Abstract
Background and Objectives
Adiponectin is an adipose tissue-derived hormone that has beneficial effects on cardiac function and has been reported to be associated with lipid metabolism, glucose metabolism, and insulin resistance. Serum levels of adiponectin are reduced in obese individuals compared with non-obese individuals. Obesity is associated with an increased incidence of atrial fibrillation (AF); however, the role of adiponectin in AF is unclear. The aim of this study is to evaluate the relationship between the plasma adiponectin level and AF.
Subjects and Methods
Sixty-one consecutive patients were prospectively enrolled for this study. Subjects were divided into two groups: patients with AF (n=30) and controls (n=31). Laboratory evaluation, including levels of plasma adiponectin, was performed and echocardiographic parameters were measured.
Results
The baseline characteristics were not different between the two groups. The plasma adiponectin level of patients in the AF group was significantly lower than in the control group (14.9±7.2 vs. 19.±8.9 µg/mL, p<0.05). In addition, when we divided the AF patients into paroxysmal and chronic AF, the plasma adiponectin level was significantly lower in patients with paroxysmal AF, compared with the control group. In multiple binary logistic regression analysis to evaluate the independent predictors for AF, adiponectin and left atrial diameter were strong independent predictors of AF.
Conclusion
In this study a lower plasma adiponectin concentration was significantly associated with that of paroxysmal AF. Hypoadiponectinemia can potentially be an important risk factor for AF.
Keywords: Adiponectin, Atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common chronic arrhythmia, associated with an adverse prognosis. AF has been associated with hypertension, ischemic heart disease (IHD), heart failure (HF), and valvular heart disease, and with different metabolic disturbances. Furthermore, AF is also a major risk factor for stroke.1)
Multiple factors are involved in the etiology of AF, including stretching, autonomic imbalance, hyperthyroidism, and inflammation.2-4) Among these, obesity and metabolic syndrome are associated with AF: patients with metabolic syndrome have a larger left atrial (LA) size than those without metabolic syndrome.5) Obesity is also a strong risk factor for the occurrence of post-operative AF in patients older than 50 years.6)
Adiponectin is a protein, secreted by adipose cells that may couple the regulation of insulin sensitivity with energy metabolism, and serve to link obesity with insulin resistance through its anti-diabetic, anti-atherogenic, and anti-inflammatory properties. Adiponectin itself is controlled in conditions of metabolic stress by a number of hormones and factors involved in the regulation of metabolic function. Most factors with a significant impact on adiponectin regulation have inhibitory effects, including catecholamines, glucocorticoids, cytokines (interleukin-6, tumor necrosis factor-α), prolactin, growth hormones, and androgen.7)
Many clinical studies have shown that adiponectin is associated with various obesity-related complications. Plasma adiponectin levels are decreased in obese subjects, and in those with type 2 diabetes, coronary artery disease, and hypertension. However, the role of adiponectin in AF has not been extensively evaluated. In the present study, we examined the correlation of plasma adiponectin levels and AF in various type of AF.
Subjects and Methods
Study population
We prospectively enrolled 30 consecutive AF patients (mean age 57±12 years), who visited Kosin University Gospel Hospital. The presence of AF was determined by a serial electrocardiogram and ambulatory monitoring. Thirty-one patients with normal sinus rhythm (NSR) were enrolled as controls. Control patients were matched on the basis of age and sex.
Subjects were excluded before enrollment if they had a history of coronary artery disease, rheumatic heart disease, cardiomyopathy, congestive HF, or significant valvular disease. Type of AF was determined, based on the episodes of AF, as follows:8),9) 1) Paroxysmal AF was defined as a history of 1 or more episodes of AF that was medically or self-terminated within 7 days; 2) Persistent and chronic AF was defined as a history of 1 or more episodes of AF over 7 days, which required pharmacological cardioversion or electrical cardioversion, to establish a NSR or continuous AF of more than 1 year in duration.
The study protocols were reviewed and approved by the institutional committee. All patients agreed to participate in the study, and informed consent was obtained from all participating subjects.
Laboratory analysis
Venous blood sampling from all subjects was performed after overnight fasting. Height and body weight were measured, and a body mass index (BMI) was calculated by weight (kg)/height (m)2. Blood samples were obtained in ethylenediaminetetraacetic acid-containing tubes, which were centrifuged to extract the plasma. The extracted plasma was stored at -70℃. The plasma adiponectin concentration was assessed using a radioimmunoassay (Linco Research, Inc., St. Charles, MO, USA). All assays were performed in duplicate. This assay is both sensitive (detection limit <1.5 µg/L) and precise (within-assay coefficient of variation of standards and unknown samples averaged 5%). Finally, the assay yielded an average adiponectin level of 14 mg/L (range 6 to 27 mg/L).
We also measured a lipid concentration, fasting glucose, fasting insulin, and high sensitivity C-reactive protein (hs-CRP), and performed an echocardiography. Total cholesterol, triglyceride (TG), and high density lipoprotein-cholesterol were determined by enzymatic methods, employing standard biochemical procedures and using a BM Hitachi automated clinical chemistry analyzer (Hitachi, Tokyo, Japan). C-reactive protein (CRP) was measured using commercially available hs-CRP assays (Dade Behring Inc. Newark, DE, USA). Fasting plasma glucose was measured by the glucose oxidase method.
Echocardiography
Two-dimensional and Doppler echocardiography was performed by an experienced sonographer, using an Acuson-Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA) with patients in the left lateral decubitus position. Transthoracic echocardiography studies were carried out according to the recommendations of the American Society of Echocardiography.10) M-mode LA dimension, right ventricular dimension, and interventricular septal dimension were measured in the parasternal long-axis view at the end-diastole. Left ventricular (LV) end diastolic dimension, LV end-systolic dimension, and ejection fraction were measured in the parasternal long-axis view.
The images were recorded digitally, and were analyzed by offline software. LV end-diastolic diameter and LA dimensions were determined by standard M-mode measurements. LV ejection fraction was calculated, using the modified Simpson's rule.
Statistical analysis
Characteristics of the study population are given as the mean±standard deviation. Correlation analysis was performed for adiponectin plasma level and risk factors for cardiac disease.
Mann-Whitney U test was used to analyze the difference in adiponectin serum level between AF patients and sinus rhythm patients. Spearman's correlation method was applied for the correlation between adiponectin and other factors. Entered multiple binary logistic regression was performed to determine the most influential factor on AF. A p<0.05 was considered significant. Data were analyzed with Statistical Package for the Social Sciences (SPSS) software (version 16.0, SPSS, Inc., Chicago, IL, USA).
Results
Baseline patient characteristics
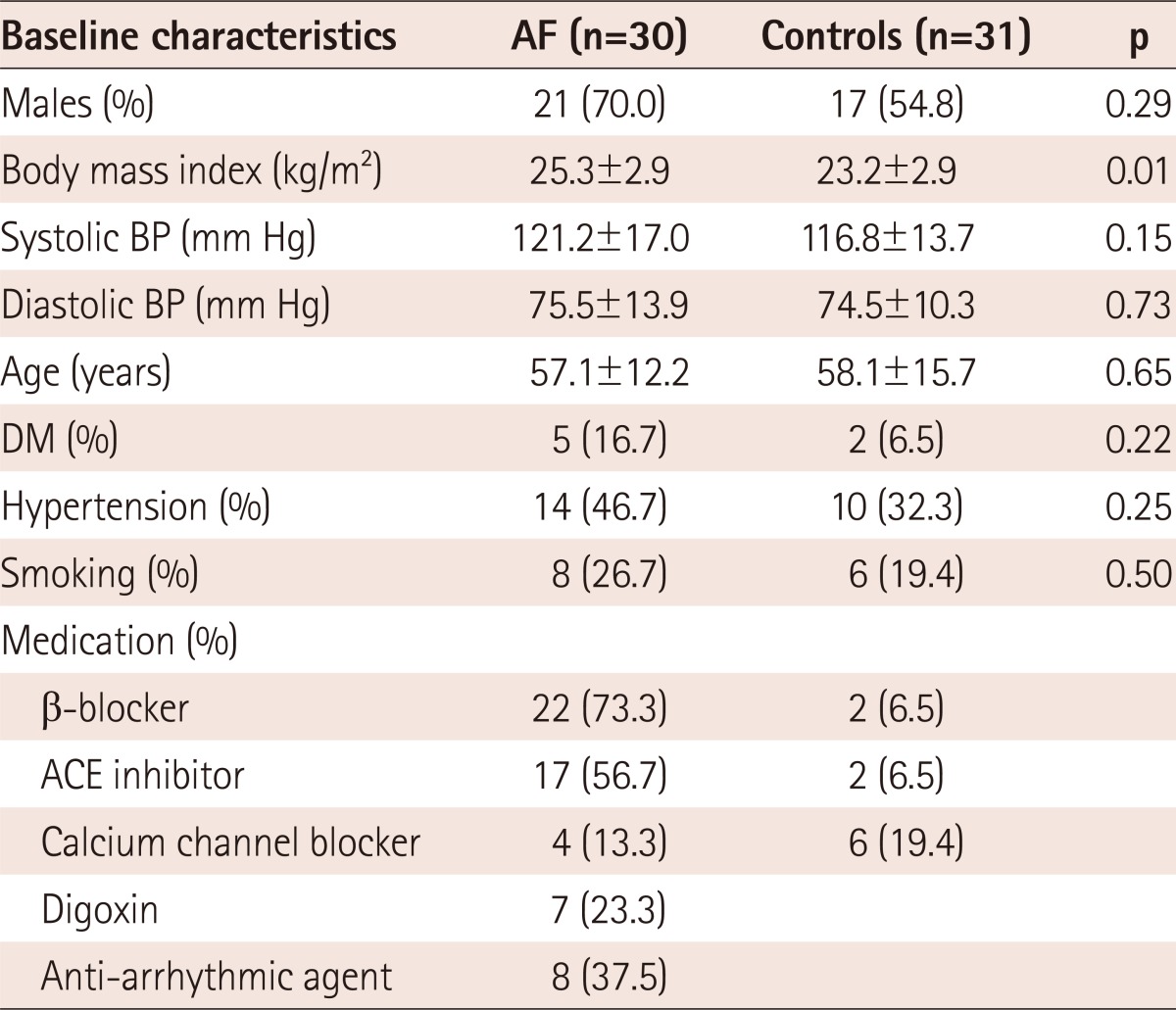
A total of 61 consecutive subjects (male : female=34 : 27; average age 57.5±14.5 years, range 18-73 years; BMI 19.9-33.5 kg/m2) were prospectively enrolled in this study. Subjects were divided into two groups: AF patients (n=30) and NSR controls (n=31). Table 1 shows the baseline characteristics for both groups. There were no significant differences in sex, BMI, blood pressure, age, or prevalence of diabetes mellitus (DM), hypertension, and smoking.
Table 1.
Baseline characteristics of subjects with AF and controls
All values are presented as number (percentage) or mean±SD. AF: atrial fibrillation, BP: blood pressure, DM: diabetes mellitus, ACE: angiotensin converting enzyme
In the AF group, 73% of patients were treated with a β-blocker, 56.7% were treated with angiotensin converting enzyme (ACE) inhibitor or an angiotensin II antagonist, and 13.3% were treated with a calcium-channel blocker. In the control group, 19.4% of patients were treated with a calcium channel blockers, 6.5% were treated with ACE inhibitor or an angiotensin II antagonist, and 6.5% were treated with a β-blocker.
Echocardiographic findings
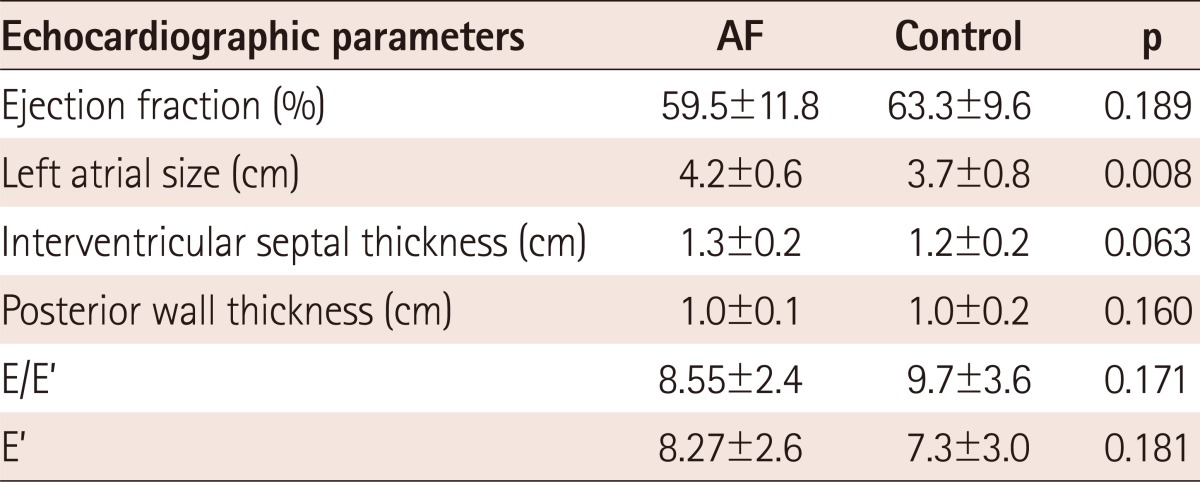
The mean LA size was significantly increased in the AF group, compared with that in the control group (4.2±0.6 vs. 3.7±0.8 cm, p=0.008) (Table 2). The mean value of other echocardiographic parameters, including EF, LV wall thickness, and E/E', were not different between the two groups.
Table 2.
Echocardiographic characteristics of AF patients and controls
Values are presented as mean±SD. AF: atrial fibrillation
If we divided the AF patients into paroxysmal and chronic AF, LA size was significantly increased in the chronic AF group (3.8±0.4 vs. 4.5±0.6 cm, p=0.028).
Laboratory findings
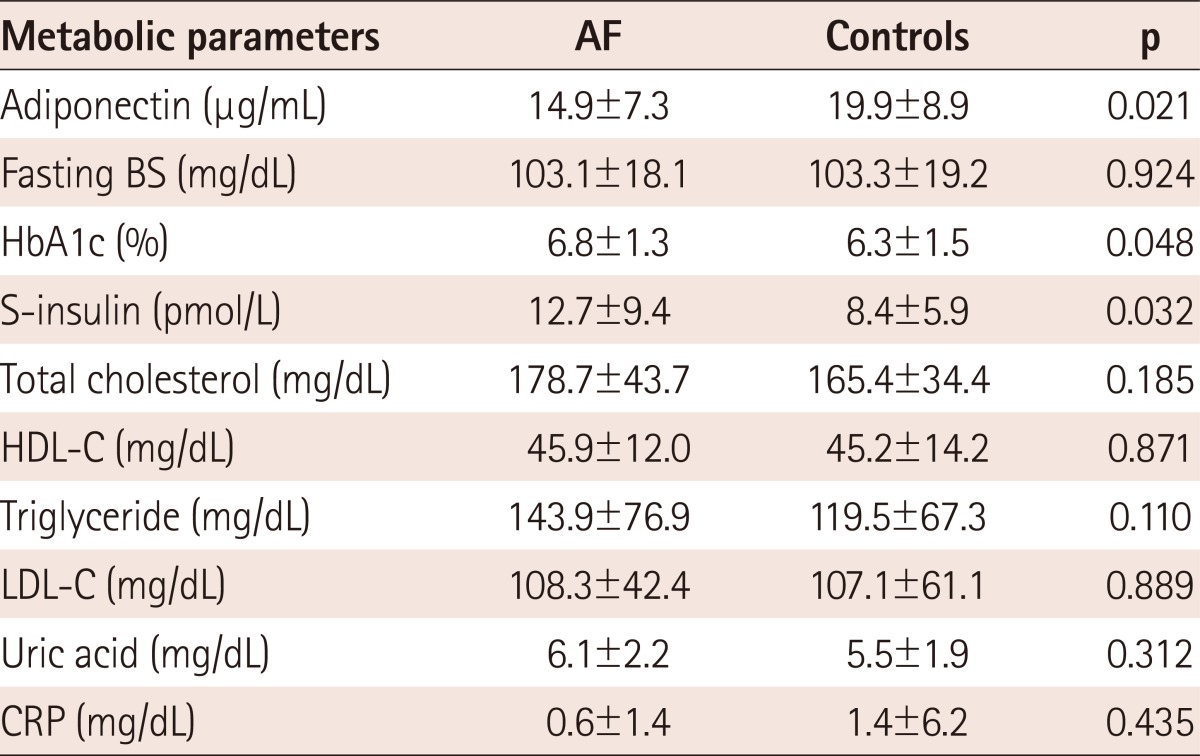
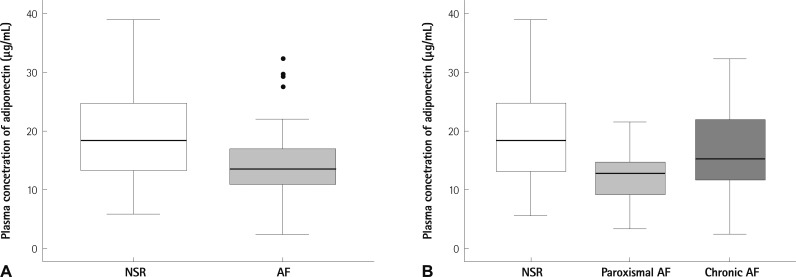
The mean adiponectin concentration was decreased in subjects with AF, compared with those in the control group (14.9±7.2 µg/mL vs. 19.9±8.9 µg/mL, p=0.021) (Table 3, Fig. 1). When we divided the AF group into paroxysmal AF and chronic AF, the adiponectin level showed a significant difference between paroxysmal AF and controls (12.2±4.9 µg/mL vs. 19.9±8.9 µg/mL, p=0.016), but not between chronic AF and controls (17.1±8.3 µg/mL vs. 19.9±8.9 µg/mL). The fasting s-insulin level was also significantly increased in AF patients (12.7±9.4 vs. 8.4±5.9 µU/mL, p=0.04) (Table 3).
Table 3.
Comparison of metabolic parameters between AF and control groups
Values are presented as mean±SD. AF: atrial fibrillation, BS: blood sugar, HbA1c: hemoglobin A1c, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, CRP: C-reactive protein
Fig. 1.
Serum adiponectin level in AF and control patients. Circulating levels of adiponectin were measured in patients with AF and in patients with NSR as controls. Box plots represent median levels with 25th and 75th percentiles of observed data, whereas whiskers show the 5th and 95th percentiles in each group. A: comparison of NSR and AF patients (p=0.021). B: comparison of NSR (n=31), paroxysmal AF (n=13), and chronic AF (n=17) (p=0.016, control vs. paroxysmal AF). AF: atrial fibrillation, NSR: normal sinus rhythm.
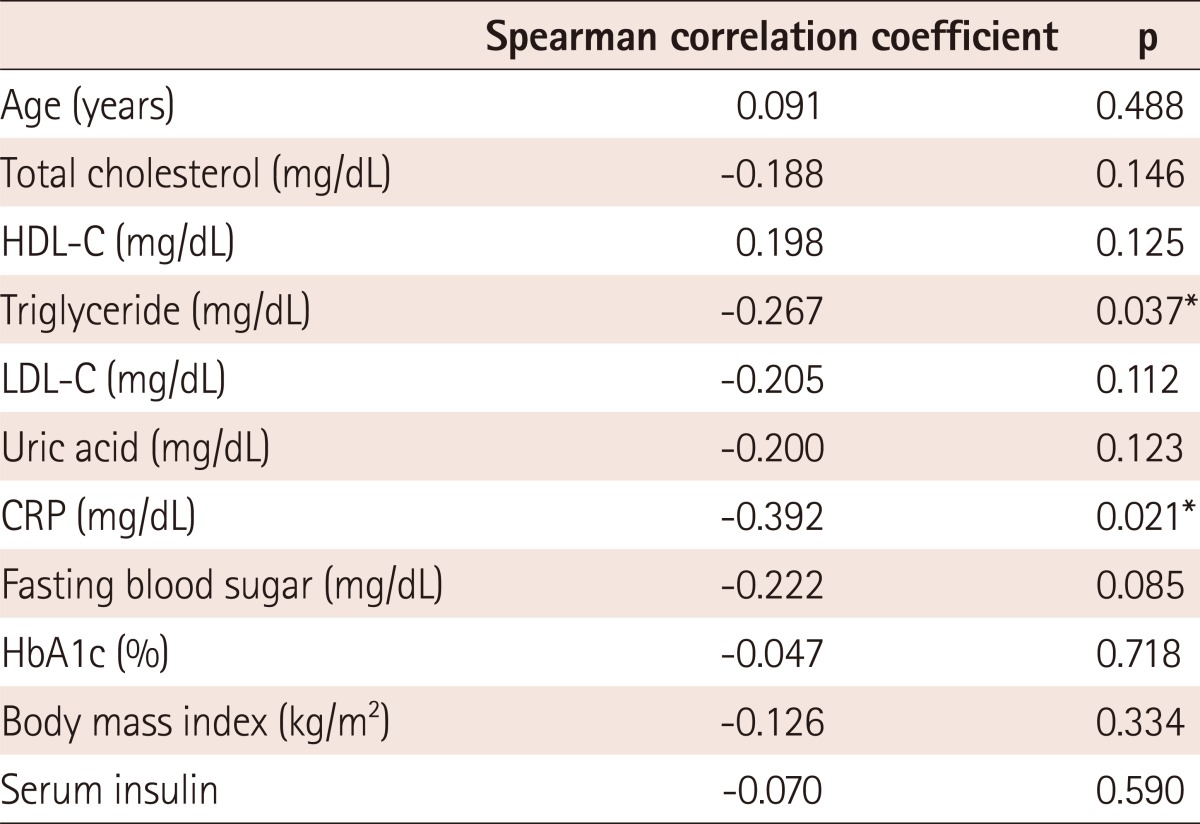
In univariate analysis, TG, and CRP were correlated with plasma adiponectin level. As shown in Table 4, there was a negative correlation between the plasma adiponectin concentration and TG (r=-0.267, p=0.037) and CRP (r=-0.392, p=0.021) levels.
Table 4.
Correlation between plasma adiponectin concentration and other parameters
*p<0.05. HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, CRP: C-reactive protein, HbA1c: hemoglobin A1c
Multiple logistic regression analysis was done to evaluate the independent predictors for AF. Adiponectin and LA diameter were strong independent predictors of AF in a binary logistic regression model (adjusted for clinical and echocardiographic parameters) (Table 5).
Table 5.
Multivariate logistic regression analysis of independent predictors for AF
Model was adjusted for clinical and echocardiographic characteristics. AF: atrial fibrillation, HR: hazard ratio, CI: confidence interval, TG: triglyceride, CRP: C-reactive protein, LA: left atrial
Discussion
The major findings of our study are: 1) The plasma concentration of adiponectin was significantly different between the AF and control groups, indicating that there may be some patho-physiological links between adiponectin and AF; 2) Adiponectin level was negatively correlated with the TG level and CRP level.; 3) Adiponectin level and LA diameter were significant independent predictors of AF; 4) There are significant changes in the adiponectin level between paroxysmal and chronic AF.
Adiponectin is a protein secreted specifically by adipose cells that may couple regulation of insulin sensitivity with energy metabolism, and are served to link obesity with insulin resistance.7) Adiponectin increases fatty acid oxidation and glucose uptake and inhibits gluconeogenesis. Decreased plasma adiponectin levels are observed in patients with DM, metabolic syndrome, and IHD, and may play a key role in the development of insulin resistance.11) In contrast, high adiponectin levels are observed in HF patients12-15) and in dialysis patients.16),17) In our study, low adiponectin levels were observed in paroxysmal AF, but not in chronic AF, indicating that the role of adiponectin is different in terms of AF disease progression.18) Our report is not fully in line with other studies. Rienstra et al.19) reported that in a community-based longitudinal study, no statistically significant association between adiponectin and incident of AF was found. These differences may be partially related with the disease progression state of AF. Shimano et al.18) reported that it is possible for the elevated adiponectin level to reflect a disconnection between adiponectin and the adiponectin receptors in AF, with a resulting increase in adiponectin secretion. Further study is needed to confirm the role of adiponectin in the disease progression of AF.
Multiple factors are involved in the etiology of AF. Among these, stretching and inflammation increase the angiotensin II level, thereby, inducing calcium overload and ectopic focal activities that initiate AF.20),21) Structural changes in the atria, in association with inflammation, might promote AF persistence. Our study showed that LA size is also a determinant of AF, together with adiponectin level. This implies that anatomical changes reflected by LA size and pathophysiological changes reflected by adiponectin may act together to promote the progress of AF. An inflammatory contribution, to at least some forms of AF, was initially suggested by the high incidence (25-40%) of AF after a cardiac surgery. Although the underlying mechanisms are not well understood, adiponectin clearly has anti-inflammatory properties that may be related to decreased adiponectin levels in AF patients in this study.
Another important mechanism related to adiponectin is the renin-angiotensin-aldosterone system (RAS). Human preadipocytes possess a functional RAS, and both undifferentiated preadipocytes and immature adipocytes secrete Angiotensin II. Expression of the RAS components seems to be related to the body weight because obese women are reported to have higher circulating levels of angiotensinogen, renin, aldosterone, and ACE than lean women. On the other hand, weight reduction reduced angiotensinogen, renin, and aldosterone levels and decreased ACE expression.22)
Thus, an upregulated adipose RAS may have deleterious local and systemic effects in obese individuals and might contribute to insulin resistance and hypertension. RAS blocking agent increased adiponectin levels with an accompanying improvements in the insulin sensitivity, but did not affect the degree of adiposity.21) Previously, Guo et al.23) reported that the treatment of losartan are accompanied by decreased adiponectin levels, which might offer potential protection in humans. Yenisecu et al.24) also reported that ramipril corrects proteinuria and increases the low adiponectin levels in patients with diabetic nephropathy. Further study is needed to confirm the role of adiponectin and ARB system.
There are several limitations of this study. First, the sample size is limited. As such, this study is underpowered to make a firm conclusion concerning the relation with adiponectin and AF. Second, although there was no statistically significant difference in the prevalence of DM, diabetes was more common in the AF group, as compared to the control patients. These differences may influence the adiponectin level. Third, some of the subjects were taking drugs, such as ACE inhibitor. However, in AF patients, with a decreased adiponectin level, the influence of ACE inhibitor on adiponectin level was not significant. In addition, several important determinants of plasma adiponectin level, such as body fat content and waist circumference, were not measured in our study.
Despite these limitations, our data implicate adiponectin as a target in reducing AF and the morbidity and mortality of atherosclerotic disease. In the healthy state, such as the control group in this study, plasma adiponectin levels may play a protective role through anti-inflammatory, anti-atherogenic, and anti-AF properties. The plasma adiponectin level was reported to be decreased in patients with risk factors for HF,12),25-29) and was decreased in AF patients in this study, compared with that of the controls. However, the plasma adiponectin level was increased in chronic AF, compared with paroxysmal AF. This is probably because the serum levels of atrial natriuretic peptide are increased in disease states, such as congestive HF and chronic AF,30) leading to increased secretion of adiponectin.25)
In conclusion, in this study, hypoadiponectinemia was significantly associated with the paroxysmal AF, but this hypoadiponectinemia cannot be shown in Chronic AF. Further investigation is needed to confirm the potential role for AF.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Goette A, Arndt M, Röcken C, et al. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 2.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 3.Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. doi: 10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 4.Henningsen KM, Therkelsen SK, Johansen JS, Bruunsgaard H, Svendsen JH. Plasma YKL-40, a new biomarker for atrial fibrillation? Europace. 2009;11:1032–1036. doi: 10.1093/europace/eup103. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou VN, Papadakis JE, Karatzis EN, Dermitzaki SI, Tsakiris AK, Skoufas PD. Impact of the metabolic syndrome on atrial size in patients with new-onset atrial fibrillation. Angiology. 2007;58:21–25. doi: 10.1177/0003319706297913. [DOI] [PubMed] [Google Scholar]
- 6.Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):I213–I219. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 7.Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol. 2007;49:531–538. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Rydén LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Aatrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 2001;104:2118–2150. [PubMed] [Google Scholar]
- 9.McNamara RL, Brass LM, Drozda JP, Jr, et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Commitee to Develop Data Standards on Atrial Fibrillation) J Am Coll Cardiol. 2004;44:475–495. doi: 10.1016/j.jacc.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 10.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance: endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Soc Echocardiogr. 2007;20:787–805. doi: 10.1016/j.echo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Celik T, Iyisoy A. A new villain of the village: hypoadiponectinemia. Int J Cardiol. 2009;137:54–55. doi: 10.1016/j.ijcard.2008.04.088. [DOI] [PubMed] [Google Scholar]
- 12.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 13.George J, Patal S, Wexler D, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420–1424. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEntegart MB, Awede B, Petrie MC, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829–835. doi: 10.1093/eurheartj/ehm033. [DOI] [PubMed] [Google Scholar]
- 15.Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 17.Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant. 2005;20:129–134. doi: 10.1093/ndt/gfh568. [DOI] [PubMed] [Google Scholar]
- 18.Shimano M, Shibata R, Tsuji Y, et al. Circulating adiponectin levels in patients with atrial fibrillation. Circ J. 2008;72:1120–1124. doi: 10.1253/circj.72.1120. [DOI] [PubMed] [Google Scholar]
- 19.Rienstra M, Sun JX, Lubitz SA, et al. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: the Framingham Offspring Study. Am Heart J. 2012;163:119–124. doi: 10.1016/j.ahj.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumagai K. Upstream approach for atrial fibrillation. Nihon Yakurigaku Zasshi. 2010;135:59–61. doi: 10.1254/fpj.135.59. [DOI] [PubMed] [Google Scholar]
- 21.Furuhashi M, Ura N, Higashiura K, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 22.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 23.Guo LL, Pan Y, Jin HM. Adiponectin is positively associated with insulin resistance in subjects with type 2 diabetic nephropathy and effects of angiotensin II type 1 receptor blocker losartan. Nephrol Dial Transplant. 2009;24:1876–1883. doi: 10.1093/ndt/gfn770. [DOI] [PubMed] [Google Scholar]
- 24.Yenicesu M, Yilmaz MI, Caglar K, et al. Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron Clin Pract. 2005;99:c115–c121. doi: 10.1159/000083929. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 27.Della Mea P, Lupia M, Bandolin V, et al. Adiponectin, insulin resistance, and left ventricular structure in dipper and nondipper essential hypertensive patients. Am J Hypertens. 2005;18:30–35. doi: 10.1016/j.amjhyper.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 29.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 30.Rossi A, Enriquez-Sarano M, Burnett JC, Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35:1256–1262. doi: 10.1016/s0735-1097(00)00515-5. [DOI] [PubMed] [Google Scholar]