Abstract
Background:
Cell line models suggest that activation of NFκB is associated with progression of prostate cancer. This pathway may be a therapeutic target if these observations translate to clinical specimens.
Methods:
Immunohistochemistry measured NFκBp65 (p65), NFκBp65 nuclear localisation signal (NLS), NFκBp65 phosphorylated at ser 276 (p65ser276), NFκBp65 phosphorylated at ser 536 (p65ser536), IκBα phosphorylated at ser 32/36 (pIκBαser32/36) and MMP-9 protein expression in 61 matched hormone naive prostate cancer (HNPC) and castrate-resistant prostate cancer (CRPC) tumours. Animal and cell models were used to investigate the role of NFκB inhibition in prostate carcinogenesis.
Results:
In HNPC tumours, NLS expression significantly associated with a shorter time to disease recurrence and disease-specific death. In CRPC tumours p65, pIκBαser32/36 and MMP-9 expression significantly associated with shorter time to death from disease recurrence and shorter disease-specific death. MMP-9 and pIκBαser32/36 expression significantly associated with metastases at recurrence and were independent of Gleason sum and prostate-specific antigen at recurrence. Expression of phosphorylated Akt was associated with increased p65 activation in mouse models and inhibition of NFκB in LNCaP cells significantly reduced cellular proliferation and induced apoptosis.
Conclusion:
These results provide further evidence that the NFκB pathway could be exploited as a target for CRPC.
Keywords: MMP-9, pIκBα, NFκB, hormone naïve prostate cancer
Prostate cancer incidence in the United Kingdom is ∼36 000 cases per year and is the second most common cause of male cancer-specific death (Jemal et al, 2009). Currently, the main therapy for locally advanced and metastatic prostate cancer is maximum androgen blockade (MAB). This combines inhibition of testicular androgen by surgical or chemical castration with the blockade of androgen action at the peripheral level using antiandrogens. However, when deprived of androgen stimulation, hormone naive prostate cancer (HNPC) cells gain the capability to survive and thrive by upregulating oncogenic pathways. After 18–24 months, MAB begins to fail, with the cancer evolving into castrate-resistant prostate cancer (CRPC). Castrate-resistant prostate cancer is associated with a poor prognosis and has a median survival of 12–18 months. Currently, there are relatively few effective treatments available for this disease.
Aberrant NFκB activation has been implicated in the pathogenesis of several human malignancies including prostate cancer. NFκB has been shown to have important roles in the control of cell growth, differentiation, and apoptosis (Chen et al, 2001; Li and Stark, 2002) and has also been shown to be involved in the initiation and progression of many cancers through its target genes. These target genes include c-Myc, cyclin D1, and interleukin 6 (IL-6) that promote cell growth, Bcl-2 that inhibits apoptosis, interleukin 8 (IL-8) and vascular endothelial growth factor (VEGF) that promote angiogenesis, and MMP-9 that promotes invasion and metastases (Huang et al, 2001; Suh and Rabson, 2004). Many different stimuli have been reported to cause nuclear localisation and transcriptional activation of NFκB via activation of the IKK complex (IKKα, IKKβ, and IKKγ/NEMO). Phosphorylation of IKKα/β results in phosphorylation of IκBα at serine 32 and serine 36. NFκB normally exists in a dormant state bound to IκBα, but upon phosphorylation of IκBα, NFκB is released from the complex and IκBα is marked for degradation. Following release, NFκB translocates from to the nucleus where it binds to the promoter region of multiple genes. IκBα has a central role in the termination of NFκB activation. Newly synthesised IκBα enters the nucleus and binds to NFκB, thus enhancing the dissociation from DNA and causing its re-exportation to the cytoplasm by means of a nuclear export sequence (NES) present on IκBα (Karin, 1999). Activation of NFκB is stimulated through several mechanisms including signal transduction pathways involving the tyrosine kinases, NFκB inducing kinase (NIK), IKK and PI3K. A key step in NFκB activation is phosphorylation of the p65 and p50 subunits. A number of phosphorylation sites in the p65 subunit have been acknowledged and are associated with the action of multiple stimulus-coupled kinases that act in both the cytoplasm and the nucleus. A key event is the phosphorylation of serine 536 of the p65 subunit. This site is phosphorylated by multiple kinases, which are activated by diverse stimuli (Karin, 1999; Karin et al, 2002; Ghosh and Karin, 2002; Hu et al, 2004).
NFκB is hypothesised to be associated with the development of CRPC. Constitutive activation of NFκB has been observed in the prostate cancer cell lines PC-3 and DU-145 that lack AR expression however, only very low levels of NFκB were seen in the LNCaP AR-positive cell line (Suh et al, 2002). These data suggest that either the presence of AR actually inhibits NFκB activity in prostate cancer or alternatively that constitutive activation of NFκB may correlate with AR loss, which in turn may contribute to compensatory cellular changes, allowing cell survival and growth in the absence of AR activation. A markedly higher NFκB activity has been observed in an androgen-independent prostate cancer xenograft model than in its androgen-dependent counterpart (Chen and Sawyers, 2002). It was found that NFκB increased expression of the AR regulated gene PSA, suggesting that NFκB is contributing to androgen-independent prostate cancer cell growth in the absence of androgen signalling.
The aim of the current study is to establish if components of the NFκB signalling cascade in a cohort of matched hormone naive and castrate-resistant prostate tumours are associated with poor prognosis and additionally determine if inhibition of this pathway could be employed as a therapeutic target.
Patients and methods
This biological marker study was performed in accordance to the reporting of tumour marker studies criteria (REMARK).
Patients
A total of 61 prostate cancer patients were included in this study (61 hormone naive tumours and their corresponding tumour samples at recurrence, therefore 122 tumours in total) who were diagnosed from 1984 to 2000. All tumours had patient identification removed, and the clinical information database was anonymised. Ethical approval was obtained from the Multicentre Research Ethics Committee for Scotland (MREC/01/0/36) and Local Research and Ethical Committees. Patients were only selected for analysis if they initially responded to hormone treatment (in the form of subcapsular bilateral orchidectomy or MAB) but subsequently relapsed (two consecutive rises in PSA) and had a hormone naive and castrate-resistant tissue sample available for analysis. Hormone naive tissue was obtained from 18 patients by TRUS-guided biopsy and the remaining by TURP. Castrate-resistant tumours were all obtained by TURP which had been carried out to relieve bladder outflow obstruction.
Clinical data available for each patient included age (median 70, interquartile range (IQR) 67–74), PSA at diagnosis (median 31 ng ml−1, IQR=7.8–109), PSA at recurrence (median 10 ng ml−1, IQR=4–11), Gleason grade at diagnosis (median 8, IQR=6–9) and Gleason grade at recurrence (median 7, IQR=7–9). All patients developed disease recurrence (median time to recurrence 2.48 years, IQR=1.76–4.43 years). At last follow-up, 40 patients had died of their disease and 15 patients had died of other causes and 6 were alive. The median follow-up for those patients still alive was 6.4 years (IQR=3.7–9.2 years). Following diagnosis, 10% of patients received surgical orchidectomy and 90% of patients received Luteinising hormone-releasing hormone (LHRH) analogue in combination with antiandrogen therapy. Following disease recurrence, 64% of patients received radiotherapy and none received taxane therapy. Clinical parameters associated with time to disease recurrence, time to death from disease recurrence and disease-specific survival are shown in Table 1.
Table 1. Overview and correlation of patient characteristics.
| Time to disease recurrence | Time to death from disease recurrence | Disease-specific survival | |
|---|---|---|---|
| Age (<70/>70/unknown) | 0.809 (29/29/3) | 0.137 (28/29/4) | 0.434 (30/29/2) |
| Gleason (<7/=7/>7/unknown) | 0.473 (14/12/28/7) | 0.026 (14/12/27/8) | 0.013 (14/12/29) |
| Metastasis at diagnosis (no/yes/unknown) | 0.182 (40/14/7) | 0.013 (40/14/7) | 0.002 (40/16/unknown) |
| PSA at diagnosis (<4/4–10/>10) unknown | 0.063 (9/7/38/7) | 0.568 (9/7/37/7) | 0.664 (9/7/37/8) |
| Metastasis at recurrence (No/Yes/Unknown) | 0.001 (10/33/unknown) | 0.012 (10/35/unknown) | |
| PSA at recurrence | 0.933 (39/18/unknown) | 0.045 (39/18/unknown) |
Abbreviation: PSA=prostate-specific antigen.
Each clinical parameter, where appropriate, has been correlated with time to disease recurrence, time to death from recurrence, and disease-specific survival (P-values).
Murine models
The mice used in this study were as previously described (Ahmad et al, 2011).
In short three types of mouse model were used: wild-type mice, PB Ptenfl/fl (conditional knockout of exons 4–5 of the Pten allele, resulting in loss of epithelial Pten protein expression) and PB Ptenfl/fl Her2KI (conditional knockout Pten allele and a Her2KI knockin allele, which results in loss of Pten expression combined with upregulation of Her 2 in prostate epithelial cells) (Ahmad et al, 2011).
Immunohistochemistry
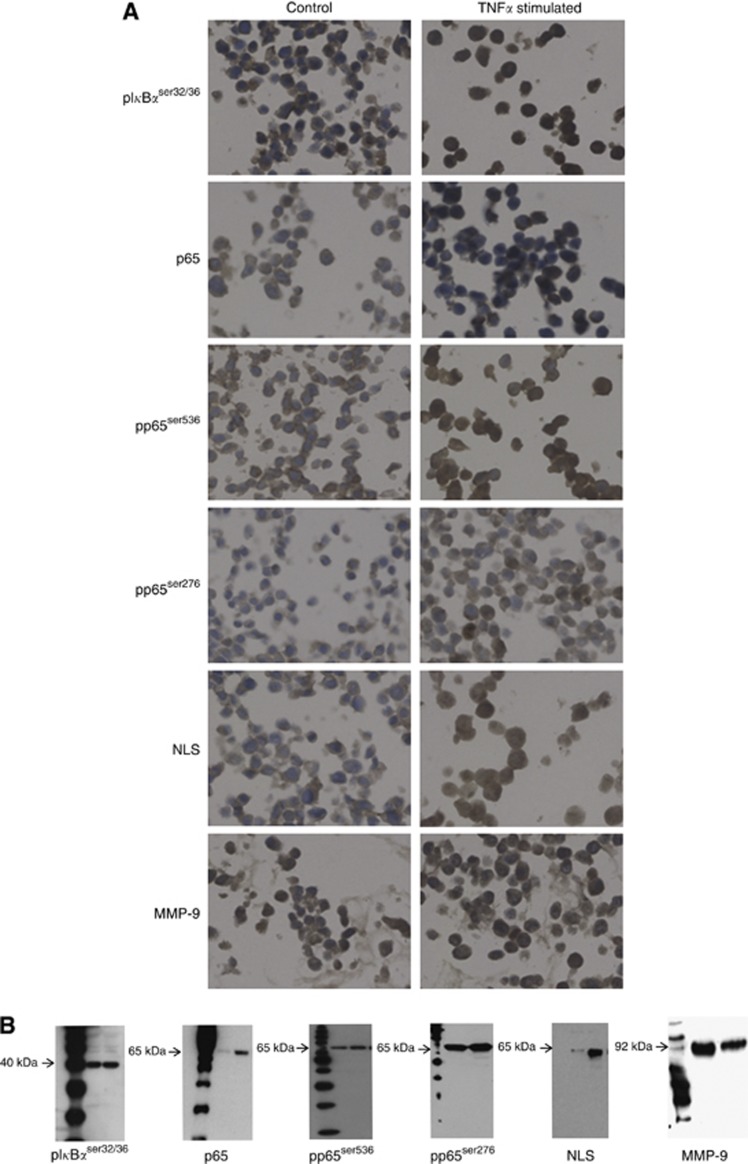
Before IHC commenced antibody specificity was confirmed by western blotting and cell pellet studies (Figure 1). All IHC was performed on 5 μm, archival formalin fixed, paraffin embedded prostate tumour sections on separate slides. Immunohistochemistry for p65, NFκBp65 phosphorylated at serine 276 (pp65ser276), NFκBp65 phosphorylated at serine 536 (pp65ser536), IκBα phosphorylated at serine 32/36 (pIκBαser32/36), p65 nuclear localisation signal (NLS) and MMP-9 was employed to assess the level of expression of each protein. Heat induced antigen retrieval was performed for all antibodies, with the exception of MMP-9. Nuclear localisation signal required Tris EDTA buffer pH 9 (5 mℳ Trizma Base, 1 mℳ EDTA, pH 9) and all others required citrate buffer pH 6 (Vector Laboratories, Burlingame, CA, USA). Endogenous peroxidase activity was blocked using 3% hydrogen peroxide and non-specific background staining was blocked using 5% horse serum in TBS for 20 min. p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), pp65ser536 (Abcam, Cambridgeshire, UK), MMP-9 (Millipore, Billerica, MA, USA), pp65ser276, pIκBαser32/36 (Cell Signalling Technology, Beverly, MA, USA) and NLS (Millipore), antibodies were used at the following concentrations: 8, 2, 20, 2, 0.5, and 0.5 μg ml−1, respectively. MMP-9 was incubated for 30 min at 25°C, p65 was incubated for 2 h at 25°C and all other antibodies were incubated overnight at 4°C. Negative controls were incubated in antibody diluent alone. Staining was developed using EnVision plus kit (Dako, Glostrup, Denmark) and 3,3-diaminobenxidine teretrahydrochloride (DAB; Vector Laboratories). Nuclei were counterstained with haematoxylin before mounting with DPX.
Figure 1.
Antibody specificity. (A) Protein expression and cellular location were observed by IHC in control LNCaP cell pellets and LNCaP cell pellets stimulated with 10 ng ml−1 TNFα for 15 min. (B) On western blots, all antibodies showed single bands of appropriate size for each protein investigated. Lysates for each antibody are as follows: pIκBαser32/36=HeLa cell extracts treated with TNFα (lane 1) and untreated (lane 2); p65, pp65ser536, pp65ser276 and NLS=LNCaP (lane 1) and LNCaP-AI (lane 2) both untreated; MMP-9=PC3 (lane 1) and LNCaP (lane 2) both untreated.
Mouse tissue staining; for each genotype we stained for NLS (Millipore) and Akt phosphorylated at serine 473 (pAktser473) (Cell Signalling Technology). Staining was carried out as described above; heat induced antigen retrieval (5 mℳ Trizma Base, 1 mℳ EDTA, pH 9). Non-specific background staining was blocked using 1 × casein in TBS for 45 min for pAktser473. Maxo Homo mouse on mouse detection kit (Stratech, Suffolk, UK) was used for NLS staining as detailed in the kit. Antibodies were incubated overnight at 4°C at 0.5 μg ml−1. pAktser473 staining was developed using Rabbit secondary (Dako).
Scoring method
Tissue staining intensity was scored blind by two independent observers using a weighted histoscore method also known as the Hscore system (Kirkegaard et al, 2006). Histoscores were calculated from the sum of (1 × % cells staining weakly positive)+(2 × % cell staining moderately positive)+(3 × % cells staining strongly positive) with a maximum score of 300. The interclass correlation coefficient (ICCC) for each protein was calculated to confirm consistency between observers and the mean of the two observers’ scores were used for analysis. Changes in protein expression between HNPC and CRPC cases were defined as an increase or decrease out with the 95% confidence interval (CI) for the difference in interobserver variation, that is, the mean difference between the histoscores that each observer assigns for protein expression plus 2 standard deviations. Change in expression of all proteins investigated is shown in Table 2.
Table 2. Protein expression in hormone naive and castrate-resistant tumours.
| HNPC (IQR) | CRPC (IQR) | P -value | ICCC | Histoscore units | |
|---|---|---|---|---|---|
| p65, c | 100 (140–180) | 100 (150–180) | 0.755 | 0.86 | 40 |
| NLS, c | 163 (139–192) | 196 (173–210) | 0.003 | 0.8 | 57 |
| NLS, n | 90 (50–124) | 102 (50–137) | 0.037 | 0.8 | 45 |
| pp65ser276, c | 0 (0–41) | 0 (0–30) | 0.010 | 0.77 | 64 |
| pp65ser276, n | 7 (50–80) | 50 (70–92) | 0.544 | 0.85 | 42 |
| pp65ser536, c | 70 (100–120) | 42 (90–123) | 0.276 | 0.83 | 80 |
| pp65ser536, n | 0 (20–73) | 0 (40–80) | 0.159 | 0.85 | 55 |
| pIκBαser32/36, c | 110 (116–196) | 133 (160–186) | 0.392 | 0.80 | 80 |
| pIκBαser32/36, n | 194 (150–220) | 196 (136–228) | 0.963 | 0.93 | 55 |
| MMP-9 | 80 (120–170) | 90 (120–160) | 0.922 | 0.83 | 80 |
Abbreviation: ICCC=interclass correlation coefficient.
The median histoscore and interquartile range (IQR) for hormone naive tumours (HNPC) and castrate-resistant tumours (CRPC) and the P-value of these values compared using a Wilcoxon sign rank test. The mean difference in observer scores plus 2 standard deviations is also shown as the number of histoscore units that is defined as a change in protein expression (change). c and n relates to protein cellular location, c=cytoplasm and n=nucleus. P before a protein indicates that the antibody detects phosphorylated protein and the number following the protein represents the site of phosphorylation.
Statistical analysis
All statistical analysis was performed using the SPSS version 18.0 (IBM, Armonk, NY, USA) for Windows. Protein expression data are shown as median and IQRs. Wilcoxon Signed Rank Tests were used to compare expression between HNPC and CRPC tumours. Survival analysis was conducted using the Kaplan–Meier method and curves were compared with the log-rank test. Hazard ratios (HRs) were calculated using Cox Regression analysis. Correlations between members of the pathway were performed using a Spearman’s rank test. The Dunnets test was performed to compare untreated and treated samples in the cell line studies.
Cell culture
Prostate cancer cell lines LNCaP and LNCaP-AI were a kind gift from Professor CN Robson (Northern Institute for Cancer Research, Newcastle, UK). LNCaP cells were routinely maintained in RPMI-1640 (Invitrogen, Paisley, UK) containing phenol red and supplemented with 10% foetal calf serum (Invitrogen), and 1% glutamine. LNCaP-AI cells have been developed using parental LNCaP cells as a model of CRPC by gradual withdrawal of androgens from the medium (Halkidou et al, 2003). These cells were routinely cultured in RPMI-1640 supplemented with 1% glutamine and 10% charcoal-stripped fetal calf serum (Sigma, Dorset, UK) known to contain negligible amount of androgens.
Cell proliferation
Proliferation was assessed using the WST-1 assay as per manufacturer's instructions (Millipore). Cells were seeded in 96-well plates at a density of 5 × 103 per well in standard culture medium, allowed to adhere overnight, and the following day treated with increasing concentrations of NFκB inhibitors 2607 and 2070 (Calbioscience International Ltd, Edinburgh, UK); ethanol was added to the control well. The assay was performed at 24 and 72 h, by adding 10 μl WST-1 reagent before dilution in Electro Coupling Solution to each well. The spectrophotometric absorbance was measured after 2 h incubation at 37°C using a 96-well microplate reader at 450 nm with reference wavelength 600 nm.
Histone/DNA ELISA for detection of apoptosis
The Cell Death Detection ELISA Kit (Roche, Palo Alto, CA, USA) was used to detect apoptosis in prostate cancer cells treated with 2607 and 2070 according to manufacturer’s protocol. Briefly, the cells were lysed for 30 min and collected by centrifugation at 200 g, 20 μl of the cytoplasmic histone/DNA fragments was transferred to a streptavadin-coated microtitre plate module. Subsequently, 80 μl of immunoreagent (Anti-histone-biotin and Anti-DNA-POD) was added to each well and incubated on an MP shaker for 2 h at room temperature. The peroxidase-conjugated anti-DNA antibody was used for the detection of immobilised histone/DNA fragments, followed by colour development with 2,2 azinobis (3-ethylbenzothiazoline-6-sulphonic acid) substrate for peroxidase. The spectrophotometric absorbance of the samples was measured using a 96-well microplate reader at 405 nm with a reference wavelength of 490 nm.
Results
Protein expression patterns
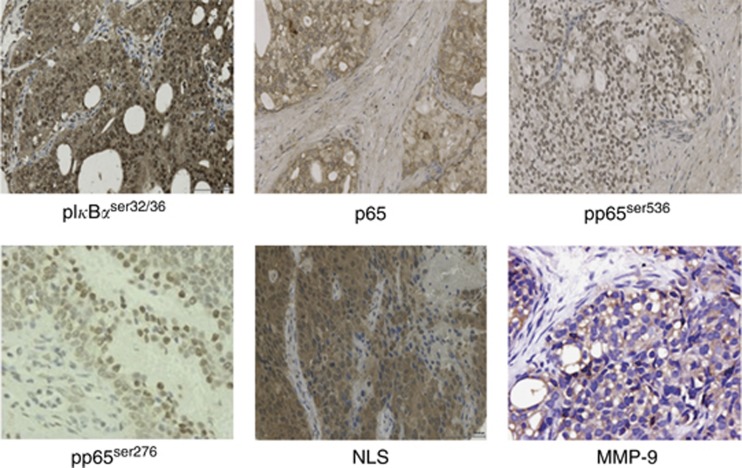
p65, NLS, pp65ser276, pp65ser536, MMP-9, and pIκBαser32/36 protein expression was observed in the cell cytoplasm. NLS, pp65ser276, pp65ser536, and pIκBαser32/36 expression was also observed in the nucleus. Expression of all proteins investigated was non-parametric, median values and IQRs for each protein at each location are provided in Table 2 for both hormone naive and castrate-resistant tumours. Immunohistochemistry images are displayed in Figure 2.
Figure 2.
Immunohistochemistry. Prostate tumours displaying immunohistochemical staining, magnification × 400.
Protein expression in the hormone naive cohort
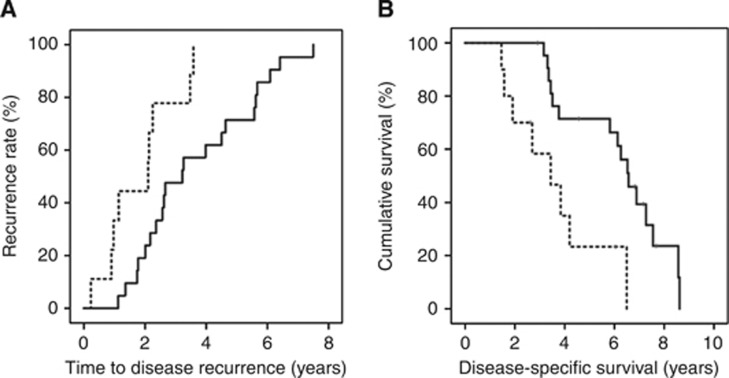
To determine if protein expression was linked to time to disease recurrence, Kaplan–Meier graphs were plotted for the hormone naive tumours expressing low levels of protein vs high levels of protein and compared using the log-rank test. Patients expressing a high level of cytoplasmic NLS (upper tertile) had a significantly shorter time to recurrence (P=0.005, Figure 3A). Patients whose tumours expressed higher levels of NLS were twice as likely to have an earlier disease recurrence than those with lower (two lower tertiles) levels (1.8 years vs 3.6 years, P=0.008, HR=1.9, 95% CI: 1.2–2.8). Additionally, patients expressing a high level of cytoplasmic NLS had a significantly shorter disease-specific survival (P=0.003, Figure 3B). Patients whose tumours expressed higher levels of NLS were twice as likely to die at an earlier stage, with a mean time to death of 3.6 years compared with 6.2 years survival for those patients who expressed lower levels (P=0.006, HR=2, 95% CI: 1.2–3.3). None of the other proteins investigated were associated with disease outcome.
Figure 3.
(A) NLS expression and time to disease recurrence. Kaplan–Meier plot demonstrates that those patients whose tumours express high NLS in the cytoplasm (broken line) have a shorter time to disease recurrence than those patients whose tumours exhibit low NLS expression (black line). (B) NLS expression and disease-specific survival. Kaplan–Meier plot demonstrates that those patients whose tumours express high NLS in the cytoplasm (broken line) have a shorter time to disease-specific death than those patients whose tumours exhibit low NLS expression (black line).
Protein expression in the castrate-resistant cohort
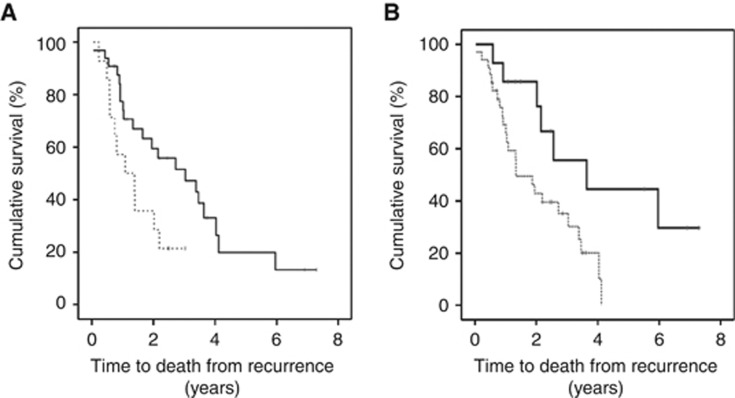
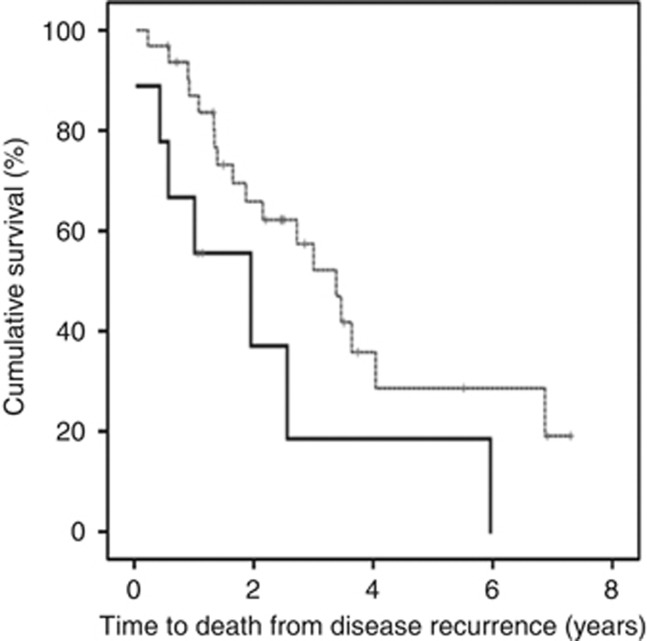
To establish if protein expression was linked to time to death from disease recurrence, Kaplan–Meier graphs were plotted for the castrate-resistant tumours expressing low levels of protein vs high levels and compared using the log-rank test. Patients expressing a high level of nuclear pIκBαser32/36 (above median) had a significantly shorter time to death from disease recurrence (P=0.028, Figure 4A). Patients whose tumours expressed high levels of pIκBαser32/36 were twice as likely to die at an earlier stage, with a mean time to death from disease recurrence of 2.3 years compared with those patients who expressed levels below the median, 4.4 years (P=0.034, HR=2.59, 95% CI: 1.1–6.2). Patients with high levels of MMP-9 (above median) also had a significantly shorter time to death from disease recurrence (P=0.015, Figure 4B). Patients whose tumours expressed high levels of MMP-9 were almost three times more likely to die at an earlier stage (P=0.020, HR=2.9, 95% CI: 1.1–7.5). These patients survived for 2 years and patients with lower levels survived for 4.2 years. This was independent of Gleason sum, presence of metastasis at recurrence and PSA at recurrence (P=0.025).
Figure 4.
(A) pIκBαser32/36 expression and time to death from disease recurrence. Kaplan–Meier plot demonstrates that those patients whose tumours express high pIκBαser32/36 in the nucleus (broken line) have a shorter time to death from disease recurrence than those patients whose tumours exhibit low pIκBαser32/36 expression (black line). (B) MMP-9 expression and time to death from disease recurrence. Kaplan–Meier plot demonstrates that those patients whose tumours express high MMP-9 (broken line) have a shorter time to death from disease recurrence than those patients whose tumours exhibit low MMP-9 expression (black line).
Correlation between clinical parameters and protein expression
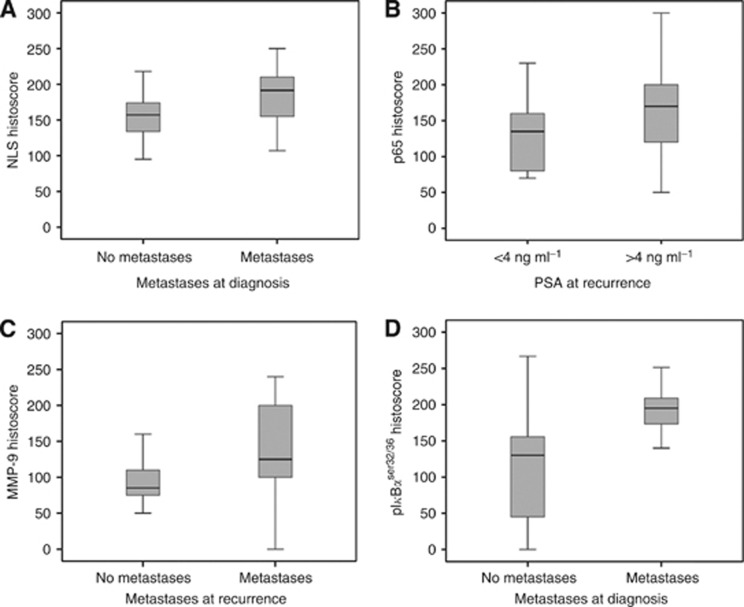
When protein expression patterns were correlated with clinical parameters, cytoplasmic NLS expression was significantly associated with metastases at diagnosis (P=0.005). The median NLS histoscore for patients without metastases was 157 (IQR=130–175) compared with 192 (IQR=154–215) for patients with metastases (Figure 5A). Expression of p65 was significantly associated with PSA at recurrence (P=0.020, Figure 5B). The median p65 histoscore for patients with PSA below 4 (ng ml−1) was 135 (IQR=80–160) vs 170 (IQR=120–200) for patients with a PSA of >4 ng ml−1. In addition, MMP-9 expression was significantly associated with the presence of metastasis at recurrence (P=0.026), the median histoscore for patients with no metastasis was 85 (IQR=72.5–115) vs 125 (IQR=100–200) for those with metastasis at recurrence (Figure 5C). Metastasis at recurrence was also significantly associated with nuclear pIκBαser32/36 (P=0.003, Figure 5D). The median histoscore for patients with no metastasis was 130 (IQR=40–161) compared with 195 (IQR=171–213) for those with metastasis at recurrence.
Figure 5.
(A) NLS expression and metastases at diagnosis. NLS expression is significantly associated with metastases at diagnosis (P=0.05). (B) p65 expression and PSA at disease recurrence. p65 expression is significantly associated with higher PSA at disease recurrence (P=0.02). (C) MMP-9 expression and metastases at disease recurrence. MMP-9 expression is significantly higher in patients with metastases at disease recurrence (P=0.026). (D) pIκBαser32/36 expression and metastases at disease recurrence. pIκBαser32/36 expression is significantly higher in patients with metastases at disease recurrence (P=0.003).
Changes in protein expression
The strength of this patient cohort is the ability to investigate whether those patients whose tumours exhibit an increase or decrease in protein expression, in the transition from hormone naive to castrate-resistant disease, are likely to have a shorter disease-specific survival after disease recurrence. Table 2 provides the cutoff histoscore selected to separate subgroups of patients. The associations observed between time to disease recurrence and disease-specific survival for each protein investigated are shown in Table 3. Cytoplasmic NLS significantly increased with the progression to castrate-resistant disease (P=0.003). The median histoscore for cytoplasmic NLS is 163 (IQR=139–192) for the hormone naive tumours compared with 196 (IQR=173–210) for the castrate-resistant tumours.
Table 3. Changes in protein expression and survival.
| Protein | Time to death from disease recurrence | Overall survival |
|---|---|---|
| p65, c | P=0.044 | P=0.081 |
| NLS, c | P=0.698 | P=0.806 |
| NLS, n | P=0.264 | P=0.002 |
| pp65ser 276, c | P=0.983 | P=0.901 |
| pp65ser276, n | P=0.428 | P=0.839 |
| pp65ser536, c | P=0.259 | P=0.009 |
| pp65ser536,n | P=0.137 | P=0.200 |
| pIκBαser32/36, c | P=0.399 | P=0.172 |
| pIκBαser32/36, n | P=0.114 | P=0.260 |
| MMP-9 | P=0.129 | P=0.022 |
Kaplan–Meier survival analysis was performed to investigate if changes in protein expression were linked to time to death from disease recurrence and overall survival.
To determine if an increase in protein expression was linked to time to death from disease recurrence and overall survival, Kaplan–Meier graphs were plotted for the castrate-resistant tumours expressing increased levels of protein vs decreased/no change in protein expression. These were compared using the log-rank test (Table 3). An increase in cytoplasmic p65 expression was significantly associated with shorter time to death from disease recurrence (P=0.004). Patients with an increase in p65 expression were 2.5 times more likely to die at an earlier stage with a survival of 1.9 years compared with 3.4 years for those with decreased/no change in expression (P=0.051, HR=2.5, 95% CI: 0.9–5.9, Figure 6). This was independent of Gleason sum, presence of metastasis at recurrence and PSA at recurrence (P=0.006).
Figure 6.
Increased p65 expression. Kaplan–Meier plot demonstrates that those patients whose tumours express an increase in p65 expression (black line) have shorter time to death from disease recurrence than those patients whose tumours exhibit decrease/no change in expression (broken line).
NLS expression correlates with low PTEN expression in HNPC and confers a poor prognosis
We have previously investigated the role of the PI3K/Akt cascade in the same patient cohort and observed members of this cascade to be significantly associated with a poorer disease-specific survival (McCall et al, 2008a, 2008b). To identify if NFκB signalling was driving tumour progression in the tumours with PTEN loss or PTEN protein expression, we investigated whether any of the significant proteins from this study correlated with PTEN loss or PTEN protein expression. Interestingly, NLS expression was associated with PTEN protein expression. Patients were stratified into three groups according to expression of NLS and PTEN: Group 1 consisted of tumours that displayed low expression of both proteins; group 2 consisted of tumours that displayed high expression of NLS protein but low expression of PTEN; group 3 consisted of tumours with low expression of NLS but high PTEN. Mean time to recurrence for patients with tumours belonging to the third group was more than double that of patients with tumours belonging to any of the other two groups: group 1=2.7 years, group 2=1.6 years, group 3=5.9 years (P=0.0014, HR=0.51, 95% CI: 0.30–0.87, Figure 7).
Figure 7.
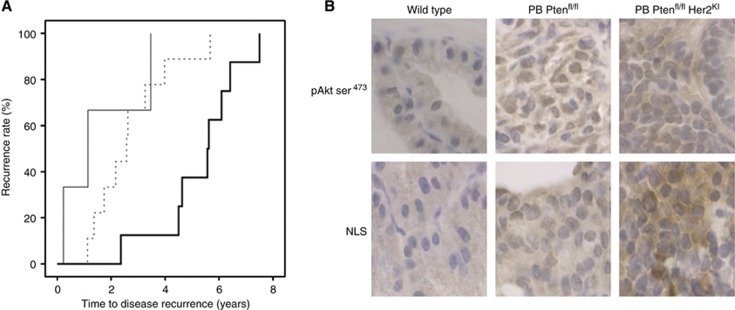
(A) Pten/NLS expression and time to disease recurrence. Kaplan–Meier plot demonstrates that those patients whose tumours express high Pten/low NLS (bold black line) have a longer time to disease recurrence than those with low Pten/low NLS (dotted line) and low Pten/high NLS (black line). (B) Ptenfl/fl Her2KI demonstrates increased NLS expression. Mouse models demonstrate that Ptenfl/fl Her2KI prostate tumours display higher NLS expression relative to both wild-type and Ptenfl/fl tumours.
NLS expression is higher in murine Ptenfl/flHer2KI prostate tumours
Mouse CaP tissue PB Ptenfl/fl that has been previously demonstrated to have epithelial loss of Pten, double mutant tumours PB Ptenfl/flHer2KI with minimal Pten expression and significant upregulation of both Her2 and Her3 and wild-type mice (Ahmad et al, 2011) was used to further investigate if high NLS expression was associated with Pten loss as seen in a subset of HNPC patients. In the previous study, it was demonstrated that larger more aggressive tumours were observed in the double mutant PB Ptenfl/flHer2KI mice compared with the wild-type and PB Ptenfl/fl mice (Ahmad et al, 2011). We observed that phosphorylated Akt and NLS expression was higher in the aggressive double mutant murine prostate tumours than expression levels in the wild-type and PB Ptenfl/fl prostates (Figure 7B).
NFκB inhibition
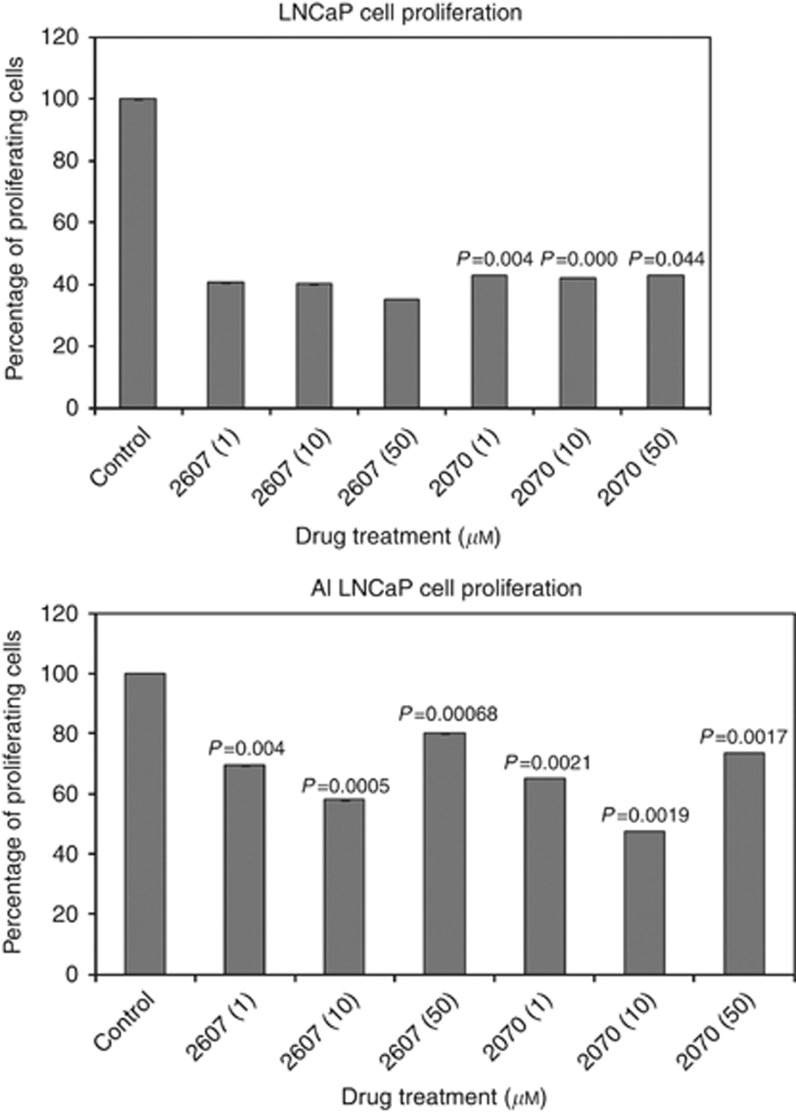
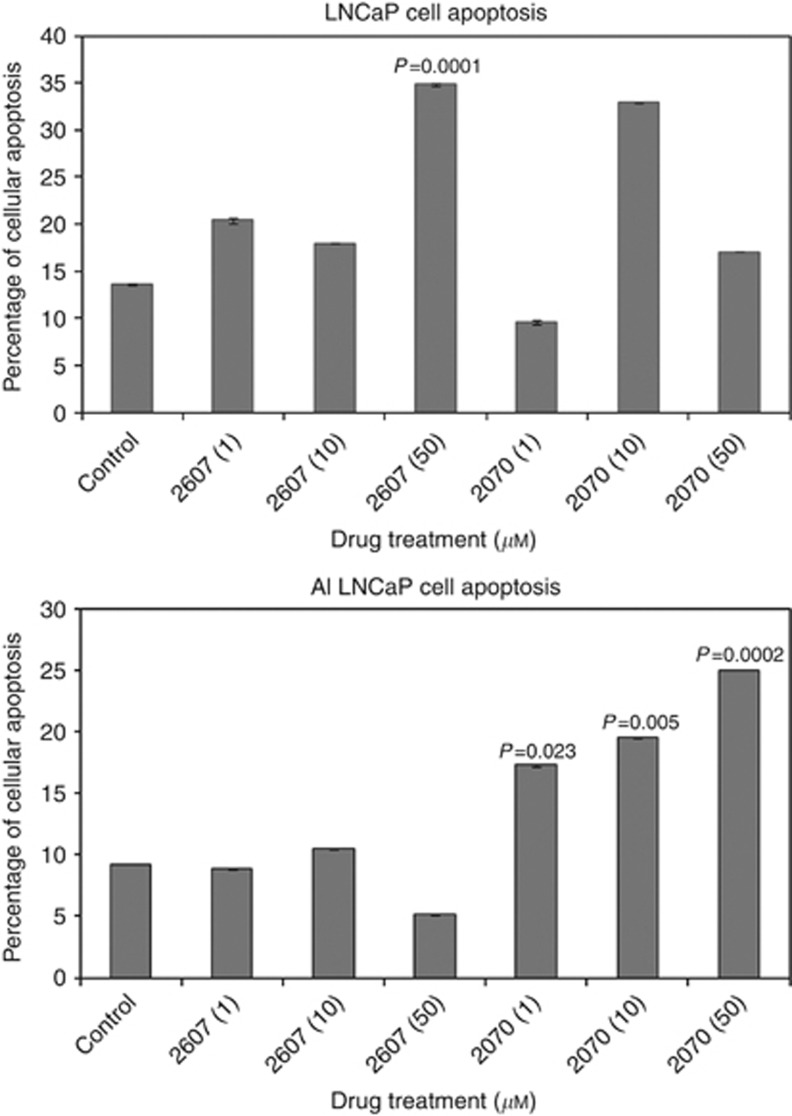
To examine the affect of NFκB inhibition, both cell lines were incubated with NFκB inhibitors 2607 and 2070 and the proliferation measured by WST assay. Both cell lines showed a dose-dependent inhibition in proliferation after 72 h (Figure 8A and B). The effect of these inhibitors on apoptosis was also investigated. In LNCaP cells 2607 significantly induced apoptosis during 24 h in response to 50 μℳ only. Apoptosis was induced in LNCaP AI cells by 2070 at all concentrations at 24 h (Figure 9A and B).
Figure 8.
Drugs 2607 and 2070 inhibit cellular proliferation. LNCaP cell proliferation is significantly reduced by drug 2070 at 72 h. LNCaP-AI cell proliferation was significantly reduced by both drugs 2607 and 2070, at 72 h.
Figure 9.
Drugs 2607 and 2070 induce apoptosis. LNCaP cell apoptosis was significantly induced at 24 h by drug 2607 (50 μℳ). LNCaP-AI cell apoptosis was significantly induced at 24 h by drug 2070.
Discussion
The NFκB signalling cascade is viewed as a hopeful anticancer target due to its role in oncogenesis and chemoresistance in many tumour types. As the biological significance of constitutive NFκB activation in prostate cancer is unclear at present, this study was undertaken to provide further evidence that the NFκB signalling cascade may be employed as a therapeutic target in prostate cancer and explore the value of p65 in predicting patient outcome and response to therapy. The phosphorylation sites chosen in the current study had been extensively reported in the literature as being associated with p65 transactivation in many cancer types, and pp65ser536 in particular due to the controversial involvement of Akt signalling (Vermeulen et al, 2003; Arun et al, 2009; Cohen et al, 2009).
We have previously reported that increased Akt signalling is observed in the progression to castrate-resistant disease (McCall et al, 2008a). Studies have demonstrated that Akt can activate NFκB via several stimuli and this in turn functions to prevent apoptosis. In particular, two studies have indicated that Akt, via TNFα signalling or in response to growth factor stimulation, stimulated NFκB nuclear translocation via the IKK complex (Kane et al, 1999; Ozes et al, 1999; Romashkova and Makarov, 1999). Another study indicated that Akt alone could not induce nuclear translocation of NFκB but synergised with PMA to induce this response (Kane et al, 1999). It has been shown that Akt signalling involves the stimulation of the transcriptional function of NFκB and that the ability of oncogenic Ras to activate NFκB transcriptional activity is dependent on Akt activity (Sizemore et al, 1999; Madrid et al, 2000). Madrid et al have also observed that the ability of Akt to stimulate the transcriptional potential of the p65 subunit of NFκB actually requires IKK and p38. Their study reported a negative correlation between activated Akt (phosphorylation of serine 473) and pp65ser536, which corroborates with others suggesting that Akt does not directly phosphorylate p65 at serine residue 536.
In the present study, it was observed that phosphorylation of p65 at serine residues 276 and 536 was a common event in prostate cancer with all samples showing some degree of expression of both phosphorylation sites in the nucleus. However, neither of these phosphorylation sites showed any significant associations with patient outcome.
Studies showing that NFκB is active (i.e., nuclear) in a number of tumours are consistent with a role for NFκB in cancer, although some tumour cell lines exhibit NFκB activity without significant nuclear accumulation suggesting a cytoplasmic role for p65. We studied the expression of the nuclear localisation signal (NLS) of p65 using an antibody that specifically recognises an epitope overlapping the NLS of the p65 subunit of the NFκB heterodimer. This epitope is covered by IκBα binding, and hence the NLS antibody selectively binds to the IκBα free, activated form of p65, allowing this site to be used as a marker of activation. Interestingly, this study found that cytoplasmic NLS expression was significantly associated with a shorter time to disease recurrence and disease-specific death and cytoplasmic NLS expression significantly increased in the transition from HNPC to CRPC. These data suggest an additional role for NFκB in the cytoplasm other than a nuclear transcription factor. Moreover, an increase in cytoplasmic p65 expression in the transition from HNPC to CRPC was significantly associated with a shorter time to death from disease recurrence and cytoplasmic p65 expression also correlated with PSA at disease recurrence. The PSA gene promoter contains a κB site (Zhang et al, 2004) and PSA is involved in prostate epithelial growth thus suggesting that p65 is associated with PSA expression.
It has been suggested that the absence of PTEN might contribute to constitutive activation of NFκB induced by the PI3K/Akt pathway. However, no direct correlation has been observed in prostate cancer cell lines. NFκB has been implicated in prostate cancer progression via two mechanisms: promotion of metastases via MMP-9 expression or promotion of androgen independence via an as yet unknown mechanism. In this study, we have observed that in patients with a combined high PTEN/low NLS protein expression have a survival advantage with a longer time to disease recurrence when compared with high NLS/low PTEN expression. This suggests that PTEN absence may contribute to constitutive NFκB signalling. Interestingly in the mouse CaP models higher NLS expression was observed in mice with double mutations in comparison with wild-type and Pten null mice, suggesting that Her2/Her3 activation combined with Pten loss is more lethal in driving prostate carcinogenesis.
When expression levels of pIκBαser32/36 were examined it was noted that high nuclear levels were significantly associated with a shorter time to death from recurrence, indicating that p65 is active in the cell. Metastasis at recurrence was significantly associated with nuclear pIκBαser32/36, further demonstrating an association between NFκB and the presence of metastasis. Numerous reports have demonstrated that in prostate cancer, as well as other tumour types, metastases directly correlates with the expression level of several angiogenic genes including; VEGF, basic fibroblast growth factor (Bfgf), IL-8 and matrix metalloproteases MMP-2 and MMP-9 (Huang et al, 2001; Andela et al, 2003). Protein expression of MMPs is associated with poor prognosis in a variety of cancers including prostate. An increase in MMP-2 and MMP-9 is associated with tumour progression but how the constitutive expression of these genes is regulated in prostate cancer is at present uncertain. It is known however that NFκB binds the MMP-9 promoter (Andela et al, 2003). This study found that MMP-9 expression was significantly associated with the presence of metastasis at recurrence and was also significantly associated with shorter time to death from recurrence independent of Gleason sum, presence of metastasis at recurrence and PSA at recurrence (P=0.025). This adds to evidence that NFκB signalling may be involved in the metastasis of prostate cancer.
In prostate cancer cells, androgen deprivation induces cell-cycle arrest and apoptotic cell death. NFκB mediates cell-cycle progression through direct binding of the cyclin D1 promoter at multiple sites and regulates progression through G1-S cell-cycle check point (Guttridge et al, 1999). The expression data of cyclin D1 in the same patient cohort were available (carried out previously by another study done in our laboratory) and the association between NFκB pathway members and cyclin D1expression was investigated. A positive correlation between nuclear pIκBαser32/36 and cyclin D1 expression (P=0.020, r2=0.516) was observed in the castrate-resistant cohort, again suggesting that this pathway is active in a subset of patients with castrate-resistant disease.
NFκB has also been shown to inhibit apoptosis by directly binding the promoter and inducing genes encoding the BCL-2 homologue BCL-XL and survivin (Zong et al, 1999). Therefore, inhibition of NFκB is an important strategy to restrain prostate cancer tumorigenesis. Due to the diverse ranges of upstream activators of NFκB, the effect of inhibiting NFκB activity directly was investigated. Inhibition of proliferation and induction of apoptosis in both LNCaP and LNCaP-AI cells was achieved by the use of two novel NFκB inhibitors. These observations support the hypothesis that NFκB could offer a therapeutic target for treatment of a subset of CRPC patients. Additionally, the suggested biomarker for prediction of patient response for these novel therapies would be p65 NLS and not phosphorylation of the sites investigated in the current study.
In summary, these findings demonstrate upregulation of the NFκB pathway in a subset of castrate-resistant patients and that p65 may be a potential prognostic marker. Inhibition of NFκB activity leads to a decrease in cellular proliferation and induced apoptosis. Thus, the NFκB pathway may be a potential target for therapy in a subset of patients with castrate-resistant disease.
Acknowledgments
This work was supported by Think Pink, and the Glasgow Royal Infirmary Endowment fund. We also thank Calscience International Ltd for donating the inhibitors used in this study.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Ahmad I, Patel R, Singh LB, Nixon C, Seywright M, Barnetson RJ, Brunton VG, Muller WJ, Edwards J, Sansom OJ, Leung HY (2011) HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc Natl Acad Sci USA 108(39): 16392–16397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andela VB, Gordon AH, Zotalis G, Rosier RN, Goater JJ, Lewis GD, Schwarz EM, Puzas JE, O’Keefe RJ (2003) NF kappa B: a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res 415: S75–S85 [DOI] [PubMed] [Google Scholar]
- Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C (2009) Nuclear NF-kappaB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res 15(19): 5974–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Sawyers CL (2002) NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol 22(8): 2862–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X (2001) New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 159(2): 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Chen Z, Lu SL, Yang XP, Arun P, Ehsanian R, Brown MS, Lu H, Yan B, Diallo O, Wang XJ, Van Waes C (2009) Attenuated transforming growth factor beta signaling promotes nuclear factor-kappaB activation in head and neck cancer. Cancer Res 69(8): 3415–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS (1999) NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19(8): 5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkidou K, Gnanapragasam VJ, Mehta PB, Logan IR, Brady ME, Cook S, Leung HY, Neal DE, Robson CN (2003) Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene 22(16): 2466–2477 [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC (2004) IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117(2): 225–237 [DOI] [PubMed] [Google Scholar]
- Huang SY, Pettaway CA, Uehara H, Bucana CD, Fidler IJ (2001) Blockade of NF-kappa B activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20(31): 4188–4197 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4): 225–249 [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A (1999) Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol 9(11): 601–604 [DOI] [PubMed] [Google Scholar]
- Karin M (1999) How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18(49): 6867–6874 [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2(4): 301–310 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM (2006) Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 48(7): 787–794 [DOI] [PubMed] [Google Scholar]
- Li X, Stark GR (2002) NFkappaB-dependent signaling pathways. Exp Hematol 30(4): 285–296 [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJG, Baldwin AS, Mayo MW (2000) Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappa B. Mol Cell Biol 20(5): 1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall P, Gemmell LK, Mukherjee R, Bartlett JM, Edwards J (2008a) Phosphorylation of the androgen receptor is associated with reduced survival in hormone-refractory prostate cancer patients. Br J Cancer 98(6): 1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J (2008b) Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer 99(8): 1296–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401(6748): 82–85 [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS (1999) NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401(6748): 86–90 [DOI] [PubMed] [Google Scholar]
- Sizemore N, Leung S, Stark GR (1999) Activation of Phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappa B p65/RelA subunit. Mol Cell Biol 19(7): 4798–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB (2002) Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 52(3): 183–200 [DOI] [PubMed] [Google Scholar]
- Suh J, Rabson AB (2004) NF-kappa B activation in human prostate cancer: Important mediator or epiphenomenon? J Cell Biochem 91(1): 100–117 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22(6): 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Charron M, Wright WW, Chatterjee B, Song CS, Roy AK, Brown TR (2004) Nuclear factor-{kappa}B activates transcription of the androgen receptor gene in sertoli cells isolated from testes of adult rats. Endocrinology 145(2): 781–789 [DOI] [PubMed] [Google Scholar]
- Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev 13(4): 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]