed to document the long-term clinical outcomes of CLPs and to provide data for determination of the management and surveillance of the CLPs.
MATERIALS AND METHODS
1. Patients
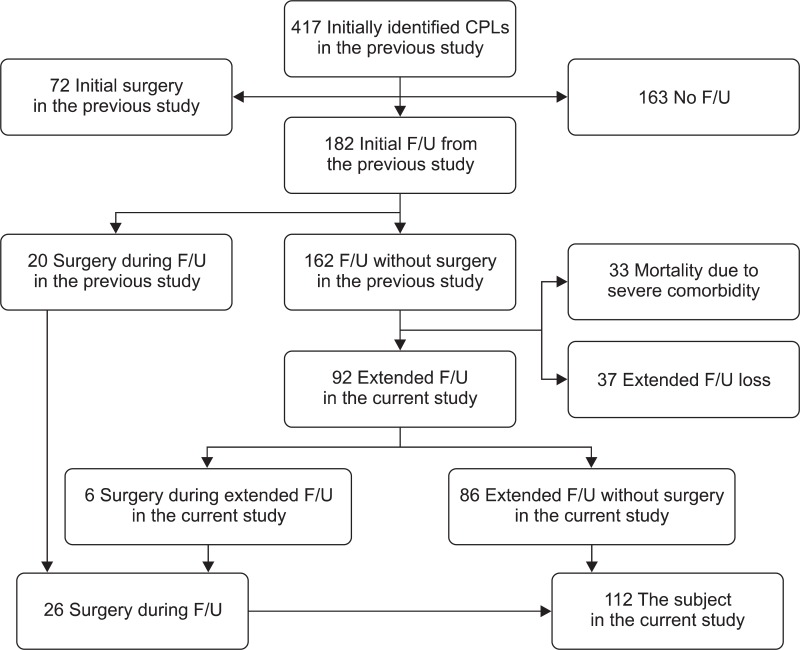
The initial identification of patients with CLP was described in the previous study2 and was as follows: Inclusion criteria were patients older than 20 years of age who were diagnosed as having CLP by abdominal ultrasonography (USG) or abdominal computed tomography (CT) from January 1998 to December 2004 and who received follow-up with repeated imaging studies for more than 3 months. Exclusion criteria were patients with evidence of pancreatitis and patients who had a history of von Hippel-Lindau disease, a polycystic disease of the kidney or liver, or cystic fibrosis. A total of 182 patients received follow-up and 20 patients among them underwent surgery in the previous study (Fig. 1).
Fig. 1.
Algorithm for the initial identification and clinical follow-up of patients with cystic lesions of the pancreas from the previous and current studies.
CLPs, cystic lesions of the pancreas; F/U, follow-up.
In the current study, an additional extended follow-up of the cohort recruited during the previous study (from January 1998 to December 2004) was performed. Among 162 patients who had received follow-up without surgery in the previous study, 33 patients died during or within 6 months after the previous study due to severe co-morbidities. Among the other 129 patients available for additional follow-up, 37 patients refused further follow-up at our institution, which resulted in a loss to follow-up. Therefore, 92 patients (71.3% of 129 patients) agreed to participate in the current study and received additional follow-up (Fig. 1). Six patients among them underwent surgery during this additional follow-up. Finally, the natural history and clinical outcome of 112 patients who underwent surgery during follow-up in both the previous and current study (26 patients) or received additional extended follow-up in the current study without surgery (86 patients) were investigated in the current study (Fig. 1).
2. Methods
All enrolled patients received the first follow-up with imaging study (CT or USG) 3 to 6 months after initial diagnosis of CLPs. In cases of absence of significant change in cystic size & feature, the interval of follow-up extended to 6 months to 1 year according to the individual clinical situation (6-month and 1-year interval at the second and third follow-up, respectively, in most patients). Magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography, or EUS was performed in patients with suspicion of malignancy or with change in cystic size or feature.
The classification and definition of clinical, radiologic, endoscopic, and pathologic data is the same as in the previous study.2 The size of the CLP was measured by axial image of CT or MRCP and was defined as the longest diameter. In patients with multiple CLPs, the largest diameter of the largest CLP was measured and recorded; in addition, the location of the CLPs was defined as the location of the largest CLP. The size of the CLP was classified as <2 cm, between 2 and 3 cm, and >3 cm, which is consistent with the classification in previous studies.1,5,11-14,21 Growth of the CLP was considered to be significant if the increase of the CLP size exceeded 1 cm. The duration of follow-up was defined as a period from the initial detection of CLP to the last imaging follow-up of the CLP (in case of surgery, the last imaging follow-up before surgery).
In the current study, a grade for co-morbidities was determined for each enrolled patient according to the Adult Co-morbidity Evaluation 2722 and was included in the baseline clinical characteristics.
Each pathologic specimen was reviewed by two pathologists. The lesions were considered to be malignant when carcinoma in situ or invasive cancer was present in any pathologic examination field and were considered to be premalignant when an adenoma or borderline malignancy was identified in cases with an intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm, solid pseudopapillary tumor, or neuroendocrine tumor of the pancreas. Serous cystadenoma (SCA), lymphangioma, pseudocyst, or other nonmalignant conditions were defined as benign lesions.
The survival status of patients was investigated through the Korean Registry of Birth and Death. For patients who were not available for clinical follow-up evaluation, in the current study, telephone contacts were attempted to obtain information about the clinical outcome. The study protocol was reviewed and approved by the Institutional Review Board of our institution.
3. Outcomes
The primary outcome in the current study was defined as malignant progression during follow-up. The secondary outcome was defined as growth of the CLP and surgical resection during follow-up and mortality related to CLPs.
4. Statistical analysis
The results are presented as the means±standard deviation. Comparisons were performed using the independent t-test for continuous variables and Pearson's chi-square test or Fisher's exact test for categorical variables. The logistic regression multivariate analysis was performed to identify independent factors associated with the final pathology of the surgical specimen (malignant vs non-malignant). The multivariate analysis was performed with a model using factors with p-values less than 0.10 in the univariate analysis. A p-value less than 0.05 was considered statistically significant.
RESULTS
1. Baseline characteristics
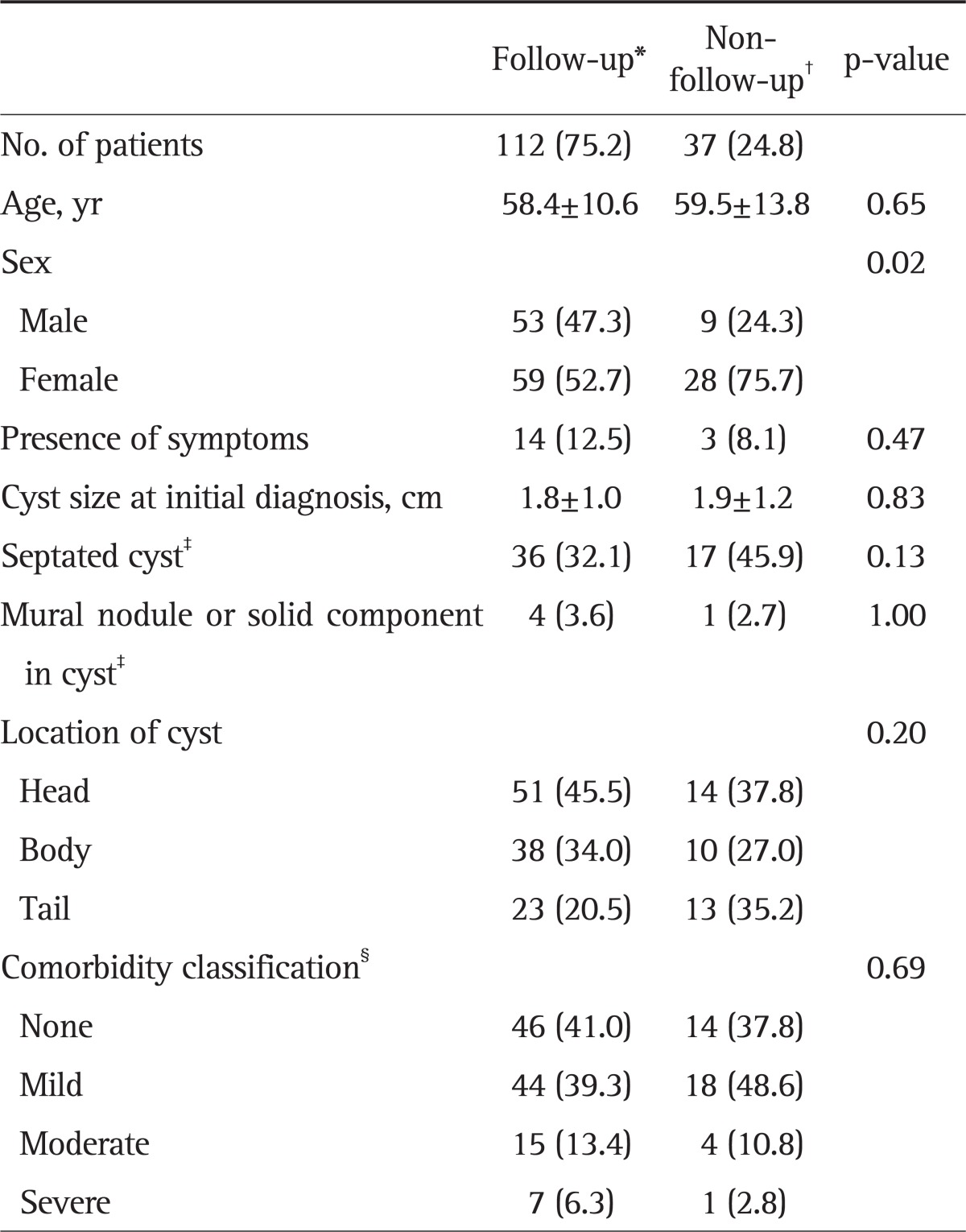
The group enrolled in the current study (112 patients) was first investigated to determine whether they were a representative sample; the baseline characteristics of this group (112 patients, follow-up group) and the 37 patients who refused further follow-up after the previous study2 (non-follow-up group) were compared. The comparison between the two groups is shown in Table 1. While male gender tended to be more common in the follow-up group, other baseline characteristics such as age, presence of symptoms, initial size of the CLP, features of the CLP, and co-morbidity grade were not significantly different between the two groups.
Table 1.
Comparative Data of Patients with or without Additional Follow-up
Data are presented as mean±SD or number (%).
*112 patients enrolled in the current study; †37 patients refusing further follow-up; ‡At initial diagnosis; §According to the Adult Comorbidity Evaluation 27.
The baseline characteristics of the 112 enrolled patients are summarized in Table 1. The mean age was 58.4±10.6 years (range, 31.0 to 81.0) and the mean initial cyst size was 1.8±1.0 cm (range, 0.3 to 7.0). At the end of the current study (February 2010), the median period of follow-up was 72.3 months (range, 5.0 to 142.8).
2. Surgical treatment and malignant progression during follow-up
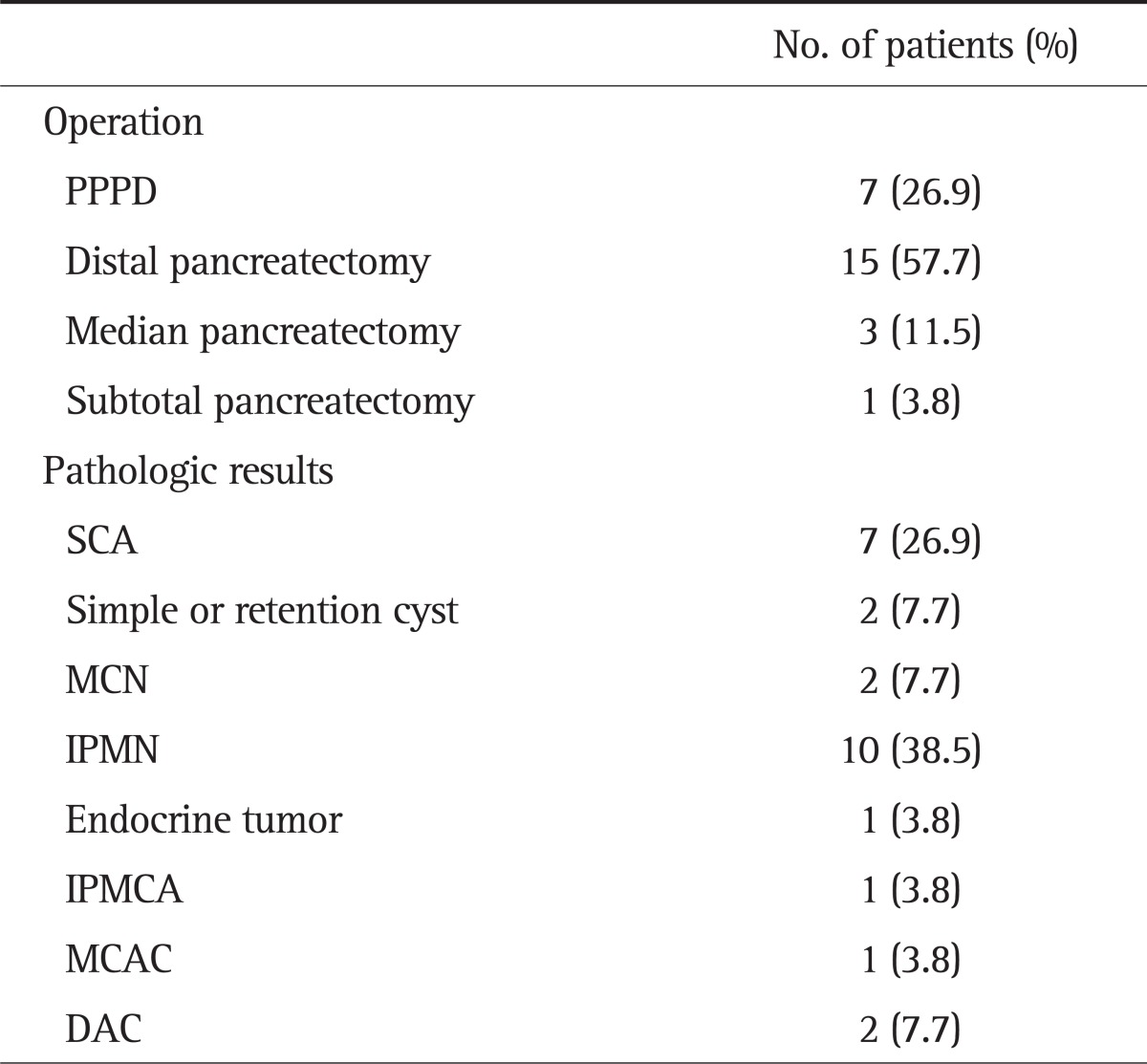
During the follow-up, a total of 26 patients (23.2% of 112 patients) underwent surgery due to an increase of the size of CLPs (11 patients), changes in features of the CLP (seven patients), pain aggravation (four patients), recurrent pancreatitis (one patient), or a simultaneous operation for another intra-abdominal tumor (three patients). The median time from the initial diagnosis to the surgical resection was 42.8 months (5 to 114 months). Operation and pathologic results are summarized in Table 2. The pathologic results revealed premalignant and benign cyst in 13 and nine patients, respectively, and malignant cysts were detected in four patients (mucinous cystadenocarcinoma, ductal adenocarcinoma [DAC], and intraductal papillary mucinous carcinoma [IPMCA]). Therefore, the rate of malignant progression during follow-up was 3.6% (four out of 112 patients).
Table 2.
Operations and Pathologic Results
PPPD, pylorus-preserving pancreaticoduodenectomy; SCA, serous cystadenoma; MCN, mucinous cystic neoplasm; IPMN, intraductal papillary mucinous neoplasm; IPMCA, intraductal papillary mucinous carcinoma; MCAC, mucinous cystadenocarcinoma; DAC, ductal adenocarcinoma.
Among 26 patients, seven patients received surgery after follow-up for more than 5 years. In these seven patients, changes in features of the CLP, growth of the CLP, which resulted in surgery, occurred after 5 years of follow-up. As a result of surgery, malignant CLPs were detected in two patients (DAC and IPMCA, respectively).
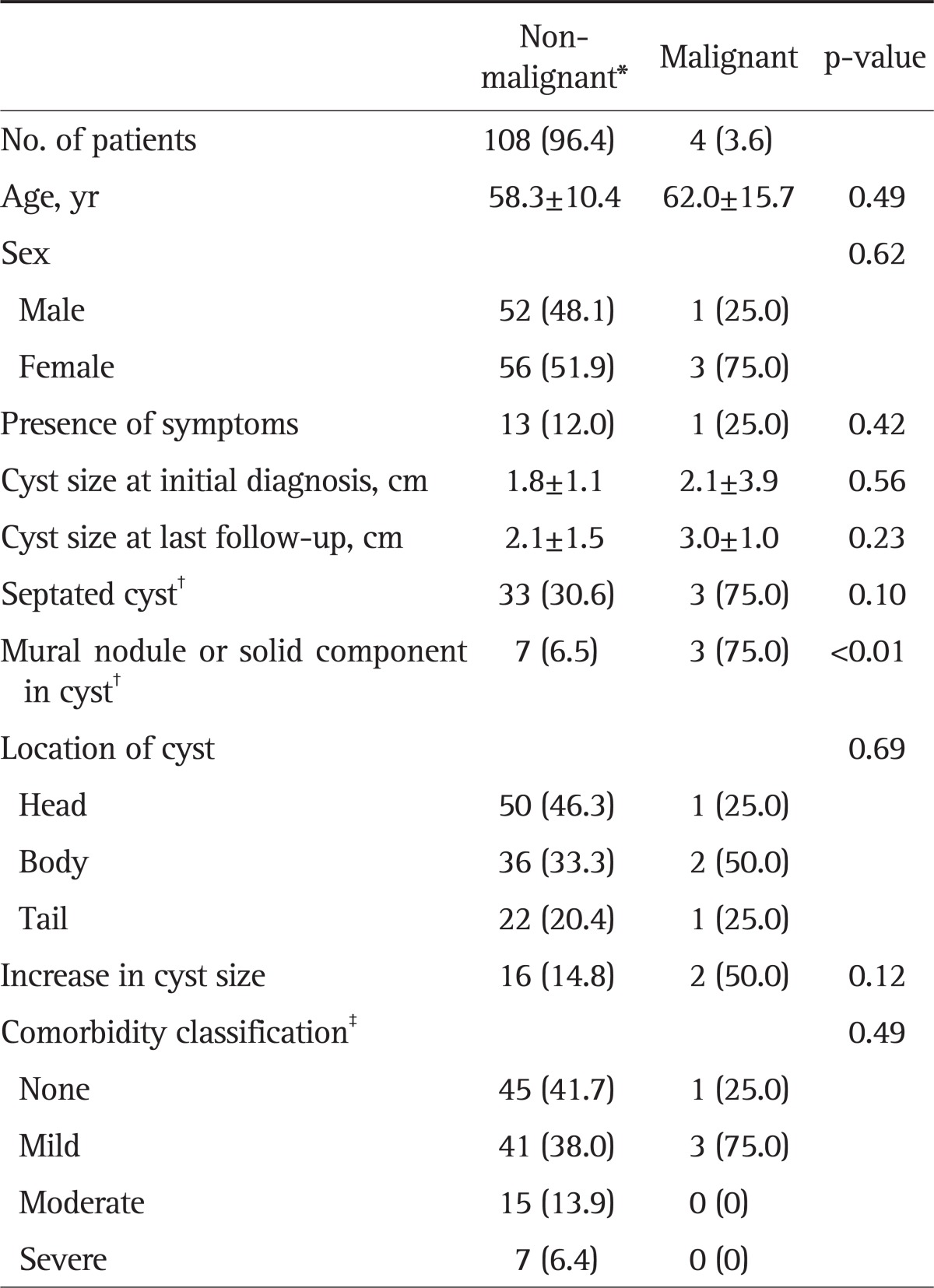
Patients were classified into two groups (malignant vs non-malignant) according to the final pathology of the surgical specimen (patients who had not received surgery were included in the non-malignant group). A comparison of the clinical characteristics of both groups is shown in Table 3. The presence of mural nodule or solid component at last follow-up was significantly associated with malignancy (p=0.002) and the risk of malignancy was 30% (three out of 10 patients) in case of the presence of mural nodule or solid component. The presence of septated cyst at last follow-up or the increase in cyst size tended to be associated with malignancy. However, this was not significant (p>0.05). Multivariate analysis revealed that the presence of mural nodule or solid component was independently associated with the risk of a malignant CLP (odds ratio, 86.716; 95% confidence interval, 4.847 to 1,551.396; p=0.002) (Table 4).
Table 3.
Comparative Data of Patients with or without Malignant Progression
Data are presented as mean±SD or number (%).
*Patients who had not undergone surgery were included in the non-malignant group; †At last follow-up; ‡According to the Adult Comorbidity Evaluation 27.
Table 4.
Results of Multivariate Analysis to Identify the Risk Factors Associated with Malignant Cysts
OR, odds ratio; CI, confidence interval.
*At last follow-up.
3. Natural history and clinical outcomes after follow-up
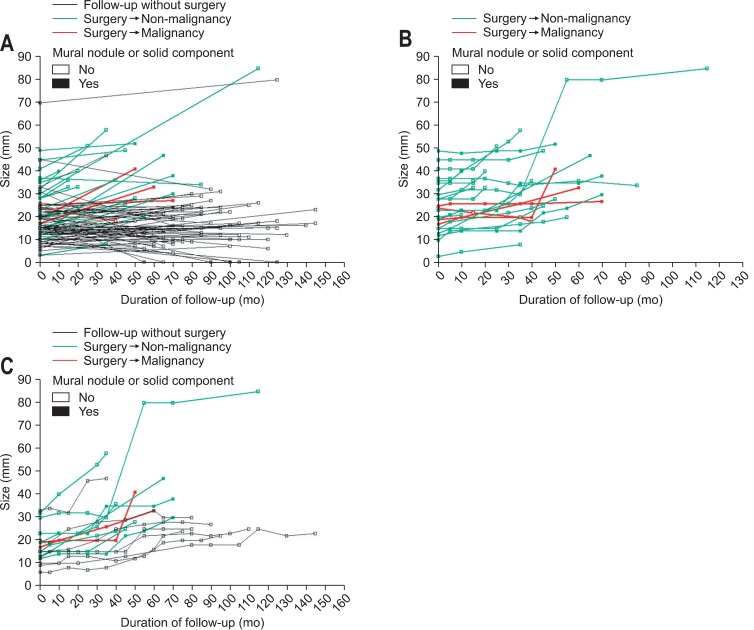
During follow-up, growth of the CLP was noted in 18 patients (16.1%) and the median duration between the initial diagnosis and the detection of the growth of the CLP in these 18 patients was 51.6 months (range, 27.6 to 117.8 months). The duration was longer than 5 years in six patients and three patients among them eventually received surgical resection. Fig. 2 shows the pattern of the change in the size of CLPs during follow-up for individual cases and represents the natural histories and clinical outcomes of the CLPs (in a total of 112 patients enrolled in the current study [Fig. 2A], in 26 patients who underwent surgery during follow-up [Fig. 2B], and in 18 patients who experienced the growth of the CLP during follow-up [Fig. 2C]). In most patients who received follow-up without surgery, the size of CLPs remained within 3 cm (Fig. 2A). In patients who underwent surgery during follow-up, the size of the CLPs at initial diagnosis and the growth of the CLPs did not seem to be associated with the malignancy (Fig. 2B). No patients experienced the growth of the CLPs within 2 years after initial diagnosis (Fig. 2C).
Fig. 2.
Patterns of the change in the size of the cyst. (A) In a total of 112 patients enrolled in the current study. (B) In 26 patients who underwent surgery during follow-up. (C) In 18 patients who experienced cyst growth during follow-up.
We investigated the natural histories and clinical outcomes of CLPs in a subgroup of 73 patients with small CLPs (less than 2 cm) at initial diagnosis. In this subgroup, the growth of the CLP during follow-up was noted in 13 patients (17.8%) and the median duration between the initial diagnosis and the detection of the growth of the CLP was 53.8 months (35.0 to 117.8 months). Nine of them (12.3%) underwent surgical resection during follow-up and two malignant cysts were detected. Therefore, the overall rate of malignant progression was 2.7%. The median duration between the initial diagnosis and surgery was 50.3 months (range, 36.2 to 72.0 months).
Among 112 patients, 4 patients had CLPs containing mural nodule or solid component at initial diagnosis (mural nodule and solid component in three and one patient, respectively). The mural nodule in two patients disappeared during follow-up. In other one patient, the size of mural nodule did not change during follow-up for 68 months. Other one patient with solid component experienced growth of the solid component 64 months after initial diagnosis. This patient received surgical resection and the pathologic results showed SCA.
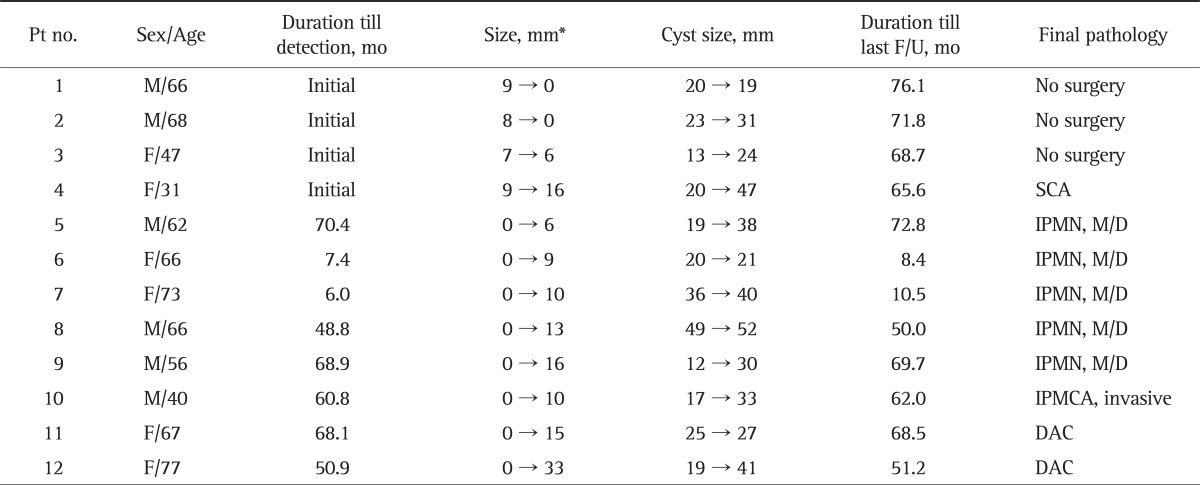
Among 112 patients, 108 patients had CLPs without mural nodule or solid component at initial diagnosis. In eight out of these 108 patients (7.4%), however, a mural nodule or solid component developed during follow-up. The duration between the initial diagnosis and detection of the mural nodule or solid component ranged from 6.0 to 70.4 months. All of these eight patients received surgical resection and the malignant CLPs were detected in three out of eight patients (37.5%). The clinical data and final pathology of a total of 12 patients with mural nodule or solid component are summarized in Table 5. The mean size of mural nodule or solid component at last follow-up tended to be higher in patients with malignancy (8.4 mm vs 19.3 mm). However, this difference was not significant (p>0.05). Other factors such as the size of mural nodule or solid component at initial diagnosis and the size of CLP at initial diagnosis or last follow-up were not associated with malignancy.
Table 5.
The Clinical Data and Final Pathology of 12 Patients with Mural Nodules or Solid Components
Pt, patient; F/U, follow-up; M, male; F, female; SCA, serous cystadenoma; IPMN, intraductal papillary mucinous neoplasm; M/D, moderate dysplasia; IPMCA, intraductal papillary mucinous carcinoma; DAC, ductal adenocarcinoma.
*The size of mural nodules or solid components.
Twenty-nine patients received EUS during follow-up and six of them underwent surgery as a result of the findings on the EUS. The EUS detected mural nodule or solid component in these six patients who had received follow-up for the presumptive diagnosis of IPMN and all of these six patients received surgery. The pathologic results revealed IPMN with moderate dysplasia in three patients, IPMCA in one patient, and DAC in two patients. Only two out of 29 patients received EUS-guided fine needle aspiration (FNA) and it did not influence clinical decisions.
According to the Korean Registry of Birth and Death and the telephone contacts (for a total of 182 patients who have received follow-up form the previous study),2 133 patients were alive and 49 patients died by the end of the current study. The causes of death were unrelated to pancreatic pathology with the exception of one patient. This one patient initially refused surgical resection for a CLP with an initial size of 3.2 cm and a solid component and died 8 months later.
DISCUSSION
The current study is an extended study of the previous study;2 it provides the results of clinical outcomes of CLPs after more than 5 years of follow-up. This study confirms the indolent nature and favorable prognosis of most CLPs as a result of long-term follow-up. However, this study also shows the potential for growth and malignant transformation of CLPs even after 5 years, which provides evidence to support long-term surveillance.
In the current study, the rate of malignant progression of CLPs during follow-up was 3.6% and the univariate and multivariate analysis showed that the presence of mural nodule or solid component was strongly associated with malignancy. Therefore, we evaluated the natural history of CLPs containing mural nodules or solid component. Although some mural nodules showed regression during follow-up, overall risk of malignancy in case of the presence of mural nodule or solid component was 30% in the current study. Furthermore the development of these intra-cystic components occurred even after follow-up for more than 5 years. Several studies also reported the variable factors associated the malignancy. Jang et al.23 presented the tumor size and the presence of a mural nodule as meaningful predictors of malignancy. However, in this study, only patients with IPMN proven pathologically were included. Brounts et al.16 reported that the presence of symptoms, male gender, and cystic loculation were associated with the risk for a malignancy. Lee et al.,1 on multivariate analysis of their 166 surgical cases, reported that the presence of weight loss, a solid component in the CLP, and common bile duct dilatation were independent predictors of a malignancy. These two studies, in contrast to the current study, included patients who underwent surgical resection immediately after the initial diagnosis.
Few studies have evaluated the natural history of CLPs for more than 5 years. Handrich et al.13 reported the clinical outcome of long-term follow-up (mean duration of 8 years) of CLPs. The number of patients, however, was only 22 and only patients with small CLPs (less than 2 cm) were included in their study. In the current study, the median duration of follow-up was 72.3 months and 81 patients (72.3%) received follow-up for more than 5 years. Among these 81 patients, CLPs in 6 patients were noted to begin growing even after 5 years of follow-up; three of these patients eventually received surgical resection. A total of seven patients underwent surgical resection after 5 years of follow-up and the pathologic results revealed malignancies in two patients. Furthermore, in all seven patients, changes in the features of the CLP or growth of the CLP occurred after 5 years of follow-up. Few studies have reported malignant change after 5 years of follow-up in CLPs with initial features of a benign lesion; this is the first study to report such findings. These results of long-term follow-up (more than 5 years) demonstrate the potential of growth and malignant transformation of CLPs even after 5 years of follow-up and provide evidence to support clinical and radiological follow-up of these lesions for more than this long-term duration.
Several studies have suggested guidelines for surveillance of CLPs. Das et al.10 reported that growth of CLPs was unlikely to occur before 2 years in CLPs 3 cm or less in size and without mural nodules; they suggested that the optimal imaging interval should be at 2 years from the initial diagnosis. Handrich et al.13 reported that 59% of patients with CLPs less than or equal to 2 cm remained unchanged over a minimum radiologic follow-up of 5 years and Lahav et al.8 suggested that asymptomatic CLPs without unfavorable EUS findings could be managed conservatively for at least a mean period of 4 years. In the current study, among 73 patients with a small CLP (<2 cm), 12.3% (nine out of 73 patients) underwent subsequent surgery. However, all subsequent surgeries occurred after 36 months of follow-up, and the risk of malignancy in this subgroup was only 2.7% (two out of 73 patients). Furthermore, none of the patients in this subgroup experienced growth of the CLP before 2 years of follow-up. According to the results of the current study, therefore, the suggestion of Das et al.10 about the optimal imaging interval (2 years from initial diagnosis) can be applied to clinical practice, especially in patients who have severe co-morbidities. However, further studies with larger number of patients and with more elaborately defined protocol will be needed to clarify this issue.
The limitations of this study include the followings. First, this study is a retrospective cohort study performed without elaborately defined protocol. However, to our knowledge, this is the first study both with duration of follow-up for more than 5 years and with the largest number of patients to date. Second, because only 92 out of 129 patients (71.3%) received additional follow-up, the absolute risk of malignant potential of CLPs might be underestimated. However, the baseline characteristics (except for gender) were not significantly different between the follow-up group and the non-follow-up group; the difference in gender was likely to be an incidental finding. Furthermore, the data was obtained from the Korean Registry of Birth and Death and the telephone contacts (for a total of 182 patients who have received follow-up from the previous study)2 to evaluate the effect of CLPs on long-term prognosis. According to the data, only one pancreas-related mortality occurred in this study. Third, because of low rate of malignancy (3.6%), the analysis for the identification of factors associated with malignancy could not be performed with sufficient accuracy in the current study; although the presence of septum and the increase in the size of CLPs tended to be associated with malignancy, these factors failed to show statistical significance. Further studies with larger number of patients (including only patients who received conservative management initially) are needed to identify the predictors of malignancy clearly. Fourth, only a small portion of patients (25.9%, 29 out of 112 patients) received EUS for the evaluation of CLPs. In fact, EUS was not available for many patients during the period of patient enrollment (from 1998 to 2004); most EUS procedures were performed after this time. EUS can be particularly helpful in demonstrating septae, solid components, and communication with pancreatic ducts, and guiding FNA for cytological examinations and fluid analysis.15,24 In this study, EUS influenced clinical decision in six patients by detecting change in the features of the CLPs.
In summary, according to the results of long-term follow-up, most CLPs, when conservative management is planned initially, have an indolent course and generally a favorable prognosis, irrespective of subsequent surgery during follow-up; although the rate of subsequent surgery for CLPs was considerable (23.2%), the overall rate of malignant progression during long-term follow-up was only 3.6%. However, in case of the development of mural nodule or solid component during follow-up, surgery should be considered because of considerable rate of malignancy. Moreover, the results of this study suggest that clinical and radiological surveillance should be maintained for at least more than 5 years because of the potential for growth and malignant transformation of CLPs even after this long-term duration.
ACKNOWLEDGEMENTS
This study was supported by research funding from the Seoul National University Bundang Hospital (grant no. 11-2009-020).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee CJ, Scheiman J, Anderson MA, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234–242. doi: 10.1007/s11605-007-0381-y. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Shin CM, Park JK, et al. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci. 2007;52:2653–2659. doi: 10.1007/s10620-006-9634-y. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–154. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Allen PJ, D'Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Jung MK, Cho CM, et al. A case of pancreatic pseudocyst with an atypical multilocular appearance on endoscopic ultrasound. Gut Liver. 2010;4:270–273. doi: 10.5009/gnl.2010.4.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahav M, Maor Y, Avidan B, Novis B, Bar-Meir S. Nonsurgical management of asymptomatic incidental pancreatic cysts. Clin Gastroenterol Hepatol. 2007;5:813–817. doi: 10.1016/j.cgh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Cadili A, Bazarrelli A, Garg S, Bailey R. Survival in cystic neoplasms of the pancreas. Can J Gastroenterol. 2009;23:537–542. doi: 10.1155/2009/139780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol. 2008;103:1657–1662. doi: 10.1111/j.1572-0241.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 11.Walsh RM, Vogt DP, Henderson JM, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670. doi: 10.1016/j.surg.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Walsh RM, Vogt DP, Henderson JM, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677–684. doi: 10.1016/j.surg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Handrich SJ, Hough DM, Fletcher JG, Sarr MG. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005;184:20–23. doi: 10.2214/ajr.184.1.01840020. [DOI] [PubMed] [Google Scholar]
- 14.Pausawasdi N, Heidt D, Kwon R, Simeone D, Scheiman J. Long-term follow-up of patients with incidentally discovered pancreatic cystic neoplasms evaluated by endoscopic ultrasound. Surgery. 2010;147:13–20. doi: 10.1016/j.surg.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brounts LR, Lehmann RK, Causey MW, Sebesta JA, Brown TA. Natural course and outcome of cystic lesions in the pancreas. Am J Surg. 2009;197:619–622. doi: 10.1016/j.amjsurg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Woo SM, Ryu JK, Lee SH, Yoon WJ, Kim YT, Yoon YB. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009;96:405–411. doi: 10.1002/bjs.6557. [DOI] [PubMed] [Google Scholar]
- 18.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93–102. doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 20.Oh HC, Kim MH, Hwang CY, et al. Cystic lesions of the pancreas: challenging issues in clinical practice. Am J Gastroenterol. 2008;103:229–239. doi: 10.1111/j.1572-0241.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 21.Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912–919. doi: 10.1148/radiol.2382041806. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 23.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 24.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]