Abstract
Allergic asthma is a complex disease characterized by eosinophilic pulmonary inflammation, mucus production and reversible airway obstruction1. Exposure to indoor allergens is a clear risk factor for asthma, but this disease is also associated with high household levels of total and Gram-negative bacteria2. The ability of bacterial products to act as adjuvants3 suggests they might promote asthma by priming allergic sensitization to inhaled allergens. In support of this idea, house dust extracts (HDEs) can activate antigen presenting dendritic cells (DC) in vitro and promote allergic sensitization to inhaled innocuous proteinsin vivo4. It is unknown which microbial products provide most of the adjuvant activity in HDEs. A screen of microbial products for their adjuvant activity in the airway revealed that the bacterial protein, flagellin (FLA) stimulated strong allergic responses to an innocuous inhaled protein. Moreover, toll-like receptor (TLR)5, the mammalian receptor for FLA5,6, was required for priming strong allergic responses to natural indoor allergens present in HDEs. In addition, the incidence of human asthma was associated with high serum levels of FLA-specific antibodies. Together, these findings suggest that household FLA promotes the development of allergic asthma by TLR5-dependent priming of allergic responses to indoor allergens.
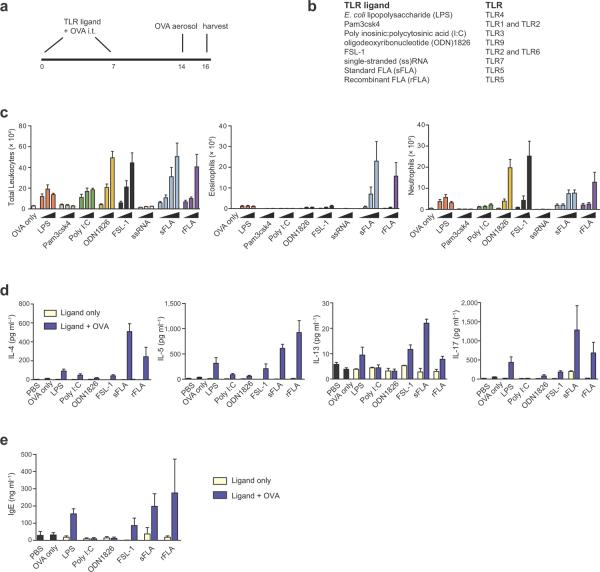
To test the adjuvant properties of microbial products, we instilled them together with chicken ovalbumin (OVA) into the airways of mice. Control animals receiving either OVA alone or microbial products alone did not become sensitized and failed to develop airway inflammation after subsequent challenge with OVA (Fig. 1, a–c and Supplementary Fig. 1a). However, instillation of some microbial products together with OVA elicited immune responses, as inferred from airway inflammation following OVA challenge (Fig. 1c). Some animals displayed an allergic form of inflammation, as evidenced by airway eosinophilia. We observed unexpectedly strong allergic responses in mice receiving OVA together with either standard FLA (sFLA) from Salmonella (S.) typhimurium, or with a more highly purified, recombinant (r)FLA produced in insect cells and lacking detectable endotoxin7. Ultrapure LPS was comparable to rFLA as an adjuvant (Supplementary Fig. 1b), suggesting that direct comparisons of adjuvant activities among TLR ligands are confounded by impurities in their preparations.
Figure 1.
Adjuvant activity of microbial products in the airway. (a) Timeline for sensitizations and challenges. (b) Microbial products used, and the TLRs that mediate signaling responses to each product. (c) Airway inflammation in sensitized mice and challenged mice: Cell number ± s.e.m. in BALF of total leukocytes (left), eosinophils (middle) and neutrophils (right).  , increasing amounts (25 ng, 125 ng or 625 ng) of microbial product used during sensitizations; sFLA also includes 1250 ng. n = 5 – 8 mice per group. (d) Pulmonary cytokines: left to right, IL-4, IL-5, IL-13 and IL-17. (e) Total serum IgE. Data are from one of three similar experiments. n = 5 – 8 mice per group. nd, not detected.
, increasing amounts (25 ng, 125 ng or 625 ng) of microbial product used during sensitizations; sFLA also includes 1250 ng. n = 5 – 8 mice per group. (d) Pulmonary cytokines: left to right, IL-4, IL-5, IL-13 and IL-17. (e) Total serum IgE. Data are from one of three similar experiments. n = 5 – 8 mice per group. nd, not detected.
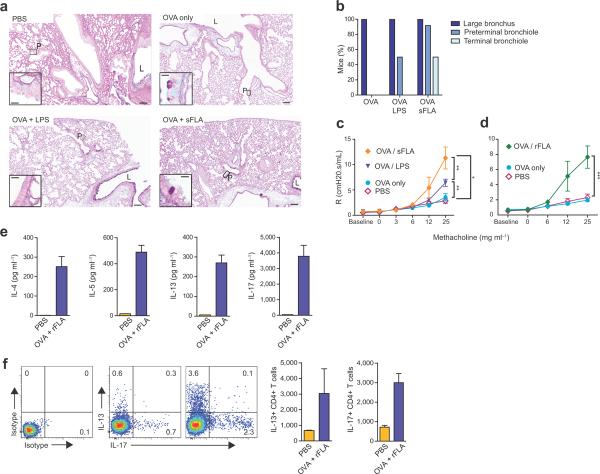
The clinical features of asthma stem largely from the actions of CD4+ T helper (TH)2 cells, which produce the cytokines interleukin (IL)-4, IL-5 and IL-13, which promote IgE synthesis, eosinophilic inflammation, and mucus and airway hyperresponsiveness (AHR), respectively. IL-17 production by TH17 cells also contributes to asthma severity by promoting neutrophil accumulation and AHR8–10. We found that pulmonary IL-4, IL-5, IL-13 and IL-17 (Fig. 1d) as well as serum IgE (Fig. 1e), were all elevated in OVA-challenged mice previously sensitized with sFLA-OVA or rFLA-OVA. Mucus was present in lungs of most mice that displayed eosinophilic responses, but it was generally restricted to the large airways. However, mice sensitized with sFLA-OVA also had mucus in preterminal and terminal bronchioles (Fig. 2 a,b). Invasive measurements of airway resistance revealed that mice sensitized with either sFLAOVA (Fig. 2c) or rFLA (Fig. 2d) developed very strong AHR after a single OVA challenge, whereas mice sensitized to LPS-OVA developed weaker AHR. In the former animals, inflammation and AHR was sustained after seven daily OVA challenges (Supplementary Fig. 2a,b). Cytokine production by T cells in draining lymph nodes (Fig. 2e) and lungs (Fig. 2f and Supplementary Fig. 2c) confirmed that inhaled FLA promotes TH2 cell differentiation in adult mice.
Figure 2.
FLA promotes asthma-like responses to OVA. (a). Periodic acid-Schiff / Alcian blue staining of mucus-producing cells in the airway. Representative, low magnification (8×) images are shown (scale bar, 50 microns). Insets (40×) show expanded images of the indicated regions (scale bar, 10 microns). L, large airway; P, preterminal bronchioles. (b) Compiled data of mucus staining. n = 8 – 10 mice per group. (c,d) Mean values ± s.e.m. of airway resistance for intubated mice inhaling air (baseline), or aerosols of PBS containing the indicated concentrations of methacholine. n ≥ 8 mice/group. (e) Cytokine concentrations in cultures of lymph nodes excised from mice sensitized as indicated. IL-4 (left), IL-5 (middle) and IL-17 (right). (f) Intracellular staining for cytokines in pulmonary T cells. Shown are representative flow plots and bar histograms of mean ± s.e.m. numbers of CD4+ cells staining for IL-13 (left) and IL-17 (right).
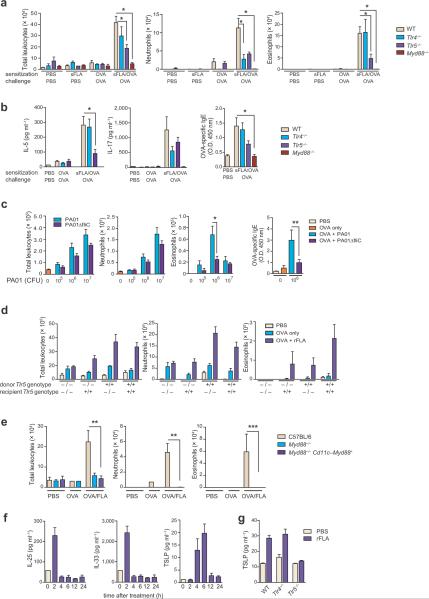
Unlike highly pure rFLA, sFLA contains only 10% FLA and is contaminated with LPS7. However, Tlr4−/− mice were readily sensitized to OVA when sFLA was used as an adjuvant, as evidenced by airway eosinophils following subsequent challenge with OVA (Fig. 3a). By contrast, Tlr5−/− mice, which cannot respond to extracellular FLA6, had lower levels of airway eosinophils and IL-5 than WT mice, and trended towards lower IL-17 and IgE (Fig. 3 a,b). The reduced allergic response of sFLA-OVA treated Tlr5−/− mice was not due to a general immune deficit because unlike Tlr4−/− mice, the former strain was readily sensitized to OVA when we used LPS as an adjuvant (Supplementary Fig. 3). The inflammasome-associated molecule, NLR family CARD domain-containing protein 4 (Nlrc4), responds to intracellular FLA11,12, but we found that Nlrc4−/− mice were efficiently sensitized by OVA/FLA and developed strong allergic responses to subsequent OVA challenge (Supplementary Fig. 4). By contrast, similarly-treated myeloid differentiation factor 88 (Myd88)−/− mice had virtually no eosinophils, neutrophils, or inflammatory cytokines (Fig. 3 a,b). Together, these data suggest that although multiple microbial products contribute to the adjuvant activity of sFLA, the primary adjuvant component in this preparation is FLA. This is consistent with the ability of rFLA, which lacks detectable endotoxin, to prime allergic responses. In addition to monomeric FLA, airway instillations of intact heat-killed Pseudomonas aeruginosa, a ubiquitous, flagellated strain of bacteria, also primed allergic responses to OVA in a FLA-dependent manner (Fig. 3c). Thus, multiple forms of FLA can prime allergic responses to inhaled OVA.
Figure 3.
FLA possesses potent, TLR5-dependent adjuvant activity in the airway. (a) Mean numbers ± s.e.m. of total leukocytes (left), neutrophils (middle) and eosinophils (right) in mice sensitized and challenged as indicated. (b) Concentrations of IL-5 (left) and IL-17 (middle) in BALF, and relative amounts of serum OVA-specific IgE (right) in mice of the indicated genotypes. (c) Left to right, total leukocytes, neutrophils, eosinophils and OVA-specific IgE in mice sensitized to OVA using heat-killed P. aeruginosa as an adjuvant. (d) Total leukocytes (left), neutrophils (middle) and eosinophils (right) in bone marrow chimeric mice generated with WT and Tlr5−/− mice, sensitized as indicated. (e) Total leukocytes (left), neutrophils (middle) and eosinophils in mice expressing a Myd88 transgene in CD11c+ cells only. Experiments were done at least twice with similar results. n = at least 6 mice per group. * P < 0.05. (f) Cytokines in BALF at the indicated time point after rFLA instillation. (g) TSLP concentration in supernatants of FLA-treated primary airway epithelial cells.
The two major DC populations in the lung (CD103+ and CD11bhi)13 expressed low, but detectable levels of Tlr5 RNA (Supplementary Fig. 5a). Alveolar macrophages expressed intermediate levels of Tlr5, and airway epithelial cells expressed the highest levels of this receptor. To investigate the requirement of different FLA-responsive cell compartments during allergic sensitization, we used WT and Tlr5−/− mice to generate reciprocal bone marrow chimeric animals (Supplementary Fig. 5b). WT mice receiving WT marrow displayed the strongest eosinophilic responses to rFLA-OVA sensitization and OVA challenge (Fig. 3d), whereas WT mice receiving Tlr5−/− marrow, as well as Tlr5−/− mice receiving WT marrow, had intermediate levels of eosinophils. Tlr5−/− mice receiving marrow from Tlr5−/− mice had almost no eosinophilic inflammation. This suggests that Tlr5 expression in radio-sensitive hematopoietic cells and radio-resistant structural cells contribute to FLA-mediated allergic sensitization, in agreement with a previous report14. Mice in which Myd88 expression is restricted to Cd11c-expressing cells15 did not become sensitized to OVA when we used rFLA as an adjuvant (Fig. 3e), although CD11c+ cells prepared from lungs of these mice were able to respond to FLA, as evidenced by production of TNF-α (Supplementary Fig. 5c). Thus, FLA responsiveness in DCs and alveolar macrophages is not sufficient for FLA-mediated allergic sensitization through the airway. Previous reports have indicated that the airway epithelium contributes to innate immune responses to FLA16,17 and to LPS-mediated TH2 responses18. We found that levels of IL-25 and IL-33 in the airway were rapidly increased within 2 h of rFLA instillation, and that thymic stromal lymphopoietin (TSLP) was increased within 4 h of treatment (Fig. 3f). Increased TSLP was also seen in supernatants of primary airway epithelial cells treated with rFLA (Fig. 3g), whereas we saw no increases for IL-25 or IL-33 (data not shown). FLA might therefore promote allergic sensitization to inhaled allergens by inducing secretion of pro-inflammatory cytokines by epithelial cells.
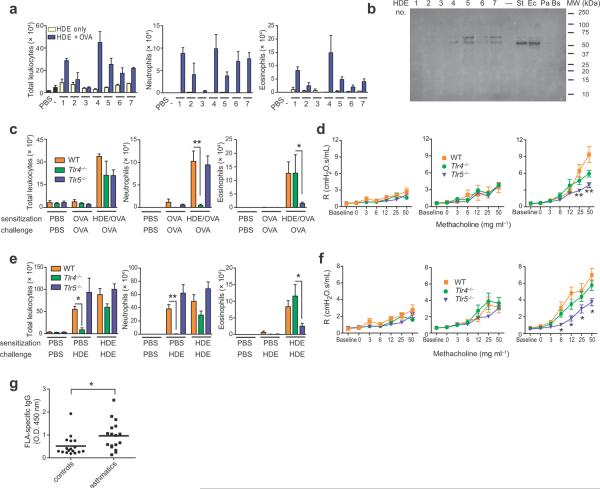
Having found that multiple forms of FLA can function as strong adjuvants in the airway, we next tested if FLA is a naturally-occurring, environmental adjuvant. Common house dust represents a readily available source of airborne material that is inhaled daily. Although they differed in potency, several different HDEs promoted allergic responses to inhaled OVA (Fig. 4a). Western blot analysis (Fig. 4b, Supplementary Fig. 6a) revealed immunoreactive proteins with the expected molecular weights of FLA (30 kDa to 65 kDa19. To investigate the individual contributions of LPS and FLA to the adjuvant activity of HDEs, we tested the abilities of Tlr4−/− and Tlr5−/− mice to undergo HDE-mediated allergic responses to OVA. Following HDE-OVA sensitization and OVA challenge, Tlr4−/− mice had fewer airway neutrophils and trended towards lower concentrations of IL-17 compared with WT mice (Fig. 4c, and Supplementary Fig. 6b). However, airway eosinophils and Th2 cytokines were not decreased in Tlr4−/− mice compared with WT mice (Fig. 4c, Supplementary Fig. 6b), indicating that LPS is dispensable for HDE-mediated allergic sensitization. By contrast, Tlr5−/− mice had fewer airway eosinophils and had lower concentrations of IL-5 than did WT or Tlr4−/− mice. HDE-OVA sensitized WT and Tlr4−/− mice developed AHR after OVA challenge, whereas this response was markedly reduced in Tlr5−/− mice (Fig. 4d). These findings suggest that FLA is a major component of HDE for priming allergic and physiologic responses to inhaled antigens.
Figure 4.
FLA is a primary adjuvant component of common house dust for priming asthma-like responses. (a) Airway inflammation in OVA-challenged mice previously sensitized by instillation of OVA together with the indicated sample of HDE. (b) Western blot of HDEs probed with an anti-S. typhimurium FLA antibody. Also loaded were FLA from S. typhimurium (St) (100 ng), E. coli (Ec) (400 ng), P. aeruginosa (Pa) (400 ng) and B. subtilis (Bs) (400 ng). (c) Airway inflammation and (d) AHR in the indicated strains of OVA-challenged mice previously sensitized to OVA using HDE #7 or given HDE #7 alone. (e) Airway inflammation and (f) AHR in mice given PBS, a single instillation of HDE #7, or two instillations of HDE #7. n = 8 mice per group. Experiments shown were done at least twice, with similar results. * P < 0.05; ** P < 0.01, vs values for WT mice. (g) Relative titers of IgG antibodies to S. typhimurium FLA in asthmatic and non-asthmatic individuals. n = 17 controls, 17 asthmatics. P value by t test.
OVA is widely used in animal models of allergic pulmonary inflammation, but it is not a clinically relevant allergen for asthma. Household dust typically contains many allergens, including those derived from dust mites, cockroaches and animal dander, and multiple instillations of HDE alone is sufficient to trigger allergic responses in mice20. Our HDEs also contained multiple allergens and various levels of endotoxin (Supplementary Table 1). We confirmed that sensitization and challenge with HDE elicited eosinophilic and neutrophilic airway inflammation (Fig. 4e). The neutrophilic inflammation was due in part to innate immune responses to LPS because WT mice displayed airway neutrophils after a single HDE instillation, whereas this response was markedly reduced in Tlr4−/− mice. Eosinophil accumulation in the airways of WT mice was dependent on adaptive immune responses because these cells were present only in HDE-challenged animals that had been previously sensitized with HDE. Like WT mice, Tlr4−/− mice displayed high levels of eosinophils after HDE challenge, whereas this response was blunted in Tlr5−/− mice. This suggests that interactions between FLA and TLR5 promote allergic sensitization to indoor allergens.
When sensitized and challenged with HDE, WT mice developed strong AHR (Fig. 4f), whereas mice exposed to HDE on a single occasion did not develop AHR. These results indicate that adaptive immune responses to HDE are required for AHR. Tlr4−/− mice also developed AHR after repeated instillation of HDE, although their responses were slightly decreased compared with WT mice. Therefore, LPS in HDEs likely contributes to, but is not essential for, HDE-induced AHR. By contrast, AHR was markedly reduced in Tlr5−/− mice, again indicating that FLA residing in HDEs promotes asthma-like responses to natural indoor allergens. We confirmed this using two additional extracts (Supplementary Fig. 6c). Although the absolute levels of inflammation and AHR varied for different HDEs, Tlr5−/− mice had reduced levels of AHR or eosinophilia for each extract tested. Tlr5−/− mice also displayed reduced levels of airway inflammation following sensitization and challenge using a cockroach antigen (Supplementary Fig. 6d).
We reasoned that if FLA:TLR5 interactions contributed to human asthma, individuals exposed to high levels of FLA might be at increased risk for developing this disease. We therefore assessed titers of antibodies to FLA in sera from mild to moderate asthmatics as an indirect measure of their exposure to FLA. Compared with age- and gender-matched subjects with healthy airways, asthmatics had significantly higher titers of antibodies to FLA (Fig. 4g, Supplementary Fig. 7a), suggesting that exposure to FLA might be a risk factor for asthma. An alternative explanation is that antibodies to FLA reflect exposure to bacterial products in general. Asthmatics also had slightly higher levels of antibodies to LPS than did control subjects, but this difference did not reach statistical significance (Supplementary Fig. 7b). When taken together with the reduced eosinophilic and AHR responses of Tlr5−/− mice to HDE-mediated allergic sensitization, our data suggest that environmental FLA participates directly in priming allergic responses to indoor allergens.
The relationship between allergic asthma and TLR signaling is complex. Whereas some studies found that exposure to endotoxin protects against the development of asthma21–23, other studies found that endotoxin levels positively correlate with asthma24,25. These contradictory conclusions might be due to differences in timing or levels of endotoxin, which can impact the nature of immune responses26. Recent evidence suggests that gene-environment interactions can also impact the effect of TLR ligands exposures on allergic asthma27–29. Although several polymorphisms have been identified in the human TLR5 gene30, it remains to be determined if gene-gene or gene-environment interactions also affect the relationship between FLA exposure and allergic asthma.
Previous reports have shown that depending on the experimental setting, FLA can prime Th1 responses31,32, Th2 responses33, or both Th1 and Th17 responses34. Direct comparison of these studies is confounded by differences in FLA preparation and routes of administration. In addition, the context of FLA presentation is likely important. Thus, soluble monomeric FLA can prime Th2 responses, whereas FLA associated with S. typhimurium primes Th1 responses35. Similarly, a fusion protein of rFLA and OVA suppresses Th2 responses in a model of murine intestinal allergy, whereas co-administration of OVA and rFLA as separate molecules can enhance responses36. Although the evolutionary advantage of FLA-mediated allergic responses in the airway is unclear, eosinophils are reported to promote clearance of some bacteria37.
There is no known single structural feature that is common to all allergens. Some possess protease activity, whereas others possess carbohydrate moieties, bind lipids or interact with a TLR signaling complex38,39. It is currently unknown whether FLA promotes sensitization to all allergens residing in house dust or only to a subset of them. Future studies that address the relationships between FLA, allergens and other TLR ligands should improve our understanding of how various environmental exposures impact the initiation of allergic responses that lead ultimately to allergic asthma.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Environmental Health Sciences. We thank the human subjects for their blood donations, B. Yingling, J. Marshburn and A. Rice of the NIEHS Clinical Research Unit and D. Beaver of Duke University for assistance with patient recruitment and serum isolation. J. Hollingsworth (Duke University) with permission from R. Medzhitov (Yale University) provided CD11c-Myd88 transgenic mice on a Myd88−/− background. S. Akira (Osaka University) provided Tlr2−/−, Tlr4−/− and Myd88−/− mice and J. Ting (U. North Carolina) provided Nlrc4−/− mice. D. Wozniak (Ohio State University) provided both strains of P. aeruginosa, and F.-S. Shu (Wayne State University) provided purified FLA from P. aeruginosa, and A. Gewirtz (Emory U.) provided FLA from E. coli. We also thank NIEHS members K. Nakano for genotyping, L. Perrow for mouse colony management, N. Flagler for histology, J. Aloor for measuring endotoxin levels in HDEs, M. Sifre and C. Bortner for help with FACS-based cell sorting, J. Ciencewicki for advice on airway epithelial cell culture and M. Fessler and S. London for suggestions on the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS R.H.W. and S.M. conducted the majority of the experiments, designed experiments and helped prepare the manuscript. G.S.W. performed some assays and animal experiments, including most airway physiology experiments. D.C.Z. and M.L.S. provided dust samples from U.S. households and M.L.S. performed the MARIA assay for allergens. J.F.F. and G.P.F. analyzed and graded lung sections for mucus production and took photographs. H.N. helped with flow cytometry and experimental design. M.K. and S.G. provided sera from asthmatic and control patients. D.N.C. conceived of the project and helped with experimental design and writing of the manuscript. All authors discussed the results and commented on the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
LITERATURE CITED
- 1.Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Ross MA, et al. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000;55:705–711. doi: 10.1034/j.1398-9995.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both TH2 adjuvant and tolerogenic activities. J Allergy Clin Immunol. 2006;117:1074–1081. doi: 10.1016/j.jaci.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. doi:10.1038/35074106 35074106 [pii] [DOI] [PubMed] [Google Scholar]
- 7. http://www.invivogen.com/family.php?ID=102&ID_cat=2&ID_sscat=9.
- 8.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 9.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RH, et al. Allergic Sensitization Through the Airway Primes Th17-dependent Neutrophilia and Airway Hyperresponsiveness. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 12.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 13.Sung SS, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 14.Sanders CJ, Moore DA, 3rd, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180:7184–7192. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 15.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. doi:10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 16.Janot L, et al. Radioresistant cells expressing TLR5 control the respiratory epithelium's innate immune responses to flagellin. Eur J Immunol. 2009;39:1587–1596. doi: 10.1002/eji.200838907. [DOI] [PubMed] [Google Scholar]
- 17.Mijares LA, et al. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. Journal of immunology. 2011;186:7080–7088. doi: 10.4049/jimmunol.1003687. doi:10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. doi:10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonough MW, Smith SE. Molecular weight variation among bacterial flagellins. Microbios. 1976;16:49–53. [PubMed] [Google Scholar]
- 20.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. Reproducibility of a novel model of murine asthma-like pulmonary inflammation. Clin Exp Immunol. 2004;136:224–231. doi: 10.1111/j.1365-2249.2004.02461.x. doi:10.1111/j.1365-2249.2004.02461.x CEI2461 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. The New England journal of medicine. 2002;347:869–877. doi: 10.1056/NEJMoa020057. doi:10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 22.El-Sharif N, Douwes J, Hoet P, Nemery B. Childhood asthma and indoor aeroallergens and endotoxin in Palestine: a case-control study. J Asthma. 2006;43:241–247. doi: 10.1080/02770900600567122. doi:10.1080/02770900600567122. [DOI] [PubMed] [Google Scholar]
- 23.Gehring U, et al. Exposure to house dust endotoxin and allergic sensitization in adults. Allergy. 2004;59:946–952. doi: 10.1111/j.1398-9995.2004.00551.x. doi:10.1111/j.1398-9995.2004.00551.x. [DOI] [PubMed] [Google Scholar]
- 24.Bolte G, et al. Early endotoxin exposure and atopy development in infants: results of a birth cohort study. Clin Exp Allergy. 2003;33:770–776. doi: 10.1046/j.1365-2222.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 25.Thorne PS, et al. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Custovic A, et al. Effect of day care attendance on sensitization and atopic wheezing differs by Toll-like receptor 2 genotype in 2 population-based birth cohort studies. The Journal of allergy and clinical immunology. 2011;127:390–397. e391–399. doi: 10.1016/j.jaci.2010.10.050. doi:10.1016/j.jaci.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eder W, et al. Opposite effects of CD 14/−260 on serum IgE levels in children raised in different environments. The Journal of allergy and clinical immunology. 2005;116:601–607. doi: 10.1016/j.jaci.2005.05.003. doi:10.1016/j.jaci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Simpson A, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. doi:10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 30.Merx S, Zimmer W, Neumaier M, Ahmad-Nejad P. Characterization and functional investigation of single nucleotide polymorphisms (SNPs) in the human TLR5 gene. Hum Mutat. 2006;27:293. doi: 10.1002/humu.9409. doi:10.1002/humu.9409. [DOI] [PubMed] [Google Scholar]
- 31.Strindelius L, Filler M, Sjoholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22:3797–3808. doi: 10.1016/j.vaccine.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Wang BZ, et al. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Didierlaurent A, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172:6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 34.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature immunology. 2008;9:769–776. doi: 10.1038/ni.1622. doi:10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham AF, et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34:2986–2995. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 36.Schulke S, et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. The Journal of allergy and clinical immunology. 2011;128:1340–1348. e1312. doi: 10.1016/j.jaci.2011.07.036. doi:10.1016/j.jaci.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 37.Yousefi S, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 38.Karp CL. Guilt by intimate association: what makes an allergen an allergen? The Journal of allergy and clinical immunology. 2010;125:955–960. doi: 10.1016/j.jaci.2010.03.002. quiz 961-952, doi:10.1016/j.jaci.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009);457:585–588. doi: 10.1038/nature07548. doi:10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead GS, et al. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med. 2007;175:243–249. doi: 10.1164/rccm.200606-839OC. Epub 2006 Nov 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam D, Ng N, Lee S, Batzer G, Horner AA. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. J Immunol. 2008;181:2925–2932. doi: 10.4049/jimmunol.181.4.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sever ML, et al. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J Allergy Clin Immunol. 2007;120:849–855. doi: 10.1016/j.jaci.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.