Abstract
We confirm the hypothesis that Agrobacterium tumefaciens-induced galls produce ethylene that controls vessel differentiation in the host stem of tomato (Lycopersicon esculentum Mill.). Using an ethylene-insensitive mutant, Never ripe (Nr), and its isogenic wild-type parent we show that infection by A. tumefaciens results in high rates of ethylene evolution from the developing crown galls. Ethylene evolution from isolated internodes carrying galls was up to 50-fold greater than from isolated internodes of control plants when measured 21 and 28 d after infection. Tumor-induced ethylene substantially decreased vessel diameter in the host tissues beside the tumor in wild-type stems but had a very limited effect in the Nr stems. Ethylene promoted the typical unorganized callus shape of the gall, which maximized the tumor surface in wild-type stems, whereas the galls on the Nr stems had a smooth surface. The combination of decreased vessel diameter in the host and increased tumor surface ensured water-supply priority to the growing gall over the host shoot. These results indicate that in addition to the well-defined roles of auxin and cytokinin, there is a critical role for ethylene in determining crown-gall morphogenesis.
Infection of sensitive plants by Agrobacterium tumefaciens is known to induce crown galls. Tumor growth is initiated by the integration and expression of the T-DNA of the bacterial Ti plasmid within the plant nDNA. The T-DNA encodes enzymes catalyzing the synthesis of high levels of auxin, cytokinin, and opines (Weiler and Spanier, 1981; Zambryski et al., 1989; Schell et al., 1994).
Aloni et al. (1995) found that an A. tumefaciens-induced crown gall caused the development of pathologic xylem in the centripetal direction within the host stem of castor bean. This pathologic xylem was characterized by narrow vessels, giant rays, and an absence of fibers. A similar anatomy was induced experimentally in stems of elm seedlings by the ethylene-releasing agent ethrel (Yamamoto et al., 1987), indicating the possible involvement of the hormone ethylene in crown-gall development. Therefore, Aloni et al. (1995) suggested that the high auxin levels induced by the T-DNA-encoded genes iaaM and iaaH (Thomashow et al., 1984, 1986) stimulate ethylene synthesis at the base of the crown gall, where high auxin streams originating in the tumor merge. Therefore, tumor-induced ethylene might affect crown-gall morphogenesis and differentiation of host tissues adjacent to the tumor.
A. tumefaciens-induced crown galls cause poor xylem development in grapevine, which impairs water flow to the young parts of the shoot above the gall (Agrios, 1988). Aloni et al. (1995) proposed the “gall-constriction hypothesis” to explain the mechanism that gives water-supply priority to the growing gall over the host shoot. The hypothesis proposes that the growing gall retards the development of its host shoot by decreasing vessel diameter in the host, substantially reducing the supply of water to the upper parts of the shoot. It was also suggested that the controlling signal that induces narrow vessels in the host is the hormone ethylene (Aloni et al., 1995), which is known to reduce vessel width (Yamamoto et al., 1987; Aloni, 1991). If this suggestion is true, one might expect that in a plant that is not sensitive to ethylene the tumor will not reduce vessel diameter, and consequently will not interrupt shoot supply and development above the gall. The working hypothesis of the present study was that we could expect normal-sized vessels in host plants that are not sensitive to ethylene. This would give water-supply priority to the host shoot over the crown gall. A limited supply of water and nutrients to the growing crown gall might therefore reduce or even inhibit tumor development.
To uncover the possible role of ethylene in crown-gall morphogenesis we induced tumors with the wild-type strain C58 of A. tumefaciens in wild-type tomato (Lycopersicon esculentum Mill.) plants and the Never ripe (Nr) mutant of tomato (Lanahan et al., 1994), which is nearly insensitive to ethylene, and studied the morphogenesis and differentiation of their crown galls and host shoots.
A preliminary account of some of the findings of the present study has been published previously (Aloni et al., 1997b).
MATERIALS AND METHODS
Plants
Wild-type and Never ripe (Nr) seeds of tomato (Lycopersicon esculentum Mill.) plants were obtained from the Monsanto Company (St. Louis, MO). Nr was originally identified as a spontaneous mutation in a single fruit in a field of cv Pearson plants (Rick and Butler, 1956). We have shown that the ethylene-insensitive phenotype is regulated by a single locus (Lanahan et al., 1994). Based on triple-response assays in seedlings, we concluded that Nr is incompletely dominant and in the homozygous state confers near but not complete ethylene insensitivity (Lanahan et al., 1994; Yen et al., 1995). It is important to note that the mutant is not completely insensitive to ethylene, and this residual responsiveness likely accounts for the ethylene-associated responses observed in this study. The tomato plants were grown in standard potting soil (Shacham, Giveat Ada, Israel) in a 24°C ± 1°C growth room at a light intensity of 150 μE m−2 s−1 from cool-white fluorescent lamps (Sylvania) with a 16-/8-h light/dark regime.
Bacteria
The wild-type strain C58 of Agrobacterium tumefaciens, obtained from the Max Planck Institut (Köln, Germany), was grown and treated as described by Aloni et al. (1995).
Crown-Gall Morphogenesis
To induce a tumor, a V-shaped wound was made with a razor blade in the middle of a young internode (ranging from 10 to 60 mm long) of 3-week- to 2-month-old tomato plants. The wound reached about one-half of the internode width, and was inoculated with the bacterial pellet. In each experiment we used internodes of similar length. The tumor experiment was repeated 5 times, with 5 to 20 repetitions per line. For studying vascular differentiation in the host and tumor, shoots of wild type and the Nr mutant were harvested after various periods ranging from 2 weeks to 3 months.
Ethylene Measurements
Ethylene evolution was studied from A. tumefaciens-induced crown galls grown on both wild-type and Nr plants. For ethylene measurements whole young plants or excised internodes from older plants were kept during the experiment in either 300- or 30-mL tubes, respectively. In preliminary measurements, an increase in ethylene production occurred during the first 2 h after wounding in 3-week-old tomato plants. Therefore, to reduce the effect of wounding caused by the cuts made in the stem for preparing the excised internodes, the ethylene measurements 21 and 28 d after infection by A. tumefaciens started 2 h after the internodes were cut from the plants (i.e. the tubes containing the internodes were kept open for the first 2 h before we started to measure ethylene evolution in closed tubes).
During the experiment the tubes were kept completely closed with rubber stoppers. For the experiments we used whole, small, 3- and 4-week-old plants 7 and 14 d after infection by A. tumefaciens, and excised internodes from 5- and 6-week-old plants 21 and 28 d after infection, respectively. Five-milliliter air samples from the closed tubes were analyzed for ethylene after 5 and 7 h. Ethylene was measured with a gas chromatograph (model 3350, Varian, Sugarland, TX) according to the method of Chalutz et al. (1984). The ethylene experiment (Table I) was repeated three times, with five or six repetitions per line.
Table I.
Evolution of ethylene from whole wild-type tomato plants and the Nr mutant 7 and 14 d after infection (DAI) by A. tumefaciens and from excised internodes 21 and 28 d after infection
| Examined Tissue | Whole
Plants
|
Excised Internodes
|
||
|---|---|---|---|---|
| 7 DAI | 14 DAI | 21 DAI | 28 DAI | |
| nL g−1 fresh wt h−1 | ||||
| Wild type | ||||
| Control | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.02 |
| Internode | – | – | 0.25 ± 0.02 | 0.38 ± 0.06 |
| A.t. galla | 0.58 ± 0.04* | 0.53 ± 0.04* | 10.87 ± 1.22* | 9.01 ± 2.38* |
| Nr | ||||
| Control | 0.19 ± 0.01 | 0.21 ± 0.02 | 0.29 ± 0.02 | 0.37 ± 0.05 |
| Internode | – | – | 0.28 ± 0.01 | 0.4 ± 0.07 |
| A.t. gall | 0.84 ± 0.04* | 0.95 ± 0.08* | 12.84 ± 1.88* | 11.14 ± 1.92* |
Values are means ± se (n = 5) of ethylene production. All differences between ethylene evolution from the controls and from plants and internodes carrying galls (*) were significant (P < 0.05). The controls were uninfected whole plants or internodes from uninfected plants, whereas the internodes measured 21 and 28 d after infection were internodes harvested from A. tumefaciens-infected plants and defined as the second internode above the crown gall.
A.t., A. tumefaciens.
Ethrel Application
To clarify the role of tumor-induced ethylene on vessel diameter in tomato stems, ethrel (2-chloroethyl-phosphonic acid; Sigma), which releases ethylene (Yamamoto et al., 1987), was applied in the form of lanolin paste in two concentrations, 1 and 5% (w/w), as a ring around the middle point of the fifth internode below the apical bud. To mark the border between the xylem formed before the experiment and that affected by ethrel, a very gentle longitudinal scratch was made at the site of ethrel application at the beginning of the experiment. The ethrel in lanolin paste was prepared by dissolving the ethrel in ethanol, which was then mixed with warm lanolin. To evaporate the ethanol, the mixture was kept warm and stirred with a magnetic stirrer for 15 min. The lanolin paste containing the ethrel and the control paste (lanolin with no ethrel) were reapplied weekly. Tissue was harvested after 5 weeks and the experiments were repeated 3 times, with 5 to 10 repetitions per line.
Tissue Preparation and Microscopy
Hand-cut sections (1–3 mm thick) of tumor and host stem tissues were cleared by boiling in 90% lactic acid for 2 to 10 min. The sections were allowed to cool for at least 1 h, stained at room temperature with a 0.4% solution of lacmoid (PolyScience, Niles, IL) in 90% lactic acid for about 60 to 75 min, and then rinsed in tap water until the red color of the tissue became blue (about 1 h). After the washing step, air bubbles within the tissue were removed by vacuum infiltration with tap water for 30 to 45 min. The sections were then transferred to 50% sodium lactate for microscopic analysis under transmitted white light (Aloni and Sachs, 1973; Aloni and Barnett, 1996).
Micrographs were reproduced from color slides taken with a light microscope (model BH2, Olympus) and an OM-2 camera (Olympus) with Fujichrome 64T (64 ASA, Fuji Photo Film Co. Ltd., Tokyo, Japan) film.
Statistics
Statistical terminology and the test of significance for vessel diameter were with the Student's t test and according to the method of Sokal and Rohlf (1969). The Scheffe test and Tukey's honesty test for multiple comparisons were used for analyzing ethylene evolution. Data were run through SPSS statistical software (Microsoft) for these analyses.
RESULTS
Ethylene Evolution from A. tumefaciens-Induced Galls
Ethylene production by whole 1-month-old plants, measured 7 d after infection by A. tumefaciens, was up to 4-fold greater than that in uninfected control plants (Table I). The same was true with whole plants studied 14 d after infection (Table I). The Nr plants with young growing galls produced more ethylene than wild-type plants with growing galls.
The evolution of ethylene from excised internodes carrying galls was up to 50-fold greater than from excised internodes of control plants when measured 21 and 28 d after infection by A. tumefaciens (Table I). An internode carrying a gall produced up to 50-fold more ethylene than an ungalled internode from the same infected plant. However, higher levels of ethylene were also noted in some of the ungalled internodes of the infected plants. Generally, we noted a positive correlation in the levels of ethylene production between a gall-carrying internode and an ungalled internode of the same plant.
The Epinastic Response of Petioles to Crown-Gall-Associated Ethylene Is Absent in the Nr Mutant
Many of the shortest internodes (usually about 10 mm long) carrying crown galls in the young wild-type tomato plants showed some epinastic response (Fig. 1a) in the leaves located immediately above and below the growing crown gall, indicating ethylene production in the tumor tissues. Conversely, in all of the young Nr mutant plants the orientation of the leaves was always normal (Fig. 1b), the same as in uninfected control plants. When long internodes (longer than 40 mm) of wild-type tomato plants were infected by A. tumefaciens, their leaves did not show an epinastic response. In experiments in which the door of the growth room was left open, allowing fast air circulation, no epinastic response could be observed on any of the A. tumefaciens-infected plants.
Figure 1.
Comparison of A. tumefaciens-induced crown galls on wild-type tomato (a and c) and Nr mutant (b and d) stems. a, Front view of a 3-week-old tumor developed on a wild-type plant showing the typical unorganized callus shape of a young crown gall and the epinastic response of the leaves both above and below the tumor. b, Front view of a 3-week-old tumor developed on the Nr mutant, characterized by a smooth surface and leaves in the normal orientation. Note that the lower half of the gall is protected by epidermis. c, Side view of a 2-month-old tumor on a wild-type stem with numerous adventitious roots (white spots) developed both above and below the crown gall (indicated with arrowheads). d, Side view of a 2-month-old tumor on the Nr mutant showing a fibrous hard gall and a stem almost free of adventitious roots. All photographs are at the same magnification (bars = 10 mm).
It was also noted that wild-type tomato plants were infected easily by A. tumefaciens, whereas it was necessary to make a larger wound in the Nr shoots to obtain a tumor of comparable size.
Limited Development of Adventitious Roots on the Tumor-Bearing Internodes of the Nr Mutant
Adventitious roots occur normally along the stems of tomato plants. Adventitious roots do not appear on the youngest internodes, and they are evident on mature internodes (usually from the fifth internode below the apical bud). Development of a crown gall substantially stimulated the development and size of numerous adventitious roots on the infected internode of the wild-type plants (Fig. 1c). Conversely, only a few small adventitious roots developed on the infected internodes of the Nr mutant plants (Fig. 1d).
Smooth Surface Characterizes the Young A. tumefaciens-Induced Crown Gall of the Nr Mutant
The crown galls developed on the wild-type stems had a substantially enlarged surface area (Fig. 1a) attributable to the unorganized callus shape of the tumor. During the early stages of tumor growth the epidermis was torn and the inner, fast-growing gall tissues were exposed, forming the typically unorganized callus appearance of a crown gall (Fig. 1a). The vascular elements in the tumor tissue extended up to the gall surface, with no epidermis to protect against water transpiration. Residual epidermis, characterized by nonfunctioning stomata with no starch granules, occurred at the borders of the host stem. These stomata remained continuously wide open because the epidermis was stretched by the fast-expanding tumor tissues beneath it. Conversely, 3-week-old crown galls that developed on the Nr mutant (Fig. 1b) were characterized by an epidermis with active stomata, containing starch granules in a density typical of intact epidermis. Only in the area where the original wound was inflicted did a relatively smooth callus structure appear. Therefore, tumors that developed on the Nr mutant had a minimum tumor surface area and most of it was protected by epidermis. In the Nr mutant the vascular tissues did not extend to the tumor surface and were protected from the atmosphere by a few cortex layers, which remained under the intact epidermis in young tumors.
The gall tissues that developed on the Nr mutant had the same green color as the host stem tissues (Fig. 1b), whereas crown-gall tissues that developed on the wild-type plants had a yellowish appearance (Fig. 1a).
Almost Normal Xylem Differentiation Occurs in the Host Stem Adjacent to the Tumor in the Nr Mutant
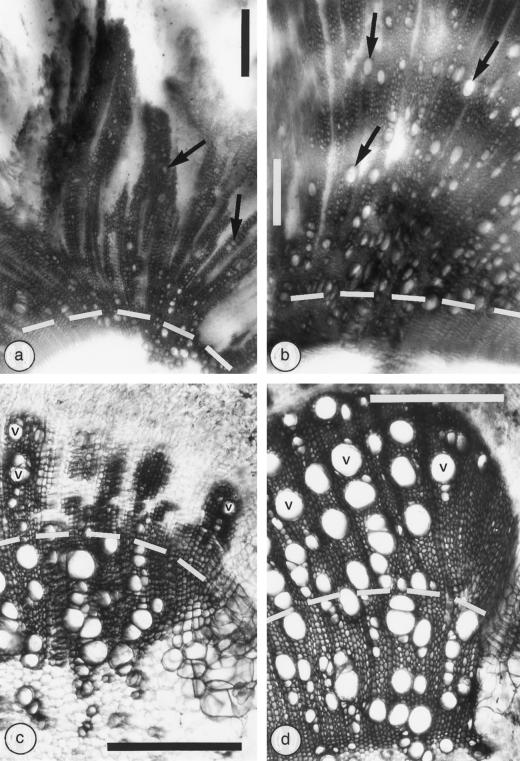
In wild-type tomato stems the vessels near the tumor were very narrow (Fig. 2a), whereas the same vessels on Nr stems were almost of normal size (relatively large) (Fig. 2b). The average diameter (measured in the radial direction) of the widest vessels in the pathologic xylem of the stem adjacent to the tumor was more than 2-fold smaller (highly statistically significant at P < 0.01 by Student's t test) in the wild-type stems than in the Nr mutant: 53 ± 4 μm versus 126 ± 5 μm, respectively (n = 25; 5 vessels from 5 plants). Beside the tumor, there was a drastic decrease in xylem production in the wild-type stems, which was characterized by wide, unlignified rays (Fig. 2a). The very large, unlignified rays in the wild-type host tissues adjacent to the tumor (Fig. 2a) made the shoot somewhat soft and breakable. Conversely, the Nr shoots had almost normal (small) lignified rays (Fig. 2b), resulting in a relatively strong stem.
Figure 2.
The effects of 6-week-old A. tumefaciens-induced crown galls on xylem differentiation in tomato host stems (a and b), and the effects of a 5-week application of 1% (w/w) ethrel on xylem differentiation in tomato stems (c and d), are shown in thick transverse sections cleared with lactic acid and stained with lacmoid. The crown galls were located above the micrographs (a and b), and the white region at the lower part of the photographs is the cleared pith. The border between the xylem formed after infection with A. tumefaciens or after ethrel application (upper part of each micrograph) and the intact xylem developed before the treatments (lower part) is delineated by a broken line. At the lower right of the ethrel-affected stems (c and d) there is a wound reaction resulting from a marking scratch done at the beginning of the experiment. a, Limited differentiation of pathologic xylem with very narrow vessels and wide rays (the clear regions in the upper part of the xylem) characterize the wild-type host. b, Massive xylem with wide vessels and almost normal rays occur in the Nr mutant. c, Limited differentiation of xylem with narrow vessels and wide rays (clear radial regions) characterize the ethrel-affected xylem. d, Wide vessels and normal lignified rays are typical of the new xylem formed in the Nr mutant after ethrel application. Arrows, Vessels affected by A. tumefaciens; v, vessels affected by ethrel. Bars = 500 μm.
There was a negative correlation between the diameter of the host vessels beside the crown gall and tumor development. Tumor growth was faster on the wild-type plants than on the Nr mutant. On some of the Nr shoots, the development of the tumor on mature internodes was very slow and tumor growth almost stopped (Fig. 1d).
The Nr shoots carrying the A. tumefaciens-induced crown galls grew faster and had larger leaves and taller stems than the wild-type infected shoots (Fig. 3A). When moderate water stress was applied, the wild-type shoots above the tumors suffered from water deficiency earlier than the Nr shoots. Leaves started to turn yellow and senesce on the wild-type shoots above the tumor, whereas most of the leaves on the Nr shoots remained green and healthy (Fig. 3B).
Figure 3.
The effects of 4-week-old A. tumefaciens-induced crown galls located at the base of the stems on shoot development and leaf senescence in tomato plants. A, Retarded wild-type shoot (left) and a typically taller Nr shoot with large leaves (right). Note that the older leaves in the wild-type plant started to turn yellow and senesce. B, Moderate water stress caused leaf senescence in the wild-type shoot (left), whereas most of the leaves remained green and healthy on the Nr shoot (right).
Ethrel Application Reduced Vessel Diameter and Xylem Production in Wild-Type Stems
Application of 1% ethrel to the stem of wild-type tomato plants substantially reduced xylem formation. The xylem produced after ethrel application was characterized by very wide rays, leaving wide, unlignified radial strips in the xylem (Fig. 2c). In addition, this xylem was clearly identified by narrow vessels, which were typically narrower than the largest vessels of the xylem formed before the treatment (Fig. 2c). Application of 5% ethrel had a stronger effect, resulting in depressed xylem production characterized by a few very narrow vessels and extremely wide, unlignified rays (not shown).
Conversely, application of 1% ethrel to Nr stems did not reduce xylem production. The xylem produced after ethrel application had very large vessels, some of them larger than the vessels formed before the treatment (Fig. 2d). In addition, the rays were narrow and lignified (Fig. 2d), as in the intact control plants. Application of 5% ethrel to Nr stems decreased xylem production but the vessels and rays had a normal shape (not shown).
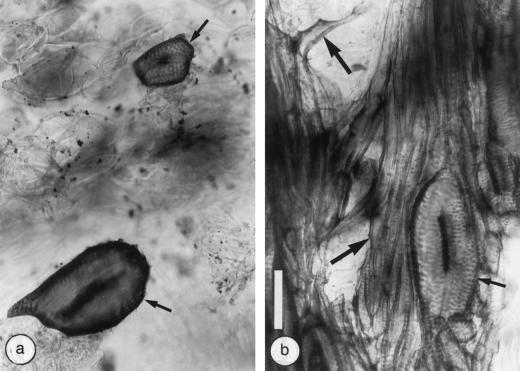
Fiber Differentiation Occurs Only in Tumor Tissues of the Nr Mutant
Crown galls that developed on wild-type shoots typically contained neither fibers nor sclereids and their vascular elements were surrounded by parenchyma cells only (Fig. 4a); therefore, these tumors (Fig. 1c) were relatively soft. Conversely, fibrous, hard crown galls developed on the Nr mutant (Fig. 1d) as a result of fiber and sclereid differentiation within the tumor tissues (Fig. 4b). Fibers were first detected in the xylem of the 3-week-old tumors and their number increased with time. Circular vessels occurred randomly in the tumors that developed on both the wild-type and Nr shoots (Fig. 4, a and b).
Figure 4.
Typical patterns of vascular tissues in 6-week-old A. tumefaciens-induced crown galls on tomato stems observed in thick, longitudinal radial sections cleared with lactic acid and stained with lacmoid. a, Circular vessels surrounded by parenchyma cells in wild-type stems. b, Circular vessels surrounded by fibers in the Nr mutant. Small arrows, Circular vessels; large arrows, fibers. Both photographs are at the same magnification (bar = 100 μm).
DISCUSSION
The findings of the present study confirm that A. tumefaciens-induced crown galls produce the hormone ethylene, as proposed by Aloni et al. (1995). Similar results have been found in A. tumefaciens-induced tumors of castor bean (by using CO2-laser-activated photoacoustic spectrometry), in which up to a 70-fold higher level of ethylene production was measured in 5-week-old crown galls than in control stem tissues (C.I. Ullrich, personal communication). Our findings show that tumor-induced ethylene is a limiting and a major controlling factor of crown-gall morphogenesis. Therefore, along with auxin and cytokinin (Weiler and Spanier, 1981; Zambryski et al., 1989; Malsy et al., 1992; Schell et al., 1994), the hormone ethylene also affects tumor morphogenesis.
The integration and expression of the T-DNA of the bacterial Ti plasmid within the plant nDNA (Zambryski et al., 1989) substantially elevate auxin concentrations in crown gall tissues, which may be 500 times higher in the tumor than in control tissues (Weiler and Spanier, 1981). Because high auxin levels are known to enhance ethylene synthesis (Yang and Hoffman, 1984; Abeles et al., 1992), it was suggested that tumor-induced auxin promotes the synthesis of ethylene in crown galls (Aloni et al., 1995). Our results confirm the results of Romano et al. (1993), which showed that many “auxin” effects in iaaM were actually ethylene effects. However, we should note that auxin also has a direct effect on vascular differentiation within the tumor, in that auxin is a limiting and controlling factor in the differentiation of both vessels and sieve tubes (Roberts et al., 1988; Aloni, 1995). It is also possible that elevated cytokinin levels, which may be 1500 times higher in the tumor than in control tissues (Weiler and Spanier, 1981; Akiyoshi et al., 1983), might also promote ethylene synthesis, because cytokinin is known to stimulate ethylene production (Wright, 1980; Mattoo and White, 1991; Cary et al., 1995). In addition, it is possible that there is a synergistic effect within crown-gall tissues whereby a combination of the elevated levels of both auxin and cytokinin boosts ethylene production in the tumor. It is also possible that information in the bacterial Ti plasmid directly promotes ethylene synthesis in the tumor, although no T-DNA gene exhibits homology to any known bacterial or plant ethylene-biosynthetic gene.
It should be noted that the anatomy of the vascular tissues in crown galls and host stems indicates that the gall tissues are the source of ethylene production (Aloni et al., 1995). The strongest effect of the tumor caused pathologic xylem only adjacent to the tumor center, a limited effect at its borders, and normal xylem in the stem away from the tumor, indicating regular levels of ethylene in the host stem (Aloni et al., 1995) (Fig. 4).
The epinastic response observed on some of the tumor-bearing wild-type shoots but not on shoots of the Nr mutant provides developmental evidence for the production of ethylene in A. tumefaciens-induced crown galls. The tumor-induced ethylene affected the orientation of the closest leaves above and below young internodes. It is possible that some of the observed epinasty in the nearby leaves might have been cause by transport of ACC (Abeles et al., 1992; Jackson, 1994) originating in the tumor. However, no epinastic response occurred when long internodes were infected, probably because of the greater distances between the tumor and the leaves, which were somewhat older than leaves on the shortest internodes. This was also true when the door to the growth room was left open to increase air flow around the plants.
Adventitious root formation is associated with increased ethylene synthesis (Liu et al., 1990; McNamara and Mitchell, 1990, 1991). Ethylene may stimulate the initiation of adventitious root primordia on tomato stems (McNamara and Mitchell, 1991). Therefore, the appearance of numerous adventitious roots both above and below the tumor on the infected internodes of the wild-type plants but not on the Nr mutant is further morphogenetic evidence for the production of ethylene in crown galls.
Application of ethrel (which releases ethylene) mimicked the effect of the tumor-induced ethylene on xylem differentiation in the host stem, decreasing vessel diameter and increasing ray size in the xylem of wild-type tomato plants, whereas no effect was observed in the stems of the Nr mutant. These results demonstrate that the tumor-induced signal for decreasing vessel diameter and increasing ray size in the host stem adjacent to the crown gall (Aloni et al., 1995) is the hormone ethylene.
This study shows that ethylene has a major role in controlling water transport to crown galls. There are two mechanisms that influenced water transport to the tumors. The first is that ethylene substantially reduced the diameter of vessels adjacent to the tumor in wild-type tomato stems, whereas almost normal-sized vessels differentiated in the Nr mutant. The rate at which water flows through a vessel is proportional to the fourth power of its radius (Poiseuille's law) (Zimmermann, 1983). Accordingly, we calculated water flow through the narrow vessels measured in the pathologic xylem beside the tumor and found a 30-fold decrease in the wild-type tomato stems compared with the Nr stems. This result confirms the gall-constriction hypothesis, which explains how a decrease in the host vessels located beside the tumor gives water-flow priority to the tumor over the host shoot (Aloni et al., 1995).
The second mechanism is that ethylene substantially increased the surface of the tumor only in the wild-type plants. The epidermis was torn by the expanding inner tumor tissues, forming the typically unorganized callus appearance of a crown gall. Furthermore, the vascular elements in the tumor tissue extended up to the gall surface, which did not have an epidermis to protect against water transpiration. Residual epidermis, characterized by continuously widely open stomata, occurred only at the borders of the host stem. Schurr et al. (1996) showed that similar changes in the enlarged tumor surface substantially increased transpiration to about 15 and 7.5 times higher at the tumor surface compared with host leaves and leaves of noninfected castor bean plants, respectively. Therefore, the combination of a decrease in vessel diameter in the host and an increased surface of the tumor, both induced by ethylene, ensures water-supply priority to fast-growing crown galls. Consequently, crown galls decrease shoot development and promote leaf senescence in wild-type host plants. On the other hand, tumors on the Nr mutant were less successful in competing with the growing leaves above the tumor, and some of the crown galls on the Nr shoots even degenerated.
Ethylene is known to depress polar auxin transport (Mattoo and Aharoni, 1988; Goren and Riov, 1989), and it seems likely that the circular vessels shown in this study indicate the sites of local interruptions of polar auxin movement. Circular vessels, which are induced by circular movement of auxin, were induced experimentally above sites where the polar auxin flow was interrupted (Sachs and Cohen, 1982; Aloni et al., 1997a).
Fibers are usually induced by both auxin and GA (Aloni, 1979, 1987). However, high levels of auxin alone might also stimulate fiber differentiation (Aloni, 1979). Fibers differentiated in the crown galls that developed on the Nr mutant, but were completely absent in the tumors that developed on the wild-type tomato shoots. There are no reports regarding fiber differentiation in crown galls developing on other plant species (Sachs, 1991), and the limited numbers of regenerative fibers in A. tumefaciens-induced crown galls that developed on castor bean shoots were observed only in the periphery, at the base of the tumor (Aloni et al., 1995). Because ethylene is known to inhibit fiber differentiation (Yamamoto et al., 1987), we suggest that the absence of fibers in crown galls developing on wild-type plants is caused by a relatively high background level of ethylene, which prevents fiber differentiation in the tumor. Only in the Nr tumor tissues, which are nearly insensitive to ethylene, could fibers differentiate. We also suggest that background ethylene might affect other reactions and functions in tumors and should therefore be considered when crown galls are studied.
Crown galls induced by A. tumefaciens produce ethylene, which is a limiting and controlling factor of tumor morphogenesis. The tumor-induced ethylene reduces vessel diameter in the host stem and enlarges the surface of the tumor, thus giving water-supply priority to the growing gall over the host shoot, resulting in fast tumor growth, retarded shoot development, and enhanced leaf senescence above the crown gall.
ACKNOWLEDGMENTS
We thank Prof. Cornelia I. Ullrich (Technische Hochschule, Darmstadt, Germany) for encouragement and for the gift of the wild-type strain C58 of A. tumefaciens (originally obtained from Dr. Z. Koncz, Max Planck Institut fur Zuchtungsforschung, Köln, Germany). We also thank the Monsanto Company for the gift of seeds of the wild-type tomato and the Nr mutant.
Footnotes
This study was supported by the research authority of Tel Aviv University, Israel.
This paper is dedicated to Prof. Judah Folkman (Harvard Medical School, Boston, MA) for his contributions on the role of angiogenesis in human and animal tumor morphogenesis.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology, Ed 2. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Agrios GN (1988) Bacterial galls. In GN Agrios, ed, Plant Pathology. Academic Press, New York, pp 483–488
- Akiyoshi DE, Morris RO, Hinz R, Mischke BS, Kosuge T, Garfinkel DJ, Gordon MP, Nester EW. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci USA. 1983;80:407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. Role of auxin and gibberellin in differentiation of primary phloem fibers. Plant Physiol. 1979;63:609–614. doi: 10.1104/pp.63.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. Differentiation of vascular tissues. Annu Rev Plant Physiol. 1987;38:179–204. [Google Scholar]
- Aloni R (1991) Wood formation in deciduous hardwood trees. In AS Raghavendra, ed, Physiology of Trees. Wiley, New York, pp 175–197
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 531–546. [Google Scholar]
- Aloni R, Alexander JD, Tyree MT. Natural and experimentally altered hydraulic architecture of branch junctions in Acer saccharum Marsh. and Quercus velutina Lam. trees. Trees. 1997a;11:255–264. [Google Scholar]
- Aloni R, Barnett JR. The development of phloem anastomoses between vascular bundles and their role in xylem regeneration after wounding in Cucurbita and Dahlia. Planta. 1996;198:595–603. doi: 10.1007/BF00262647. [DOI] [PubMed] [Google Scholar]
- Aloni R, Pradel KS, Ullrich CI. The three-dimensional structure of vascular tissues in Agrobacterium tumefaciens-induced crown galls and in the host stems of Ricinus communis. Planta. 1995;196:597–605. [Google Scholar]
- Aloni R, Sachs T. The three-dimensional structure of primary phloem systems. Planta. 1973;113:343–353. doi: 10.1007/BF00387317. [DOI] [PubMed] [Google Scholar]
- Aloni R, Wolf A, Feigenbaum P, Klee HJ. Evidence that tumor-induced ethylene controls the morphogenesis of Agrobacterium tumefaciens-induced crown galls on tomato stems. Isr J Plant Sci. 1997b;45:255. doi: 10.1104/pp.117.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E, Mattoo AK, Solomos T, Anderson JD. Enhancement by ethylene of cellulysin-induced ethylene production by tobacco leaf discs. Plant Physiol. 1984;74:99–103. doi: 10.1104/pp.74.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren R, Riov J. Interference by ethylene with the abscission regarding effects of auxin in citrus leaves. In: Osborne DJ, Jackson MB, editors. Cell Separation in Plants. Berlin: Springer-Verlag; 1989. pp. 191–200. [Google Scholar]
- Jackson MB. Root-to-shoot communication in flooded plants: involvement of abscisic acid, ethylene and 1-aminocyclopropane-1-carboxylic acid. Agron J. 1994;86:775–782. [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. The Never ripe mutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Muckherjee I, Reid DM. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. III. The role of ethylene. Physiol Plant. 1990;78:268–276. [Google Scholar]
- Malsy S, van Bel AJE, Kluge M, Hartung W, Ullrich CI. Induction of crown galls by Agrobacterium tumefaciens (strain 58) reverses assimilate translocation and accumulation in Kalanchoë daigremontiana. Plant Cell Environ. 1992;15:519–529. [Google Scholar]
- Mattoo AK, Aharoni N. Ethylene and plant senescence. In: Nooden LD, Leopold AD, editors. Senescence and Aging in Plants. San Diego, CA: Academic Press; 1988. pp. 241–280. [Google Scholar]
- Mattoo AK, White WB. Regulation of ethylene biosynthesis. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 21–42. [Google Scholar]
- McNamara ST, Mitchell CA. Adaptive stem and adventitious root response of two tomato genotypes to flooding. Hortscience. 1990;25:100–103. [Google Scholar]
- McNamara ST, Mitchell CA. Roles of auxin and ethylene in adventitious root formation by flood-resistant tomato genotype. Hortscience. 1991;26:57–58. [Google Scholar]
- Rick C, Butler L. Phytogenetics of the tomato. Adv Genet. 1956;8:267–382. [Google Scholar]
- Roberts LW, Gahan PB, Aloni R. Vascular Differentiation and Plant Growth Regulators. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Romano C, Cooper M, Klee H. Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell. 1993;5:181–189. doi: 10.1105/tpc.5.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. Callus and tumor development. In: Sachs T, editor. Pattern Formation in Plant Tissues. Cambridge, UK: Cambridge University Press; 1991. pp. 38–55. [Google Scholar]
- Sachs T, Cohen D. Circular vessels and the control of vascular differentiation in plants. Differentiation. 1982;21:22–26. [Google Scholar]
- Schell J, Koncz C, Spena A, Palme K, Walden R (1994) The role of phytohormones in plant growth and development. In Proceeding of the XV International Botany Congress, Tokyo/Yokohama, Japan, pp 38–48
- Schurr U, Schuberth B, Aloni R, Pradel KS, Schmundt D, Jahne B, Ullrich CI. Structural and functional evidence for xylem-mediated water transport and high transpiration in Agrobacterium tumefaciens-induced tumors of Ricinus communis. Bot Acta. 1996;109:405–411. [Google Scholar]
- Sokal RR, Rohlf FJ (1969) Biometry, the Principals and Practice of Statistics in Biological Research. Freeman & Co, San Francisco, pp 166–172
- Thomashow LS, Reeves S, Thomashow MF (1984) Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc Natl Acad Sci USA: 81: 5071–5075 [DOI] [PMC free article] [PubMed]
- Thomashow MF, Hugly S, Buchholz WG, Thomashow LS. Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science. 1986;231:616–618. doi: 10.1126/science.3511528. [DOI] [PubMed] [Google Scholar]
- Weiler EW, Spanier K. Phytohormones in the formation of crown gall tumors. Planta. 1981;153:326–337. doi: 10.1007/BF00384251. [DOI] [PubMed] [Google Scholar]
- Wright STC. The effect of plant growth regulator treatments on the level of ethylene emanating from excised turgid and wilted leaves. Planta. 1980;148:381–388. doi: 10.1007/BF00388127. [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Angeles G, Kozlowski TT. Effect of ethrel on stem anatomy of Ulmus americana seedlings. IAWA Bull. 1987;8:3–9. [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yen H-C, Lee S, Tanksley SD, Lanahan MB, Klee HJ, Giovannoni JJ. The tomato Never-ripe locus regulates ethylene-induced gene expression and is linked to a homolog of the Arabidopsis ETR1 gene. Plant Physiol. 1995;107:1343–1353. doi: 10.1104/pp.107.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P, Tempé J, Schell J (1989) Transfer and function of T-DNA genes from Agrobacterium Ti and Ri plasmids in plants. Cell 56: 193–201 [DOI] [PubMed]
- Zimmermann MH. Xylem Structure and the Ascent of Sap. Berlin: Springer-Verlag; 1983. [Google Scholar]