Abstract
Over the past years the number of medical registries has increased sharply. Their value strongly depends on the quality of the data contained in the registry. To optimize data quality, special procedures have to be followed. A literature review and a case study of data quality formed the basis for the development of a framework of procedures for data quality assurance in medical registries. Procedures in the framework have been divided into procedures for the co-ordinating center of the registry (central) and procedures for the centers where the data are collected (local). These central and local procedures are further subdivided into (a) the prevention of insufficient data quality, (b) the detection of imperfect data and their causes, and (c) actions to be taken / corrections. The framework can be used to set up a new registry or to identify procedures in existing registries that need adjustment to improve data quality.
Several developments in healthcare, such as progress in information technology and increasing demands for accountability, have led to an increase in the number of medical registries over recent years. We define a medical registry as a systematic collection of a clearly defined set of health and demographic data for patients with specific health characteristics, held in a central database for a predefined purpose (based on Solomon et al.1). The specific patient characteristics (e.g., the presence of a disease or whether an intervention has taken place) determine which patients should be registered. Medical registries can serve different purposes—for instance, as a tool to monitor and improve quality of care or as a resource for epidemiological research.2 One example is the National Intensive Care Evaluation (NICE) registry, which contains data from patients who have been admitted to Dutch intensive care units (ICUs) and provides insight into the effectiveness and efficiency of Dutch intensive care.3
To be useful, data in a medical registry must be of good quality. In practice, however, quite frequently incorrect patients are registered or data items can be inaccurately recorded or not recorded at all.4–8 To optimize the quality of medical registry data, participatory centers should follow certain procedures designed to minimize inaccurate and incomplete data. The objective of this study was to identify causes of insufficient data quality and to make a list of procedures for data quality assurance in medical registries and put them in a framework. By data quality assurance we mean the whole of planned and systematic procedures that take place before, during, and after data collection, to guarantee the quality of data in a database. Our proposed framework for procedures for data quality assurance is intended to serve as a reference during the start-up of a registry. Furthermore, comparing current procedures in existing medical registries with the proposed procedures in the framework should allow the identification of possible adjustments in the organization to improve data quality.
Methods
Literature Review
To gain insight into the concept of data quality, we searched the literature for definitions. For the development of the data quality framework we searched the literature for (a) types and causes of data errors and (b) procedures that can minimize the occurrence of data errors in a registry database. An automated literature search was done using the Medline and Embase databases. The following text words and MeSH headings were used in this search: “data quality,” “registries,” “data collection,” “validity,” “accuracy,” “quality control,” and combinations of these terms. The automated search spanned the years from 1990 to 2000. To supplement the automated search, a manual search was done for papers referenced by other papers, papers and authors known by reputation, and papers from personal databases and the Internet. To search the Internet we used the same terms as for the literature search. The manual search was not restricted to the medical domain or to a specified time period. Papers were considered relevant if they described the analysis of data quality in a registry database or a trial database in terms of types and frequencies of data errors or causes of insufficient data quality. In addition, we selected papers that described procedures for the control and the assurance of data quality in registry or trial databases. We considered registry and trial databases as systematic collections of a prespecified set of data items for all people with a specific characteristic of interest (e.g., patients admitted to intensive care) for a predefined purpose, such as evaluative research.
Methods for the Case Study
We analysed the types and causes of data errors that may occur in a registry by performing a case study at two ICUs that had collected data for the NICE registry for at least one year. The NICE dataset contains 96 variables representing characteristics of the patients and the outcome of ICU treatment. It includes demographic data, admission and discharge data and all variables necessary to calculate severity of illness scores and mortality risks according to the prognostic models APACHE II and III,9,10 SAPS II,11 MPM II,12 and LODS.13 Thirty-nine variables are categorical (e.g. presence of chronic renal insufficiency prior to ICU admission), 48 are numerical (e.g., highest systolic blood pressure during the first 24 hours of ICU admission), 7 are dates/times and 2 are character strings (Appendix 1▶).
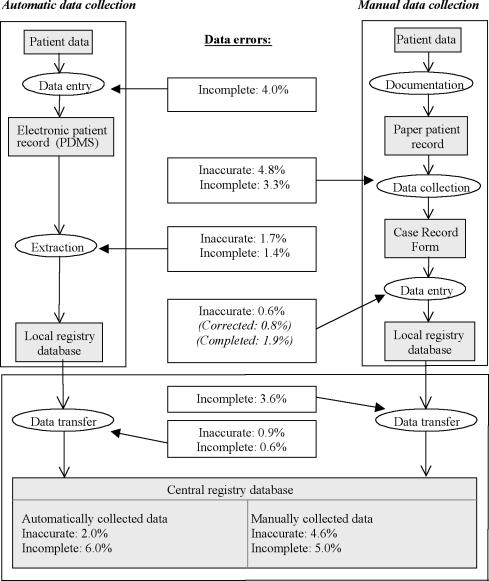
One of the ICUs in the case study collected data automatically by extracting the data from their electronic patient data management system (PDMS)14 into a local registry database. The other ICU collected the data manually by filling in case record forms (CRFs) that were manually entered into a local registry database. Each month the data from the local databases from both ICUs are transferred to the central registry database at the NICE coordinating center. Data flows are shown in Figure 1▶.
Figure 1 .
Results from the case study: types and percentages of newly occurring data errors at the different steps in the data collection process.
For each ICU we retrieved from the central registry database the records of 20 randomly selected patients that had been admitted in September or October of 1999. To evaluate the accuracy and the completeness of the data we compared the data from the central registry database, the local database and the CRFs with the gold standard data. The gold standard data were re-abstracted from the paper patient record or the PDMS by one of the authors (DA). Registered values were found to be inaccurate if (1) a categorical value was not equal to the gold standard value or (2) a numerical value deviated from the gold standard value more than acceptable (e.g., a deviation in systolic blood pressure of > 10 mmHg below or above the gold standard systolic blood pressure). The appendix contains a complete list of variables and criteria. A data item was found to be incomplete when it was not registered, even though it was available in the paper-based record or the PDMS.
By means of a structured interview with the physicians responsible for the data collection process, we gained insight into the local organization of the data collection. This information and discussion of discovered data errors helped us to identify the causes of insufficient data quality. The causes of insufficient data quality that we found through the literature review and the case-study were grouped according to their place in the data collection process.
Quality Assurance Framework
Procedures for data quality assurance were collected through literature review as described before. According to their characteristics, the procedures were placed in a framework that maps with the grouping of data error causes, obtained by the literature review and case study.
Results
Data Quality Definitions from Literature
According to the International Standards Organisation (ISO) definition, quality is “the totality of features and characteristics of an entity that bears on its ability to satisfy stated and implied needs” (ISO 8402-1986, Quality-Vocabulary) Similarly, in the context of a medical registry, data quality can be defined as “the totality of features and characteristics of a data set, that bear on its ability to satisfy the needs that result from the intended use of the data.” Many researchers point out that data quality is an issue that needs to be assessed from the data users’ perspective. For example, according to Abate et al.,15 data are of the required quality if they satisfy “the requirements stated in a particular specification and the specification reflects the implied needs of the user.”
The review of relevant literature yielded a large number of distinct data quality attributes that might determine usability. Most of the data quality attributes in literature had ambiguous definitions or were not defined at all. In some cases, multiple terms were used for one single data quality attribute in different articles. The two most frequently cited data quality attributes were “accuracy” and “completeness.”6,7,15–31 From all data quality attributes found in literature, these two are the most relevant in the context of the case study. Based on the definitions found in literature, we formulated clear, unambiguous definitions for (1) data accuracy (the extent to which registered data are in conformity to the truth) and (2) data completeness (the extent to which all necessary data that could have been registered have actually been registered).
Types and Causes of Data Errors
Literature Review
Review of relevant literature4,17–19,32–38 resulted in a number of types of data errors. Van der Putten et al.36 divides data errors into interpretation errors, documentation errors and coding errors. Other authors divide data errors into systematic (type I) errors and random (type II) errors.17 Knatterud et al.35 additionally mentions bias as a category of data errors, which can cause random as well as systematic errors. Causes of systematic data errors include programming errors,32,34 unclear definitions for data items,4,33,36 or violation of the data collection protocol.34,35 Random data errors, for instance, can be caused by inaccurate data transcription and typing errors18,32,35,39 or illegible handwriting in the patient record.19,34 Clarke32 describes only two causes of data errors, inaccurate data transcription and programming errors in the software used. These two causes of errors are most frequently cited in literature. Inaccurate data transcription occurs during the actual data collection process. Alternatively, programming errors are part of the procedures that precede the actual data collection process. Other examples are the lack of clear definitions for data items and guidelines for data collection4,33,36 or insufficient training of participants in using the data definitions and guidelines.34,37
We have now identified several possible types and causes of data errors through literature review. To analyse the types and causes of data errors in real practice we performed a case study at the NICE registry.
Case-study of Data Quality in the NICE Registry
Figure 1▶ displays the frequencies of inaccurate and incomplete data as they occurred during the different steps in the data collection process. Because some variables were not documented in the PDMS, for all patients, 4.0% of the data was not available in the extraction source. After the extraction of the data from the PDMS another 1.4% of the data was incomplete because the extraction software lacked queries for some data items. Of the extracted data, 1.7% was inaccurate because of programming errors in the extraction software. Because of programming errors in the software used for transferring data into the central registry database, another 0.6% of the data was incomplete and 0.9% was incorrect. For example, one extraction query did not return the longest prothrombin time in the first 24 hours of ICU admission but in the entire ICU stay. Finally, the central registry database contained 2.0% inaccurate and 6.0% incomplete data for the hospital with automatic data collection.
In the hospital using manual data collection, after transcription of data from the paper patient record to the CRF 4.8% of the data was inaccurate and 3.3% was incomplete. We identified several causes of these errors, such as inaccurate transcription of the data and inaccurate calculations of derived variables such as daily urinary output and alveolar-arterial oxygenation difference. A relatively frequent error cause was nonadherence to data definitions. Contrary to the definitions, data outside the first 24 hours of ICU admission were frequently registered. The next step in the data collection process is the manual entry of the data from the CRFs into the local registry database. Inaccurate typing was a relatively infrequent cause of data errors (0.6%). During entry of data in the local database, some data that were inaccurate or incomplete on the CRFs were corrected or completed. Programming errors in the software used for transferring the data into the central registry database caused another 3.6% incomplete data items. Finally, the central registry database contained 4.6% inaccurate and 5% incomplete data for the hospital with manual data collection.
The causes of inaccurate data that we found through the literature review and the case study, and the types of data errors that they cause are presented in Table 1▶. The causes are grouped according to the related stage in the registration process and subdivided into causes at central and at local level. Central level refers to the coordinating center that sets up the registry and transfers data sets from all participating centers into the central registry database. Local level refers to the sites where the actual data collection takes place.
Table 1 .
Causes of Insufficient Data Quality in Medical Registries at the Different Stages in the Registry Process and Type (Systematic or Random) of Data Errors
| Central coordinating centere | Data errors | Local sites | Data errors |
|---|---|---|---|
| Set up and organisation of registry | |||
| Unclear / ambiguous data definitions | Systematic | Illegible handwriting in data source | Random |
| Unclear data collection guidelines | Systematic | Incompleteness of data source | Systematic |
| Poor CRF lay-out | Systematic/random | Unsuitable data format in source | Systematic |
| Data dictionary not available to data collectors | Systematic/random | ||
| Poor interface design | Systematic/random | Lack of motivation | Random |
| Data overload | Random | Frequent shift of personnel | Random |
| Programming errors | Systematic | Programming errors (data entry module/extraction software) | Systematic |
| Data collection | |||
|---|---|---|---|
| No control over adherence to guidelines and data definitions | Systematic | Non-adherence to data definitions | Systematic |
| Non-adherence to guidelines | Systematic | ||
| Insufficient data checks | Systematic/random | Calculation errors | Systematic/random |
| Typing errors | Random | ||
| Insufficient data checks at data entry | Systematic/random | ||
| Transcription errors | Random | ||
| Incomplete transcription | Random | ||
| Confusing data corrections on CRF | Random | ||
| Quality improvement | |||
|---|---|---|---|
| Insufficient control over correction of detected | Systematic/random | No correction of detected data errors data errors locally | Systematic/random |
| Lack of a clear plan for quality improvement | Systematic/random | ||
Proposed Framework with Procedures for Data Quality Improvement
Many different quality assurance procedures have been discussed in literature. Whitney et al.38 discuss data quality in longitudinal studies. They make a distinction between quality assurance procedures and quality control procedures. Quality assurance consists of activities undertaken before data collection to ensure that the data are of the highest possible quality at the time of collection. Examples of quality assurance procedures are a clear and extensive study design and training of data collectors. Quality control takes place during and after data collection and is aimed at identifying and correcting sources of data errors. Some examples of quality control procedures are completeness checks and site visits.38
Knatterud et al.35 describe guidelines for standardisation of quality assurance in clinical trials. According to the authors, quality assurance should include prevention, detection, and action, from the beginning of the data collection through publication of the study results. Important aspects of prevention are the selection and training of adequate and motivated personnel and the design of a data collection protocol. Detection of data errors can be achieved through routinely monitoring the data, which means that they are compared with data in another independent data source. Finally, action implies that data errors are corrected and causes of data errors are resolved.
To standardize data collection in a registry, the data items that need to be collected should be provided with clear data definitions, and standardized guidelines for data collection methods must be designed.18,19,32–35,38 Many authors recommend training of persons involved in the registry.5,18,33–35,38,40–42 Issues in training sessions are the scope of the registry, the data collection protocol and data definitions. Participants should be trained centrally to guarantee standardization of the data collection procedures across participating centers.
From two studies20,43 it appeared that data quality improves if the CRF contains less open-ended questions. To reduce the chance of errors occurring in the data during collection several authors recommend collecting the data in space close to the original data source as soon as the data are available.19,40,44,45 Ideally, the data should be entered by the clinician or obtained directly from the relevant electronic data source (e.g., a laboratory system).19
Several different methods can be applied to detect errors at data entry. For example the registry data can be entered twice, preferably by two independent persons. Detected inconsistencies, that indicate data-entry errors should be checked and re-entered.19,38,41,44,46 Automatic domain or consistency checks on the data at data entry, data extraction or data transfer, can also detect anomalous data.8,19,32,38,42,44,45 Not all data errors can be detected through automatic data checks. Data errors that are still within the predefined range will not be uncovered. Therefore, in addition to the automatic checking of the data, a visual check of the entered data is recommended.8,44 Analyses of the data (e.g., simple cross-tabulation) could also help to uncover anomalies in data patterns.32,35,44 The coordinating center of a registry can control data quality by visiting the participating centres and performing data audits. These audits imply that a sample of the data from the central registry database is being compared with the original source data (e.g., in the paper patient record).34,35,38,42
Based on causes of insufficient data quality from the case study and on experiences described in literature we made a list of procedures for data quality assurance. These procedures have been placed in a framework (Table 2▶) in which quality assurance procedures are divided into “central” and “local” procedures. Central and local procedures are further subdivided into three phases: (a) the prevention of insufficient data quality, (b) the detection of inaccurate or incomplete data and their causes and (c) corrections or actions to be taken to improve data quality. This grouping for the data quality assurance procedures resembles the grouping of causes of data errors in Table 1▶. Preventive procedures are aimed at the causes of inaccurate data due to deficiencies in the set-up or the organization of the registry. Detection of inaccurate data takes place during the local data collection process and during the transfer of data into the central database. Finally detected data inaccuracies and their causes should lead to actions that improve data quality.
Table 2 .
Framework of Procedures for the Assurance of Data Quality in Medical Registries
| Central coordinating centre | Local sites |
|---|---|
| Prevention during set up and organisation of registry | |
| At the onset of the registry | At the onset of participating in the registry |
| compose minimum set of necessary data items | assign a contact person |
| define data & data characteristics in data dictionary | check developed software for data entry and for extraction |
| draft a data collection protocol | check reliability and completeness of extraction sources |
| define pitfalls in data collection | standardise correction of data items |
| compose data checks | Continuously |
| create user friendly case record forms | train (new) data collectors |
| create quality assurance plan | motivate data collectors |
| In case of new participating sites | make data definitions available |
| perform site visit | place date & initials on completed forms |
| train new participants | keep completed case record forms |
| Continuously | data collection close to the source and as soon as possible |
| motivate participants | use the registry data for local purposes |
| communicate with local sites | In case of changes (e.g., in data set) |
| In case of changes (e.g., in data set) | adjust data dictionary, forms, software, etc. |
| adjust forms, software, data dictionary, protocol, training material, etc. | communicate with data collectors |
| communicate with local sites | |
| Detection during data collection | |
|---|---|
| During import of data into the central database | Continuously |
| perform automatic data checks | visually inspect completed forms |
| Periodically and in case of new participants | perform automatic data checks |
| perform site visits for data quality audit (registry data <> source data) and review local data collection procedures | check completeness of registration |
| Periodically | |
| check inter- and intraobserver variability | |
| perform analyses on the data | |
| Actions for quality improvement | |
|---|---|
| After data import and data checks | After receiving quality reports |
| provide local sites with data quality reports | check detected errors |
| control local correction of data errors | correct inaccurate data & fill in incomplete data |
| After data audit or variability test | resolve causes of data errors |
| give feedback of results and recommendations | After receiving feedback |
| resolve causes of data errors | implement recommended changes |
| communicate with personnel | |
Discussion
The definitions of data quality that we found clearly give “the data requirements that proceed from the intended use” a pivotal position. Thus, the intended use of registry data determines the necessary properties of the data. For example, in a registry that is used to calculate incidence rates of diseases, it is essential to include all existing patient cases. In other cases (e.g., registries used for case-control studies), it is essential to record correctly characteristics of registered patients, such as diagnoses; the exact number of included patients is of minor importance.17
Definitions of data quality and data quality attributes in literature are frequently unclear, ambiguous or unavailable. Before designing a plan for quality assurance of registry data, a clear description of what attributes constitute data quality is necessary. Additionally, standard definitions of data quality and data quality attributes are necessary to be able to compare data quality among registries or within a registry at different points in time.
Investigating the causes of data errors is a prerequisite for the reduction of errors. The literature search that we performed was not conducted fully in accordance with the methodology of a systematic review. Nevertheless, we believe that we captured most of the relevant articles. In addition to the literature search, we performed a case study. We analyzed the types and causes of data errors in the NICE registry at the different steps in the data collection process, from the patient record to the central registry database. We did not question the quality of data documented in the patient record, either electronic or paper-based. Nevertheless, ample evidence in the literature indicates that the patient record is generally not completely free of data errors.47–49 For our case study, however, the paper-based patient record or the PDMS was the most reliable source available. The case study showed missing values for some variables (e.g., admission source, ICU discharge reason) for each patient in the PDMS, because these variables were not configured in the PDMS. This was a relatively large source of systematically missing data for the hospital with automatic data collection. The paper-based records also missed some of the data that were obligatory in the registry. This however was a minor cause of randomly missing data in the registry.
From our case study, it appeared that in case of automatic data collection, data errors are mostly systematic and caused by programming errors. The disadvantage of these programming errors is that they can cause a large number of data errors. On the other hand, once the programming error is detected, this cause can easily be resolved. In case of manual data collection, errors appeared to be mostly random. Most data errors occurred during recording of the data on the CRFs due to inaccurate transcription or non-adherence to data definitions. The fact that in this case most data errors occur during transcription of data to the CRF corresponds to the results of other studies.39 The percentages of inaccurate and incomplete data in case of manual data collection are comparable to those found in other studies.7,39,42 The literature search yielded no articles presenting results of data quality analysis for automatically extracted data to compare with the results of our case study.
It is unrealistic to aim for a registry database that is completely free of errors. Some errors will remain undetected and uncorrected regardless of quality assurance, editing, and auditing. Implementation of procedures for data quality assurance can merely lead to an improvement of data quality. As evident from the literature review, the assurance of data quality can entail many different procedures. Procedures were selected for our framework when they were practically feasible and when they seemed likely to prevent, detect, or correct frequently occurring errors. This implies that the procedures in the framework can be expected to be effective in improving data quality.
Procedures in the framework are divided into “central” and “local” procedures. This division was applied because most registries consist of several participating local centers that collect the data and send it to the central coordinating center that sets up and maintains the registry. Prud’homme et al.42 similarly described quality assurance procedures separately for all parties involved in a trial, such as the clinical centers, the coding center, and the data coordination center. Central and local procedures in our framework are further subdivided into the prevention of insufficient data quality, detection of (causes of) insufficient data quality and actions to be taken. Knatterud et al.35 and Wyatt19 made similar divisions. The framework of procedures for data quality assurance fits in the table with causes of data errors. If, for example, the local causes of data errors lie mainly in the data collection phase, quality assurance procedures from the local detection section in the framework should be implemented.
For the development of the framework we took two methods of data collection into consideration, manual and automatic data collection. We did not consider the use of alternative data entry methods such as the OCR/OMR scanning of registry data. Since manual and automatic data collection are the two most commonly used methods, the framework should be applicable for reviewing the organization in most medical registries.
The developed quality assurance framework for medical registries will also be useful for reviewing procedures and improving data quality in clinical trials. Good clinical practice guidelines for clinical trials state that procedures for quality assurance have to be implemented at every step in the data collection process.50 To ensure the quality of data in clinical trials, the framework described in this article could be a suitable addition to the good clinical practice guidelines.
We believe that the framework proposed in this article can be a helpful tool for setting up a high quality medical registry. Our experiences with the NICE registry and with three other national and international registries support this opinion. In existing registries it can be a useful tool for identifying adjustments in the data collection process. Further research, such as pre- and postmeasurements of data quality, should be conducted to determine whether implementation of the framework in a registry in fact reduces the percentages of data errors.
Acknowledgments
The authors thank Martien Limburg and Jeremy Wyatt for their valuable comments on this manuscript.
Appendix
Appendix 1▶
Appendix 1 .
List of NICE variables, the severity-of-illness-models in which they are used, their data type, and the criteria used for analysis of data accuracy. Data accuracy was analysed by comparing the registry data to the gold standard (g.s.) data.
| Admission data | Used in | Type | Criterium |
|---|---|---|---|
| Hospital number | — | Numerical | Must be consistent with g.s. |
| ICU number | — | Numerical | Must be consistent with g.s. |
| Admission number | — | Numerical | Must be consistent with g.s. |
| Patient number | — | Numerical (encrypted) | Must be consistent with g.s. |
| Surname | — | String (encrypted) | Must be consistent with g.s. |
| Maiden name | — | String (encrypted) | Must be consistent with g.s. |
| Date of birth | — | Date | Must be consistent with g.s. |
| Age | APACHE II & III, SAPS II, MPM0, 24 | Numerical | Must be consistent with g.s. |
| Gender | — | Categorical | Must be consistent with g.s. |
| Length | — | Numerical | Deviation from g.s. must not exceed 10 centimetres. |
| Weight | — | Numerical | Deviation from g.s. must not exceed 10 kilograms. |
| Hospital admission date | — | Date | Must be consistent with g.s. |
| ICU admission date | — | Date | Must be consistent with g.s. |
| ICU admission time | Time | Deviation from g.s. must not exceed 1 hour. | |
| Referring specialty | — | Categorical | Must be consistent with g.s. |
| Admission source | — | Categorical | Must be consistent with g.s. |
| Admission type | APACHE II & III, SAPS II, MPM0, 24 | Categorical | Must be consistent with g.s. |
| Planned admission | — | Categorical (yes/no) | Must be consistent with g.s. |
| ASA score at admission | — | Categorical | Must be consistent with g.s. |
| Diagnostic data | Used in | Type | Criterium |
|---|---|---|---|
| Cardio Pulmonary Resuscitation | MPM0 | Categorical (yes/no) | Must be consistent with g.s |
| Dysrhytmia | MPM0 | Categorical (yes/no) | Must be consistent with g.s. |
| Cerebrovascular accident | MPM0 | Categorical (yes/no) | Must be consistent with g.s. |
| Gastrointestinal bleeding | MPM0 | Categorical (yes/no) | Must be consistent with g.s. |
| Intracranial mass effect | MPM0, 24 | Categorical (yes/no) | Must be consistent with g.s. |
| Chronic renal insufficiency | MPM0 | Categorical (yes/no) | Must be consistent with g.s. |
| Metastatic neoplasm | APACHE III, SAPS II, MPM0, 24 | Categorical (yes/no) | Must be consistent with g.s. |
| AIDS | APACHE III, SAPS II | Categorical (yes/no) | Must be consistent with g.s. |
| Chronic dialysis | APACHE II | Categorical (yes/no) | Must be consistent with g.s. |
| Haematological malignancy | APACHE III, SAPS II | Categorical (yes/no) | Must be consistent with g.s. |
| Cirrhosis | APACHE II & III, MPM0, MPM24 | Categorical (yes/no) | Must be consistent with g.s. |
| Chronic cardiovascular insufficiency | APACHE II | Categorical (yes/no) | Must be consistent with g.s. |
| Respiratory insufficiency | APACHE II | Categorical (yes/no) | Must be consistent with g.s. |
| Immunological insufficiency | APACHE II & III | Categorical (yes/no) | Must be consistent with g.s. |
| Mechanical ventilation at admission | MPM0 | Categorical (yes/no) | Must be consistent with g.s. |
| Mechanical ventilation during first 24 hours | SAPS II, LODS II, MPM24 | Categorical (yes/no) | Must be consistent with g.s. |
| Confirmed infection | MPM24 | Categorical (yes/no) | Must be consistent with g.s. |
| Acute renal failure | APACHE II &III, MPM0, 24 | Categorical (yes/no) | Must be consistent with g.s. |
| APACHE II Reason for admission | APACHE II | Categorical | Must be consistent with g.s. |
| Glasgow Coma Scale | Used in | Type | Criterium |
|---|---|---|---|
| Eye reaction at admission | MPM0 | Categorical | Must be consistent with g.s. |
| Eye reaction after the first 24 hours of admission | MPM24 | Categorical | Must be consistent with g.s. |
| Lowest eye reaction in the first 24 hours | APACHE II & III, SAPS II, LODS II | Categorical | Must be consistent with g.s. |
| Motor reaction at admission | MPM0 | Categorical | Must be consistent with g.s. |
| Motor reaction after the first 24 hours of admission | MPM24 | Categorical | Must be consistent with g.s. |
| Lowest motor reaction in first 24 hours | the APACHE II & III, SAPS II, LODS II | Categorical | Must be consistent with g.s. |
| Verbal reaction at admission | MPM0 | Categorical | Must be consistent with g.s. |
| Verbal reaction after the first 24 hours of admission | MPM24 | Categorical | Must be consistent with g.s. |
| Lowest verbal reaction in the first 24 hours of admission | APACHE II & III, SAPS II, LODS II | Categorical | Must be consistent with g.s. |
| Physiologic & laboratory data | Used in | Type | Criterium |
|---|---|---|---|
| Highest heartrate within 1 h after admission | MPM0 | Numerical | Deviation from g.s. must not exceed10 beats/min. |
| Lowest heartrate in the first 24 hours | APACHE II& III, SAPS II, LODS II | Numerical | Deviation from g.s. must not exceed 10 beats/min. |
| Highest heartrate in the first 24 hours | APACHE II& III, SAPS II, LODS | Numerical | Deviation from g.s. must not exceed 10 beats/min. |
| Lowest respiratory rate in the 24 hours | APACHE II & III | Numerical | Must be exactly equal to g.s. |
| Highest respiratory rate in the first 24 hours | APACHE II & III | Numerical | Must be exactly equal to g.s. |
| Lowest systolic blood pressure within 1 h after admission | MPM0 | Numerical | Deviation from g.s. must not exceed 10 mmHg. |
| Lowest systolic blood pressure in the first 24 hours | SAPS II, LODS II | Numerical | Deviation from g.s. must not exceed 10 mmHg. |
| Highest systolic blood pressure in the first 24 hours | SAPS II, LODS II | Numerical | Deviation from g.s. must not exceed 10 mmHg. |
| Lowest mean blood pressure in the first 24 hours | APACHE II & III | Numerical | Deviation from g.s. must not exceed 10 mmHg. |
| Highest mean blood pressure in the first 24 hours | APACHE II & III | Numerical | Deviation from g.s. must not exceed 10 mmHg. |
| Lowest body temperature in the first 24 hours | APACHE II & III, SAPS II | Numerical | Deviation from g.s. must not exceed 0.5 degrees Celsius. |
| Highest body temperature in the first 24 hours | APACHE II & III, SAPS II | Numerical | Deviation from g.s. must not exceed 0.5 degrees Celsius. |
| Longest prothrombin time in the first 24 hours | LODS II, MPM24 | Numerical | Must be exactly equal to g.s. |
| Lowest urine output in 8 hours | MPM24 | Numerical | Deviation g.s. must not exceed 0.250 Liter. |
| Urine output in the first 24 hours | APACHE III, SAPS II, LODS II | Numerical | Deviation from g.s. must not exceed 1 Liter. |
| Vasoactive drugs | MPM24 | Categorical (yes/no) | Should be consistent with g.s. |
| Lowest PaO2 in the first 24 hours | MPM24 | Numerical | Must be exactly equal to g.s. |
| Lowest PaO2 / FIO2 in the first 24 hours | SAPS II, LODS II | Numerical | Must be exactly equal to g.s. |
| FIO2 to calculate the highest A-aDO2 in the first 24 hours | APACHE II | Numerical | Must be taken from sample that results in highest A-aDO2 according to g.s. |
| PaO2 to calculate the highest A-aDO2 in the first 24 hours | APACHE II & III | Numerical | Must be taken from sample that results in highest A-aDO2 according to g.s. |
| PaCO2 to calculate the highest A-aDO2 in the first 24 hours | APACHE II | Numerical | Must be taken from sample that results in highest A-aDO2 according to g.s. |
| Highest A-aDO2 in the first 24 hours | APCHE II & III | Numerical | Must be exactly equal to g.s. |
| PH from same bloodgas sample as A-aDO2 | APACHE II & III | Numerical | Must be taken from sample that results in highest A-aDO2 according to g.s. |
| Lowest white blood cell count in the first 24 hours | APACHE II & III, SAPS II, LODS II | Numerical | Must be exactly equal to g.s. |
| Highest white blood cell count in the first 24 hours | APACHE II & III, SAPS II, | Numerical LODS II | Must be exactly equal to g.s. |
| Lowest serum creatinine level in the first 24 hours | APACHE II & III, LODS II | Numerical | Must be exactly equal to g.s. |
| Highest serum creatinine level in the first 24 hours | APACHE II & III, LODS II | Numerical | Must be exactly equal to g.s. |
| Lowest serum potassium level in the first 24 hours | APACHE II, SAPS II | Numerical | Must be exactly equal to g.s. |
| Highest serum potassium level in the first 24 hours | APACHE II, SAPS II | Numerical | Must be exactly equal to g.s. |
| Lowest serum sodium level in the first 24 hours | APACHE II & III, SAPS II | Numerical | Must be exactly equal to g.s. |
| Highest serum sodium level in the first 24 hours | APACHE II & III, SAPS II | Numerical | Must be exactly equal to g.s. |
| Lowest serum bicarbonate level in the first 24 hours | APACHE II & III, SAPS II | Numerical | Must be exactly equal to g.s. |
| Highest serum bicarbonate level in the first 24 hours | APACHE II & III, SAPS II | Numerical | Must be exactly equal to g.s. |
| Highest serum urea level in the first 24 hours | the APACHE III, SAPS II, LODS II | Numerical | Must be exactly equal to g.s. |
| Highest bilirubin level in the first 24 hours | APACHE III, SAPS II, LODS II | Numerical | Must be exactly equal to g.s. |
| Lowest haemoglobine level in the first 24 hours | APACHE II & III | Numerical | Must be exactly equal to g.s. |
| Hightest haemoglobine level in the first 24 hours | APACHE II & III | Numerical | Must be exactly equal to g.s. |
| Lowest serum albumin level in the first 24 hours | APACHE III | Numerical | Must be exactly equal to g.s. |
| Highest serum albumin level in the first 24 hours | APACHE III | Numerical | Must be exactly equal to g.s. |
| Lowest level of platelets in the first 24 hours | LODS II | Numerical | Must be exactly equal to g.s. |
| Lowest glucose level in the first 24 hours | APACHE III | Numerical | Must be exactly equal to g.s. |
| Lowest glucose level in the first 24 hours | APACHE III | Numerical | Must be exactly equal to g.s. |
| Discharge data | Used in | Type | Criterium |
|---|---|---|---|
| ICU discharge date | — | Date | Must be consistent with g.s. |
| ICU discharge time | — | Time | Deviation from g.s. must not exceed 1 hour. |
| Discharge destination | — | Categorical | Must be consistent with g.s. |
| Reason for discharge | — | Categorical | Must be consistent with g.s. |
| Patient died at ICU | — | Categorical (yes/no) | Must be consistent with g.s. |
| Patient died in hospital | — | Categorical (yes/no) | Must be consistent with g.s. |
| Hospital discharge date | — | Date | Must be consistent with g.s. |
References
- 1.Solomon D, et al.. Evaluation and implementation of public-health registries. Public Health Rep. 1991;106(2):142–50. [PMC free article] [PubMed] [Google Scholar]
- 2.van Romunde L, Passchier J. Medische registraties: doel, methoden en gebruik [in Dutch]. Ned Tijdschr Geneeskd. 1992;136(33):1592–5. [PubMed] [Google Scholar]
- 3.de Keizer N, Bosman R, Joore J, et al. Een nationaal kwaliteitssysteem voor intensive care [in Dutch]. Medisch Contact. 1999;54(8):276–9. [Google Scholar]
- 4.Goldhill DR, Sumner A. APACHE II, data accuracy and outcome prediction. Anaesthesia. 1998;53(10):937–43. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzoni L, Da Cas R, Aparo UL. The quality of abstracting medical information from the medical record: The impact of training programmes. Int J Qual Health C. 1999;11(3):209–13. [DOI] [PubMed] [Google Scholar]
- 6.Seddon D, Williams E. Data quality in the population-based cancer registration: An assessment of the Merseyside and Cheshire Cancer Registry. Brit J Cancer 1997;76(5):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrie J, Marsh D. Quality of data in the Manchester orthopaedic database. Br Med J. 1992;304:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horbar JD, Leahy KA. An assessment of data quality in the Vermont-Oxford Trials Network database. Control Clin Trials. 1995;16(1):51–61. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 10.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults [see comments]. Chest. 1991;100(6):1619–36. [DOI] [PubMed] [Google Scholar]
- 11.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270(24):2957–63. [DOI] [PubMed] [Google Scholar]
- 12.Lemeshow S, Teres D, Klar J, et al. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478–86. [PubMed] [Google Scholar]
- 13.Le Gall JR, Klar J, Lemeshow S, et al. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996; 276(10):802–10. [DOI] [PubMed] [Google Scholar]
- 14.Weiss Y, Sprung C. Patient data management systems in critical care. Curr Opin Crit Care. 1996;2:187–92. [Google Scholar]
- 15.Abate M, Diegert K, Allen H. A hierarchical approach to improving data quality. Data Qual J. 1998;33(4):365–9. [Google Scholar]
- 16.Tayi G, Ballou D. Examining data quality. Commun ACM. 1998;41(2):54–7. [Google Scholar]
- 17.Sørensen HT, Sabroe S, Olsen J. A framework for evaluation of secondary data sources for epidemiological research. Int J Epidemiol. 1996;25(2):435–42. [DOI] [PubMed] [Google Scholar]
- 18.Hilner JE, McDonald A, Van Horn L, et al. Quality control of dietary data collection in the CARDIA study. Control Clin Trials. 1992;13(2):156–69. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt J. Acquisition and use of clinical data for audit and research. J Eval Clin Pract. 1995;1(1):15–27. [DOI] [PubMed] [Google Scholar]
- 20.Teperi J. Multi method approach to the assessment of data quality in the Finnish Medical Birth Registry. J Epidemiol Community Health. 1993;47(3):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosking J, Newhouse M, Bagniewska A, Hawkins B. Data collection and transcription. Control Clin Trials. 1995; 16:66S–103S. [DOI] [PubMed] [Google Scholar]
- 22.AHIMA. The American health information management association practice brief: Data Quality Management Model. June, 1998. Available at http://www.ahima.org/journal/pb/98-06.html. Accessed Sept. 15, 2001.
- 23.DISA. DOD Guidelines on Data Quality Management. 2000. Available at http://datadmn.disa.mil/dqpaper.html. Accessed Aug. 15, 2001.
- 24.Shroyer AL, Edwards FH, Grover FL. Updates to the Data Quality Review Program: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1998; 65(5):1494–7. [DOI] [PubMed] [Google Scholar]
- 25.Golberg J, Gelfand H, Levy P. Registry evaluation methods: A review and case study. Epidemiol Rev. 1980;2:210–20. [DOI] [PubMed] [Google Scholar]
- 26.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33(4):365–9. [DOI] [PubMed] [Google Scholar]
- 27.Grover FL, Shroyer AL, Edwards FH, et al. Data quality review program: the Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg 1996;62(4):1229–31. [DOI] [PubMed] [Google Scholar]
- 28.Wand Y, Wang R. Anchoring data quality dimensions in ontological foundations. Communications of the ACM. 1996; 39(11):86–95. [Google Scholar]
- 29.Maudsley G, Williams EM. What lessons can be learned for cancer registration quality assurance from data users? Skin cancer as an example. Int J Epidemiol. 1999;28(5):809–15. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu R, Khalil O. Data quality in the database systems course. Sept, 1998. Available at http://www.dataquality.com/998mathieu.htm. Accessed Sept. 15, 2001.
- 31.Kaomea P. Valuation of data quality: A decision analysis approach. Sept, 1994. Available at http://web.mit.edu/tdqm/www/papers/94/94-09.html. Accessed Aug. 15, 2001.
- 32.Clarke PA. Data validation. In Clinical Data Management. Chichester, John Wiley & Sons, 1993, pp 189–212.
- 33.Clive RE, Ocwieja KM, Kamell L, et al. A national quality improvement effort: Cancer registry data. J Surg Oncol. 1995; 58(3):155–61. [DOI] [PubMed] [Google Scholar]
- 34.Gassman JJ, Owen WW, Kuntz TE, et al. Data quality assurance, monitoring, and reporting. Control Clin Trials. 1995;16(2 Suppl):104S–136S. [DOI] [PubMed] [Google Scholar]
- 35.Knatterud GL, Rockhold FW, George SL, et al. Guidelines for quality assurance in multicenter trials: a position paper. Control Clin Trials. 1998;19(5):477–93. [DOI] [PubMed] [Google Scholar]
- 36.van der Putten E, van der Velden JW, Siers A, Hamersma EA. A pilot study on the quality of data management in a cancer clinical trial. Control Clin Trials. 1987;8(2):96–100. [DOI] [PubMed] [Google Scholar]
- 37.Stein HD, Nadkarni P, Erdos J, Miller PL. Exploring the degree of concordance of coded and textual data in answering clinical queries from a clinical data repository [see comments]. J Am Med Inform Assoc. 2000;7(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitney CW, Lind BK, Wahl PW. Quality assurance and quality control in longitudinal studies. Epidemiol Rev. 1998; 20(1):71–80. [DOI] [PubMed] [Google Scholar]
- 39.Vantongelen K, Rotmensz N, van der Schueren E. Quality control of validity of data collected in clinical trials. EORTC Study Group on Data Management (SGDM). Eur J Cancer Clin Oncol. 1989;25(8):1241–7. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Kon H. Toward total data quality management (TDQM). June, 1992. Available at http://web.mit.edu/tdqm/papers/92-02.html. Accessed Aug. 15, 2001.
- 41.Neaton J, Duchene AG, Svendsen KH, Wentworth D. An examination of the efficiency of some quality assurance methods commonly employed in clinical trials. Stat Me.d 1990; 9(1–2):115–23, discussion, 124. [DOI] [PubMed] [Google Scholar]
- 42.Prud’homme GJ, Canner PL, Cutler JA. Quality assurance and monitoring in the Hypertension Prevention Trial. Hyper-tension Prevention Trial Research Group. Control Clin Trials. 1989;10(3 Suppl):84S–94S. [DOI] [PubMed] [Google Scholar]
- 43.Gissler M, Teperi J, Hemminki E, Merilainen J. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23(1):75–80. [DOI] [PubMed] [Google Scholar]
- 44.Blumenstein BA. Verifying keyed medical research data. Stat Med. 1993;12(17):1535–42. [DOI] [PubMed] [Google Scholar]
- 45.Christiansen DH, Hosking JD, Dannenberg AL, Williams OD. Computer-assisted data collection in multicenter epidemiologic research. The Atherosclerosis Risk in Communities Study. Control Clin Trials. 1990;11(2):101–15. [DOI] [PubMed] [Google Scholar]
- 46.Day S, Fayers P, Harvey D. Double data entry: what value, what price? Control Clin Trials. 1998;19(1):15–24. [DOI] [PubMed] [Google Scholar]
- 47.Aronsky D, Haug PJ. Assessing the quality of clinical data in a computer-based record for calculating the pneumonia severity index. J Am Med Inform Assoc. 2000;7(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan W, Wagner M. Accuracy of data in computer-based patient records. J Am Med Inform Assoc. 1997;5:342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner M, Hogan W. The accuracy of medication data in an outpatient electronic medical record. J Am Med Inform Assoc. 1996;3:234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. WHO Technical Reports Series. Guidelines for Good Clinical Practice (GCP) for Trials on Pharmaceutical Products. 1995, Annex 3.