Abstract
Background
Considerable evidence suggests that cigarette smoking is associated with a higher risk of colorectal cancer. What is unclear, however, is the impact of quitting smoking on risk attenuation and whether other risk factors for colorectal cancer modify this association.
Methods
We performed a pooled analysis of 8 studies, including 6,796 colorectal cancer cases and 7,770 controls to evaluate the association between cigarette smoking history and colorectal cancer risk, and to investigate potential effect modification by other risk factors.
Results
Current smokers (OR=1.26, 95% CI=1.11–1.43) and former smokers (OR=1.18, 95% CI=1.09–1.27), relative to never smokers, showed higher risks of colorectal cancer. Former smokers remained at higher colorectal cancer risk, relative to never smokers, for up to about 25 years after quitting. The impact of time since quitting varied by cancer subsite: the excess risk due to smoking decreased immediately after quitting for proximal colon and rectal cancer, but not until about 20 years post-quitting for distal colon cancer. Further, we observed borderline statistically significant additive interactions between smoking status and BMI (relative excess risk due to interaction [RERI]=0.15, 95% CI:−0.01–0.31, P=0.06) and significant additive interaction between smoking status and fruit consumption (RERI=0.16, 95% CI: 0.01–0.30, P=0.04).
Conclusion
Colorectal cancer risk remained increased for about 25 years after quitting smoking, and the pattern of decline in risk varied by cancer subsite. BMI and fruit intake modified the risk associated with smoking.
Impact
These results contribute to a better understanding of the mechanisms through which smoking impacts colorectal cancer etiology.
Keywords: smoking, colorectal cancer, smoking status, time since quitting smoking, multiplicative and additive interaction, body mass index, vegetable and fruit intake
Introduction
Colorectal cancer is the third most common cancer in men and the second most common cancer in women worldwide.(1) Almost 60% of the cases occur in developed countries.(1) The wide variation in colorectal cancer incidence across countries and the dramatic increase in colorectal cancer incidence with economic development after 1900 indicate that lifestyle and environment play prominent roles in the development of this disease.(2–4) One lifestyle factor that may play a role in such geographic variation and temporal patterns of colorectal cancer incidence is cigarette smoking. Whereas cigarette consumption is now decreasing in developed countries, it is continuing to increase in many developing countries (e.g. China and India). (5)
Although some earlier studies (6–8) did not detect a significant association between smoking and colorectal cancer, many studies provide support that cigarette smoking is a risk factor.(9–17) Two recent meta-analyses suggested that current and former smokers have about an 18% higher risk of colorectal cancer compared to never smokers.(9, 10) However, the impact of time since quitting smoking is still not well understood. In particular, there remains some question as to how quickly the risk of colorectal cancer decreases after quitting smoking and whether the excess risk due to smoking could be completely eliminated. The answer to this question is important for public health, including screening decisions. Previous meta-analyses (9, 10) were based on summary statistics extracted from published articles, and therefore they could not uniformly categorize variables (such as time since quitting smoking) and control for other smoking related variables and potential confounders which may lead to less precise estimates. More precise estimates of the association between smoking and colorectal cancer risk are important to aid understanding of the biological mechanism underlying the association between smoking and colorectal cancer. Furthermore, it is not known whether factors associated with risk of colorectal cancer, such as BMI, sex, fruit and vegetables consumption, or use of non-steroidal anti-inflammatory drugs (2, 18) modify the association between smoking and risk of colorectal cancer. An appropriately powered analysis of such interactions requires individual-level data and large sample sizes.
In this study, we used the data from the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) (19) to examine the association between cigarette smoking and risk of colorectal cancer, including assessment of the impact of time since quitting smoking, and to investigate interactions between cigarette smoking and other lifestyle factors.
Materials and Methods
Study population
The GECCO study is supported by the US National Cancer Institute and it is comprised of well-characterized prospective cohorts and case-control studies of colorectal cancer. (19) Details of studies have been described previously (19) and are provided in Supplementary Material 1. Five cohort studies (the Health Professionals Follow-up Study (HPFS) (15); the Nurses’ Health Study (NHS) (14); the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (20); the VITamins and Lifestyle Study (VITAL) (21); and the Women’s Health Initiative (WHI) (22)) and three population-based case-control studies (the Colon Cancer Family Registry (CCFR) (23); the Diet, Activity and Lifestyle Survey (DALS) (24, 25); and the Ontario Familial Colorectal Cancer Registry (OFCCR) (26)) were included in this analysis. Subjects who were included in both the CCFR and the OFCCR were excluded from the CCFR. All participants gave informed consent, and studies were approved by the Institutional Review Board.
All colorectal cancers were invasive colorectal adenocarcinomas, confirmed by medical records, pathologic reports, or death certificates. Colorectal cancer cases had International Classification of Diseases, 9th edition (ICD 9) site codes of 153.0–153.4, 153.6–153.9, and 154.0–154.1. Cases arising from the cohort studies were included in this analysis with a matched set of controls. Details on matching criteria are described in the supplemental material. Inclusion was restricted to those with available DNA because GECCO is focused on genetic and environmental factors related to colorectal cancer. Subjects using pipes, cigars, or snuff were excluded.
Before exclusions, the eight studies comprised data from 7,310 cases and 8,113 controls. We excluded participants with missing information on smoking (223 cases and 76 controls) and appendix cancer cases (27 cases). Because the majority of study participants self-reported non-Hispanic white race / ethnicity (96.5% non-Hispanic white, 0.3% Hispanic, 1.1% African American, 1.1% Asian, 0.3% American Indian, 0.4% others, 0.3% missing), we restricted our analysis to non-Hispanic white participants. After these exclusions, 6,796 cases of colorectal cancer and 7,770 controls remained in the analysis.
Statistical analysis
The descriptions of the smoking-related variables used in this study were provided in Supplementary Material 2. We used a two-stage pooled approach to evaluate the association between smoking and risk of colorectal cancer: (1) using multiple logistic regression models to calculate study-specific odds ratios (OR) and the corresponding 95% confidence intervals (CI); and (2) using an inverse variance-weighted random-effects meta-analysis approach (27) to pool the study-specific ORs to generate summary ORs. For the analyses of smoking status and pack-years, the following covariates were adjusted: age at reference time, sex, BMI(<25, 25–<30, ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), alcohol intake (0–1 g/day, 1<−28 g/day, >28 g/day, when available), and study site (if applicable); for the analyses of time since quitting smoking and age at cessation, multiple logistic regression models included the aforementioned covariates as well as categorized pack-years of smoking (never smoker, ≤20, 21–40, 41–60, >60 pack-years); for the analyses of smoking intensity and smoking duration, we additionally adjusted for smoking duration and smoking intensity, respectively. Additional adjustment for other variables, including family history of colorectal cancer, history of sigmoidoscopy/colonoscopy, use of NSAIDs, physical activity, and dietary variables (i.e. total energy, red meat, processed meat, dietary fiber, vegetables, and fruits), did not appreciably alter our estimates and were not included in final models. Trend tests were performed for pack-years, time since quitting smoking, age at cessation, smoking intensity, and smoking duration by evaluating these variables as continuous variables (for pack-years, smoking intensity, and smoking duration, never smokers were assigned to 0; for time since quitting smoking, current smokers were assigned to 0 and never smokers were excluded; for age at cessation, never and current smokers were excluded). We also performed analyses by cancer subsite, colon (ICD 9: 153.0–153.4, 153.6, 153.7, or 153.9) vs. rectum (ICD 9: 154.0 or 154.1), and for colon cancer, we further stratified by proximal (ICD 9: 153.0, 153.1, 153.4, or 153.6) vs. distal colon (ICD 9: 153.2, 153.3, or 153.7) cancer. All cases in DALS are colon cancers, and hence, it was not included in analyses of rectal cancer. We stratified by study design (case-control vs. cohort study) to evaluate whether summary ORs were affected by study design, and conducted leave-one study-out analyses (omitting each study in turn and redoing meta-analysis) to examine if a single study dominated the summary ORs.
We used nonparametric regression analysis through fitting a restricted cubic spline (28, 29) to logistic regression models to examine colorectal cancer risk as a function of time since quitting and accounting for the possibly nonlinear relationship. We treated time since quitting as a continuous variable with current smokers assigned to 0 and used as the reference group (never smokers were excluded). For this analysis, all studies were merged into a single dataset with adjustment for study and the knots were established through automatically stepwise selection. Likelihood ratio tests were used to test nonlinearity by comparing spline models to a linear model.(29)
To assess whether there were multiplicative interaction effects on the risk of colorectal cancer between smoking status (ever vs. never smoker) and risk factors including BMI (<25, ≥25 kg/m2), sex (male, female), fruit and vegetable consumption (both dichotomized at sex- and study- specific medians [servings/day]), and use of NSAIDs (yes/no), we conducted analyses in logistic regression models: a) stratified by the potential effect modifiers; b) including multiplicative interaction terms of the potential modifiers and smoking status.
To evaluate additive interaction effects, we used linear odds-ratio models with interaction terms between the potential effect modifiers as listed above and smoking status (30). We used an inverse variance-weighted random-effects meta-analysis approach (27) to pool study specific coefficient estimates of interaction terms. Wald tests were performed to test whether summary estimates were equal to 0 and Wald-type confidence intervals were computed. In linear odds-ratio models, the estimated coefficients of interaction terms are estimators of relative excess risk due to interaction (RERI) (30, 31), a measure of additive interaction. In the calculation of RERI, we used never smoker, BMI <25 kg/m2, male, fruit and vegetable consumption greater than or equal to the sex- and study- specific median (servings/day), and any NSAID use as the reference groups. Studies that were restricted to one sex (HPFS, NHS, and WHI) were excluded in interaction analyses with sex.
In all pooled analyses, we calculated I2 to estimate the percentage of total variation across studies due to heterogeneity beyond chance (32) and Q statistics to test heterogeneity across studies. (33) All statistical tests were two-sided. All analyses were performed using R software version 2.14 and SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina).
Results
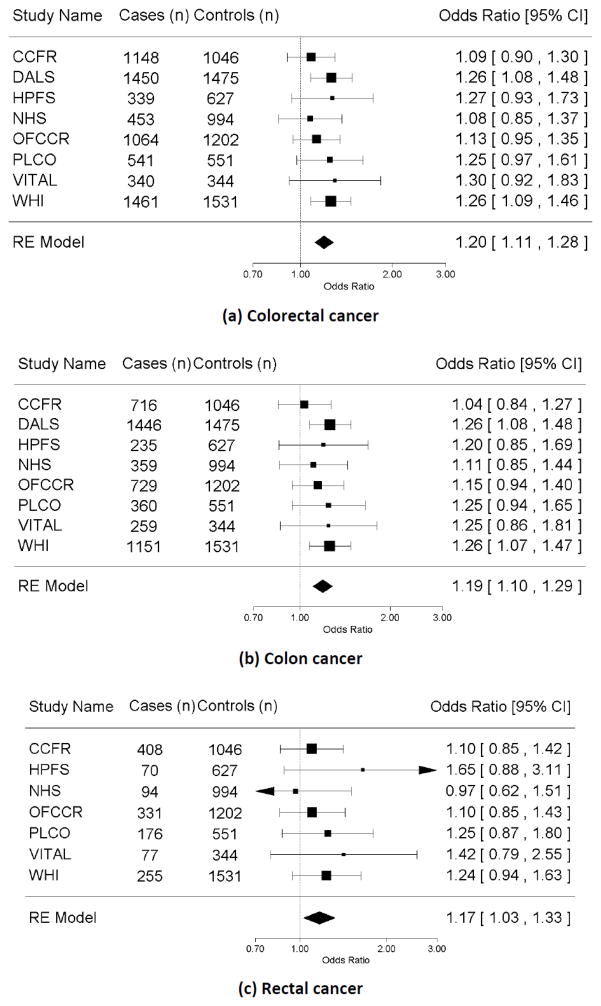
The basic characteristics of each study involved in this analysis are described in Table 1. The fraction of ever smokers across studies varied from 53% to 64% among cases and from 48% to 59% among controls. Our pooled analysis showed that the risk of colorectal cancer was 20% higher for ever smokers compared with never smokers (OR=1.20, 95% CI=1.11–1.28; I2=0, Pheterogeneity =0.82; Table 2 and Figure 1a). The results did not differ by cancer subsite (colon vs. rectal cancer [P=0.98]; proximal vs. distal colon cancer [P=0.99], Table 2, 3 and Figure 1b, 1c). We observed elevated risk of colorectal cancer with increased pack-years of smoking overall and when stratified by colon and rectal cancers.
Table 1.
Description of the characteristics by study
| Study | Design | Case, n | Control, n | Smoking, n (%)
|
Age, years Mean (SD) |
Sex, %men | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Former | Current | |||||||||||
| case | control | case | control | case | control | case | control | case | control | ||||
| CCFR | Case-control | 1148 | 1046 | 517 (45) | 493 (47) | 414 (36) | 405 (39) | 217 (18) | 148 (14) | 54 (11) | 57 (11) | 53 | 44 |
| DALS | Case-control | 1450 | 1475 | 592 (41) | 701 (48) | 650 (45) | 577 (39) | 208 (14) | 197 (13) | 64 (10) | 64 (5) | 56 | 56 |
| HPFS | Cohort | 339 | 627 | 133 (39) | 288 (46) | 190 (56) | 305 (49) | 16 (5) | 34 (5) | 67 (8) | 66 (8) | 100 | 100 |
| NHS | Cohort | 453 | 994 | 192 (42) | 443 (45) | 196 (43) | 431 (43) | 65 (14) | 120 (12) | 60 (7) | 60 (6) | 0 | 0 |
| OFCCR | Case-control | 1064 | 1202 | 442 (42) | 497 (41) | 530 (50) | 589 (49) | 92 (9) | 116 (10) | 61 (9) | 62 (9) | 40 | 55 |
| PLCO | Cohort | 541 | 551 | 235 (43) | 272 (49) | 249 (46) | 240 (44) | 57 (11) | 39 (7) | 64 (5) | 64 (5) | 57 | 57 |
| VITAL | Cohort | 340 | 344 | 124 (36) | 159 (46) | 186 (55) | 165 (48) | 30 (9) | 20 (6) | 67 (6) | 67 (6) | 55 | 55 |
| WHI | Cohort | 1461 | 1531 | 683 (46) | 799 (52) | 667 (46) | 643 (42) | 111 (8) | 89 (6) | 66 (7) | 66 (6) | 0 | 0 |
| Total | 6796 | 7770 | 2918 (45) | 3652 (43) | 3082 (43) | 3355 (47) | 796 (12) | 763 (10) | 62 (10) | 63 (9) | 39 | 40 | |
Table 2.
Association of smoking-related variables and risk of colorectal cancer, colon and rectal cancer
| Exposure | Colorectal cancer
|
Colon cancer
|
Rectal cancer a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% C.I.) e | I2, % | Cases | OR (95% C.I.) e | I2, % | Cases | OR (95% C.I.) e | I2, % | |
| Smoking status | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| Ever smoker | 3878 | 4118 | 1.20 (1.11, 1.28) | 0 | 2989 | 1.19 (1.10, 1.29) | 0 | 813 | 1.17 (1.03, 1.33) | 0 |
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| Former smoker | 3082 | 3355 | 1.18 (1.09, 1.27) | 0 | 2393 | 1.19 (1.09, 1.29) | 0 | 630 | 1.12 (0.98, 1.28) | 0 |
| Current smoker | 796 | 763 | 1.26 (1.11, 1.43) | 8 | 596 | 1.21 (1.06, 1.37) | 0 | 183 | 1.37 (1.11, 1.68) | 0 |
| Pack-years of smoking | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| ≤20 | 1631 | 1941 | 1.08 (0.99, 1.18) | 0 | 1215 | 1.06 (0.96, 1.16) | 0 | 375 | 1.10 (0.94, 1.28) | 0 |
| (20, 40] | 1066 | 1061 | 1.28 (1.15, 1.42) | 0 | 837 | 1.28 (1.15, 1.44) | 0 | 207 | 1.21 (1.00, 1.46) | 0 |
| (40, 60] | 592 | 583 | 1.29 (1.12, 1.48) | 5 | 469 | 1.29 (1.12, 1.49) | 0 | 117 | 1.35 (1.06, 1.73) | 0 |
| >60 | 400 | 336 | 1.37 (1.16, 1.62) | 1 | 321 | 1.32 (1.09, 1.60) | 10 | 73 | 1.40 (1.03, 1.90) | 0 |
| P trend b | 2.5×10−8 | 1.7×10−7 | 0.01 | |||||||
| Time since quitting (years) f | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| Current smoker | 796 | 763 | 1.36 (1.12, 1.64) | 0 | 596 | 1.29 (1.05, 1.58) | 0 | 183 | 1.50 (1.05, 2.13) | 0 |
| (0, 15) | 871 | 762 | 1.47 (1.21, 1.78) | 1 | 681 | 1.37 (1.07, 1.76) | 22 | 182 | 1.51 (1.05, 2.17) | 0 |
| [15,25) | 835 | 836 | 1.31 (1.07, 1.60) | 0 | 652 | 1.30 (1.05, 1.61) | 0 | 169 | 1.29 (0.90, 1.85) | 0 |
| [25,35) | 677 | 784 | 1.15 (0.85, 1.55) | 39 | 518 | 1.04 (0.68, 1.57) | 62 | 135 | 1.09 (0.73, 1.64) | 0 |
| ≥35 | 488 | 744 | 0.74 (0.47, 1.18) | 70 | 395 | 0.73 (0.45, 1.17) | 67 | 85 | 0.70 (0.40, 1.22) | 25 |
| P trend c | 1.7×10−3 | 0.01 | 3.1×10−5 | |||||||
| Age at cessation (years) f | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| <40 | 1081 | 1326 | 1.03 (0.79, 1.34) | 8 | 804 | 0.95 (0.68, 1.33) | 28 | 257 | 1.17 (0.71, 1.92) | 8 |
| [40,50) | 744 | 755 | 1.28 (0.96, 1.70) | 23 | 587 | 1.21 (0.86, 1.69) | 32 | 142 | 1.35 (0.86, 2.11) | 0 |
| ≥50 | 1055 | 1063 | 1.31 (1.01, 1.70) | 26 | 861 | 1.25 (0.92, 1.70) | 36 | 172 | 1.28 (0.86, 1.93) | 0 |
| P trend d | 5.9×10−3 | 7.8×10−3 | 0.30 | |||||||
| Smoking intensity (cig/day) g | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| <20 | 1529 | 1732 | 1.28 (1.11, 1.48) | 0 | 1158 | 1.23 (1.06, 1.43) | 0 | 332 | 1.44 (1.10, 1.88) | 0 |
| =20 | 1212 | 1211 | 1.30 (1.09, 1.55) | 31 | 946 | 1.29 (1.09, 1.52) | 16 | 247 | 1.40 (1.05, 1.86) | 15 |
| >20 | 1022 | 1035 | 1.28 (1.10, 1.49) | 9 | 798 | 1.27 (1.09, 1.48) | 0 | 210 | 1.31 (0.93, 1.83) | 30 |
| P trend b | 0.60 | 0.59 | 0.63 | |||||||
| Smoking duration (years) h | ||||||||||
| Never smoker | 2918 | 3652 | 1.0 | 2266 | 1.0 | 598 | 1.0 | |||
| <10 | 499 | 663 | 0.94 (0.78, 1.13) | 6 | 372 | 0.96 (0.77, 1.20) | 19 | 118 | 0.84 (0.61, 1.15) | 0 |
| [10,20) | 756 | 875 | 1.07 (0.93, 1.24) | 0 | 559 | 1.08 (0.92, 1.27) | 0 | 179 | 1.02 (0.79, 1.33) | 0 |
| [20,30) | 844 | 827 | 1.29 (1.11, 1.50) | 9 | 640 | 1.30 (1.11, 1.52) | 7 | 189 | 1.23 (0.96, 1.59) | 0 |
| [30,40) | 852 | 834 | 1.29 (1.12, 1.48) | 0 | 672 | 1.30 (1.13, 1.51) | 0 | 164 | 1.15 (0.87, 1.51) | 10 |
| ≥40 | 805 | 805 | 1.28 (1.10, 1.49) | 9 | 649 | 1.27 (1.09, 1.48) | 0 | 141 | 1.31 (0.93, 1.83) | 30 |
| P trend b | 0.01 | 0.03 | 0.01 | |||||||
: For rectum cancer, all studies were included except DALS;
: included never smokers and assign them to 0;
: excluded never smokers and assign current smokers to 0;
: among former smokers;
: adjusted for age, sex, BMI (<25, 25–<30), ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), alcohol intake (0–1 g/day, 1<−28 g/day, >28 g/day, when available), and study site (if applicable);
: additionally adjusted for pack-years of smoking (never smoker, ≤20, 21–40, 41–60, >60 pack-years);
: additionally adjusted for smoking duration (never smoker, <10, 10–19, 20–29, 30–39, ≥40 years);
: additionally adjusted for smoking intensity (never smoker, <20, =20, >20 cigarettes per day).
Figure 1.
Forest plot for smoking status (ever vs. never) and risk of (a) colorectal cancer, (b) colon cancer, and (c) rectum cancer ; adjusted for age, sex, BMI (<25, 25–<30), ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), alcohol intake (0–1 g/day, 1<−28 g/day, >28 g/day, when available), and study site (if applicable); RE model: random effect model.
Table 3.
Association of smoking-related variables and risk of proximal and distal colon cancer
| Exposure | Proximal colon cancer | Distal colon cancer | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | OR (95% C.I.) d | I2, % | Cases | OR (95% C.I.) d | I2, % | |
| Smoking status | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| Ever smoker | 1633 | 1.18 (1.08, 1.30) | 0 | 1258 | 1.18 (1.07, 1.31) | 0 |
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| Former smoker | 1313 | 1.17 (1.06, 1.29) | 0 | 1008 | 1.20 (1.08, 1.34) | 0 |
| Current smoker | 320 | 1.26 (1.08, 1.47) | 0 | 250 | 1.10 (0.93, 1.31) | 0 |
| Pack-years of smoking | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| ≤20 | 671 | 1.07 (0.95, 1.20) | 0 | 511 | 1.05 (0.92, 1.20) | 0 |
| (20, 40] | 456 | 1.28 (1.12, 1.47) | 0 | 354 | 1.25 (1.08, 1.46) | 0 |
| (40, 60] | 255 | 1.28 (1.07, 1.53) | 2 | 202 | 1.33 (1.10, 1.61) | 0 |
| >60 | 165 | 1.15 (0.87, 1.52) | 28 | 139 | 1.40 (1.11, 1.77) | 0 |
| P trend a | 4.5×10−4 | 2.1×10−5 | ||||
| Time since quitting (years) e | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| Current Smoker | 320 | 1.23 (0.95, 1.60) | 2 | 250 | 1.24 (0.94, 1.62) | 0 |
| (0, 15) | 341 | 1.16 (0.80, 1.68) | 40 | 318 | 1.51 (1.15, 1.96) | 0 |
| [15,25) | 337 | 1.10 (0.84, 1.44) | 0 | 294 | 1.47 (1.11, 1.96) | 0 |
| [25,35) | 293 | 0.91 (0.59, 1.39) | 43 | 210 | 1.16 (0.79, 1.70) | 22 |
| ≥35 | 255 | 0.69 (0.40, 1.18) | 60 | 130 | 0.74 (0.43, 1.25) | 46 |
| P trend b | 0.04 | 0.01 | ||||
| Age at cessation (years) e | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| <40 | 432 | 0.87 (0.58, 1.29) | 44 | 355 | 1.15 (0.81, 1.63) | 0 |
| [40,50) | 314 | 1.05 (0.74, 1.49) | 36 | 257 | 1.53 (1.10, 2.12) | 0 |
| ≥50 | 493 | 1.24 (0.98, 1.60) | 20 | 342 | 1.51 (1.13, 2.01) | 0 |
| P trend c | 0.04 | 0.05 | ||||
| Smoking intensity (cig/day) f | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| <20 | 639 | 1.21 (1.01, 1.45) | 0 | 478 | 1.22 (0.99, 1.50) | 0 |
| =20 | 530 | 1.36 (1.14, 1.62) | 0 | 388 | 1.19 (0.97, 1.45) | 0 |
| >20 | 411 | 1.22 (1.00, 1.50) | 13 | 360 | 1.25 (1.02, 1.54) | 0 |
| P trend a | 0.85 | 0.36 | ||||
| Smoking duration (years) g | ||||||
| Never smoker | 1266 | 1.0 | 933 | 1.0 | ||
| <10 | 211 | 1.00 (0.79, 1.26) | 0 | 153 | 0.94 (0.72, 1.22) | 0 |
| [10,20) | 297 | 1.01 (0.83, 1.23) | 0 | 242 | 1.13 (0.91, 1.40) | 0 |
| [20,30) | 335 | 1.23 (1.02, 1.48) | 0 | 289 | 1.40 (1.10, 1.78) | 23 |
| [30,40) | 361 | 1.23 (1.03, 1.47) | 0 | 289 | 1.36 (1.11, 1.65) | 0 |
| ≥40 | 372 | 1.22 (1.00, 1.50) | 13 | 253 | 1.25 (1.02, 1.54) | 0 |
| P trend a | 0.23 | 0.02 | ||||
: included never smokers and assign them to 0;
: excluded never smokers and assign current smokers to 0
: among former smokers;
: adjusted for age, sex, BMI (<25, 25–<30), ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), alcohol intake (0–1 g/day, 1<−28 g/day, >28 g/day, when available), and study site (if applicable);
: additionally adjusted for pack-years of smoking (never smoker, ≤20, 21–40, 41–60, >60 pack-years);
: additionally adjusted for smoking duration (never smoker, <10, 10–19, 20–29, 30–39, ≥40 years);
: additionally adjusted for smoking intensity (never smoker, <20, =20, >20 cigarettes per day).
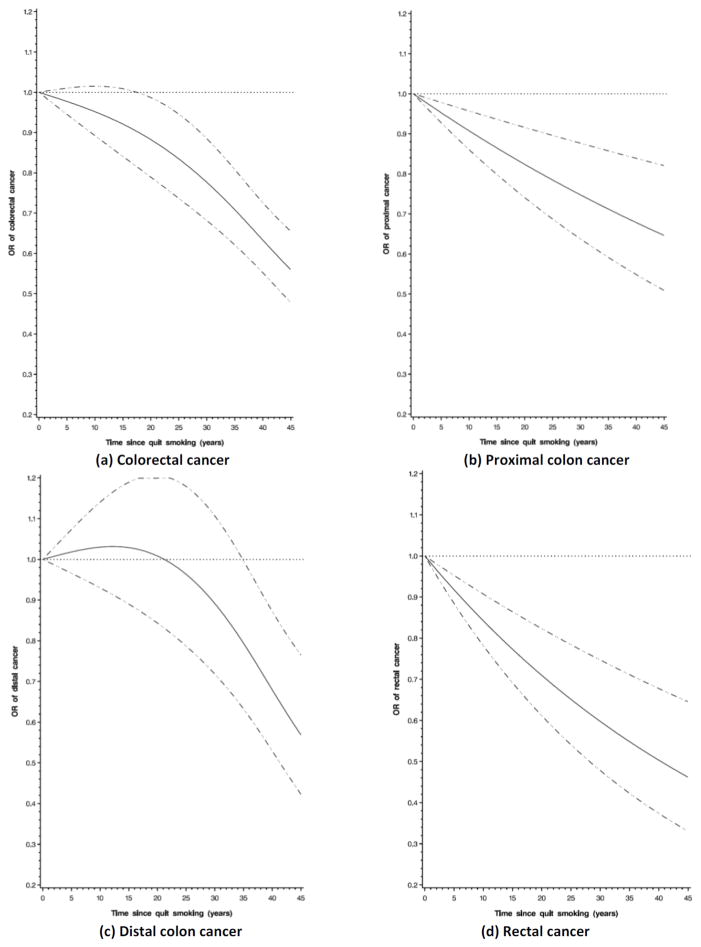
Compared to never smokers, former smokers had statistically significant higher risks of colorectal cancer and colon cancer for up to about 25 years after quitting (Table 2; Supplementary Figure 1). We observed similar trends for colon and rectal cancer, although risk of those quitting smoking 15–25 years was not statistically significant for rectal cancer; however, this is probability due to limited power as risk estimates were similar for colon and rectal cancer. When further stratified by subsite within the colon, risk reduced after a short time since quitting for proximal colon cancer, while, for distal colon cancer, the risk estimates remained statistically significant up to 25 years after quitting smoking (Table 3). To further investigate the association between time since quitting smoking and risk, we ran the nonparametric regression model among smokers only (using current smokers as the reference group). We found that risk declined immediately after quitting smoking for colorectal cancer (Figure 2a). Subsite stratification showed a similar pattern for proximal colon and rectal cancer (Figure 2b, 2d) whereas risk did not decline until about 20 years for distal colon cancer (P for nonlinearity=0.002, Figure 2c). We found between-study heterogeneity in the highest categories of time since quitting smoking for colorectal and colon cancer (I2≥60%, Pheterogeneity≤0.02). When excluding one study at a time from this meta-analysis, exclusion of VITAL reduced heterogeneity the most (for colorectal cancer: I2=41%, Pheterogeneity=0.12, and for colon cancer I2=30%, Pheterogeneity=0.19 for colon cancer), whereas summary risk estimates did not change substantially (OR=0.90, 95%CI= 0.64–1.26 for colorectal cancer; OR=0.91, 95% CI=0.66–1.27 for colon cancer).
Figure 2.
Nonparametric regression curve for the association between time since quit smoking and risk of (a) colorectal cancer, (b) proximal colon cancer, (c) distal colon cancer, and (d) rectal cancer; never smokers were excluded; current smoker was assigned to 0 and used as reference group; stratified by study and additionally adjusted for age, sex, BMI (<25, 25–<30), ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), and pack-years (≤20, 21–40, 41–60, >60 pack-years); solid line is regression curve and dotted line is 95% confidence interval).
If former smokers quit smoking before age of the 40 years we did not observe an elevated risk of colorectal cancer relative to never smokers, whereas colorectal cancer risk was increased in those with older ages at cessation (Table 2). These results were similar for colon and rectal cancer. Risk of colorectal cancer did not vary by smoking intensity. Risk of colorectal cancer was significantly increased in ever smokers who smoked for at least 20 years but was not increased for those who smoked less than 20 years. A similar result was observed for colon and rectal cancer, although results for rectal cancer were not statistically significant and, overall, showed a less clear trend. We observed a borderline statistically significant additive interaction between smoking and BMI (P =0.06) and a statistically significant additive interaction between smoking and fruit consumption (P=0.04) (Table 4). Compared with normal-weight never smokers, the pooled RERI is 0.15 (95% CI,−0.01–0.31; I2=0, Pheterogeneity=0.93); that is, 15% of the excess risk of colorectal cancer for ever smokers with BMI≥25 kg/m2 was attributable to the interaction between smoking and BMI. Compared with never smokers with high fruit consumption, the pooled RERI is 0.16 (95% CI, 0.01–0.30; I2=0, Pheterogeneity=0.79); that is, 16% of the excess risk of colorectal cancer among ever smokers with low fruit consumption was attributable to the interaction between smoking and lower fruit consumption. When we stratified the analysis by other environmental risk factors of interest, the association between colorectal cancer and smoking status (ever vs. never) was stronger among overweight and obese participants and those with low fruit consumption. No other statistically significant interactions (additive or multiplicative) were observed. Because the associations with smoking status were similar across cancer sites, we did not perform interaction analyses by cancer site.
Table 4.
Meta-analysis for the interaction effects between smoking status (ever vs. never) and variables possible or established risk factors of colorectal cancer
| Risk factor of colorectal cancer | Case | Control | OR (95% C.I.) 2 | I2, % | P value for multiplicative interaction b,c | RERI (95% CI) b,d | I2, % | P value for additive interaction b,d |
|---|---|---|---|---|---|---|---|---|
| BMI<25 | 2375 | 3137 | 1.14 (1.10, 1.27) | 0 | 0.210 | 0.15 (−0.01, 0.31) | 0 | 0.06 |
| BMI≥25 | 4293 | 4472 | 1.24 (1.13, 1.35) | 0 | ||||
| Male a | 2341 | 2454 | 1.23 (1.09, 1.38) | 0 | 0.496 | −0.05 (−0.25, 0.16) | 0 | 0.66 |
| Female | 2202 | 2164 | 1.18 (1.08, 1.29) | 0 | ||||
| Fruit consumption (<median) | 3114 | 3404 | 1.27 (1.14, 1.41) | 0 | 0.112 | 0.16 (0.01, 0.30) | 0 | 0.04 |
| Fruit consumption (≥median) | 3272 | 3951 | 1.13 (1.03, 1.25) | 0 | ||||
| Vegetables consumption (<median) | 2825 | 3156 | 1.18 (1.06, 1.32) | 0 | 0.492 | −0.02 (−0.19, 0.15) | 0 | 0.81 |
| Vegetables consumption (≥median) | 3618 | 4235 | 1.21 (1.10, 1.34) | 8 | ||||
| Any NSAID usage (Yes) | 2201 | 3195 | 1.23 (1.09, 1.39) | 0 | 0.900 | 0.14 (−0.04, 0.32) | 0 | 0.14 |
| Any NSAID usage (No) | 4539 | 4492 | 1.21 (1.11, 1.32) | 0 |
: In interaction analysis with sex, studies only including one sex were excluded (HPFS, NHS, and WHI);
: Adjusted for age, sex, BMI (<25, 25–<30), ≥30 kg/m2), education (high school graduate or less, some college or technical school, and college graduate or higher), alcohol intake (0–1 g/day, 1<−28 g/day, >28 g/day, when available), and study site (if applicable).
: Multiplicative interaction effects were evaluated by use of logistic regression models with interactive terms.
: Additive interaction effects were examined by use of linear odds ratio models with interactive terms; in the calculation of RERI, the reference groups are nerve smoker, BMI (<25 kg/m2), male, fruit consumption (≥ sex, study specific median [servings/day]), vegetables consumption (≥ sex, study specific median [servings/day]), any NSAID use (Yes), and alcohol intake (≤1 g/day).
Discussion
In our large pooled analysis, we confirmed results from previous studies showing that smoking is associated with increased risk of colorectal cancer. Excess risks remained up to about 25 years after quitting smoking, but risk starts to decline immediately after quitting smoking for proximal colon and rectal cancer and about 20 years later for distal colon cancer. Further, we observed marginal statistically significant additive interactions of smoking with both BMI and fruit consumption.
There remains debate in the literature about the impact of time since quitting smoking on risk of colorectal cancer. Some studies have suggested that excess risk of colorectal cancer persists indefinitely among former smokers (14–16, 34), whereas other studies have suggested that the higher risk of colorectal cancer for former smokers is attenuated and eventually becomes comparable to that of never smokers (11, 12); however, results are not consistent on when the risk starts to decline and when the excess risk is fully eliminated. When we evaluated this questions consistently across studies, we found that compared to current smokers, former smokers experienced a lower risk of colorectal cancer soon after quitting, although they still had a higher risk compared to never smokers up to about 25 years since quitting. Further, we observed differences in this pattern by cancer subsite: risk started to decline among former smokers right after quitting smoking for proximal colon and rectal cancer and about 20 years later for distal colon cancer. Growing evidence suggests that there are the substantial subsite differences in colorectal cancer by genetic etiology, gene expression, molecular pathogenesis, and protein profiles.(2, 35, 36) These disparities may contribute to the observed different associations with time since quitting by cancer subsite. In particular, recent studies have indicated that smoking is more strongly associated with a particular molecular phenotype of colorectal tumors, those that are microsatellite instability (MSI) high and possess mutations in the BRAF gene (37, 38), as well as with the relevant precursor lesions.(39) As these tumors are seen more frequently in the proximal than in the distal colon (35), smoking cessation may benefit proximal more than distal tumors. As we observed, however, our failure to find different risks associated with smoking in the distal and proximal colon suggests that additional factors may be involved. Further research is required to explore the mechanism underlying the difference in our findings by cancer subsite. Our large pooled analysis suggests that the risk in former smokers remains increased for a long time compared to never smokers.
It has been suggested that pack-years of smoking, a combination of smoking intensity and duration, may misrepresent the individual effects of these two characteristics because they may not equally contribute to disease risk.(40, 41) Thus, we evaluated the effects of smoking intensity and duration separately while controlling one variable for the other. Our results suggested that both duration and intensity increased colorectal cancer risk and that patterns with both variables appeared nonlinear. This non-linear plateau effect is consistent with some previous studies (12, 42) and has been observed for other cancers (e.g. lung, liver, kidney, pancreas, and bladder cancer (43, 44)). This finding may point to potential molecular mechanisms such as saturation of smoking-derived carcinogen activation pathways.(45, 46)
We were able to investigate interactions of smoking with various environmental risk factors. We observed statistical evidence for additive interaction between fruit intake and smoking status on risk of colorectal cancer. An interaction with plant foods has been reported for other cancers as well (e.g. lung cancer (47) and pancreatic cancer (48)). The potential biological mechanism for this interaction may be that anticarcinogenic components in fruits modify the effects of smoking through reducing DNA damage and mutation from smoking carcinogens (49). We also found a borderline statistically significant additive interaction between BMI and smoking status. The biologic mechanism for the interaction between BMI and smoking status is unclear, but possible explanations include the pro-oxidant and inflammatory effects of increased insulin, glucose, insulin-like growth factors (IGF), and related compounds that accompany overweight and obesity which, in turn, may enhance the rate of accumulation of DNA damage due to smoking (50), and that immunosuppressive effects of specific free fatty acids (FFA) from adipocytes may increase the susceptibility to cancer triggered by smoking.(51) However, given the marginal significance of our findings, it will be important that these results are replicated in other large studies, such as available in the Cohort Consortium.(52) We note that when exploring interactions on the multiplicative scale we observed no interaction. Rothman and others (53, 54) have remarked that assessment of interaction should mainly be based on an additive scale and it has been illustrated that under causal pie models biological interaction results in departure from additivity of disease rates.(55)
This pooled analysis has several strengths, including the large sample size and the availability of individual-level data from each study on detailed smoking exposures, major confounders, and potential effect modifiers. The availability of individual data permitted us to consistently and flexibly evaluate exposure-disease relationship, potential confounding, and interaction effects. We observed little evidence for heterogeneity and risk estimates overall did not vary substantially between studies. Our results were not dominated by a single study and did not vary by study design (case-control vs. cohort studies).
There are also some limitations to this analysis. Because we restricted the analysis to non-Hispanic white participants with available DNA as the parent-study from which these data were drawn (GECCO) is focused on genetic and environmental factors, it is likely that our study populations do not represent the full range of social-economic status or racial and ethnic groups. However, effect estimates of smoking status and the relationship with pack-years are consistent with those from previous meta-analyses.(9, 10) Additionally, similar association between CRC and smoking was observed in Asians.(56, 57) Case-control studies could be affected by recall bias. However, studies showed that recalled information on tobacco use is valid and reliable (58, 59) and furthermore, results from case-control and cohort studies were similar. The reference time at which smoking exposure was assessed for HPFS and NHS was at time of blood draw rather than time of enrollment. Accordingly, prevalent cases may bias smoking effect estimates in the two studies. Nevertheless, dropping prevalent cases (n=91) in these two studies did not influence our results. Due to the difference in study design, current smoking was defined differently in cohort vs. case-control studies. However, this has not led to obvious heterogeneity in results. We adjusted for BMI as a potential confounder in our study but BMI could be either a confounder or a mediator of the association between smoking and CRC given the impact of smoking on BMI. However, the results without adjustment for BMI are similar to those with BMI adjustment and our conclusions don’t change. When evaluating additive interaction, we used asymptotic variance estimates from linear odds ratio models in meta-analysis approach and calculated Wald-type confidence intervals for pooled estimates of additive interaction effects. Some researchers indicated that Wald-type confidence interval based on asymptotic variance may have poor coverage at typical sample size and likelihood-based confidence interval may be preferred.(60, 61) However, studies showed that in large sample sizes or at disease prevalence below 10%, Wald-type confidence interval works well and is similar to likelihood-based confidence interval.(30, 62)
In summary, our findings confirmed previous results of positive association between smoking and colorectal cancer. We evaluated the effect of time since quitting smoking in detail and found that the increased risk persisted for about 25 years after quitting smoking; however, risk started to decline immediately after quitting smoking for proximal colon and rectal cancer and about 25 year later for distal colon cancer. The observed effect modification of smoking and colorectal cancer by BMI and fruit consumption, if replicated in future independent studies, could contribute to better understanding of the mechanisms and potentially improving strategies for colorectal cancer prevention.
Supplementary Material
Acknowledgments
HPFS, NHS: We would like to acknowledge Carolyn Guo who assisted in programming for NHS and HPFS, the participants, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA,WA, WY.
PLCO: The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, at the National Cancer Institute, the screening center investigators and staff of the PLCO Cancer Screening Trial, Mr. Thomas Riley and staff at Information Management Services, Inc., and Ms. Barbara O’Brien and staff at Westat, Inc. for their contributions to the PLCO Cancer Screening Trial. Most importantly, we acknowledge the study participants for their contributions to making this study possible.
WHI: The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
Grant support
CCFR was supported by the National Cancer Institute, National Institutes of Health under RFA # CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and P.I.s. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. The following Colon CFR centers contributed data to this manuscript and were supported by the following sources: Australasian Colorectal Cancer Family Registry (U01 CA097735), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800), Seattle Colorectal Cancer Family Registry (U01 CA074794) and the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783). The OFCCR was also supported by a GL2 grant from the Ontario Research Fund, the Canadian Institutes of Health Research, and the Cancer Risk Evaluation (CaRE) Program grant from the Canadian Cancer Society Research Institute. TJH and BWZ are recipients of Senior Investigator Awards from the Ontario Institute for Cancer Research, through generous support from the Ontario Ministry of Research.
DALS is supported by the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (R01 CA48998).
GECCO is supported by the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (U01 CA137088). C.M.H. was supported by a training grant from the National Cancer Institute, Institutes of Health, U.S. Department of Health and Human Services (R25 CA094880) Funding for the genome-wide scan of DALS, PLCO, and WHI was provided by the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (R01 CA059045).
HPFS is supported by the National Institutes of Health (P01 CA 055075, R01 137178, and P50 CA 127003).
NHS is supported by the National Institutes of Health (R01 137178, P50 CA 127003, and P01 CA 087969).
PLCO is supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Control samples were derived from the Prostate, Lung, Colon and Ovarian Screening Trial, which was supported by the Intramural Research Program of the National Cancer Institute.
VITAL is supported in part by the National Institutes of Health (K05 CA154337) from the National Cancer Institute and Office of Dietary Supplements.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Conflicts of Interest:
Andrew T. Chan declares a minor conflict of interest in his role as a consultant/advisory board member of Bayer HealthCare, Pfizer Inc. and Millenium Pharmaceuticals
References
- 1.Ferlay JSH, Bray F, Forman D, Mathers C, Parkin DM. Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10 [Internet] 2008. GLOBOCAN 2008 v1.2. [Google Scholar]
- 2.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. 3. Oxford ; New York: Oxford University Press; 2006. [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Bingham S, Riboli E. Diet and cancer--the European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4:206–15. doi: 10.1038/nrc1298. [DOI] [PubMed] [Google Scholar]
- 5.PROJECTIONS OF TOBACCO PRODUCTION, CONSUMPTION AND TRADE TO THE YEAR 2010. FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS; 2003. [Google Scholar]
- 6.Nyren O, Bergstrom R, Nystrom L, Engholm G, Ekbom A, Adami HO, et al. Smoking and colorectal cancer: a 20-year follow-up study of Swedish construction workers. Journal of the National Cancer Institute. 1996;88:1302–7. doi: 10.1093/jnci/88.18.1302. [DOI] [PubMed] [Google Scholar]
- 7.Wakai K, Hayakawa N, Kojima M, Tamakoshi K, Watanabe Y, Suzuki K, et al. Smoking and colorectal cancer in a non-Western population: a prospective cohort study in Japan. Journal of epidemiology / Japan Epidemiological Association. 2003;13:323–32. doi: 10.2188/jea.13.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doll R, Gray R, Hafner B, Peto R. Mortality in relation to smoking: 22 years’ observations on female British doctors. British medical journal. 1980;280:967–71. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 10.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. International journal of cancer Journal international du cancer. 2009;124:2406–15. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 11.Hannan LM, Jacobs EJ, Thun MJ. The association between cigarette smoking and risk of colorectal cancer in a large prospective cohort from the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:3362–7. doi: 10.1158/1055-9965.EPI-09-0661. [DOI] [PubMed] [Google Scholar]
- 12.Leufkens AM, Van Duijnhoven FJ, Siersema PD, Boshuizen HC, Vrieling A, Agudo A, et al. Cigarette smoking and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:137–44. doi: 10.1016/j.cgh.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Luchtenborg M, White KK, Wilkens L, Kolonel LN, Le Marchand L. Smoking and colorectal cancer: different effects by type of cigarettes? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1341–7. doi: 10.1158/1055-9965.EPI-06-0519. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. Journal of the National Cancer Institute. 1994;86:192–9. doi: 10.1093/jnci/86.3.192. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. Journal of the National Cancer Institute. 1994;86:183–91. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 16.Sturmer T, Glynn RJ, Lee IM, Christen WG, Hennekens CH. Lifetime cigarette smoking and colorectal cancer incidence in the Physicians’ Health Study I. Journal of the National Cancer Institute. 2000;92:1178–81. doi: 10.1093/jnci/92.14.1178. [DOI] [PubMed] [Google Scholar]
- 17.Heineman EF, Zahm SH, McLaughlin JK, Vaught JB. Increased risk of colorectal cancer among smokers: results of a 26-year follow-up of US veterans and a review. International journal of cancer Journal international du cancer. 1994;59:728–38. doi: 10.1002/ijc.2910590603. [DOI] [PubMed] [Google Scholar]
- 18.Food, Nutrition and Physical Activity and the Prevention of Colorectal Cancer. World Cancer Research Fund / American Institute for Cancer Research; 2011. Continuous Update Project Interim Report Summary. [Google Scholar]
- 19.Peters U, Hutter CM, Hsu L, Schumacher FR, Conti DV, Carlson CS, et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum Genet. 2011 doi: 10.1007/s00439-011-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled clinical trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 21.White E, Patterson RE, Kristal AR, Thornquist M, King I, Shattuck AL, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. American journal of epidemiology. 2004;159:83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 22.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 24.Slattery ML, Friedman GD, Potter JD, Edwards S, Caan BJ, Samowitz W. A description of age, sex, and site distributions of colon carcinoma in three geographic areas. Cancer. 1996;78:1666–70. doi: 10.1002/(sici)1097-0142(19961015)78:8<1666::aid-cncr5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Slattery ML, Potter J, Caan B, Edwards S, Coates A, Ma KN, et al. Energy balance and colon cancer--beyond physical activity. Cancer research. 1997;57:75–80. [PubMed] [Google Scholar]
- 26.Zanke BW, Greenwood CM, Rangrej J, Kustra R, Tenesa A, Farrington SM, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nature genetics. 2007;39:989–94. doi: 10.1038/ng2089. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. American journal of epidemiology. 2006;163:1053–64. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 28.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge ; New York: Cambridge University Press; 2003. [Google Scholar]
- 29.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Statistics in medicine. 2007;26:3735–52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 30.Richardson DB, Kaufman JS. Estimation of the relative excess risk due to interaction and associated confidence bounds. American journal of epidemiology. 2009;169:756–60. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Slattery ML, Potter JD, Friedman GD, Ma KN, Edwards S. Tobacco use and colon cancer. International journal of cancer Journal international du cancer. 1997;70:259–64. doi: 10.1002/(sici)1097-0215(19970127)70:3<259::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. International journal of oncology. 2010;37:707–18. doi: 10.3892/ijo_00000720. [DOI] [PubMed] [Google Scholar]
- 36.Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12:755–62. [PubMed] [Google Scholar]
- 37.Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2745–50. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. Journal of the National Cancer Institute. 2010;102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1012–8. [PubMed] [Google Scholar]
- 40.Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. American journal of epidemiology. 2002;156:813–23. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 41.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer research. 2003;63:6556–62. [PubMed] [Google Scholar]
- 42.Limburg PJ, Vierkant RA, Cerhan JR, Yang P, Lazovich D, Potter JD, et al. Cigarette smoking and colorectal cancer: long-term, subsite-specific risks in a cohort study of postmenopausal women. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2003;1:202–10. doi: 10.1053/cgh.2003.50030. [DOI] [PubMed] [Google Scholar]
- 43.Vineis P, Kogevinas M, Simonato L, Brennan P, Boffetta P. Levelling-off of the risk of lung and bladder cancer in heavy smokers: an analysis based on multicentric case-control studies and a metabolic interpretation. Mutation research. 2000;463:103–10. [PubMed] [Google Scholar]
- 44.Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: intensity patterns in the alpha-tocopherol, beta-carotene cancer prevention study in Finnish men. American journal of epidemiology. 2008;167:970–5. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 45.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 46.Lutz WK. Dose-response relationships in chemical carcinogenesis: superposition of different mechanisms of action, resulting in linear-nonlinear curves, practical thresholds, J-shapes. Mutation research. 1998;405:117–24. doi: 10.1016/s0027-5107(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 47.Buchner FL, Bueno-de-Mesquita HB, Ros MM, Overvad K, Dahm CC, Hansen L, et al. Variety in fruit and vegetable consumption and the risk of lung cancer in the European prospective investigation into cancer and nutrition. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2278–86. doi: 10.1158/1055-9965.EPI-10-0489. [DOI] [PubMed] [Google Scholar]
- 48.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Vegetable intake and pancreatic cancer risk: the multiethnic cohort study. American journal of epidemiology. 2007;165:138–47. doi: 10.1093/aje/kwj366. [DOI] [PubMed] [Google Scholar]
- 49.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. The American journal of medicine. 1994;97:5S–13S. doi: 10.1016/0002-9343(94)90292-5. discussion 22S–8S. [DOI] [PubMed] [Google Scholar]
- 50.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. The Journal of nutrition. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 51.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. The American journal of clinical nutrition. 2007;86:s867–71. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 52.National Cancer Institute. Cohort Consortium. http://epigrantscancergov/Consortia/worldmaphtml.
- 53.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 54.Koopman JS. Interaction between discrete causes. American journal of epidemiology. 1981;113:716–24. doi: 10.1093/oxfordjournals.aje.a113153. [DOI] [PubMed] [Google Scholar]
- 55.Rothman KJ. Epidemiology : an introduction. New York, N.Y: Oxford University Press; 2002. [Google Scholar]
- 56.Otani T, Iwasaki M, Yamamoto S, Sobue T, Hanaoka T, Inoue M, et al. Alcohol consumption, smoking, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan Public Health Center-based prospective study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12:1492–500. [PubMed] [Google Scholar]
- 57.Ho JW, Lam TH, Tse CW, Chiu LK, Lam HS, Leung PF, et al. Smoking, drinking and colorectal cancer in Hong Kong Chinese: a case-control study. International journal of cancer Journal international du cancer. 2004;109:587–97. doi: 10.1002/ijc.20018. [DOI] [PubMed] [Google Scholar]
- 58.Brigham J, Lessov-Schlaggar CN, Javitz HS, Krasnow RE, Tildesley E, Andrews J, et al. Validity of recall of tobacco use in two prospective cohorts. American journal of epidemiology. 2010;172:828–35. doi: 10.1093/aje/kwq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brigham J, Lessov-Schlaggar CN, Javitz HS, McElroy M, Krasnow R, Swan GE. Reliability of adult retrospective recall of lifetime tobacco use. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:287–99. doi: 10.1080/14622200701825718. [DOI] [PubMed] [Google Scholar]
- 60.Moolgavkar SH, Venzon DJ. General relative risk regression models for epidemiologic studies. American journal of epidemiology. 1987;126:949–61. doi: 10.1093/oxfordjournals.aje.a114733. [DOI] [PubMed] [Google Scholar]
- 61.Greenland S. Additive risk versus additive relative risk models. Epidemiology. 1993;4:32–6. doi: 10.1097/00001648-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Vanderweele TJ, Vansteelandt S. A weighting approach to causal effects and additive interaction in case-control studies: marginal structural linear odds models. American journal of epidemiology. 2011;174:1197–203. doi: 10.1093/aje/kwr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.