Abstract
Background/Aims
Early intestinal mucosal damage plays an important role in severe acute pancreatitis (AP). Previous studies have shown that intestinal permeability (IP), serum endotoxin and cytokines contribute to the early intestinal barrier dysfunction in AP. This study explored the predictive capacity of IP, endotoxemia and cytokines as prognostic indicators in AP patients.
Methods
Eighty-seven AP patients were included in the study. The patients were classified into three groups according to the Balthazar computed tomography severity index (CTSI). We compared the biochemical parameters, including IP, serum endotoxin level and cytokine level among the three groups. The associations of IP with serum endotoxin, cytokines, CTSI, and other widely used biochemical parameters and scoring systems were also examined.
Results
IP, serum endotoxin, interleukin (IL-6) and tumor necrosis factor (TNF)-α had a positive correlation with the CTSI of AP. Endotoxin, IL-6, TNF-α, CTSI, the Ranson/APACHE II score, the duration of hospital stay, complications and death significantly affect IP in the AP patients.
Conclusions
We believe that IP with subsidiary measurements of serum endotoxin, IL-6 and TNF-α may be reliable markers for predicting the prognosis of AP. Further studies that can restore and preserve gut barrier function in AP patients are warranted.
Keywords: Acute pancreatitis, Intestinal permeability, Endotoxins, Balthazar computed tomography severity index, Cytokines
INTRODUCTION
The intestine is most externally exposed to the environment among internal organs. It not only exerts basic actions for the uptake, digestion, absorption, anabolism and excretion of food, but also plays an important role in immunological function by forming an effective barrier to inhibit the absorption of harmful materials such as bacteria, toxin, antigens and cytokines related to inflammation.1 Although the mechanism of intestinal barrier damage in acute pancreatitis (AP) is complicated and has not yet been elucidated, it has been reported that intestinal barrier damage occurs as a result of intestinal ischemia secondary to local and systemic inflammatory reactions and hypovolemic shock in the early stage of AP which increases intestinal permeability (IP) and causes endotoxemia.2 Products and toxins from the intestinal flora or microbial organisms enter blood circulation following intestinal barrier damage, which can cause sepsis and multi-organ failure which are the main causes of death in patients with AP.3 It has recently been demonstrated that endotoxins, mast cells and inflammatory mediators, including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and platelet-activating factor, contribute to the development of intestinal barrier damage in early phase of AP.4-8
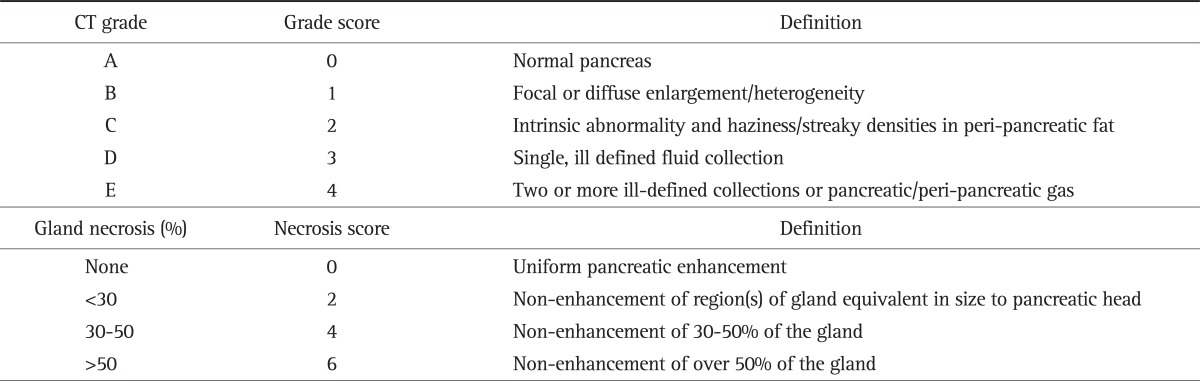
Balthazar computed tomography severity index (CTSI) is a 10 point scoring system derived from assessing the degree of pancreatic and peri-pancreatic inflammation (0 to 2 points), the presence and number of peri-pancreatic fluid collections (0 to 2 points), the presence and degree of pancreatic parenchymal non-enhancemenet or necrosis (0 to 6 points).9 The CTSI is superior to the Ranson score, Acute Physiology and Chronic Health Evaluation (APACHE) II or III score and serum C-reactive protein (CRP) concentration in predicting AP outcome.10,11
This study was conducted to investigate whether an increase of IP and endotoxemia may be related to prognostic indicators such as the CTSI and to examine whether inflammatory mediators contribute intestinal barrier damage in AP patients through the relationship between IP, endotoxemia and serum TNF-α/IL-6.
MATERIALS AND METHODS
1. Patients
A total of 118 patients with AP who were admitted to Kangbuk Samsung Hospital between July 2007 and February 2010 were prospectively studied in the study. Consent was given by each patient after a complete description of the study protocol. AP was diagnosed when subjects had clinical symptoms of AP including acute abdominal pain, a three- or more fold increase in serum amylase above the normal value and suggestive computed tomography (CT) findings of AP. Exclusion criteria were as follows: 1) chronic gastrointestinal disease at admission, 2) past medical history of operations other than appendectomy, 3) renal disease, 4) diabetes mellitus, 5) ingestion of drugs (aspirin, non-steroidal anti-inflammatory drug, steroid or misoprostol) that are known to affect IP, 6) consumption of materials (glutamine, bovine colostrums, nucleic acid or omega-3 unsaturated fatty acid) during the 7 days of the test that may affect IP, 7) a 24-hour glomerular filtration rate of ≤50 mL/hr, and 8) previous history of hypersensitivity reaction to radioisotope.
In a total of 118 patients, 31 were excluded by exclusion criteria, and 87 were included in the study. Patients were diagnosed with alcoholic AP when 1) they apparently ingested large amounts of alcohol for a long time, 2) they showed no biliary stone by hepatobiliary ultrasonography (US), CT or endoscopic retrograde cholangiopancreatography (ERCP), and 3) other etiologic factors were not suspected. The cause of AP was considered as biliary stone when a stone or a biliary sludge of the gallbladder or common bile duct was confirmed with abdominal US, CT, ERCP, or laparotomy. The cause of AP was considered idiopathic when no specific causes (which included serum triglycerides, calcium, phosphate, infectious serological screen, drug, trauma, autoimmune, and anormaly) were identified.12 The study proposal was approved by the Institutional Review Board of our hospital.
2. Methods
The IP test was performed within 72 hours of the onset of AP symptoms. All patients underwent abdominal CT before the IP test. The serum levels of endotoxin, IL-6, and TNF-α were measured within 24 hours of admission. The serum CRP test was performed at admission and 48 hours after admission. The routine complete blood cell (CBC) test and blood chemistry were performed before the IP test. AP severity was estimated with obtaining the Ranson and APACHE-II scores 48 hours later. The CTSI was calculated at 72 hours after admission with assigning numerical values to the Balthazar CT grades (A-E) and necrosis categories, and summing up these numbers (Table 1). All CT scores were calculated from the dictated reports as provided by a single departmental radiologist at the time the CT scan was performed. The grades were grouped as follows: group I, CTSI 0 to 2; group II, CTSI 3 to 6; and group III, CTSI 7 to 10. Complications were defined as organ failure (shock, pulmonary insufficiency, renal failure and gastrointestinal bleeding) and/or local complications (necrosis, abscess and pseudocyst) according to the Atlanta criteria.12
Table 1.
Balthazar CT Severity Index
Balthazar computed tomography (CT) severity index, necrosis score+grade score.
Modified from Balthazar et al. Radiology 1990;174:331-336, with permission.9
The IP test was performed with lactulose and mannitol. After fasting for at least 8 hours, a mixture of 100 mL of water, 10 g of lactulose, and 5 g of mannitol was administered following urination. Thereafter, 2 mL of 8-hour urine was stored at -20℃ until needed. The recovered amount of lactulose in urine was measured by solid-phase enzyme-linked immunosorbent assay (ELISA) using a K-LACTUL kit (Incorporating reagents for use in the procedure described by ISO Method II285:2004; Megazyme International Ireland Ltd., Wicklow, Ireland). CRP was measured using a Behring Nephelometer Analyzer II (Dade Behring, Marbug, Germany). CBC was measured using a LH 750 Analyzer (Beckmancoulter Inc., Fullerton, CA, USA). Blood chemistry was performed using an Advia1650 (Bayer Healthcare Co., Ltd., Tarrytown, NY, USA).
3. Statistical analysis
Statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±SD. One-way ANOVA was applied to compare parametric variables between the three aforementioned groups. Nonparametric variables were compared using the χ2 test. Pearson's and Speaman's correlations were used to investigate the relationship between IP and clinical variables. The associations between IP and correlated variables were analyzed in multivariate approach. A p-value of <0.05 was considered statistically significant.
RESULTS
1. Clinical characteristics of the three groups as classified with the CTSI
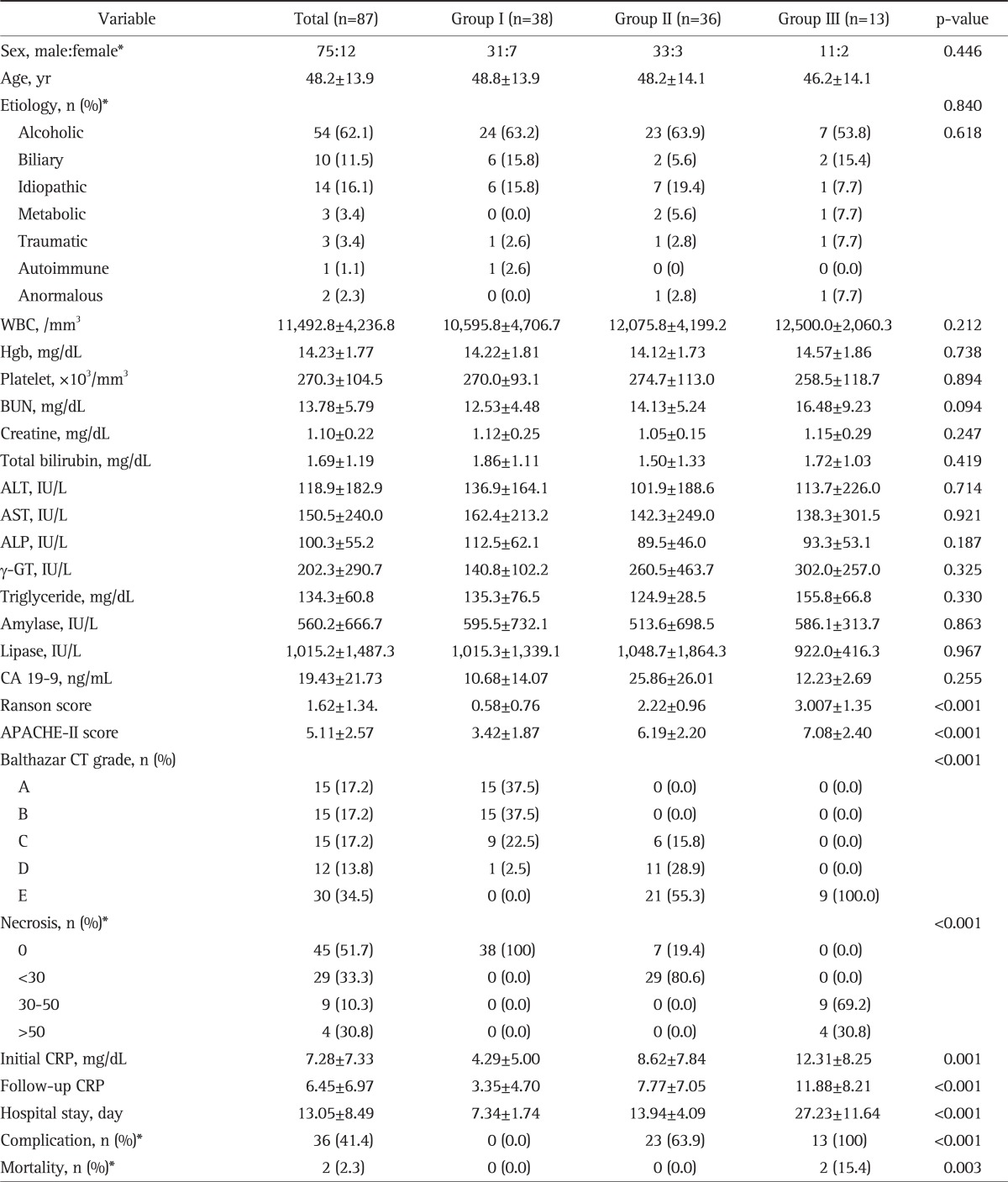
A total of 87 patients (75 males and 12 females) with AP were included in the study. The mean age of the patients was 48.2±13.9 years. As for the causes of AP, 54 patients had alcoholic, 10 patients biliary, 14 idiopathic, three metabolic, three traumatic, one autoimmune, and two anomalous AP. There were no significant differences in age, sex, and the causes of AP between the three groups. The serum CRP levels (at admission and 48 hours after admission), an indicator of acute inflammatory reactions, were significantly higher in patients with a higher CTSI, but leukocyte counts were not. There were no significant differences between the three groups in laboratory indices of biliary obstruction or liver function including triglyceride, ALT, AST, gamma-GT, ALP, CA19-9, amylase, lipase, and total bilirubin. There were no significant differences between the three groups in laboratory indices of severe AP including renal function test results and hemoglobin. The duration of hospital stay, the rate of complication and death, Ranson and APACHE-II scores were significantly higher in patients with higher CTSI (Table 2).
Table 2.
Clinical Characteristics according to the CT Severity Index of the Study Patients
Data are presented as mean±SD. All comparisons were performed using the one-way ANOVA. Group I, CT severity index 0-2; Group II, CT severity index 3-6; Group III, CT severity index 7-10.
CT, computed tomography; WBC, white blood cell; BUN, blood urea nitrogen; ALT, alanine aminotranferease; AST, aspartate aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transferase; CRP, C-reactive protein; APACHE-II, acute physiology and chronic health evaluation scoring system-II.
*Chi-square tests.
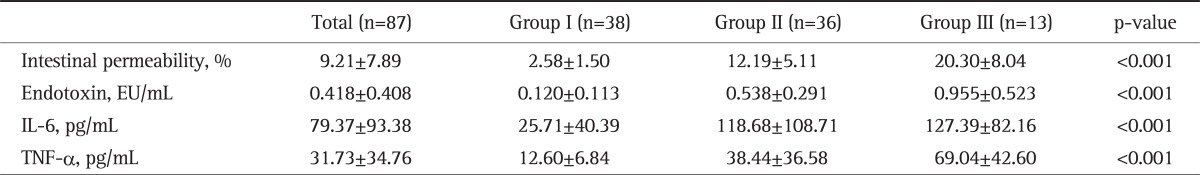
2. IP, endotoxins and cytokines of the three groups as classified with the CTSI
IP and serum endotoxin were higher in patients with a higher CTSI. IP was 2.58±1.50% in group I, 12.19±5.11% in group II and 20.30±8.04% in group III, and the differences were statistically significant (p<0.001). The serum endotoxin was 0.120±0.113 EU/mL in group I, 0.538±0.291 EU/mL in group II, and 0.955±0.523 EU/mL in group III, and the differences were statistically significant (p<0.001). The serum levels of IL-6 and TNF-α were also significantly higher in patients with a higher CTSI. The serum IL-6 level was 25.71±40.39 pg/mL in group I, 118.68±108.71 pg/mL in group II, and 127.39±82.16 pg/mL in group III, and the differences were statistically significant (p<0.001). The serum TNF-α level was 12.60±6.84 pg/mL in group I, 38.44±36.58 pg/mL in group II, and 69.04±42.60 pg/mL in group III, and the differences were statistically significant (p<0.001) (Table 3).
Table 3.
Intestinal Permeability, Endotoxin Level and Cytokine Level according to the CT Severity Index of the Study Patients
Data are presented as mean±SD. All comparisons were performed using the one-way ANOVA test. Group I, CT severity index 0-2; Group II, CT severity index 3-6; Group III, CT severity index 7-10.
CT, computed tomography; IL, interleukin; TNF, tumor necrosis factor.
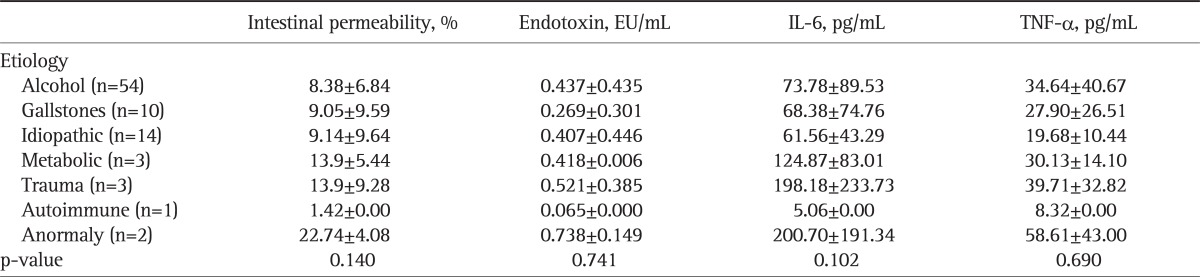
3. IP, endotoxin and cytokines according to the causes of AP
There were no significant differences according to the cause of AP in IP, endotoxin, IL-6 and TNF-α (p>0.05) (Table 4).
Table 4.
Intestinal Permeability, Endotoxin Level and Cytokine Level according to the Etiology of the Study Patients
Data are presented as mean±SD. All the tests were performed using the one-way ANOVA test.
IL, interleukin; TNF, tumor necrosis factor.
4. Association between IP and clinical variables
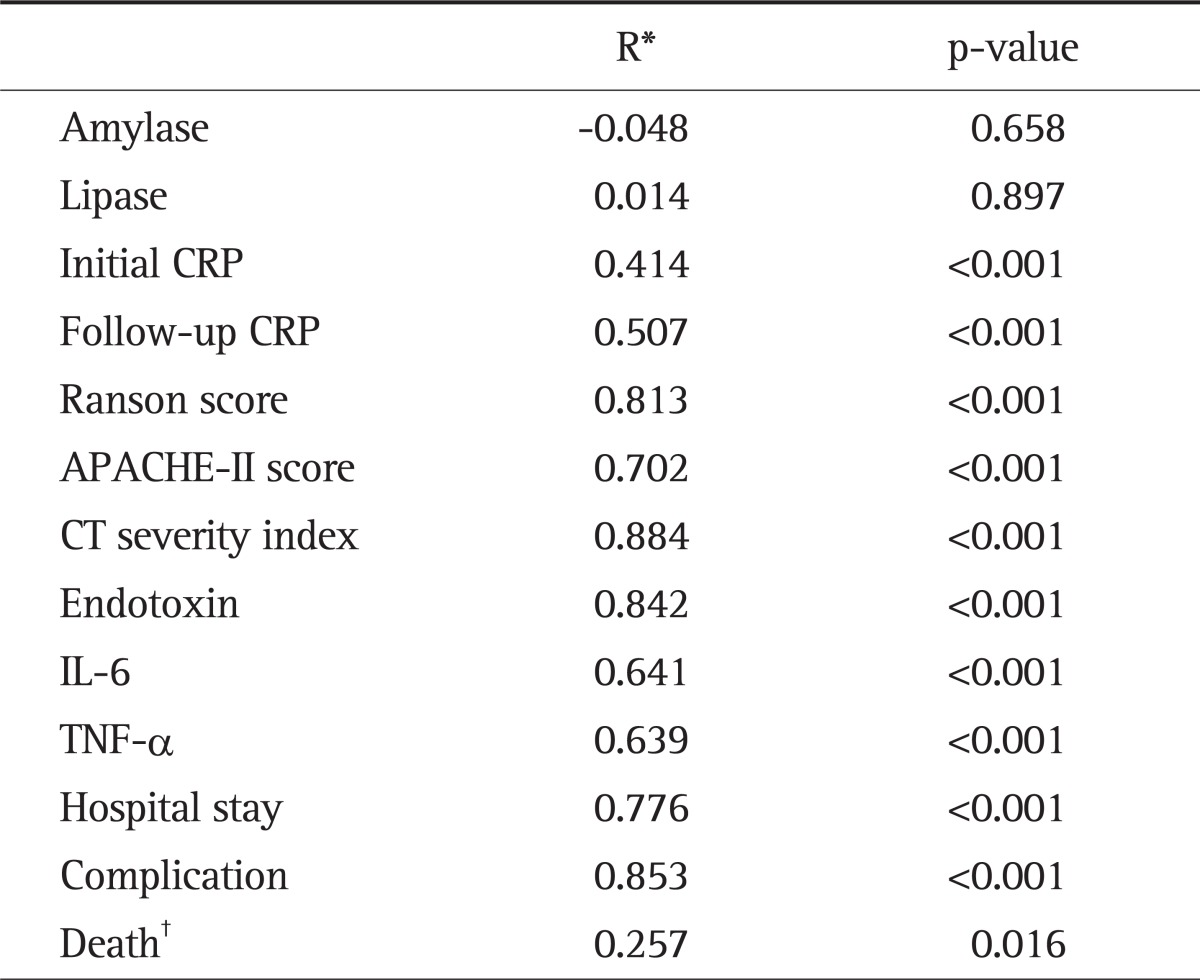
The associations between IP and endotoxin, IL-6, TNF-α or other prognostic indicators of AP were investigated using Pearson's and Spearman's correlation analyses. In all patients, IP showed positive correlations with endotoxin, IL-6, TNF-α, CRP measured at admission or 48 hours after admission, CTSI, Ranson score, APACHE-II score, the duration of hospital stay, the presence of complication and death (p<0.05). However, there was no significant correlation between IP and amylase or lipase (p>0.05) (Table 5).
Table 5.
Correlation between Intestinal Permeability and the Clinical Characteristics
All the tests were performed using the Pearson's correlation.
CRP, C-reactive protein; APACHE-II, acute physiology and chronic health evaluation scoring system-II; CT, computed tomography; IL, interleukin; TNF, tumor necrosis factor.
*R, correlation coefficient; †Spearman's correlation.
5. Clinical characteristics affecting IP
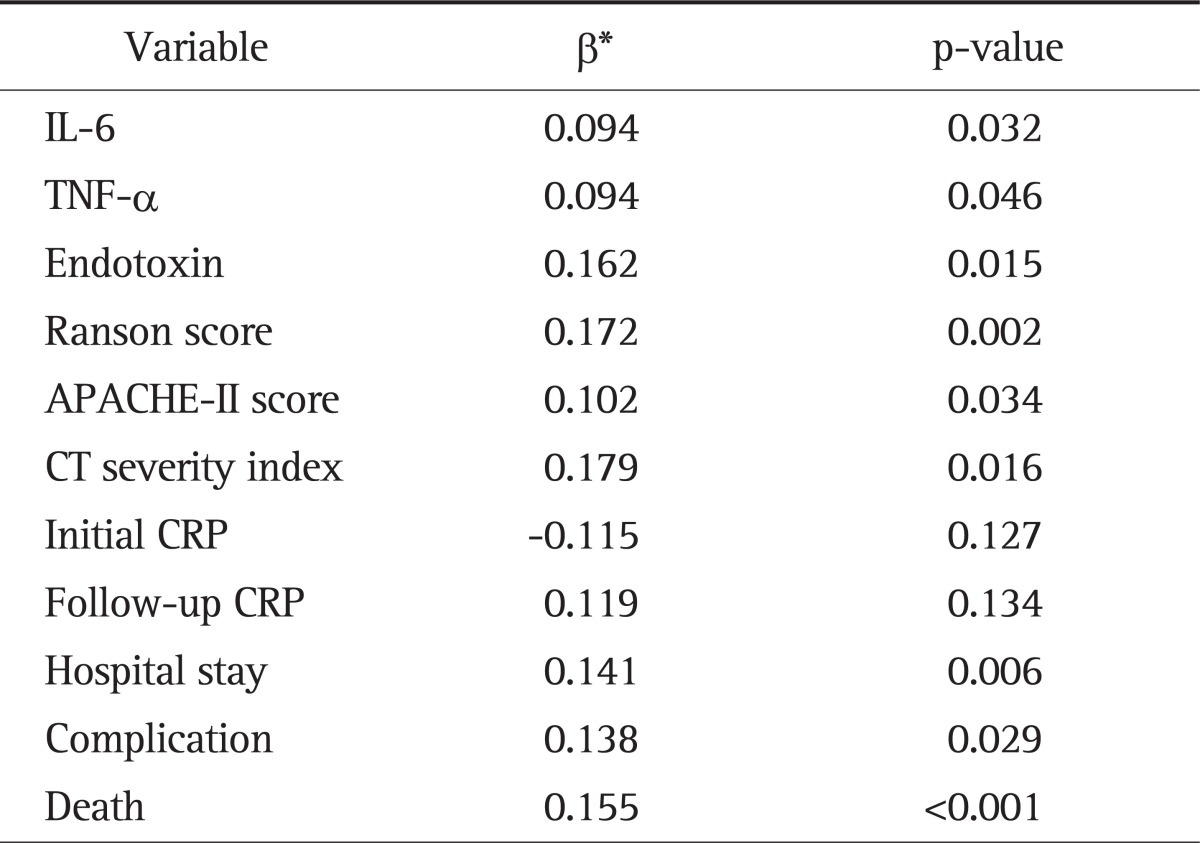
The associations between IP and correlated variables were analyzed in the multi-variate approach. In the total patients, the β-value was 0.162 (p=0.015) for endotoxin, 0.094 (p=0.032) for IL-6, 0.094 (p=0.046) for TNF-α, 0.179 (p=0.016) for CTSI, 0.172 (p=0.002) for the Ranson score, 0.102 (p=0.034) for the APACHE-II score, 0.141 (p=0.006) for the duration of hospital stay, 0.138 (p=0.029) for the presence of complication and 0.155 (p<0.001) for death, all of which significantly affected IP. However, the β-value was -0.115 (p=0.127) for CRP at admission and 0.119 (p=0.134) for CRP 48 hours after admission, which did not show significant correlations with IP (R2=0.921) (Table 6).
Table 6.
Multiple Linear Regression Analysis of the Correlated Variables for Intestinal Permeability
Statistical significances were tested by multiple linear regression analysis (adjusted R2=0.921).
IL, interleukin; TNF, tumor necrosis factor; APACHE-II, acute physiology and chronic health evaluation scoring system-II; CT, computed tomography; CRP, C-reactive protein.
*Standardized regression coefficients.
DISCUSSION
Intestinal barrier damage was suggested as one of important mechanisms for sepsis or multi-organ failure in various diseases.13 Capillary endothelial damage secondary to increased blood acidity occurs in patients with a marked decrease of intestinal microcirculation as in AP, which leads to intestinal barrier damage.14 Acute complications of AP such as erosion, ulcer, bleeding of the intestinal mucosa, diarrhea and malnutrition due to decreased intestinal absorption capacity occur after intestinal barrier damage.15
Bacterial translocation is a phenomenon in which normal enteric organisms move to various organs other than the intestine due to increased IP through severe intestinal barrier damage. It is regarded as a mechanism of pancreatic infection, and gram-negative bacteria are its important causative pathogens.16 Especially, the inflow of endotoxin produced by enteric organisms into blood is called intestinal endotoxemia.17 Particularly, increased IP secondary to intestinal barrier damage in severe pancreatitis is induced within 28 to 72 hours of the development of pancreatitis and thus causes systemic endotoxemia, consequently resulting in multi-organ failure or death.18
Intestinal barrier damage cannot be diagnosed with endoscopic examinations or radiologic imaging studies that are currently being used to observe gross and anatomical lesions in clinical settings. The IP test, a functional test, can clinically diagnose intestinal barrier damage and figure out relationships with intestinal behaviors/diseases due to increased IP such as bacterial translocation, endotoxemia. IP is usually expressed as a percentage of the excreted amount in urine to the administered amount of a certain test drug, and the normal value is <2%.19 In clinical practice, clinicians most commonly use polysaccharides, such as lactulose, which are relatively large molecules and pass through the intercellular space of the intestinal mucosa and monosaccharides, such as lamnose or mannitol, which are relatively small molecules and move into the cell interior.20 We used this test in the study.
The first objective of this study was to examine whether increased IP due to intestinal barrier damage and endotoxemia would affect prognostic predictors of AP including the CTSI. The duration of hospital stay, the presence of complication, death and the established prognostic predictors such as CRP at admission and 48 hours after admission, Ranson and APACHE-II scores were significantly different according to the CTSI, satisfying the preferential hypothesis in this study that the CTSI can be a prognostic predictors of AP. In addition, IP and blood endotoxin were significantly higher in high CTSI groups than low groups with AP, indicating that patients with increased IP and endotoxin at early stages tend to have severe AP and serious complications. Juvonen et al.16 have reported that IP increases as AP becomes more severe, which is similar to our result. In addition, IP had positive correlations with CTSI, Ranson, or APACHE-II score, CRP at admission or 48 hours after admission, the duration of hospital stay, the presence of complication and death. Among them, CTSI, Ranson, or APACHE-II score, the duration of hospital stay, the presence of complication and death affect IP significantly. Based on these results, it is conceivable that early intestinal barrier damage is more severe in patients with severe AP than in those with mild AP and IP test in patients with AP may predict the severity of AP.
The second objective of this study was to investigate the relationships of increased IP with serum endoxin, TNF-α, and IL-6. Endotoxemia tended to become more severe in proportion to IP and also significantly affected IP. This result suggests that reduction of IP decreases the severity of endotoxemia and thus protects the intestinal mucosa. IL-6, an important inflammatory mediator is primarily secreted from activated mononuclear phagocytic cells. Galloway et al.21 have demonstrated that serum IL-6 levels are higher in AP patients with complications than in those without. It has been documented that IL-6 can predict the severity of AP, end-organ failure, overall mortality, and admission duration.22 However, there is little evidence to support that IL-6 is a useful predictor of the prognosis of AP in clinical practice. Demydov et al.23 have reported that TNF-α is mainly secreted from macrophages and is increased in patients with AP. It has been shown that TNF-α plays a crucial role in the pathogenesis of sepsis in patients with AP.24 TNF-α increases the permeability of endothelial cells, decreases epithelial barrier function and thus induces intestinal barrier damage.4 In our study, the serum levels of IL-6 and TNF-α became higher as the CTSI of AP increased. Our study supports the hypothesis that increased IP due to intestinal barrier damage has a positive correlation with the serum levels of IL-6 and TNF-α. It also shows that both IL-6 and TNF-α significantly affect IP. Based on the result, it is thought that treatment modalities for decreasing the serum levels of IL-6 and TNF-α help restore intestinal barrier function and reduce the severity of AP and further studies were needed.
The results of this study are subject to at least three limitations. First, this study has a limitation due to its small sample size and short study period. Second, severe AP patients with renal failure were excluded from this study because decreased renal function can affect IP. Therefore, the number of severe AP patients with renal complications was relatively low. Third, this study mainly included patients with alcoholic pancreatitis. Persson et al.25 have documented that alcohol ingestion can increase IP and cause endotoxemia by inducing intestinal barrier damage. Since this study found that there were no significant differences in IP, endotoxin, and cytokines between alcoholic AP and AP due to other causes, the aforementioned problem can be minimized. Despite these limitations, this is the first study that revealed the correlations of IP with endotoxemia, cytokines, the duration of hospital stay, the presence of complication, death and known predictors of the prognosis of AP such as serum CRP, Ranson/APACHE-II scores, and CTSI. This study also indicates that the early assessment of IP, which is non-invasive indicator of intestinal barrier damage without complicated tests, can predict the prognosis of AP. Further studies with a larger sample size and a prospective design are needed to confirm our results.
In conclusion, high IP resulting from intestinal barrier damage in early phase of AP is associated with the severity of AP and also have a direct relationship with endotoxemia. Thus, the assessment of IP and serum endotoxin level may be reliable and simple methods to predict the prognosis of AP. In addition, the subsidiary measurement of IL-6 and TNF-α can be helpful. Furthermore, based on these results, it is expected that preservation and restoration of intestinal barrier function will reduce the morbidity and mortality of sepsis due to severe AP.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 2.Juvonen PO, Tenhunen JJ, Heino AA, et al. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand J Gastroenterol. 1999;34:308–314. doi: 10.1080/00365529950173744. [DOI] [PubMed] [Google Scholar]
- 3.Cicalese L, Sahai A, Sileri P, et al. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127–1132. doi: 10.1023/a:1010786701289. [DOI] [PubMed] [Google Scholar]
- 4.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- 5.Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 7.Dib M, Zhao X, Wang X, Andersson R. Mast cells contribute to early pancreatitis-induced systemic endothelial barrier dysfunction. Pancreatology. 2002;2:396–401. doi: 10.1159/000065087. [DOI] [PubMed] [Google Scholar]
- 8.Leveau P, Wang X, Sun Z, Börjesson A, Andersson E, Andersson R. Severity of pancreatitis-associated gut barrier dysfunction is reduced following treatment with the PAF inhibitor lexipafant. Biochem Pharmacol. 2005;69:1325–1331. doi: 10.1016/j.bcp.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 10.Gürleyik G, Emir S, Kilicoglu G, Arman A, Saglam A. Computed tomography severity index, APACHE II score, and serum CRP concentration for predicting the severity of acute pancreatitis. JOP. 2005;6:562–567. [PubMed] [Google Scholar]
- 11.Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253–260. doi: 10.1097/00004836-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Bradley EL., 3rd A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 13.Gloor B, Müller CA, Worni M, et al. Pancreatic infection in severe pancreatitis: the role of fungus and multiresistant organisms. Arch Surg. 2001;136:592–596. doi: 10.1001/archsurg.136.5.592. [DOI] [PubMed] [Google Scholar]
- 14.Hotz HG, Foitzik T, Rohweder J, et al. Intestinal microcirculation and gut permeability in acute pancreatitis: early changes and therapeutic implications. J Gastrointest Surg. 1998;2:518–525. doi: 10.1016/s1091-255x(98)80051-6. [DOI] [PubMed] [Google Scholar]
- 15.Ammori BJ. Gut barrier dysfunction in patients with acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:411–412. doi: 10.1007/s005340200050. [DOI] [PubMed] [Google Scholar]
- 16.Juvonen PO, Alhava EM, Takala JA. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol. 2000;35:1314–1318. doi: 10.1080/003655200453683. [DOI] [PubMed] [Google Scholar]
- 17.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 18.Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122–129. doi: 10.1097/00006676-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 20.Cox MA, Lewis KO, Cooper BT. Measurement of small intestinal permeability markers, lactulose, and mannitol in serum: results in celiac disease. Dig Dis Sci. 1999;44:402–406. doi: 10.1023/a:1026679123148. [DOI] [PubMed] [Google Scholar]
- 21.Galloway SW, Kingsnorth AN. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: relationship to endotoxin, interleukin 6 and disease severity. Br J Surg. 1994;81:312. doi: 10.1002/bjs.1800810261. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki T, Hoshino M, Hayakawa T, et al. Interleukin-6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas. 1997;14:1–8. doi: 10.1097/00006676-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Demydov VM, Zaporozhchenko BS, Demidov SM. Changes of cytokine levels in the serum in patients with acute pancreatitis as an early symptom for diagnosis. Klin Khir. 2003:29–32. [PubMed] [Google Scholar]
- 24.Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21(10 Suppl):S447–S463. doi: 10.1097/00003246-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 25.Persson J, Berg NO, Sjolund K, Stenling R, Magnusson PH. Morphologic changes in the small intestine after chronic alcohol consumption. Scand J Gastroenterol. 1990;25:173–184. doi: 10.3109/00365529009107940. [DOI] [PubMed] [Google Scholar]