Abstract
The aim of this study was to explore the cytochrome P450 2E1 (CYP2E1) RsaI/PstI polymorphism and risk of esophageal cancer (EC) in mainland Chinese populations. A systematic search of PubMed, EMBASE, Web of Science, CBM, CNKI and VIP databases for publications on the CYP2E1 RsaI/PstI polymorphism and risk of EC was performed. and the genotype data were analyzed in a meta-analysis. Odds ratios (ORs) with relevant 95% confidence intervals (CIs) were estimated to assess the association. Sensitivity analysis, test of heterogeneity and assessment of publication bias were performed. The search yielded 17 studies including 18 trails involving 1,663 cases and 2,603 controls. The meta-analyses showed a significant association between the CYP2E1 RsaI/PstI polymorphism and risk of EC in the mainland Chinese population (c2 vs. c1: OR=0.64; 95% CI, 0.50–0.81; P<0.001; c2/c2 vs. c1/c1: OR=0.73; 95% CI, 0.57–0.93; c2/c2 vs. c1/c1+c1/c2: OR=0.76; 95% CI, 0.60–0.96; P=0.02; c1/c2 vs. c1/c1: OR=0.54; 95% CI, 0.38–0.75; P<0.001; c1/c2+c2/c2 vs. c1/c1: OR=0.48; 95% CI, 0.34–0.70; P<0.001). An increased cancer risk in all genetic models was identified following stratification by ethnicity, source of controls and tumor type. In conclusion, in all genetic models, the association between the CYP2E1 RsaI/PstI polymorphism and risk of EC in the mainland Chinese population was significant. This meta-analysis suggests that the CYP2E1 RsaI/PstI polymorphism is a risk factor for EC, and the c2 allele is a factor that lowers the possibility of EC in the mainland Chinese population and this association did not change due to ethnic differences in genetic backgrounds and the environment.
Keywords: cytochrome P450 2E1, polymorphism, esophageal cancer, mainland Chinese, meta-analysis
Introduction
Esophageal cancer (EC) ranks as the eighth most common malignancy and the sixth most common cancer-related cause of mortality worldwide, which is characterized by a high incidence and a striking worldwide geographic variation. In fact there exists a so-called ‘Asian esophageal cancer belt’, an area that stretches from the Caucasian mountains across Northern Iran, Afghanistan, Kazakhstan, Uzbekistan and Turkmenistan, into northern China, and China, Iran and Japan have the highest rate of EC in the world (1–3). These high incidence areas are associated with poverty or poverty-related diseases, and current evidence also reveals that the incidence of EC tends to decrease when wealth and accessibility to health care increases (4,5).
However, the cause and pathogenesis of EC remains unclear. Histopathologically, EC is classified into two main types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). A higher incidence rate of EAC occurs in Western countries, while ESCC occurs more often in Oriental countries, particularly in Mainland China (1,2,6). The possible risk factors for developing EC have been reported to be alcohol consumption, smoking, coffee consumption, low socioeconomic status, poor oral health, hot drinks, high consumption of nitrosamines, diet deficient in antioxidants and other environmental factors (1–3,5,7,8). Recent studies reveal that genetic alterations are also considered as new risk factors for EC (9,10).
Numerous studies have shown that the cytochrome P450 superfamily are catalyzing enzymes for carcinogens. Cytochrome P450 2E1 (CYP2E1), a member of the CYP450 superfamily and an ethanol-inducible enzyme, is involved in the metabolic activation of numerous low molecular weight compounds, including N-nitrosamines, aniline, vinyl chloride and urethane (11–14). RsaI/PstI polymorphisms in the promoter gene region are believed to affect the transcriptional activity of the gene, and occur as a wild-type homozygous genotype (c1/c1), a heterozygous genotype (c1/c2) and a variant homozygous rare genotype (c2/c2), and the frequency of the variant c1 allele was observed to be much higher in patients with esophageal diseases than that of healthy individuals (15–17).
The first study on the association between EC and the CYP2E1 RsaI/PstI polymorphism was conducted in 1996, but the result failed to reveal a significant association. The second study in Japan also failed to identify a significant difference between healthy controls and patients with EC (18). However, certain subsequent studies on Chinese individuals indicated that a significant association exists between the CYP2E1 RsaI/PstI polymorphism and the risk of EC, yet certain studies revealed different or even contradictory findings (16,17,19–33).
A previous meta-analysis was carried out concerning EC and the CYP2E1 RsaI/PstI polymorphism, of which 11 published studies demonstrated that the CYP2E1 RsaI/PstI c2 allele may be a protective factor for EC among Asian populations (34). In the included 7 Chinese studies, the authors only searched PubMed and did not categorize subgroups according to the source of controls, which makes their studies unsuitable for reference for mainland Chinese populations. Therefore, we conducted a meta-analysis of case-control studies in order to review and summarize the epidemiological evidence, and aimed to precisely detect the correlation between the CYP2E1 RsaI/PstI polymorphism and the risk of EC among Chinese individuals.
Materials and methods
Literature search
Strictly following the proposed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (35) guidelines, our study aims to report the present review and meta-analysis systematically. Initially, we identified published and unpublished studies which tested the association between the CYP2E1 RsaI/PstI polymorphism and risk of EC by searching the following databases from their creation until January 10th, 2012: PubMed, EMBASE, Web of Science, the Chinese Biological Medicine Database (CBM), China National Knowledge Infrastructure (CNKI) and the Chinese scientific periodical database of VIP information (VIP). Terms used in the search were as follows: i) esophag* and oesophag*; ii) cancer, carcinoma, adenocarcinoma, neoplasm, neoplasia and neoplastic; iii) cytochrome p450 2E1, cytochrome p450II1, CYP2E1, CYPIIE1; iv) polymorphism; v) China and Chinese, without restrictions. In addition, we also reviewed the reference lists of retrieved manuscripts and recent reviews.
Study selection
We included any study that met with the following criteria: i) case-control study design; ii) association between CYP2E1 RsaI/PstI polymorphism and risk of EC was investigated; iii) diagnosis of ESCC and EAC were either histologically, pathologically or cytologically confirmed; and iv) the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs), or the number of events required to calculate them were reported. Two investigators independently evaluated the eligibility of all studies retrieved from the databases on the basis of the predetermined selection criteria. Disagreements were resolved by discussion or in consultation with a third investigator.
Data extraction
Two reviewers independently extracted data for the study characteristics by using a standardized data-collection form. Data were recorded as follows: first author’s last name, year of publication, place of origin, study period and duration of follow-up, characteristics of cancer cases, source of controls, matching criteria, number of cases and controls, number of different genotypes in cases and controls, Hardy-Weinberg equilibrium (HWE) and minor allele frequency in controls. Any disagreements were resolved by consensus.
Statistical analysis
We computed a pooled OR and 95% CI for the risk allele using the Comprehensive Meta Analysis software (version 2.1) to generate forest plots to determine whether there was a statistical association between cases and controls and to assess heterogeneity of the included studies. HWE was tested by a Chi-square test at a significance level of P<0.05. Heterogeneity was quantifiably evaluated using the Chi-square based Cochran’s Q statistic (36) and the I2 statistic, which yields results ranging from 0 to 100% (I2=0–25%, no heterogeneity; I2=25–50%, moderate heterogeneity; I2=50–75%, large heterogeneity; I2=75–100%, extreme heterogeneity) (37). If heterogeneity existed, the random-effects model was used, otherwise, the fixed-effects model was used. In addition, we investigated the influence of a single study to the overall risk estimate by removing each study in turn to test the robustness of the main results. Subgroup analysis was also conducted if significant heterogeneity was identified (such as Han individuals vs. ethnic minorities). If possible, potential publication bias was assessed by visual inspection of the funnel plots of the primary outcome (38).
Results
Identification of eligible studies
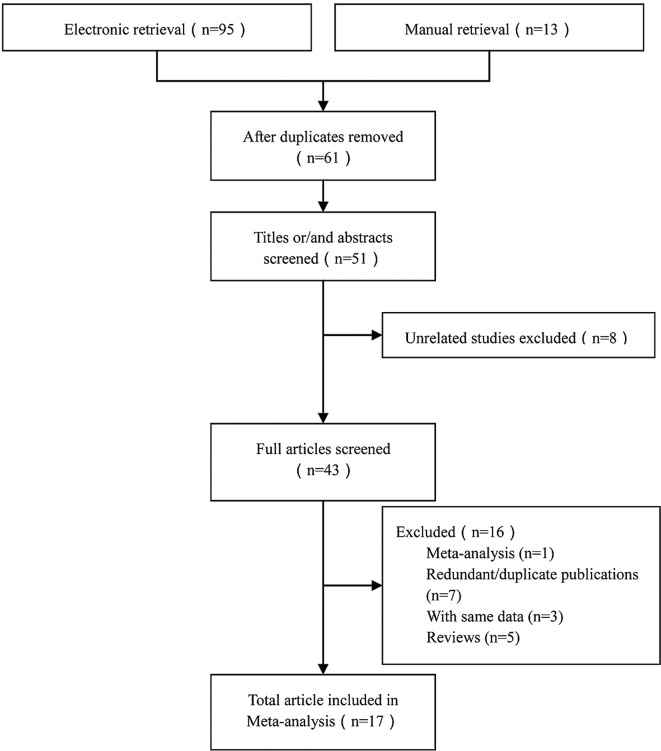
Of the 108 records retrieved initially, 17 studies including 18 trails were identified for the association between the CYP2E1 RsaI/PstI polymorphism and risk of EC, including a total of 1,663 cases and 2,603 controls (16,17,19–33). A flow chart for the study selection process is presented in Fig. 1. Duplicates (the same study searched from different databases) were excluded using the Endnote X3 software.
Figure 1.
Summary of the study selection process.
Characteristics of the studies
The detailed characteristics of the included studies are summarized in Table I. Of these studies, 7 were published in English (16,17,19,22,28–30) and 7 in Chinese (21,23–25,27,31,33), 2 were doctoral dissertations (20,32) and 1 was a master’s thesis both in English and Chinese (26). The sample sizes ranged from 45 to 480. All of the cases were histologically, pathologically or cytologically confirmed as EC. Of the cases, 6 studies clearly confirmed the presence of ESCC (17,19,28,29,31,32), while one had both ESCC and EAC (16). Controls were mainly healthy populations and matched according to age, gender or were cancer-free tissues. Of the controls 6 were hospital-based (HB) (20,23–25,27,31), 10 were population-based (PB) (16,17,19,21,22,26,28,29,32,33) and one was both (30). There were two groups of Kazakhs (Xinjiang Province) (19,30) and 16 groups of Han individuals (16,17,20–29,31–33). The genotypes were analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP); genotype distributions in the controls of all studies were in accordance with HWE, with the exception of 4 studies (17,19,23,29).
Table I.
Characteristics of the studies included in this meta-analysis.
| Author (Refs.) | Site | Group | Source | Sample | Genotype
|
Genotyping method | P-value (HWE) for controls | Adjustment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| c1/c1 | c1/c2 | c2/c2 | ||||||||
| Lin et al (16) | Linxian County, Henan Province | T | ESCC and EAC | 45 | 36 | 6 | 3 | PCR-RFLP | 0.345 | Age, gender |
| C | PB | 45 | 20 | 22 | 3 | |||||
| Tan et al (17) | Linxian County, Henan Province | T | ESCC | 150 | 107 | 31 | 12 | PCR-RFLP | 0.009 | Age, gender, smoking |
| C | PB | 150 | 66 | 77 | 7 | |||||
| Shi (20) | Linzhou City, Henan Province | T | EC | 116 | 74 | 37 | 5 | PCR-RFLP | 0.922 | Age, gender, smoking, fermented vegetable |
| C | HB | 106 | 46 | 48 | 12 | |||||
| Xi’an City, Shaanxi Province | T | EC | 71 | 39 | 28 | 4 | PCR-RFLP | 0.368 | ||
| C | HB | 62 | 30 | 24 | 8 | |||||
| Gao et al (21) | Huai’an City, Jiangsu Province | T | EC | 144 | 83 | 44 | 13 | PCR-RFLP | 0.525 | Age, gender, tea, smoking, alcohol |
| C | PB | 233 | 140 | 75 | 14 | |||||
| Shi et al (23) | Wuhan City, Hubei Province | T | EC | 98 | 72 | 19 | 7 | PCR-RFLP | 0.039 | Age, gender |
| C | HB | 120 | 54 | 45 | 21 | |||||
| Gao et al (22) | Huai’an City, Jiangsu Province | T | EC | 93 | 55 | 31 | 7 | PCR-RFLP | 0.20 | Age, gender, smoking, drinking, dietary habits |
| C | PB | 196 | 121 | 62 | 13 | |||||
| Shi et al (24) | Nanjing City, Jiangsu Province | T | EC | 78 | 48 | 24 | 6 | PCR-RFLP | 0.141 | Age, gender, smoking, alcohol |
| C | HB | 118 | 60 | 42 | 16 | |||||
| Yin et al (25) | Huai’an City, Jiangsu Province | T | EC | 106 | 52 | 49 | 5 | PCR-RFLP | 0.411 | Age, gender |
| C | HB | 106 | 55 | 45 | 6 | |||||
| Lu et al (19) | Kazakh, Xinjiang Autonomous Region | T | ESCC | 104 | 81 | 20 | 3 | PCR-RFLP | <0.001 | Age, gender |
| C | PB | 104 | 25 | 74 | 5 | |||||
| Li (26) | Taiyuan City, Shanxi Province | T | EC | 60 | 32 | 18 | 10 | PCR-RFLP | 0.781 | Age, gender, smoking, drinking habits |
| C | PB | 199 | 101 | 69 | 29 | |||||
| Liu et al (28) | Huai’an City, Jiangsu Province | T | ESCC | 77 | 34 | 33 | 10 | PCR-RFLP | 0.91 | Age, gender |
| C | PB | 79 | 45 | 29 | 5 | |||||
| Dong et al (27) | Gansu Province | T | EC | 120 | 84 | 26 | 10 | PCR-RFLP | 0.428 | Age, gender |
| C | HB | 120 | 57 | 44 | 19 | |||||
| Xia et al (31) | Jingjiang City, Jiangsu Province | T | ESCC | 45 | 30 | 12 | 3 | PCR-RFLP | 0.708 | Not reported |
| C | HB | 45 | 26 | 17 | 2 | |||||
| Qin et al (30) | Kazakh, Xinjiang Autonomous Region | T | EC | 120 | 94 | 23 | 3 | PCR-RFLP | 0.29 | Age, gender, dietary habits |
| C | PB and HB | 240 | 128 | 90 | 22 | |||||
| Guo et al (29) | Lanzhou City, Gansu Province | T | ESCC | 80 | 57 | 16 | 7 | PCR-RFLP | <0.001 | Age, gender |
| C | PB | 480 | 225 | 180 | 75 | |||||
| Yang (32) | Feicheng City, Shandong Province | T | ESCC | 27 | 19 | 8 | 0 | PCR-RFLP | 0.442 | Age, gender, alcohol, education, smoking |
| C | PB | 44 | 31 | 11 | 2 | |||||
| Zhang and Wu (33) | Gansu Province | T | EC | 129 | 75 | 40 | 14 | PCR-RFLP | 0.443 | Age, gender |
| C | PB | 156 | 70 | 56 | 21 | |||||
Characteristics of studies included in this meta-analysis. T, case subjects; C, control subjects; PB, population-based; HB, hospital-based; ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; EC, esophageal cancer; HWE, Hardy-Weinburg equilibrium; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Meta-analyses
The main results of the heterogeneity test and meta-analysis are listed in Table II.
Table II.
Main results of the heterogeneity test and subgroups meta-analyses.
| Heterogeneity
|
Meta-analyses
|
|||||
|---|---|---|---|---|---|---|
| Genetic model | Study or subgroup | P-value | I2 (%) | OR (95% CI) | P-value | |
| c2 vs. c1 | Total | <0.001 | 80 | 0.64 (0.50–0.81) | 0.0003 | |
| Ethnicity | Han | <0.001 | 72 | 0.71 (0.57–0.89) | 0.002 | |
| Kazakh | 0.12 | 58 | 0.28 (0.17–0.46) | <0.001 | ||
| Source of control | PB | <0.001 | 85 | 0.65 (0.45–0.53) | 0.02 | |
| HB | 0.02 | 59 | 0.62 (0.46–0.82) | 0.0007 | ||
| Both | Single study | 0.35 (0.23–0.55) | <0.001 | |||
| Tumor type | EC | <0.001 | 76 | 0.69 (0.53–0.89) | 0.005 | |
| ESCC | <0.001 | 84 | 0.56 (0.33–0.93) | 0.03 | ||
| c2/c2 vs. c1/c1 | Total | 0.03 | 42 | 0.70 (0.56–0.89) | 0.003 | |
| Ethnicity | Han | 0.04 | 42 | 0.75 (0.59–0.95) | 0.02 | |
| Kazakh | 0.35 | 0 | 0.32 (0.13–0.80) | 0.01 | ||
| Source of control | PB | 0.30 | 16 | 1.02 (0.76–1.38) | 0.89 | |
| HB | 0.69 | 0 | 0.44 (0.30–0.66) | <0.001 | ||
| Both | Single study | 0.23 (0.07–0.80) | 0.02 | |||
| Tumor type | EC | 0.05 | 46 | 0.63 (0.48–0.82) | 0.0008 | |
| ESCC | 0.19 | 31 | 0.94 (0.61–1.46) | 0.80 | ||
| c1/c2 vs. c1/c1 | Total | <0.001 | 81 | 0.54 (0.38–0.75) | 0.0003 | |
| Ethnicity | Han | <0.001 | 72 | 0.62 (0.46–0.84) | 0.002 | |
| Kazakh | 0.002 | 90 | 0.18 (0.05–0.68) | 0.01 | ||
| Source of control | PB | <0.001 | 88 | 0.52 (0.30–0.92) | 0.02 | |
| HB | 0.05 | 53 | 0.59 (0.42–0.85) | 0.004 | ||
| Both | Single study | 0.35 (0.20–0.59) | <0.001 | |||
| Tumor type | EC | 0.004 | 62 | 0.66 (0.50–0.88) | 0.004 | |
| ESCC | <0.001 | 87 | 0.38 (0.18–0.82) | 0.01 | ||
| c2/c2 vs. c1/c1+c1/c2 | Total | 0.12 | 29 | 0.73 (0.58–0.92) | 0.008 | |
| Ethnicity | Han | 0.15 | 27 | 0.78 (0.61–0.99) | 0.04 | |
| Kazakh | 0.38 | 0 | 0.34 (0.13–0.85) | 0.02 | ||
| Source of control | PB | 0.47 | 0 | 1.03 (0.76–1.38) | 0.54 | |
| HB | 0.8 | 0 | 0.49 (0.33–0.72) | 0.0003 | ||
| Both | Single study | 0.25 (0.07–0.87) | 0.03 | |||
| Tumor type | EC | 0.14 | 33 | 0.66 (0.50–0.87) | 0.003 | |
| ESCC | 0.31 | 16 | 0.96 (0.62–1.49) | 0.87 | ||
| c1/c2+c2/c2 vs. c1/c1 | Total | <0.001 | 85 | 0.48 (0.34–0.70) | 0.0001 | |
| Ethnicity | Han | <0.001 | 81 | 0.56 (0.40–0.79) | 0.001 | |
| Kazakh | 0.003 | 85 | 0.17 (0.05–0.59) | 0.009 | ||
| Source of control | PB | <0.001 | 88 | 0.56 (0.33–0.95) | 0.03 | |
| HB | <0.001 | 82 | 0.42 (0.23–0.75) | 0.004 | ||
| Both | Single study | 0.32 (0.19–0.52) | <0.001 | |||
| Tumor type | EC | <0.001 | 82 | 0.55 (0.37–0.82) | 0.003 | |
| ESCC | <0.001 | 87 | 0.41 (0.21–0.85) | 0.02 | ||
The main results of the heterogeneity test and meta-analysis. OR, odds ratio; CI, confidence interval; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; PB, population-based; HB, hospital-based.
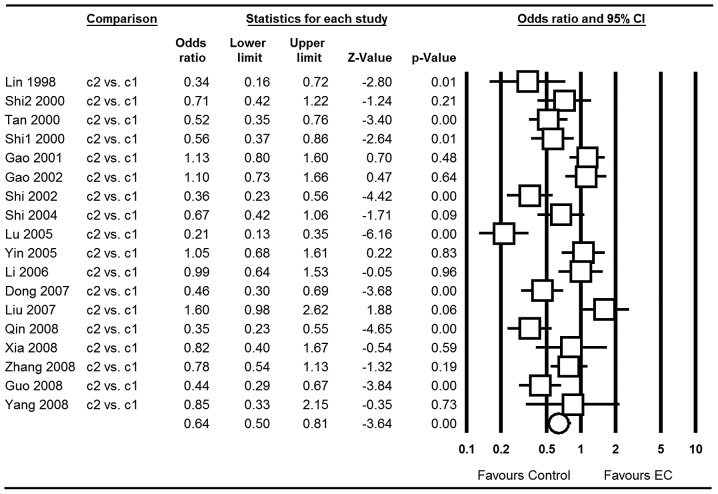
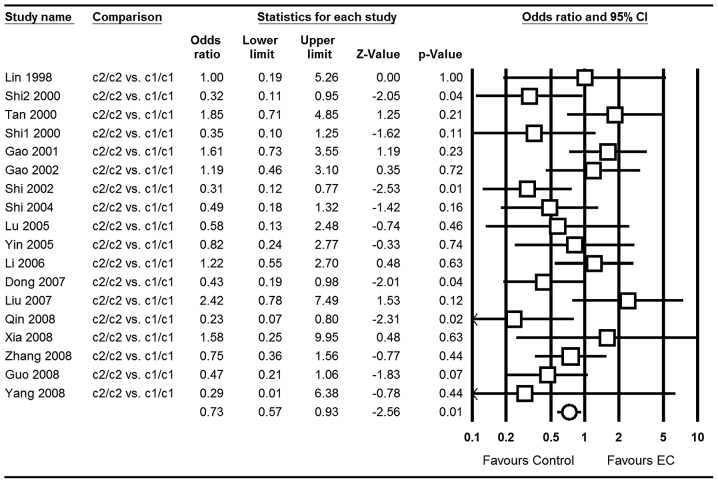
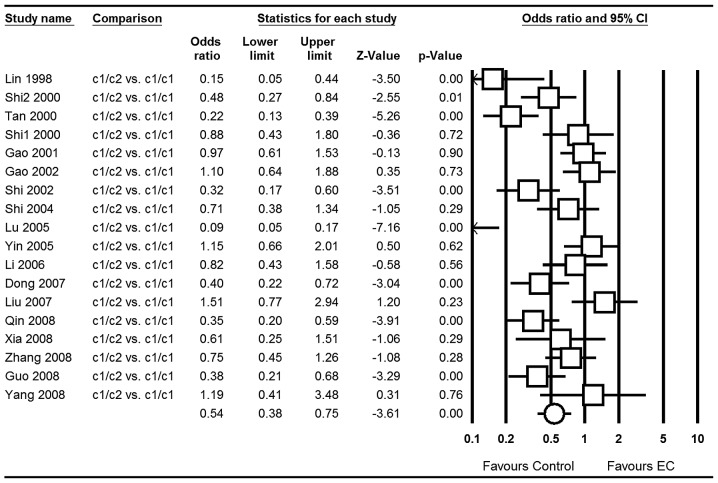
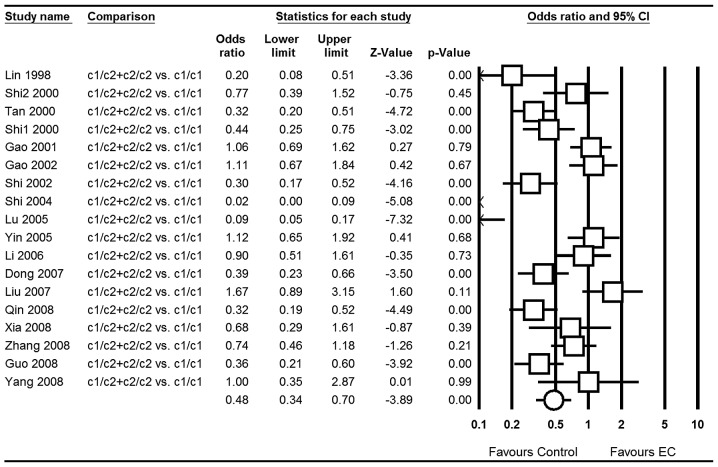
Our meta-analyses gave a significant association of the CYP2E1 RsaI/PstI polymorphism with EC risk [for the allele contrast c2 vs. c1: OR=0.64; 95% CI, 0.50–0.81; P<0.001 (Fig. 2); for c2/c2 vs. c1/c1: OR=0.73; 95% CI, 0.57–0.93; P=0.01 (Fig. 3); for c2/c2 vs. c1/c1+c1/c2: OR=0.76; 95% CI, 0.60–0.96; P=0.02 (Fig. 4); for c1/c2 vs. c1/c1: OR=0.54; 95% CI, 0.38–0.75; P<0.001 (Fig. 5); for the dominant model c1/c2+c2/c2 vs. c1/c1: OR=0.48; 95% CI, 0.34–0.70; P<0.001 (Fig. 6)] in total populations.
Figure 2.
Forest plot of EC associated with CYP2E1 RsaI/PstI for the c2 allele compared with the c1 allele in total. CI, confidence interval; EC, esophageal cancer.
Figure 3.
Forest plot of EC associated with CYP2E1 RsaI/PstI for the c2/c2 genotype compared with the c1/c1 genotype in total. CI, confidence interval; EC, esophageal cancer.
Figure 4.
Forest plot of EC associated with CYP2E1 RsaI/PstI for the c2/c2 genotype compared with the c1/c1+c1/c2 genotype in total. CI, confidence interval; EC, esophageal cancer.
Figure 5.
Forest plot of EC associated with CYP2E1 RsaI/PstI for the c1/c2 genotype compared with the c1/c1 genotype in total. CI, confidence interval; EC, esophageal cancer.
Figure 6.
Forest plot of EC associated with CYP2E1 RsaI/PstI for the c1/c2+c2/c2 genotype compared with the c1/c1 genotype in total. CI, confidence interval; EC, esophageal cancer.
When stratified by ethnicity, all genetic models also produced statistically significant results. When studies were stratified for control source, an association was detected for all genetic models, with the exception of PB in c2/c2 vs. c1/c1 and c2/c2 vs. c1/c1+c1/c2 (both P>0.05). In the stratified analysis by tumor type, the results were similar to the control source; the ESCC type showed no association in c2/c2 vs. c1/c1 and c2/c2 vs. c1/c1+c1/c2 (both P>0.05).
Sensitivity analysis
The majority of studies indicated that the frequency distributions of genotypes in the controls were in accordance with HWE, whereas deviations from HWE were observed in 4 studies of the PstI/RsaI polymorphism (all P<0.05) (17,19,23,29). However, the corresponding pooled ORs were not substantially altered whether or not these studies were included (Table III). In addition, sensitivity analysis indicated that no single study influenced the pooled OR qualitatively and this suggests the stability of the result (c2 vs. c1 for example; Fig. 7).
Table III.
Sensitivity analysis after elimination of the studies where frequency distributions of genotypes in the controls were inconsistent with HWE.
| Heterogeneity
|
Meta-analyses
|
|||||
|---|---|---|---|---|---|---|
| Genetic model | Subgroup | P-value | I2 (%) | OR (95% CI) | P-value | Model |
| c2 vs. c1 | Total | <0.001 | 80 | 0.64 (0.50, 0.81) | 0.0003 | Random |
| AE | <0.001 | 71 | 0.76 (0.60, 0.96) | 0.02 | Random | |
| c2/c2 vs. c1/c1 | Total | 0.03 | 42 | 0.70 (0.56, 0.89) | 0.003 | Fixed |
| AE | 0.08 | 37 | 0.75 (0.57, 0.98) | 0.03 | Fixed | |
| c1/c2 vs. c1/c1 | Total | <0.001 | 81 | 0.54 (0.38, 0.75) | 0.0003 | Random |
| AE | 0.001 | 62 | 0.71 (0.54, 0.93) | 0.01 | Random | |
| c2/c2 vs. c1/c1+c1/c2 | Total | 0.12 | 29 | 0.73 (0.58, 0.92) | 0.008 | Fixed |
| AE | 0.19 | 24 | 0.77 (0.59, 1.00) | 0.05 | Fixed | |
| c1/c2+c2/c2 vs. c1/c1 | Total | <0.001 | 85 | 0.48 (0.34, 0.70) | 0.0001 | Random |
| AE | <0.001 | 80 | 0.62 (0.43, 0.89) | 0.01 | Random | |
Sensitivity analysis after elimination of the studies for which the frequency distributions of genotypes in the controls were inconsistent with HWE. AE, after elimination; HWE, Hardy-Weinburg equilibrium; OR, odds ratio; CI, confidence interval.
Figure 7.
Sensitivity analysis based on c2 vs. c1; a single study was removed each turn. CI, confidence interval; EC, esophageal cancer.
Publication bias
Funnel plot based on c2/c2 vs. c1/c1 (the genetic model was pooled using a fixed-effects model) was chosen to assess publication bias. The symmetrical shape of the funnel plots (Fig. 8) implied that slight bias of the studies occurred.
Figure 8.
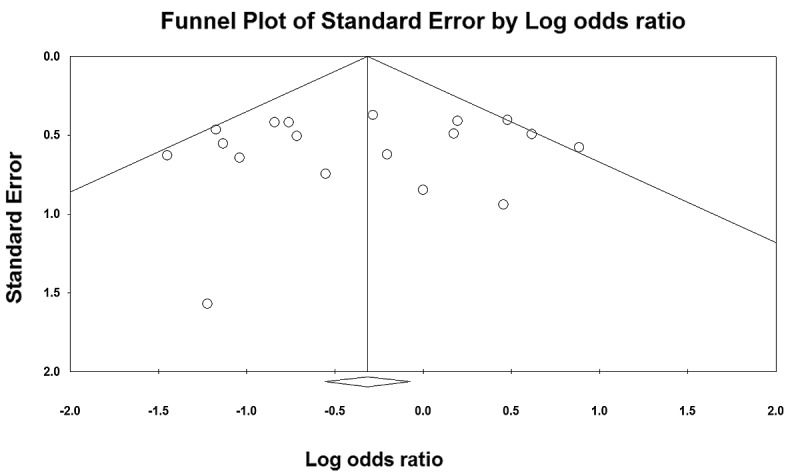
Funnel plot based on the c2/c2 vs. c1/c1+c1/c2 genetic model.
Discussion
EC is an increasingly common cancer with a poor prognosis, which is likely to be caused by multi-factors, including environmental risk and genetic factors (3,7–9). In recent years, environmental and genetic susceptivity, and their interactions, were used to evaluate the risks of EC, but the results were inconsistent and this difference may be due to geographical distribution. Environmental risk factors were once regarded as the major cause of EC, but an epidemiological study on immigrants moving to Changzhi City in Shanxi Province after a century of residing in Linzhou City in Henan Province (>200 km apart and the environment is different), observed that the detection rate of ESCC in the immigrant population was similar to that of the residents in Linzhou City. It indicated that changes in environment and time do not affect the incidence rate of ESCC, since genetic factors play an important role (39).
Unlike other factors, the positive association between family history of EC risk is consistent with previous studies, which suggests a genetic susceptibility in EC pathogenesis (40–50). A meta-analysis in 2003 involved 13 studies to evaluate risk factors of EC in China and revealed that family history was a significant factor (OR=4.0; 95% CI, 2.29–6.99) (51). The majority of studies concluded that this is caused by various hereditary susceptibilities to gene-related tumors. As for genetic susceptibility, it has been reported that gene polymorphisms of metholenetetrahydrofolate reductase (MTHFR) (52), cytochrome P450 1A1 (CYP1A1) (52), glutathione S-transferase M1 (GSTM1) (52), GSTT1 (53), CYP2A6 (54), CYP2E1 (34), human 8-oxoguanine glycosylase 1 (hOGG1) (55), X-ray repair cross-complementing group 1 (XRCC1) (56), xeroderma pigmentosum group D (XPD) (56), p53 (57) and others, are correlated with EC.
The CYP2E1 gene that encodes the CYP2E1 enzyme has been mapped to chromosome 10q24.3-qter, and is an important member of the cytochrome P450 superfamily. CYP2E1 is a naturally ethanol-inducible enzyme mainly involved in the metabolic activation of low molecular weight compounds, such as N-nitrosamines and in alcohol metabolism (11–14). The RsaI/PstI polymorphisms in the promoter gene region are reported to affect the transcriptional activity of the gene. Numerous large-sample and unbiased epidemiological studies of CYP2E1 RsaI/PstI polymorphisms could confirm it as a predisposition gene for EC risk, particularly in China (15–33). Molecular biological studies have also demonstrated that the rare allele of the RsaI/PstI polymorphism in the CYP2E1 gene is associated with increased transcriptional activity (58), which may play an important role in EC development.
Our meta-analysis summarized all the available data on the association between the CYP2E1 RsaI/PstI polymorphism and EC risk, including a total of 4,266 subjects. The results clearly suggested that there was a significant association between the CYP2E1 RsaI/PstI polymorphism and EC susceptibility. The RsaI/PstI c2 allele is a factor which lowers the possibility of EC, which may change and increase the ability to activate mutagens and carcinogens (OR=0.64; 95% CI, 0.50–0.81).
When subgroups were analyzed by ethnicity, the c2 allele was considered as a decreased risk factor in both Han and Kazakh subgroups, suggesting the ethnic differences in genetic backgrounds and the environment they lived in was non-related factor, which was in accordance with Wang et al (39).
In mainland China, ESCC is one of the most common malignancies, and has a great geographic variation of occurrence; the Northwest of China shows an exceptionally high occurrence (2). However, the meta-analysis of ESCC subgroup failed to identify any significant association in c2/c2 vs. c1/c1 (OR=0.94; 95% CI, 0.61–1.46; P=0.80) and c2/c2 vs. c1/c1+c1/c2 (OR=0.96; 95% CI, 0.62–1.49, P=0.87) genetic models. This phenomenon could have resulted since the included studies did not indicated the tumor type were all ESCC, with the exception of Lin et al (16) (both ESCC and EAC). Thus, future studies should clearly report the cancer type included.
Similar results also appeared in the PB controls in the c2/c2 vs. c1/c1 (OR=1.02; 95% CI, 0.76–1.38; P=0.89) and c2/c2 vs. c1/c1+c1/c2 (OR=1.03; 95% CI, 0.76–1.38; P=0.54) genetic models, and this could be since the HB studies have certain biases for such controls and may only represent a sample of an ill-defined reference population, and may not be representative of the general population; or it may be that numerous subjects in the PB controls were susceptible individuals. Therefore, the use of proper and representative PB control subjects is important to reduce biases in such genetic studies.
The sensitivity of the frequency distributions of genotypes in the controls were inconsistent with HWE, and the stability of results by deleting one study each time suggested that they were not substantially altered and the results were stable. Hence, the heterogeneity of the studies did not substantially lower the statistical validity of the study and the CYP2E1 RsaI/PstI polymorphism is clearly associated with EC risk in Chinese individuals.
Compared with the previous meta-analysis (34), this meta-analysis grouped subgroups with more accuracy than before, and contained more studies and a more accurate association estimation. Certain limitations of our meta-analysis must be acknowledged. Firstly, heterogeneity among the studies, resulting from different defined controls or other factors, may influence the results of this analysis. In certain studies, the controls were selected randomly from a healthy or normal population, but in other studies controls were selected from HB cancer-free patients. In addition, the matching criteria of the control group differed in age and gender. The variant risks (eg. gender, living habits) of EC in these different populations may affect the results. Secondly, it is well-known that a single gene has only a moderate effect on EC development. The combinations of certain genotypes may be the more discriminating factor than a single locus genotype. In our meta-analysis, just as the previous one, the connection between disequilibrium and haplotype analysis was not performed. Thirdly, although primary studies had adjusted for covariates, the ORs of this meta-analysis were obtained without correction, while a more precise analysis should be performed if individual data were available, which would allow for the adjustment by the covariates, including age, gender, ethnicity, smoking and other factors. Fourthly, the sample size was still relatively small; thus, we could not fully assess the effects. Finally, a potential limitation of any meta-analysis is the ‘file-drawer’ effect, in which studies with negative results may remain unpublished, and this may bias the literature toward positive findings. Although we endeavored to search for unpublished studies and the funnel plots also did not detect obvious publication bias, we still cannot confirm all the relevant studies were included.
In conclusion, evidence from the included studies suggests that the CYP2E1 RsaI/PstI polymorphism plays an important role in EC development for mainland Chinese individuals. It also suggests that the c2 allele significantly decreases the susceptibility to EC. For future study, studies with larger sample sizes, stricter selection of patients, well-matched PB controls and clearly reported cancer types are required. In addition, the potential gene-gene and gene-environment interactions of the CYP2E1 RsaI/PstI polymorphism and EC should be further investigated.
Acknowledgments
This study was partly supported by grants from the Foundation of Hubei University of Medcine (2011 CZX01) and Taihe Hospital (2010 D22), without commercial or for-profit organizations.
References
- 1.Szumiło J. Epidemiology and risk factors of the esophageal squamous cell carcinoma. Pol Merkur Lekarski. 2009;26:82–85. [PubMed] [Google Scholar]
- 2.Zheng S, Vuitton L, Sheyhidin I, Vuitton DA, Zhang Y, Lu X. Northwestern China: a place to learn more on oesophageal cancer. Part one: behavioural and environmental risk factors. Eur J Gastroenterol Hepatol. 2010;22:917–925. doi: 10.1097/MEG.0b013e3283313d8b. [DOI] [PubMed] [Google Scholar]
- 3.Umar SB, Fleischer DE. Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5:517–526. doi: 10.1038/ncpgasthep1223. [DOI] [PubMed] [Google Scholar]
- 4.Guo P, Li K. Trends in esophageal cancer mortality in China during 1987–2009: age, period and birth cohort analyzes. Cancer Epidemiol. 2012;36:99–105. doi: 10.1016/j.canep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ibiebele TI, Hughes MC, Whiteman DC, Webb PM, Australian Cancer Study Dietary patterns and risk of oesophageal cancers: a population-based case-control study. Br J Nutr. 2011;107:1207–1216. doi: 10.1017/S0007114511004247. [DOI] [PubMed] [Google Scholar]
- 6.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwano H, Kato H, Miyazaki T, et al. Genetic alterations in esophageal cancer. Surg Today. 2005;35:7–18. doi: 10.1007/s00595-004-2885-3. [DOI] [PubMed] [Google Scholar]
- 10.Hagymási K, Tulassay Z. Risk factors for esophageal cancer, and possible genetic background. Orv Hetil. 2009;150:407–413. doi: 10.1556/OH.2009.28558. (In Hungarian). [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Ishizaki H. Deuterium isotope effect on the metabolism of N-nitrosodimethylamine and related compounds by cytochrome P4502E1. Xenobiotica. 1992;22:1165–1173. doi: 10.3109/00498259209051870. [DOI] [PubMed] [Google Scholar]
- 12.Raucy JL, Kraner JC, Lasker JM. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol. 1993;23:1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- 13.Ingelman-Sundberg M, Ronis MJ, Lindros KO, Eliasson E, Zhukov A. Ethanol-inducible cytochrome P4502E1: regulation, enzymology and molecular biology. Alcohol Alcohol Suppl. 1994;2:131–139. [PubMed] [Google Scholar]
- 14.Pentiuk OO, Kachula SO, Herych OKh. Cytochrome P4502E1. Polymorphism, physiological function, regulation, and role in pathology. Ukr Biokhim Zh. 2004;76:16–28. (In Ukrainian). [PubMed] [Google Scholar]
- 15.Lucas D, Ménez C, Floch F, et al. Cytochromes P4502E1 and P4501A1 genotypes and susceptibility to cirrhosis or upper aerodigestive tract cancer in alcoholic caucasians. Alcohol Clin Exp Res. 1996;20:1033–1037. doi: 10.1111/j.1530-0277.1996.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin DX, Tang YM, Peng Q, Lu SX, Ambrosone CB, Kadlubar FF. Susceptibility to esophageal cancer and genetic polymorphisms in glutathione S-transferases T1, P1, and M1 and cytochrome P450 2E1. Cancer Epidemiol Biomarkers Prev. 1998;7:1013–1018. [PubMed] [Google Scholar]
- 17.Tan W, Song N, Wang GQ, et al. Impact of genetic polymorphisms in cytochrome P450 2E1 and glutathione S-transferases M1, T1, and P1 on susceptibility to esophageal cancer among high-risk individuals in China. Cancer Epidemiol Biomarkers Prev. 2000;9:551–556. [PubMed] [Google Scholar]
- 18.Hori H, Kawano T, Endo M, Yuasa Y. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and human esophageal squamous cell carcinoma susceptibility. J Clin Gastroenterol. 1997;25:568–575. doi: 10.1097/00004836-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lu XM, Zhang YM, Lin RY, et al. Relationship between genetic polymorphisms of metabolizing enzymes CYP2E1, GSTM1 and Kazakh’s esophageal squamous cell cancer in Xinjiang, China. World J Gastroenterol. 2005;11:3651–3654. doi: 10.3748/wjg.v11.i24.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi QL. Genetic susceptibility, environment risk factors and their interaction in esophageal cancer. 2000 PhD dissertation, (Available at: http://www.globethesis.com/?t=1104360185996737). [Google Scholar]
- 21.Gao CM, Takezaki T, Sugimura H, et al. The Impact of CYP2E1 RsaI, GSTT1 and GSTM1 polymorphisms on the risk of esophageal cancer. China Cancer. 2001;10:346–349. (In Chinese). [Google Scholar]
- 22.Gao C, Takezaki T, Wu J, et al. Interaction between cytochrome P-450 2E1 polymorphisms and environmental factors with risk of esophageal and stomach cancers in Chinese. Cancer Epidemiol Biomarkers Prev. 2002;11:29–34. [PubMed] [Google Scholar]
- 23.Shi Y, Zhou XW, Zhou YK, Ren S. Analysis of CYP2E1, GSTMl genetic polymorphisms in relation to human lung Cancer and esophageal carcinoma. J Huazhong Univ Sci Tech. 2002;31:14–17. (In Chinese). [Google Scholar]
- 24.Shi RH, Wang W, Yu LZ, Huang XY, Chen ZQ, Zhao ZQ. The association between cytochrome P-450 2E1 and Glutathione enzyme gene P1 gene polymorphisms and esophageal cancer susceptibility. Chin J Digestive Endoscopy. 2004;21:392–394. (In Chinese). [Google Scholar]
- 25.Yin LH, Pu YP, Song YH, Hu X, Liu YZ, Kai HT. Polymorphisms of susceptible genes for esophageal cancer risk in Huaian population in Jiangsu province. Tumor. 2005;25:357–361. (In Chinese). [Google Scholar]
- 26.Li H. Case-control study of the risk factors to esophageal carcinoma. 2006 MSc thesis. (Available at: http://globethesis.com/?t=2144360185452638).
- 27.Dong CX, Wu J, Jin Y, Zhang J. Correlation between genetic polymorphism of CYP2E1, GSTM1 and esophageal cancer in Gansu. Chin J Gastroenterol Hepatol. 2007;16:115–118. (In Chinese). [Google Scholar]
- 28.Liu R, Yin LH, Pu YP. Association of combined CYP2E1 gene polymorphism with the risk for esophageal squamous cell carcinoma in Huai’an population, China. Chin Med J (Engl) 2007;120:1797–1802. [PubMed] [Google Scholar]
- 29.Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z, Guo QH. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol. 2008;14:1444–1449. doi: 10.3748/wjg.14.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin JM, Yang L, Chen B, et al. Interaction of methylenetetrahydrofolate reductase C677T, cytochrome P4502E1 polymorphism and environment factors in esophageal cancer in Kazakh population. World J Gastroenterol. 2008;14:6986–6992. doi: 10.3748/wjg.14.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia H, Zhou J, Chen HJ. The research on the relationship between CD44, CYP2E1 genetic polymorphisms and esophageal cancer. Chin Oncol. 2008;18:195–198. (In Chinese). [Google Scholar]
- 32.Yang YF. A study on risk factors and biomarkers associated with the different stages of esophageal squamous cell carcinogenesis in Feicheng county. 2008 PhD dissertation. (Available at: http://www.globethesis.com/?t=1114360245996153).
- 33.Zhang B, Wu J. Correlation between genetic polymorphism of CYP2E1 and susceptibility of esophageal cancer in Gansu Province. J Chengde Med Coll. 2008;25:245–248. (In Chinese). [Google Scholar]
- 34.Niu Y, Yuan H, Leng W, Pang Y, Gu N, Chen N. CYP2E1 RsaI/PstI polymorphism and esophageal cancer risk: a meta-analysis based on 1,088 cases and 2,238 controls. Med Oncol. 2011;28:182–187. doi: 10.1007/s12032-010-9455-x. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LD, Liu B, Feng CW, et al. Histopathological comparison of the lesions at esophagus, gastric cardia and antrum on the subjects from Linzhou, Henan and the Linzhou migrated from Changzhi, Shanxi. J Zhengzhou Univ (Medical Sciences) 2006;41:5–9. [Google Scholar]
- 40.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 41.Morita M, Saeki H, Mori M, Kuwano H, Sugimachi K. Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery. 2002;131(Suppl 1):S1–S6. doi: 10.1067/msy.2002.119287. [DOI] [PubMed] [Google Scholar]
- 42.Akbari MR, Malekzadeh R, Nasrollahzadeh D, et al. Familial risks of esophageal cancer among the Turkmen population of the Caspian littoral of Iran. Int J Cancer. 2006;119:1047–1051. doi: 10.1002/ijc.21906. [DOI] [PubMed] [Google Scholar]
- 43.Dhillon PK, Farrow DC, Vaughan TL, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93:148–152. doi: 10.1002/ijc.1294. [DOI] [PubMed] [Google Scholar]
- 44.Garavello W, Negri E, Talamini R, et al. Family history of cancer, its combination with smoking and drinking, and risk of squamous cell carcinoma of the esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:1390–1393. doi: 10.1158/1055-9965.EPI-04-0911. [DOI] [PubMed] [Google Scholar]
- 45.Tavani A, Negri E, Franceschi S, La Vecchia C. Risk factors for esophageal cancer in lifelong nonsmokers. Cancer Epidemiol Biomarkers Prev. 1994;3:387–392. [PubMed] [Google Scholar]
- 46.Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–328. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li JY, Ershow AG, Chen ZJ, et al. A case-control study of cancer of the esophagus and gastric cardia in Linxian. Int J Cancer. 1989;43:755–761. doi: 10.1002/ijc.2910430502. [DOI] [PubMed] [Google Scholar]
- 48.Morita M, Kuwano H, Nakashima T, et al. Family aggregation of carcinoma of the hypopharynx and cervical esophagus: special reference to multiplicity of cancer in upper aerodigestive tract. Int J Cancer. 1998;76:468–471. doi: 10.1002/(sici)1097-0215(19980518)76:4<468::aid-ijc4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Tang L, Sun G, et al. Etiological study of esophageal squamous cell carcinoma in an endemic region: a population-based case control study in Huaian, China. BMC Cancer. 2006;6:287. doi: 10.1186/1471-2407-6-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Taylor PR, Li JY, et al. Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People’s Republic of China. Cancer Causes Control. 1993;4:195–202. doi: 10.1007/BF00051313. [DOI] [PubMed] [Google Scholar]
- 51.Tian F, Cheng QS, Yu LL. Meta-analysis of risk factors of esophageal cancer in China. Journal of the Fourth Military Medical University. 2003;24:2196–2198. (In Chinese). [Google Scholar]
- 52.Liu YX, Wang B, Wan MH, Tang WF, Huang FK, Li C. Meta-analysis of the relationship between the metholenetetrahydrofolate reductase C677T genetic polymorphism, folate intake and esophageal cancer. Asian Pac J Cancer Prev. 2011;12:247–252. [PubMed] [Google Scholar]
- 53.Moaven O, Raziee HR, Sima HR, et al. Interactions between glutathione-S-transferase M1, T1 and P1 polymorphisms and smoking, and increased susceptibility to esophageal squamous cell carcinoma. Cancer Epidemiol. 2010;34:285–290. doi: 10.1016/j.canep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Tong Z, ZhuGe J, Yu Y. Researches on the polymorphism of cytochrome P450 2A6. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2002;19:424–427. (In Chinese). [PubMed] [Google Scholar]
- 55.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer. 2001;95:140–143. doi: 10.1002/1097-0215(20010520)95:3<140::aid-ijc1024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Zhang J, Zhao Y, et al. The Arg194Trp polymorphism in the XRCC1 gene and cancer risk in Chinese mainland population: a meta-analysis. Mol Biol Rep. 2011;38:4565–4573. doi: 10.1007/s11033-010-0588-y. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Wang F, Shan S, et al. Genetic polymorphism of p53, but not GSTP1, is association with susceptibility to esophageal cancer risk - a meta-analysis. Int J Med Sci. 2010;7:300–308. doi: 10.7150/ijms.7.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5’-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991;110:559–565. doi: 10.1093/oxfordjournals.jbchem.a123619. [DOI] [PubMed] [Google Scholar]