Abstract
Background:
The clinical course of breast cancer patients with brain metastases (BM) as only metastatic site (brain-only metastatic breast cancer (BO-MBC)) has been insufficiently explored.
Methods:
All breast cancer patients with BM treated at our institution between 1990 and 2011 were identified. For each patient, full information on follow-up and administered therapies was mandatory for inclusion. Oestrogen receptor, progesterone receptor and Her2 status were determined according to standard protocols. Statistical analyses including computation of survival probabilities was performed.
Results:
In total, 222 female patients (26% luminal; 47% Her2; 27% triple negative) with BM of MBC were included in this study. In all, 38/222 (17%) BM patients did not develop extracranial metastases (ECM) during their disease course and were classified as BO-MBC. Brain-only-MBC was not associated with breast cancer subtype or number of BM. The median overall survival of BO-MBC patients was 11 months (range 0–69) and was significantly longer than in patients with BM and ECM (6 months, range 0–104; P=0.007). In all, 7/38 (18%) BO-MBC patients had long-term survival of >3 years after diagnosis of BM and long-term survival was significantly more common in BO-MBC patients as compared with BM patients with ECM (P<0.001).
Conclusions:
Brain-only metastatic behaviour occurs in around 17% of breast cancer with BM and is not associated with breast cancer subtype. Exploitation of all multimodal treatment options is warranted in BO-MBC patients, as these patients have favourable prognosis and long-term survival is not uncommon.
Keywords: metastatic breast cancer, brain metastases, brain-only metastatic behaviour, prognosis, overall survival, breast cancer subtypes
Metastatic breast cancer (MBC) is the leading cause of cancer-related death in women (De Vita et al, 2012). The overall survival (OS) of patients with MBC constantly improved over the past decades mainly due to advances in systemic treatment (Kiely et al, 2011). Despite these advances, the development of brain metastases (BM) remains a severe and devastating complication decreasing quality of life and increasing morbidity and mortality (Weil et al, 2005). The incidence seems to be rising as up to 40% of patients will develop BM during their course of disease (Weil et al, 2005; Pestalozzi et al, 2006). Treatment options for patients with BM are limited. Local treatment approaches include surgery, radiosurgery and radiotherapy depending on number of BM, status of systemic disease and performance status of the patient (Lin et al, 2004). Although some trials postulate a positive impact of systemic treatment, the true effect of systemic therapy approaches on BM remains unclear (Bartsch et al, 2007, 2012; Lin et al, 2008). Survival of BM patients varies with breast cancer subtypes, however, the median OS remains limited ranging from 3.5 to a maximum of 25.3 months (Sperduto et al, 2012).
Despite the general poor prognosis, we observed patients with BM from MBC with long-term survival of >36 months after the first diagnosis of BM at our institution. As some patients presented with isolated brain metastatic disease in the absence of extracranial disease (brain-only (BO) MBC) during their course of disease, we hypothesised that patients with BO metastatic behaviour might be a distinct entity.
We undertook this study to define the clinical characteristics and course of patients with BO-MBC and to compare it to patients with BM and extracranial metastatic disease.
Materials and Methods
Patients
We identified all breast cancer patients with BM treated at our institution between 1990 and 2011. Diagnosis of BM was performed by cranial computed tomography and/or cranial magnetic resonance imaging (MRI). All patients were treated according to the current evidence-based standard of care for systemic disease as well as for BM. Treatment approaches included radiosurgery, (whole brain) radiation therapy, surgical approaches, targeted as well as endocrine therapy and chemotherapeutic agents. For each patient, full information on clinical characteristics, follow-up and administered therapies was mandatory for inclusion.
Breast cancer subtypes
Oestrogen receptor (ER), progesterone receptor (PR) and Her2 status were assessed by immunohistochemistry (ERα antibody, clone 1D5, Dako A/S, Glostrup, Denmark; and PR antibody, Dako A/S, Herceptest; Dako A/S) with a fully automated multi-modal slide-staining system (Ventana Benchmark ULTRA, Ventana, Tucson, AZ, USA). Oestrogen receptor, PR and Her2 status were determined according to standard protocols (Wolff et al, 2007; Hammond et al, 2010). Breast cancer subtypes were defined as luminal subtype in the presence of ER and/or PR expression and the absence of Her2 expression, as Her2 subtype in the presence of Her2 expression regardless of ER and PR expression, and as triple-negative subtype in the absence of ER, PR and Her2 expression.
Study endpoints
Brain-only-MBC patients were defined as breast cancer patients with BM and the absence of extracranial metastases (ECM) during the entire course of the disease. We assessed the incidence of BO-MBC in relation to clinical characteristics including patient age, breast cancer subtypes (Her2-positive, triple-negative and luminal subtypes), number of BM and evaluated OS times. Overall survival was defined as time from first diagnosis of BM by computed tomography and/or MRI scan till death of any cause.
Statistical analysis
For correlation of two parameters the χ2 test was used. Two-tailed P-values ⩽0.05 were considered to indicate statistical significance. For univariate survival analysis the Kaplan–Meier product limit method was used. To test differences between curves the log-rank test was applied. For multivariable survival analysis a Cox regression model was used. All statistics were calculated using statistical package for the social sciences (SPSS) 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patients characteristics
In total, 222 female patients with a median age of 49 years at first diagnosis of breast cancer (range 26–79) and a median age of 53 years at first diagnosis of BM (range 26–83) were included in this study. All patients presented with symptomatic BM as none of the patients underwent screening for BM. Median time from first diagnosis of breast cancer to diagnosis of BM was 46.9 months (range 0–200). In all, 98/222 (44%) had neurosurgical resection and 22/222 (10%) radiosurgery as first local treatment approach for BM. In total, 180/222 (81%) were treated with whole brain radiotherapy (WBRT). In all, 73/180 (41%) patients received WBRT as adjuvant therapy after neurosurgical resection, 30/180 (17%) after radiosurgery and 77/180 (43%) as the only local treatment approach for BM. Overall, 85/180 (38%) received systemic therapy after diagnosis of BM. Table 1 lists further patients characteristics.
Table 1. Patients’ characteristic.
|
BM and ECM (
n
=184)
|
BO (
n
=38)
|
||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | % | χ2 Test/t-test |
| Age at first diagnosis of breast cancer (years) | |||||
| Median | 48.5 | 52 | 0.92 | ||
| Range | 26–79 | 34–79 | |||
| Breast cancer subtype | |||||
| Luminal | 45 | 24.5 | 13 | 34.2 | 0.19 |
| Her2-positive | 92 | 50.0 | 13 | 34.2 | |
| Triple-negative | 47 | 25.5 | 12 | 31.6 | |
| Stage IV at first diagnosis | |||||
| Yes | 26 | 14.2 | 1 | 2.6 | 0.5 |
| No | 157 | 85.8 | 37 | 97.4 | |
| Chemotherapy before diagnosis of BM | |||||
| Yes | 169 | 91.8 | 33 | 86.8 | 0.33 |
| No | 15 | 8.2 | 5 | 13.2 | |
| Her2-targeted therapy before BM | |||||
| Yes | 70 | 76.1 | 7 | 53.8 | 0.90 |
| No | 22 | 23.9 | 6 | 46.2 | |
| Age at first diagnosis of BM (years) | |||||
| Median | 52.5 | 54.5 | 0.48 | ||
| Range | 26–81 | 36–83 | |||
| Time to BM from first diagnosis of breast cancer (months) | |||||
| Median | 41.0 | 30.0 | 0.02 | ||
| Range | 0–200 | 0–116 | |||
| Number of BM | |||||
| Mean | 3 | 2 | 0.36 | ||
| Range | 1–20 | 1–8 | |||
| Karnofsky performance status at first diagnosis of BM | |||||
| >70 | 167 | 90.8 | 36 | 94.7 | 0.43 |
| <70 | 17 | 9.2 | 2 | 5.3 | |
| DS-GPA | |||||
| Class I | 43 | 23.4 | 6 | 15.8 | |
| Class II | 81 | 44.0 | 18 | 47.4 | |
| Class III | 51 | 27.7 | 12 | 31.6 | 0.78 |
| Class IV | 9 | 4.9 | 2 | 5.3 | |
| Metastatic pattern at first diagnosis of BM | |||||
| Visceral metastases | 129 | 58.1 | 0 | 0 | — |
| Lung metastases | 86 | 38.7 | 0 | 0 | |
| Liver metastases | 79 | 35.6 | 0 | 0 | |
| Lymph metastases only | 5 | 2.3 | 0 | 0 | |
| Bone metastases only | 7 | 3.2 | 0 | 0 | |
| First-line treatment for BM | |||||
| Radiosurgery | 19 | 10.3 | 3 | 7.9 | 0.002 |
| Chemotherapy | 1 | 0.5 | 1 | 2.6 | |
| Surgery | 71 | 38.6 | 27 | 71.1 | |
| WBRT | 88 | 47.8 | 7 | 18.4 | |
| Best supportive care | 5 | 2.7 | 0 | 0 | |
| Chemotherapy after diagnosis of BM | |||||
| Yes | 77 | 41.8 | 8 | 21.1 | 0.016 |
| No | 107 | 58.2 | 30 | 78.9 | |
| Systemic progression after treatment for BM | |||||
| Yes | 84 | 45.7 | 0 | 0 | — |
| No | 100 | 54.3 | 38 | 100 | |
| BM progression | |||||
| Yes | 62 | 33.7 | 14 | 36.8 | 0.71 |
| No | 122 | 66.3 | 24 | 63.2 | |
| OS from diagnosis of BM (months) | |||||
| Median | 6 | 11 | 0.007 | ||
| Range | 0–104 | 0–69 | |||
| Survival beyond 3 years | |||||
| Yes | 5 | 2.7 | 7 | 18.4 | <0.001 |
| No | 179 | 97.3 | 31 | 81.6 | |
Abbreviations: BM=brain metastases; BO=brain-only; DS-GPA=diagnosis-specific graded prognostic assessment; ECM=extracranial metastases; OS=overall survival; WBRT=whole brain radiotherapy.
Metastatic pattern
In total, 60/222 (27%) patients had BM as first site of recurrence. Of 60 patients with BM as first site of recurrence, 22 (37%) developed further systemic metastases during their course of disease. In all, 38/222 (17%) of BM patients did not develop ECM during their disease course (median follow-up time 7 months; range 0–104) and were classified as BO-MBC cases. Table 1 lists further details on the metastatic pattern.
Brain only metastatic pattern
Brain-only metastatic behaviour was neither associated with breast cancer subtype (P=0.198; χ2 test) nor with number of BM (P=0.110; χ2 test). Further, prior trastuzumab-based therapy did not correlate with BO metastatic behaviour (P=0.090; χ2 test). Distribution of diagnosis-specific graded prognostic assessment (GPA) class did not differ between the BO-MBC cohort and patients with extracranial disease (P=0.784; χ2 test). Patients with BO-MBC were more likely to have neurosurgical resection as first-line therapy for BM (P=0.002; χ2 test) and less likely to receive chemotherapy after diagnosis of BM compared with patients with ECM (P=0.016; χ2 test).
Overall survival
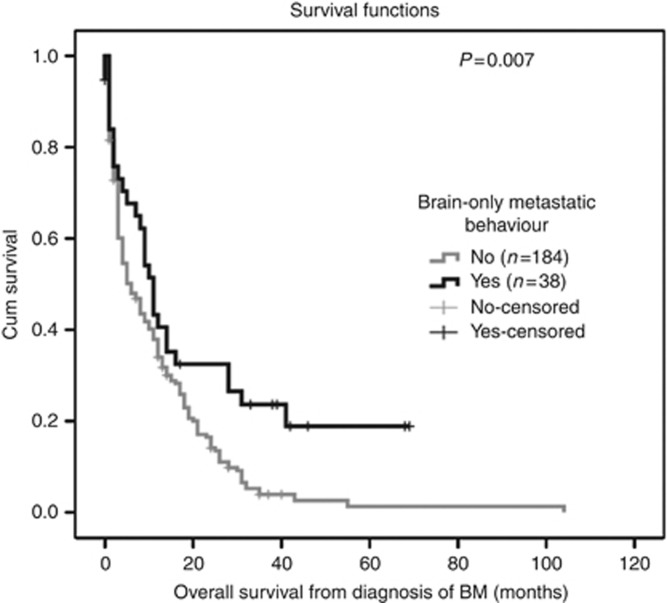
The median OS from diagnosis of BM in the entire cohort was 11.8 months (range 0–104). Overall survival in luminal subtype was 9 months, 7 months in Her2 subtype and 6 months in triple-negative subtype (P=0.47; log-rank test). At a median follow-up of 7 months after first diagnosis of BM (range 0–104) 202/222 (91%) patients had died. The median OS of BO-MBC patients was 11 months (95% CI 8.5–13.5) and was therefore significantly longer than in patients with BM and ECM (6 months; 95% CI 3.8–8.2; P=0.007, log-rank test) (Figure 1). In multivariable analysis with diagnosis-specific GPA and number of BM, BO metastatic behaviour remained a significant prognostic factor of OS (hazard ratio 0.6; P=0.029; Cox regression model). In the BO-MBC cohort, Karnofsky performance status >70 (P=0.02; log-rank test), single BM (P<0.001; log-rank test) and ER expression (P=0.014; log-rank test) were associated with favourable OS in univariate analysis and included into multivariate analysis. In multivariate analysis Karnofsky performance status >70 (hazard ratio 0.07; P=0.01; Cox regression model) and single BM (hazard ratio 0.13; P=0.003; Cox regression model) remained significant (Table 2). In all, 7/38 (18%) BO-MBC patients had long-term survival of >3 years after diagnosis of BM. Compared with patients with the presence of extracranial disease, long-term survival was significantly more common in BO-MBC patients (P<0.001; χ2 test).
Figure 1.
Overall survival from diagnosis of BM in patients with BO metastatic behaviour (11 months; 95% confidence interval (CI) 8.47–13.59) compared with patients with present ECM (6 months; 95% CI 3.81–8.19).
Table 2. Multivariate survival analysis in BO-MBC cohort.
| Hazard ratio | CI 95% | P -value | |
|---|---|---|---|
| Karnofsky performance score at first diagnosis of BM | 0.07 | 0.01–0.50 | 0.01 |
| Number of BM | 0.13 | 0.04–0.50 | 0.003 |
| ER expression | 0.49 | 0.22–1.1 | 0.78 |
Abbreviations: BM=brain metastases; BO-MBC=brain-only metastatic breast cancer; CI=confidence interval; ER=oestrogen receptor.
Discussion
Our data show that BO-MBC is a distinct clinical breast cancer entity with favourable median OS time of 11 months compared with 6 months in BM patients with additional ECM. Interestingly, we observed survival of >36 months in 7/38 (18%) patients with BO-MBC, indicating that long-time survival is possible and not uncommon in this patient population. Our data stress that intensive therapy with exploitation of all multimodal treatment approaches is warranted in breast cancer patients presenting with metastatic disease confined to the central nervous system. This conclusion is well in line with the situation in non-small cell lung cancer, where stage IV patients with exclusive oligometastatic cerebral disease and limited primary tumour also constitute a good prognosis subgroup that can be treated with curative intent (Pfannschmidt and Dienemann, 2010). High Karnofsky index, the presence of only one BM and positive ER expression were favourable prognostic factors in our cohort of BO-MBC patients and may help to adapt clinical management strategies.
The pathobiological explanation for BO metastatic involvement in breast cancer remains unclear. Previous studies have shown that the Her2-positive and the triple-negative breast cancer subtypes are characterised by relatively high incidences of BM (Kennecke et al, 2010). However, in our study we found no correlation of breast cancer subtype with BO metastatic behaviour. The distribution of breast cancer subtypes within our BO-MBC cohort was equalised, as approximately one third of the patients belonged to the Her2-positive, one third to the triple-negative and one third the luminal subtype, respectively. It is interesting to note that prior trastuzumab-based therapy did not correlate with BO metastatic behaviour, although trastuzumab is thought to favour the development of BM owing to its inability to cross the blood-brain barrier (Bendell et al, 2003; Musolino et al, 2011). Patients with triple-negative and luminal breast cancer subtypes developed BM significantly earlier during their disease course than patients with Her2-positive disease in our study (Berghoff et al, 2012). Further studies are needed to clarify the molecular mechanisms of the selective brain tropism of metastatic spread in some breast cancer patients. As postulated by the ‘seed and soil’ hypothesis, the development of this distinct metastatic behaviour may be explained by the interaction between specific tumour cells (‘the seed’) and the microenvironment of the brain (‘the soil’) (Fidler, 2011).
So far, only a few studies focusing on BM as first site of recurrence were conducted (Boogerd et al, 1993, 1997; Niwinska et al, 2011; Dawood et al, 2012). Dawood et al (2012) documented a high incidence of BM as first site of recurrence in a population of triple-negative breast cancer patients with stage I to III, but in contrast to our study, no further analysis of the clinical course after diagnosis of BM was performed. Boogerd et al (1993) showed that breast cancer patients with single BM in the absence of ECM have improved OS after intensive local treatment compared with BM patients with ECM at first diagnosis of BM. However, in this study no differentiation of breast cancer subtypes and characterisation of prognostic factors was performed. To our knowledge, our study is the first to investigate the incidence and clinical course of contemporary breast cancer patients with BM as first site of recurrence with a focus on patients with BO-MBC.
However, our study has some limitations that have to be considered in the interpretation of the data. First, only retrospectively collected data were available for our analysis and we included patients diagnosed and treated with MBC over a long period (1990–2011). Changes in clinical management such as the introduction of new therapy standards (e.g., trastuzumab, lapatinib for Her2-positive MBC) or diagnostic procedures (e.g., cranial MRT) during this period may have influence our results. However, the date of diagnosis of both groups, BO-MBC and BM with ECM, was distributed evenly over the entire study period making a bias arising from differences in clinical management improbable. In any case, analysis of data from prospective clinical trials might be useful to validate our findings.
In our series, 60/222 (27%) patients had BM as first site of recurrence and more than one third of these patients, i.e., 22/60 (37%) developed ECM after diagnosis of BM. Overall, 38/222 (17%) patients experienced BO metastatic disease in the absence of ECM during their course of disease. Our data show that patients with BO metastatic behaviour represent a distinct clinical entity with a better survival prognosis from diagnosis of BM compared with BM patients with additional ECM. We could not identify any factors predicting for BO metastatic behaviour, but identified high Karnofsky index, the presence of only one BM and positive ER status as favourable prognostic factors in BO-MBC patients. As long-term survival is not uncommon and was achieved in a fifth of BO-MBC patients, exploitation of all multimodal treatment options is warranted in patients with BM as first site of recurrence. Future studies are needed to clarify the role of systemic therapies with novel targeted agents in relation to established local therapy approaches like neurosurgery, radiosurgery and radiotherapy.
Acknowledgments
We thank Irene Leisser for technical assistance with preparation of tissue specimens. This study was performed within the PhD thesis project of Anna Sophie Berghoff in the PhD program ‘Clinical Neuroscience (CLINS)’ at the Medical University Vienna.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Bartsch R, Berghoff A, Pluschnig U, Bago-Horvath Z, Dubsky P, Rottenfusser A, DeVries C, Rudas M, Fitzal F, Dieckmann K, Mader RM, Gnant M, Zielinski CC, Steger GG (2012) Impact of anti-HER2 therapy on overall survival in HER2-overexpressing breast cancer patients with brain metastases. Br J Cancer 106(1): 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch R, Rottenfusser A, Wenzel C, Dieckmann K, Pluschnig U, Altorjai G, Rudas M, Mader RM, Poetter R, Zielinski CC, Steger GG (2007) Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol 85(3): 311–317 [DOI] [PubMed] [Google Scholar]
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97(12): 2972–2977 [DOI] [PubMed] [Google Scholar]
- Berghoff A, Bago-Horvath Z, De Vries C, Dubsky P, Pluschnig U, Rudas M, Rottenfusser A, Knauer M, Eiter H, Fitzal F, Dieckmann K, Mader RM, Gnant M, Zielinski CC, Steger GG, Preusser M, Bartsch R (2012) Brain metastases free survival differs between breast cancer subtypes. Br J Cancer 106(3): 440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogerd W, Hart AA, Tjahja IS (1997) Treatment and outcome of brain metastasis as first site of distant metastasis from breast cancer. J Neurooncol 35(2): 161–167 [DOI] [PubMed] [Google Scholar]
- Boogerd W, Vos VW, Hart AA, Baris G (1993) Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol 15(2): 165–174 [DOI] [PubMed] [Google Scholar]
- Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM (2012) Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer 118(19): 4652–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita F, Giuliani F, Silvestris N, Rossetti S, Pizzolorusso A, Santabarbara G, Galizia G, Colucci G, Ciardiello F, Orditura M (2012) Current status of targeted therapies in advanced gastric cancer. Expert Opin Ther Targets 16(Suppl 2): S29–S34 [DOI] [PubMed] [Google Scholar]
- Fidler IJ (2011) The role of the organ microenvironment in brain metastasis. Semin Cancer Biol 21(2): 107–112 [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16): 2784–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20): 3271–3277 [DOI] [PubMed] [Google Scholar]
- Kiely BE, Soon YY, Tattersall MH, Stockler MR (2011) How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol 29(4): 456–463 [DOI] [PubMed] [Google Scholar]
- Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22(17): 3608–3617 [DOI] [PubMed] [Google Scholar]
- Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW, Li X, Gelman R, Burstein HJ, Kasparian E, Kirsch DG, Crawford A, Hochberg F, Winer EP (2008) Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26(12): 1993–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, Michiara M, Silini EM, Ardizzoni A (2011) Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer 117(9): 1837–1846 [DOI] [PubMed] [Google Scholar]
- Niwinska A, Pogoda K, Murawska M, Niwinski P (2011) Factors influencing survival in patients with breast cancer and single or solitary brain metastasis. Eur J Surg Oncol 37(7): 635–642 [DOI] [PubMed] [Google Scholar]
- Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E, Pagani O, Simoncini E, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A (2006) Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17(6): 935–944 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt J, Dienemann H (2010) Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 69(3): 251–258 [DOI] [PubMed] [Google Scholar]
- Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4): 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167(4): 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1): 118–145 [DOI] [PubMed] [Google Scholar]