Abstract
Many biologic agents that were first approved for the treatment of malignancies are now being actively investigated and used in a variety of autoimmune diseases such as rheumatoid arthritis (RA), antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, systemic lupus erythematosus (SLE), and Sjogren’s syndrome. The relatively recent advance of selective immune targeting has significantly changed the management of autoimmune disorders, and in part, can be attributed to the progress made in understanding effector cell function and their signaling pathways. In this review, we will discuss the recent FDA approved biologic therapies that directly target immune cells as well as the most promising investigational drugs affecting immune cell function and signaling for the treatment of autoimmune disease.
Keywords: Autoimmune disease, Autoimmunity, Biologic therapy, Rheumatoid arthritis, Systemic lupus erythematosus, ANCA associated vasculitis, Sjogren’s syndrome, B cell, T cell, Tyrosine kinase inhibitors, chemokine receptors, Cellular targeting
INTRODUCTION
There have been important advances in our understanding of the pathogenesis of autoimmune disease over the past two decades, which have led to an expanding array of biologic therapeutics targeting selective immune responses. The goal has been to suppress disease with minimal toxicity and global immunosuppression. Successful cytokine neutralization therapy, such as anti-tumor necrosis factor-α (TNFα), anti-interleukin 6 (IL-6), and anti-interleukin 1 (IL-1), have changed the practice and management of autoimmune disease for clinical immunologists. In this review, we will move beyond anti-cytokine based therapy and highlight several new, exciting cellular targets in autoimmune disease. Particular focus will be placed on the mechanistic rationale for each drug and a brief discussion of the patient trials supporting their use. To classify the vast field of biologics currently in the clinical and pre-clinical arena, we have further categorized them into extracellular (B cell, T cell, and chemokine receptor) and intracellular (mitogen activated protein kinases and the tyrosine kinases) targets.
EXTRACELLULAR TARGETS IN AUTOIMMUNITY
B Lymphocyte Targets
B lymphocytes play an important role in pathophysiology of autoimmune disease, lending rationale to the therapeutic targeting of B cells both directly and indirectly. B cells have numerous roles in autoimmunity, most notably differentiating into plasma cells and producing autoantibodies. Autoantibodies activate immune cells and the secretion of pro-inflammatory cytokines such as IL-6, IL-10, and TNFα, which lead to tissue damage (Figure 1) [1]. Additionally, B cells act as antigen presenting cells providing co-stimulation for T cells, which then further activate the pro-inflammatory cascade [2]. In rheumatoid arthritis (RA), B cells from the synovial membrane produce rheumatoid factor (RF), which is associated with aggressive articular and extra-articular disease [3]. In systemic lupus erythematosus (SLE), immune dysregulation and breaks in self-tolerance result in increased production of autoantibodies such as anti-double-stranded DNA antibodies, which are pathogenic and associated with lupus nephritis [4, 5]. This section will highlight B cell targeted therapy in autoimmunity that is further subcategorized into direct B cell depleting agents or indirect B cell functional inhibitors.
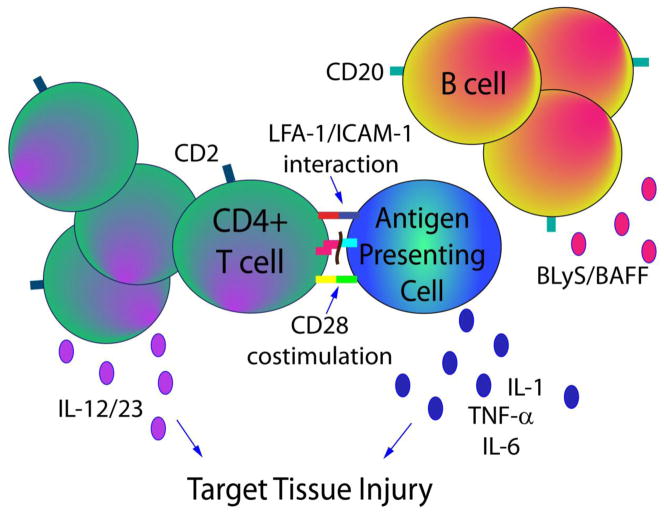
Figure 1. FDA approved biologic therapies in rheumatic autoimmune disease.
In addition to cytokine neutralization (IL-1, IL-6, TNFα, IL-12/23), direct cellular targeting is being used successfully in autoimmune disease. Rituximab targets B-cell surface marker CD20 resulting in B cell depletion, whereas belimumab targets the BLyS pathway that ultimately affects B cell maturation and survival. With respect to T cell effector function, abatacept blocks CD28 co-stimulation with CD80/86 after antigen presentation and T cell receptor engagement. In contrast, alefacept (CD2 antagonism with a LFA-3 fusion protein) and efalizumab (LFA-1/ICAM-1 inhibition) decrease T cell activation or migration, respectively.
B Cell Depletion
Although the exact pathogenesis of autoimmune diseases such as SLE, RA, Sjogren’s syndrome, and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis is not completely understood, there is abundant evidence that B cells play a critical role in the pathogenesis of each of these diseases [5–8]. Monoclonal antibodies directed against the specific B cell surface markers CD20 or CD22 result in B cell depletion by complement-mediated cytotoxicity and antibody dependent cell-mediated cytotoxicity [3, 9]. Rituximab is a chimeric antibody against CD20 which was first approved for treatment of patients with CD20 positive B cell non-Hodgkin lymphoma and is now used in various autoimmune diseases including RA, SLE, Sjogren’s syndrome, ANCA associated vasculitis, and hepatitis C virus (HCV) related and non-HCV related cryoglobulinemia [10, 11] (Figure 1 and Table 1). CD20 is an attractive target as it is expressed on greater than 95% of B cells, including immature to mature B cells and memory B cells, but it is not present on stem cells, pro-B cells, or plasma cells[12]. In contrast, CD22 is a transmembrane sialoadhesion molecule that is expressed specifically on the surface of activated B cells and memory cells, but like CD20, it is not expressed on differentiated plasma cells [13, 14]. CD22 is an inhibitory co-receptor, which negatively regulates B cell receptor (BCR)-induced calcium signaling [13]. B cells from CD22-deficient mice have enhanced apoptosis and are reduced in number in the bone marrow and circulation, suggesting that CD22 plays an important role in B cell development and survival [15].
Table 1.
Approved and promising cellular targets under clinical investigation in autoimmunity.
| Extracellular Target | Predominant Cell Type | Drug name | Clinical Status | Disease | Citation(s) |
|---|---|---|---|---|---|
| CD20 | B cell | Rituximab | FDA Approved | RA/GPA/MPA | (10), (16), (17) (18), (25), (29), (30) |
| RCT | Cryoglobulinemic Vasculitis/Sjogren’s Syndrome | (37), (38), (41) | |||
| B Cell | Ofatumumab | Phase III | RA | (49), (50) | |
| CD22 | B Cell | Epratuzumab | Phase III*** | SLE | (53) (56) |
| Phase I/II | Sjogren’s syndrome | (57) | |||
| BLyS/BAFF | B Cell | Belimumab | FDA approved | SLE | (64), (65), (66) |
| Blisibimod | Phase II | SLE | (67), (69) | ||
| Tabalumab | Phase III**** | SLE/RA | (67) | ||
| CD28 | T Cell | Abatacept | FDA approved | RA/JIA | (75), (76), (77), (78) (79), (80), (81) |
| Phase II | SLE/Psoriatic Arthritis | (82), (84) | |||
| CD2 | T Cell | Alefacept | FDA approved | Psoriasis | (85) |
| Alefacept + MTX | RCT | Psoriatic Arthritis | (88) | ||
| CD11a | T Cell | Efalizumab | FDA approved* | Psoriasis | (89) |
| Phase II** | Psoriatic Arthritis | (90) | |||
| CD25 | T Cell | Daclizumab | Phase II | Posterior and JIA associated Uveitis/Multiple Sclerosis | (95), (96), (97), (99) |
| CCR5 | T Cell and Myeloid cells | Maraviroc | Phase II** | RA | (108) |
| AZD5672 | Phase II** | RA | (109) | ||
| CCR1 | T Cell and Myeloid cells | CCX354-C | Phase II | RA | (115) |
| Intracellular Target | Predominant Cell Type | Drug name | Clinical Status | Disease | Citation(s) |
| Jak | B cell & T Cell | Tofacitinib | Phase III | RA | (140), (141), (142), (143) |
| Syk | B cell & T Cell | Fostamatinib | Phase III | RA | (147), (148), (149) |
| ABL/C-Kit | Fibroblast | Imatinib | Phase I/II | SSc | (160), (161), (162) |
Efalizumab has been voluntarily withdrawn from the market.
Failed to reach primary endpoint of ACR20.
Phase III trials discontinued due to medication supply interruptions.
Phase III trials ongoing.
Rituximab
Rituximab in Rheumatoid Arthritis
RA affects roughly 0.5–1% of the population and is a chronic, systemic autoimmune disease characterized by synovitis and systemic inflammation that can lead to functional impairment, disability, and increased mortality [8]. Rituximab has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of RA in patients who have had an inadequate response to methotrexate or are intolerant to anti-TNFα therapy [16, 17] (Figure 1 and Table 1). Rituximab is also uniquely recommended for use in RA patients with treated malignancy, in part based on its specificity of targeting B cells solely while leaving other arms of the immune system intact. In specific, rituximab was recently reviewed as safe for RA patients who have been treated for non-melanoma, solid-tumor malignancy beyond five years and was the only biologic recommended for use in patients with treated melanoma or lymphoproliferative malignancy. This was based on grade C level of evidence [18]. Additionally, the Rituximab Consensus Expert Committee, an international group of experts and patient representatives, have recently published an in-depth review and consensus statement on rituximab in RA including recommended dosage, screening, vaccination, and safety considerations. These recommendations were based on numerous studies and randomized controlled trials, which support the use of rituximab in RA [10].
Rituximab in ANCA-Associated Vasculitis
ANCA-associated vasculitis (AAV) encompasses a group of systemic autoimmune vasculitidies that affect small and medium sized vessels and include granulomatosis with polyangiitis (GPA; formerly Wegener’s granulomatosis) [19], microscopic polyangiitis (MPA), and Churg-Strauss syndrome (CSS). AAV is a multisystem autoimmune disease characterized by pauci-immune, necrotizing, crescentic glomerulonephritis and often pulmonary involvement including interstitial lung disease, pulmonary nodules, and pulmonary alveolar hemorrhage. ANCA directed against proteinase 3 (PR3-ANCA) are seen predominantly in GPA while anti-myeloperoxidase antibodies (MPO-ANCA) are present in both MPA and CSS [20]. However, there is overlap between the autoantibodies in these vasculitidies, and a small percentage of patients are ANCA negative. High dose glucocorticoids and cyclophosphamide have been the standard of care for AAV and are effective in inducing remission in 70–90% of patients [21, 22]. However, 25% of patients experience a serious adverse event or unsustained remission [23], and at least 5% of patients are refractory to standard treatment [24]. Thus, the need for alternative treatments and an increased understanding of disease pathogenesis led to interest in B cell targeted therapies, such as rituximab.
Rituximab in combination with glucocorticoids was recently approved by the European Medicine Agency and the FDA for the treatment of adults with GPA or MPA [25] and is the first FDA approved treatment for these diseases (Table 1). B cell activation correlates with AAV disease activity [26], and ANCAs have been shown to be directly pathogenic. [27]. Thus, targeting CD20 positive B cells, which are thought to be plasma cell precursors, could reduce pathogenic antibodies and also affect pro-inflammatory cytokine production, antigen presentation to T cells, and T cell activation [28].
In addition to pre-clinical and early phase clinical trials, the evidence for using rituximab in AAV stems largely from two land mark randomized controlled trials, RAVE and RITUXVAS [29, 30]. The RAVE trial was a multicenter, randomized, double-blind, non-inferiority trial of rituximab compared to oral cyclophosphamide in 197 patients with newly diagnosed or relapsing MPO or PR3-ANCA positive GPA and MPA. Both groups received high dose intravenous methylprednisolone followed by an oral prednisone taper over 5 months. The primary end point was remission of disease at 6 months as defined by a Birmingham Vasculitis Activity Score (BVAS) score of 0 and a successful prednisone taper. Secondary end-points included rates of disease flares, cumulative steroid dose, rates of adverse events, and short form-36 (SF-36) score. Sixty-four percent of the patients in the rituximab group compared to 52% in the control group reached the primary end-point, thus meeting criteria for non-inferiority (p<0.0001). In a subgroup analysis, rituximab was more efficacious in patients with relapsing AAV compared to traditional cyclophosphamide, and there were no significant differences in the number of disease or non-disease adverse events between groups.
RITUXVAS was a multicenter, open-label, two-group parallel-designed, randomized controlled trial comparing rituximab with intravenous cyclophosphamide as induction therapy in 44 patients with AAV. Results were similar to the RAVE trial in that rituximab-based therapy was not inferior to cyclophosphamide. The primary end points were remission at 12 months as defined by BVAS score of 0 and severe adverse events. Seventy six percent of patients in the rituximab group and 82% of patients in the control group reached the primary end-point of sustained remission (p=0.68). Although rituximab therapy would seemingly be less toxic than standard cyclophosphamide, there were similar rates of adverse events and deaths in both groups [30].
Although, rituximab has been shown to be non-inferior to standard cytotoxic therapy for AAV, questions remain regarding maintenance therapy. Two retrospective studies addressing this question have reported that rituximab was tolerated over the long term, but did not completely prevent relapse [31, 32]. A prospective randomized controlled trial is needed to further investigate the optimal long-term maintenance therapy for AAV.
Rituximab in Cryoglobulinemic vasculitis
Rituximab has also recently been shown to be efficacious in HCV-related cryoglobulinemic vasculitis. Cryoglobulinemic vasculitis is a systemic inflammatory condition of small and medium vessels that commonly manifests as skin ulcerations, purpura, glomerulonephritis, and neuropathy [33]. Cryoglobulinemic vasculitis is mediated by immune complexes that precipitate at temperatures less than 37°C and are generated by the clonal expansion of B cells that produce IgM and/or IgG rheumatoid factor. Often this is in the setting of chronic infection such as hepatitis C but also can occur from lymphoproliferative B cell disorders or other autoimmune diseases [34]. The standard of care for HCV-related cryoglobulinemia has been antiviral agents, glucocorticoids, and cytotoxic drugs; however, this regimen can be ineffective, poorly tolerated, and have serious adverse side effects [35, 36]. The rationale for rituximab use in HCV-cryoglobulinemia is to directly target the pathologic clonal B cell population. Two recent randomized controlled trials by De Vita et al. [37] and Sneller et al. [38] found treatment with rituximab to be significantly more efficacious than standard immunosuppression in patients with HCV-related cryoglobulinemia who had failed or had contraindications to antiviral therapy.
Rituximab in Sjogren’s syndrome
Rituximab has also been examined in Sjogren’s syndrome, which is a chronic, systemic autoimmune disorder of secretory exocrine glands characterized by lymphocytic infiltration resulting in dry mucosal surfaces of the mouth, eyes, nose, and vagina [39]. Sjogren’s syndrome may be primary or secondary related to other autoimmune diseases such as RA, SLE, or scleroderma. B cell hyperactivity with production of autoantibodies IgG and IgM rheumatoid factor (RF), anti-nuclear antibody (ANA), anti-SSa, and anti-SSb are the hallmark of primary Sjogren’s syndrome [6], suggesting that B cell targeted therapies would be efficacious in this disorder. Rituximab was examined in a randomized, double blind, placebo-controlled trial of 30 patients with primary Sjogren’s syndrome as defined by the American College of Rheumatology [40]. Patients receiving Rituximab had improvement in whole saliva flow, multidimensional fatigue inventory score, and visual analog scale for sicca symptoms, and the treatment proved to be safe over the 48-week follow-up [41]. There are case reports of using rituximab to successfully treat Sjogren’s related neuropathy [42]; however, there have not been clinical trials addressing neuropathy or other more rare manifestations of primary Sjogren’s syndrome to date.
Rituximab in Systemic Lupus Erythematosus
B lymphocytes are also central players in the pathogenesis of systemic lupus erythematosus, a complex multisystem chronic autoimmune disease characterized by vasculitis, autoantibody production, immune dysregulation, loss of self-tolerance, and immune complex deposition [43]. The clinical manifestations of SLE are quite variable from skin disease, synovitis, and cytopenias to severe life threatening glomerulonephritis, cerebritis, pericarditis, or pneumonitis. Despite the significant improvement in the 5-year survival of SLE patients with severe disease, SLE patients continue to have increased morbidity, decreased quality of life, and increased health care expenditures [44]. Current therapies for SLE include glucocorticoids, antimalarials, cytotoxic agents, and immunosuppressants. A systematic review of the use of rituximab identified 188 cases between 2002–2007 and found that 91% of patients showed a significant improvement in a variety of SLE disease manifestations, including the severe form of lupus nephritis [45]. Much has been learned about the role of B cells in SLE through the use of B cell depleting agents [46], but despite the success of rituximab in case studies, two large randomized, double blind, placebo controlled trials of rituximab in systemic lupus, LUNAR and EXPLORER, had disappointing results and did not show significant efficacy compared to standard immunosuppression [47, 48]. However, in both studies, all patients were treated with substantial amounts of other immunosuppression, which could have confounded results. Given the positive supporting case report data for rituximab in lupus, these negative clinical trials raise several questions about the role of B cell depletion therapy in lupus, including whether specific patient subtypes, demographics, or disease presentations would best respond or whether combination B cell depletion and functional inhibition should be considered. As the overall response rate was only 50% percent in both the rituximab and standard of care group for both trials, there is a clear need for on-going investigation in this challenging disease.
Other B cell Depleting Agents
Ofatumumab
Ofatumumab is a human IgG1 lytic monoclonal antibody that binds to a different epitope from rituximab on the CD20 cell surface protein. Ofatumumab was found to be effective in RA patients who previously failed disease modifying anti-rheumatic drug (DMARD) therapy in a phase I/II placebo-controlled, dose escalating trial. A greater number of patients in all treatment doses of ofatumumab achieved ACR20 (the American College of Rheumatology composite measure of 20% improvement in RA disease activity) compared to placebo, and 70% had moderate to good EULAR response criteria (the change in Disease Activity Score or DAS) at 24 weeks. The most notable serious adverse event was mild-to-moderate infusion reactions after the first infusion [49]. A follow-up phase III study in 260 RA patients with active disease, despite methotrexate, was also successful in that 50% of patients randomized to ofatumumab achieved an ACR20 compared to 27% of placebo patients (p<0.001), and 67% of ofatumumab treated patients had a good or moderate EULAR response of 67% compared to 41% placebo.
Serious adverse events were similar (5% treated, 3% placebo) [50]. Ofatumumab is therefore a promising new therapeutic target, with the exception of a significantly increased risk of initial infusion reactions that do appear to wane with subsequent exposure (<1% on second dose infusions) [50]. Long term safety endpoints are still under active investigation and are yet to be determined. This is of particular importance given that B cell depleting therapies such as rituximab do confer an associated increased risk of progressive multifocal leukoencephalopathy (PML) in subsets of substantially immunocompromised patients [51].
Epratuzumab
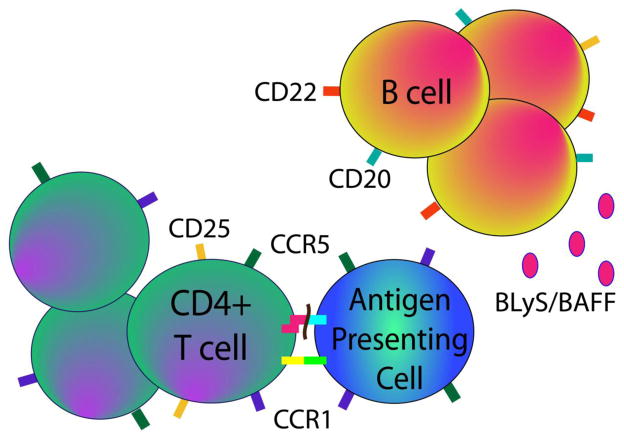
Epratuzumab is a humanized monoclonal antibody, which targets CD22 on the extracellular surface of B cells (Figure 2). Epratuzumab negatively modulates B cell receptor activation and results in modest antibody-dependent cellular cytotoxicity, but not complement-dependent cytotoxicity [52]. Although the antibody-dependent cellular cytotoxicity effects of epratuzumab are modest, a 35% decrease in the peripheral B cell count in SLE patients has been observed [53]. Later studies have shown that epratuzumab also affects the expression of adhesion molecules and the migration of B cells toward the CXCL12 chemokine (also known as SDF-1 or stromal cell derived factor-1)[54]. Epratuzumab was first studied in non-Hodgkin lymphoma (NHL) and other B cell malignancies, [55] but because of the central role of B cells in the pathogenesis of autoimmune disease, it has recently been studied in SLE and Sjogren’s syndrome as well (Table 1). The initial phase II trial of epratuzumab in SLE was an open-label single center study of 14 adult patients with moderately active SLE. At six months, 93% of patients experienced improvement in at least one BILAG (British Isles Lupus Assessment Group) B or C level disease activity score. The 35% of B cells that depleted did not repopulate during the 6 months after treatment and was well tolerated [53]. Two subsequent randomized controlled phase III trials of epratuzumab in active SLE patients were unfortunately discontinued due to medication supply interruptions. In these combined studies, 90 patients were randomized to placebo or epratuzumab. At 48 weeks, a modest reduction in BILAG scores was noted in the epratuzumab group compared to placebo. Additionally steroid doses were decreased and quality of life measures were improved in the treatment arm compared to placebo [56].
Figure 2. Investigational extracellular therapeutic targets in autoimmunity.
Given the success of B cell neutralization by depletion (rituximab) or maturation arrest (belimumab), several other exploratory drugs targeting CD20 (ofatumumab) or the BLyS signaling pathway (blisibimod, tabalumab) are in clinical trials for autoimmunity. Additionally, epratuzumab, which binds B cell-expressed CD22, negatively modulates B-cell receptor activation. Targeting the activation and migration of multiple inflammatory cell types similarly shows safety and promise in autoimmune disease. Daclizumab inhibits CD25 and thus affects cellular activation, and chemokine receptor antagonists to CCR1 and CCR5 affect migration of proinflammatory cells into target tissues.
Epratuzumab has also been studied as an open-labeled phase I/II study in primary Sjogren’s syndrome. Sixteen adult patients with primary Sjogren’s syndrome received epratuzumab, and at 6 months, 67% reached the primary end-point of a greater than 20% reduction in 2 parameters: Schirmer’s test, whole salivary flow rate, fatigue, sedimentation rate, and IgG levels [57]. Furthermore, epratuzumab had an acceptable safety profile suggesting it could be an attractive future therapy for primary Sjogren’s syndrome. However, further safety data and larger clinical trials will need to be performed in both SLE and Sjogren’s syndrome before strong conclusions can be drawn.
B Cell Function Inhibitors
Indirect or functional B cell inhibition can be achieved by targeting cytokine pathways involved in B cell maturation and survival. The B-lymphocyte stimulator (BLyS) pathway within the TNF superfamily includes two cytokine members (BLyS, also known as B cell activating factor (BAFF), and proliferation-inducing ligand APRIL), as well as three BAFF receptors (BAFF-R or BR3, B cell maturation antigen (BCMA), and transmembrane activator and calcium-modulating cyclophilin interactor (TACI)). The BLyS pathway plays an important role in B-cell differentiation and selection [58], and the association with autoimmunity was discovered from the finding that BLyS transgenic mice develop autoimmune illness similar to SLE and Sjogren’s syndrome [59]. Furthermore, BLyS levels have been shown to be elevated in human patients with SLE and Sjogren’s syndrome [60, 61], and several studies show a correlation with autoantibody production in lupus patients [58].
Belimumab
Belimumab is a human IgG1λ monoclonal antibody that binds to soluble human BLyS and inhibits its activity [62]. BLyS inhibition specifically targets the transitional and naïve B cell populations and has less effect on memory B cells and plasma cells [63]. Two international, randomized, placebo-controlled phase III trials, BLISS-52 (n=865) and BLISS-76 (n=819), compared belimumab to placebo with background standard therapy in autoantibody positive adult SLE patients [64, 65]. Both studies had similar designs enrolling ANA or dsDNA antibody positive SLE patients with active disease, but excluded severe central nervous system and renal disease patients. At 48 weeks and 72 weeks respectively, patients treated with the higher dose of 10mg/kg of belimumab reached the primary endpoint in both BLISS-52 and BLISS-76 trials. In March of 2011, it was FDA approved for treating autoantibody-positive SLE patients with active disease despite treatment with standard immunosuppressives, corticosteroids, anti-malarials and nonsteroidal anti-inflammatory medications. A post hoc analysis examining the disease activity across multiple organ systems noted the greatest improvement was in the musculoskeletal and mucocutaneous systems [66]. As belimumab is only indicated for the treatment of lupus despite standard immunosuppression, the practical application and indications for optimal belimumab use have not been yet clearly defined. Further studies investigating the efficacy of belimumab in severe renal, CNS, and hematologic disease as well as other subpopulations of lupus patients are still needed.
Blisibimod and Tabalumab
Blisibimod, also known as A-623 or AMG-623, is a peptibody BAFF (BLyS) antagonist which binds both soluble and cell surface bound BAFF (Figure 2). Blisibimod, given subcutaneously, targets BAFF, but not APRIL [67]. Treatment with AMG-623 in animal studies of murine collagen induced arthritis (CIA) and NZB/NZW F1 lupus prone mice resulted in decreased B cell numbers and improvement in arthritis and lupus [68]. The results from the unpublished PEARL-SC phase IIb trial of blisibimod in SLE patients were recently released and revealed statistically significant improvement in blisibimod-treated patients compared to those receiving placebo at 20 weeks [69]. Further studies are underway (NCT01395745, NCT01305746) (Table 1).
Tabalumab, also known as LY2127399, is a non-synthetic subcutaneous BAFF antagonist which binds both soluble and membrane bound BAFF, but not APRIL[67], and is of great interest for both SLE and RA patients (Figure 2). Several clinical trials for both RA (NCT00689728, NCT00785928, NCT01202773) and SLE (NCT01196091, NCT01205438, NCT01488708) are underway (Table 1).
T Lymphocyte and Co-stimulatory Targets
Activated T cells play a significant role in the inflammatory process in autoimmune disease. The synovial inflammatory cell infiltrate in RA contains abundant T cells, which proliferate and induce the production of inflammatory cytokines such as IL-17 in T helper 17 subsets, and IL-17, IL-23, TNFα, IL-1 and IL-6 by other inflammatory cells [70] (Figure 1). Activated T cells are also found in significant numbers in the inflamed skin and joints in psoriatic arthritis [71], and T cells stimulate B cells leading to increased production of autoantibodies in SLE [5].
The activation of T cells requires 2 signals from antigen-presenting cells (APCs); an antigen specific signal and a co-stimulatory signal [72] (Figure 1). The co-stimulatory signal results in full T cell activation when CD80 or CD86 on the APCs binds to CD28 on T cells. Normal physiologic down-regulation of activated T cells occurs when endogenous cytotoxic T-lymphocyte antigen-4 (CTL4) competes with CD28 and binds to CD80 or CD86, thus inhibiting the co-stimulatory signal [73].
Abatacept
Abatacept is an intravenously administered, fully humanized fusion protein consisting of the extracellular domain of CTLA-4 and the Fc portion of IgG1 [74]. Abatacept is FDA approved for the treatment of RA and juvenile idiopathic arthritis (JIA) and is currently being studied in psoriatic arthritis and SLE (Table 1). ATTEST was a phase III, multi-center, randomized, double-blind, placebo-controlled trial of 431 adult RA patients with an inadequate response to methotrexate who were randomized to abatacept, infliximab (anti-TNFα), or placebo with continued background methotrexate. At one year, abatacept and infliximab groups were similar with an ACR20 response of 72.4% and 55.8%, an ACR50 of 45.5% and 36.4%, and an ACR70 of 26.3% and 20.6%, respectively. Notably, abatacept had fewer serious adverse events, infections, and infusion reactions compared to infliximab [75]. In other investigations, abatacept has shown significant clinical improvements in RA patients who have previously failed TNF inhibition [76] or who have early disease but poor prognostic factors (positive rheumatoid factor (RF), positive anti-cyclic citrullinated protein (CCP), and bone erosions)[77]. The two year out-comes of the AGREE trial of early RA found sustained disease remission and inhibition of radiographic progression in the abatacept plus methotrexate group [78]. Similarly, abatacept was efficacious in treating undifferentiated inflammatory arthritis in the ADJUST trial with improvement in erosion, osteitis, and synovitis scores on MRI at 2 years[79]. Abatacept has recently been studied in subcutaneous form in the phase IIIb ATTUNE study. 123 RA patients treated initially with intravenous abatacept successfully switched over to weekly subcutaneous abatacept without lack of efficacy or intolerance [80]. Abatacept is safe when combined with methotrexate, but should not be combined with anti-TNFα inhibitors based on the increased rate of serious infections [81].
Based on the many positive RA trials and mechanistic rationale, abatacept has also been studied in psoriatic arthritis and SLE. A phase II trial of 170 psoriatic arthritis patients resulted in a significantly higher ACR20 response of 42% when compared to 19% in the placebo group [82]. Early studies of murine lupus found treatment with CTLA-4 blockade resulted in decreased lupus nephritis and prolonged survival [83]; however, primary end points of a phase IIb trial in human SLE were not met. Additionally serious adverse events were higher in the abatacept group at 19.8% compared to placebo 6.8% [84].
Alefacept
Alefacept is a fusion protein of lymphocyte function associated antigen (LFA-3) with the Fc fragment of IgG1 and was the first biologic approved by the FDA for the treatment of moderate to severe psoriasis [85] (Table 1). Alefacept inhibits T cell activation and thus modulates the T cell mediated inflammatory response by blocking the interaction of LFA-3 on antigen presenting cells with the CD2 receptor on T lymphocytes [86] (Figure 1). Additionally, alefacept can trigger T cell apoptosis via the immunoglobulin G (IgG) domain, which interacts with CD16 on natural killer cells [87]. Alefacept has been studied in psoriatic arthritis in combination with methotrexate in a randomized, placebo controlled trial of 185 psoriatic arthritis patients. At 24 weeks, 54% of patients randomized to alefacept plus methotrexate compared to 23% of patients treated with placebo plus methotrexate reached the primary end-point of an ACR20 response. There were no incidences of opportunistic infection or malignancy and the overall incidence of serious adverse events was quite low at 1.6% [88].
Efalizumab
Efalizumab is a T cell modulator which blocks the activation, adhesion, and migration of T cells and is FDA approved for the treatment of moderate to severe plaque psoriasis (Table 1). Efalizumab is a recombinant humanized monoclonal IgG1 antibody directed against the CD11a subunit of LFA-1 [89] (Figure 1). Although approved for the treatment of psoriasis, a phase II trial in psoriasis patients with concomitant inflammatory arthritis failed to meet the primary endpoint of an ACR20 response [90]. Interestingly, a retrospective case series identified 16 psoriasis patients who developed inflammatory arthritis while on efalizumab therapy [91], so this particular target may not generalize to being therapeutic in autoimmune-mediated joint disease. Finally, efalizumab has been voluntarily withdrawn from the market due to 3 cases of progressive multifocal leukoencephalopathy (PML)[51].
Daclizumab
Daclizumab is a humanized monoclonal antibody directed against the alpha subunit of the IL-2 receptor (CD25), which is located on activated T cells, NK cells, and a subset of B cells [92, 93] (Figure 2). Daclizumab was first used in treatment of acute renal transplant rejection [94] and has since been used successfully in JIA-associated active anterior uveitis and non-infectious intermediate and posterior uveitis [95–97]. Daclizumab treatment was studied in a CIA inflammatory arthritis model using rhesus monkeys and found to decrease joint inflammation and joint erosion [98]. Several small studies have also shown the efficacy of daclizumab in relapsing-remitting multiple sclerosis, and a phase II trial is currently ongoing [99] (Table 1).
Chemokine/Chemokine Receptor Targets
Chemokines and chemokine receptors contribute to inflammation via mediating inflammatory cell recruitment, cell migration, and angiogenesis [100, 101]. Chemokines can be classified into four superfamily groups including CXC, CC, C, and CX3C based on the number and spacing of cysteines in the amino acid sequence. Chemokine receptors, classified as CXCR, CCR, CR, CX3CR respectively, are G protein-coupled receptors and thus make for attractive drug targets [102]. A variety of inflammatory chemokines are increased in RA synovial fluid. Moreover, infiltrating monocytes and macrophages in rheumatoid synovium display increased expression of CCR1, CCR2, and CCR5 chemokine receptors [103, 104], which provides rationale for drug targets that modulate the trafficking of proinflammatory cells in autoimmunity to target tissues.
CCR5
CCR5 is a chemokine receptor upregulated in the RA synovium that functions in the trafficking of monocytes, macrophages, and activated T cells, and it is expressed on myeloid, Th1, and osteoclast cells [102, 105]. Animal studies of CCR5 antagonism in CIA rhesus monkeys showed clinical and serological improvement [106] lending rationale for CCR5 antagonism in human RA. Maraviroc, a human CCR5 antagonist, which is approved for treatment of HIV[107], was recently studied as phase IIa trial in RA. It was well tolerated, but the trial was halted due to the lack of efficacy [108]. Similarly, AZD5672, another oral small molecule CCR5 antagonist, was studied in phase II trials of active RA with background methotrexate use and failed to reach the primary endpoint of an ACR20 after 12 weeks [109]. Thus, CCR5 targeting alone has not demonstrated clinical benefit beyond current agents in use, albeit there could be a rationale for studying CCR5 antagonism in combination with other biologics given its safety profile to date.
CCR1
CCR1, a receptor for the chemokines CCL3, CCL5, CCL7, CCL14, CCL15, is expressed on monocytes and macrophages, and has a variety of functions including leukocyte trafficking and T cell activation [102, 110]. In preclinical animal studies, CCR1 antagonism showed clinical improvement in synovitis and joint damage in murine CIA [111], and ex vivo mechanistic studies demonstrated its ability to inhibit monocyte chemotactic activity in RA synovial fluid samples [112]. Early proof of concept phase I studies of an oral CCR1 antagonist in RA patients found decreased synovial macrophages and CD4+ and CD8+ T-cells and a trend toward clinical improvement compared to placebo [113]. However, there have been mixed results in subsequent trials. CCR1 antagonists MLN3897 [110] and CP-481 [114] in RA and BX471 in multiple sclerosis [110] did not show clinical benefits, but the most recent clinical trial in RA, CARAT-2, did demonstrate clinical activity [115]. This randomized, placebo controlled trial of the CCR1 inhibitor, CCX354-C, was a 12 week study of 160 patients with active RA despite 16 weeks of methotrexate. The ACR20 response was 43% for 100mg twice daily and 52% for 200mg daily treatment dose compared to 39% for placebo. Thus, CCR1 antagonism may be a valid therapeutic target for the treatment of RA, but clearly different chemical compounds and/or neutralization of the target protein have varied clinical outcomes. Future clinical trials will be needed to further support its use in RA or other autoimmune disorders.
INTRACELLULAR TARGETS
Mitogen Activated Protein Kinases
Mitogen activated protein kinase (MAPK) signal transduction pathways are highly conserved regulatory pathways that translate diverse extracellular stimuli to a variety of cellular processes including cell survival, apoptosis, proliferation, migration and differentiation. The four main or conventional MAP kinase pathways include the extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun-amino-terminal kinase 1 to 3 (JNK1, JNK2, JNK3), p38 (α, β, γ, and δ), and ERK5 [116–118]. MAPKs are sequentially activated by MAPK kinases (MAPKK or MEK) and MAPK kinase kinases (MAPKKK or MEKK). ERK1/2, JNK, and p38 have been shown to be activated in RA synovium within and around mononuclear cell infiltrates, supporting their role in the pathogenesis of inflammatory arthritis. ERK was also noted in fibroblasts and synovial lymphocytes, and JNK expression was similarly present but less pronounced. In addition to mononuclear cells, p38 was also expressed in the endothelial cells of synovial microvessels [119].
ERK
Extracellular signal-regulated kinases (ERKs) were the first recognized mammalian MAPK and are important in T cell activation. Inhibition of ERK phosphorylation decreased nociceptive pain behavior in a complete Freund’s adjuvant (CFA) monoarthritis model in rats [120]. T cells from RA patients had increased ERK pathway responsiveness similar to observations in the genetically manipulated spontaneous SKG mouse model of RA. Treatment with the MEK1/2 inhibitor, U0126, in mouse models delayed the onset and decreased the severity of arthritis [121], but further work has not extended beyond these few pre-clinical studies to date.
JNK
The c-Jun amino terminal kinases (JNKs) are composed of three isotypes, JNK1, JNK2 and JNK3, which have different tissue expression patterns and functions. JNKs are activated in RA synovium and are involved in cytokine production, cell proliferation, angiogenesis, and migration [122, 123]. JNK1 was found to contribute to disease pathogenesis in an animal model of arthritis where JNK1-, but not JNK2-deficient mice, had decreased joint damage [124]. Furthermore, JNK-1 inhibition with JNK-specific peptide inhibitor, D-JNKl1, similarly inhibited the development of arthritis in mice[125], but further studies in humans are lacking.
p38
The p38 signaling cascade is activated in the rheumatoid synovium, and p38 inhibitors proved efficacious in animal models of arthritis. However, despite at least 22 different phase I/II clinical trials, p38 inhibitors have not shown to be efficacious in human RA and have a variety of toxicity issues as reviewed by Hammaker et al. [126].
MEK 1/2, MKK3/6, MKK4/7
Given the limitations of p38 inhibitors, a promising strategy has been to target the upstream kinases involved in those signaling pathways. The upstream kinases for p38 are MKK3 and MKK6. MKK4 and MKK7 regulate JNK signal transduction, and MEK1 and MEK2 activate the ERK signaling cascade [127, 128]. Several MEK 1/2 inhibitors have been studied in phase I/II trials of malignancy, including non-small cell lung cancer, breast, colon and pancreatic cancers, but despite strong pre-clinical evidence supporting their use, these first generation MEK inhibitors have not shown significant antitumor efficacy [129, 130]. To examine the role of MEK inhibition in autoimmune diseases, PD184352, a potent oral MEK-1 and MEK-2 inhibitor was studied in a CIA mouse model and resulted in decreased clinical arthritis scores in a dose-dependent manner. In the same study, PD184352 reduced the IL-1α-induced pERK levels in human RA synovial fibroblasts as well as protected against proteoglycan loss in articular cartilage in a rabbit IL-1α-induced arthritis model [131].
Targets directed toward the upstream kinases involved in activating the JNK pathway are still in pre-clinical trials and have not yet progressed to clinical human studies. The rationale for targeting MKKs instead of downstream JNK is based on studies that demonstrated the expression of MKK-4 and MKK-7 in RA synovial tissue [132] and that MKK-7 activates JNK in fibroblast-like synoviocytes after IL-1 and TNFα stimulation [133]. Recent studies have shown the direct inhibition of MKK-7 in K/BxN serum transfer arthritis mice models reduces the severity of arthritis, synovial inflammation, bone erosion, and cartilage damage compared to control mice [134].
Inhibition of the p38 upstream kinases, MKK3 or MKK6, are also being investigated as an alternative to direct p38 inhibition to avoid potential toxicity and complications associated with direct p38 inhibition. In studies of MMK3- and MKK6-deficient mice, arthritis was reduced in the K/BxN serum autoantibody induced model [135, 136]. The pre-clinical data therefore supports the rationale for targeting upstream MKK signaling, and we would expect to see future studies in this area.
Tyrosine Kinases
Janus Kinase
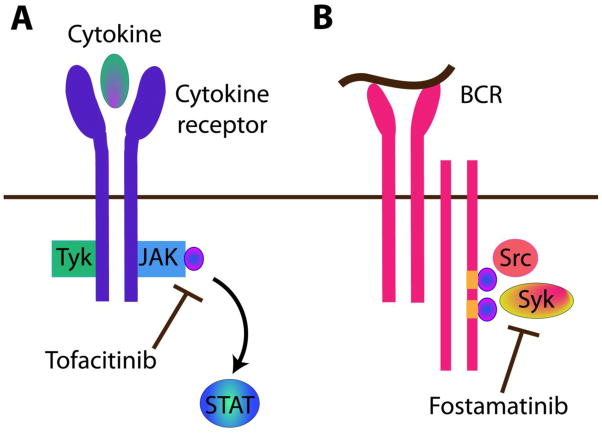
The Janus Kinase (JAK) family of tyrosine kinases plays a central role in cytokine signaling (Figure 3A). Type I and type II cytokine receptors, including interferon (INF) and IL-2, require tyrosine phosphorylation in order to mediate signal transduction. The JAK family was identified as the signaling pathway that induces tyrosine phosphorylation after cytokine receptor stimulation. There are four JAK proteins including JAK1, JAK2, JAK3 and Tyk2. [137]. While JAK1- and JAK2-deficient mice are embryonic lethal, JAK3-deficient mice are viable, and JAK3 appears more specific for immune function. JAK3 associates with the common gamma chain, gamma(c) receptor, which is one part of the IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 cytokine receptors. Mutations of the gamma(c) receptor in humans result in severe combined immunodeficiency (SCID), and JAK3-deficient mice similarly develop SCID with normal myeloid and erythroid cell precursors [138]. Tyk2 signals Type 1 INF responses, and Tyk2-deficient animal models have shown increased susceptibility to various opportunistic infections[138]. Consequently, though JAK3-deficiency had broadly demonstrated immune suppression in mice, its restriction to immune function made it a plausible candidate to test inhibitors for the treatment of autoimmunity.
Figure 3. Promising intracellular therapies in autoimmunity.
A. Two large phase III clinical trials using the oral JAK3 inhibitor tofacitinib have shown promising results in rheumatoid arthritis (RA). JAK3 signals downstream of the common gamma chain receptor, which is the part of the IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 cytokine receptors, and subsequently activates STAT mediated signal transduction. B. Three phase III clinical trials of the oral Syk kinase inhibitor fostamatinib (R788) have been performed in RA with varied results. Syk is required for B cell receptor (BCR) signaling and a subset of pre T cell receptor signaling. After antigen binding, sequential activation of the immunoreceptor tyrosine-based activation motifs (ITAMs) by Src family kinases, followed by Syk mediated double phosphorylation of ITAM, leads to downstream kinase and cellular activation.
Tofacitinib
Tofacitinib is an oral JAK3 antagonist that has proved efficacious in CIA mouse models and adjuvant-induced arthritis in rats [139]. A phase II placebo-controlled, dose-escalating study of tofacitinib in 264 RA patients who failed methotrexate and an anti-TNFα inhibitor revealed significant improvement in ACR20 response rates compared to placebo [140]. The most common adverse events were headache and nausea, and infection rates were similar (26–30%) [140]. Subsequently, a phase IIb dose ranging study randomized 384 active RA patients with inadequate responses to DMARD therapy to receive tofacitinib, adalimumab (anti-TNFα), or placebo. At 24 weeks, 70.5% of patients in the 10mg twice-daily tofacitinib group, compared to 35.9% in the adalimuamb group and 22% in the placebo group, reached an ACR20 response [141].
Very recently, two phase III clinical trials published in the New England Journal of Medicine also reported favorable results for tofacitinib monotherapy [142, 143] (Table 1). In the phase III trial, 611 patients with active RA who failed DMARD or biologic therapy were randomized to tofacitinib versus placebo. At 3 months, patients in both the 5 and 10mg twice daily tofacitinib groups reached the primary end-point with ACR20 response rates of 59.9% and 65.7% respectively, compared to 26.7% for the placebo group. There was a similar risk of adverse events over the first 3 months of the trial; however, tofacitinib was associated with an increased risk of serious infection in 6 patients. The most common adverse events included headache, upper respiratory infection, and diarrhea but there was also an increase in low density lipoprotein (LDL) levels and a mild decrease in the neutrophil count of patients treated with tofacitinib [142]. The second phase III study of 717 patients with active RA patients on stable doses of methotrexate were randomized to receive similar oral tofacitinib doses, subcutaneous adalimumab (anti-TNFα), or placebo. The ACR20 response rates were similar between the two tofacitinib dose groups (51.5–52.6%) and adalimumab (47.2%), but greater than the 28.3% response rate in the placebo group. There were also a greater number of patients with improvement in health assessment questionnaire disability index (HAQ-DI) at 6 months and an increased number of patients with low disease activity as measured by the DAS28-4ESR being less than 2.6. Again, there was evidence of mild decreased neutrophil counts and elevated LDL levels in the tofacitinib group compared to adalimumab or placebo. There were similar rates of adverse events in all groups ranging from 47–52%, but there was an increased rate of serious adverse events and serious infection in the tofacitinib group. Specifically, 3.4–4% of patients in the tofacitinib groups had infection including 2 cases of pulmonary tuberculosis compared to 1.5% in the adalimumab group over the entire study [143]. Based on these large-scale clinical trials, tofacitinib has a promising future in the treatment of RA. However, the risk of infection and the clinical significance of the laboratory abnormalities, notably the elevated LDL, will need to be further investigated prior to gaining regulatory approval.
Spleen Tyrosine Kinase
Spleen tyrosine kinase (Syk) is an intracellular tyrosine kinase involved in the signaling pathways mediated by various inflammatory cells including macrophages, neutrophils, B cells, T cells, and mast cells. Syk is required for B cell antigen receptor (BCR) signaling and pre-T cell receptor (pre-TCR) signaling [144] (Figure 3B). Furthermore Syk has been detected in RA synovial tissue and in RA fibroblast-like synoviocytes and was shown to play a role in TNF and matrix metalloproteinase production [145]. Pre-clinical trials of Syk inhibitor R788/R406 in a rodent CIA model reported suppressed synovitis, arthritis, bone erosion, and pannus formation in the treated group[146].
Fostamatinib
The three randomized controlled clinical trials of the oral Syk kinase inhibitor fostamatinib (R788) in RA have shown mixed results. An initial 12 week phase II, dose-escalating, placebo controlled trial of 189 active RA patients despite methotrexate who were randomized to twice daily R788 versus placebo showed 67% (100mg group) and 72% (150mg group) ACR 20 responses compared 38% in the placebo group, and IL-6 and MMP-3 levels were reduced. The main side effects were dose-dependent diarrhea and neutropenia [147]. A second six-month phase II randomized controlled trial of 457 active RA patients on methotrexate also reached its primary endpoint with a 67% ACR20 response in the 100mg twice daily group versus 35% in the placebo group, and side effects in the treatment group were similar to the prior study [148]. A final phase II three month randomized controlled trial of active RA patients that did not respond to biologic agents did not show a difference between the ACR20, 50 or 70 between the treatment and placebo groups, albeit the placebo ACR20 rate was quite high at 37% [149]. Further clinical trials are underway (NCT01197521, NCT01197534, NCT01197755) and will hopefully clarify the efficacy and safety of fostamatinib, and the role it may play in treatment for RA.
Syk inhibition is also being investigated in preclinical trials in lupus prone mice since Syk plays a key role in BCR and T cell receptor (TCR) signaling pathways [150], which are thought to contribute to SLE pathogenesis [151, 152]. Notably, the B cell receptor and Fc receptor signaling pathways involve sequential activation of immunoreceptor tyrosine-based activation motif (ITAM) by Src family kinases, followed by Syk-mediated double phosphorylation of ITAM, and further activation of Src kinases [144] (Figure 3B). Pre-clinical data reveal that the oral syk kinase inhibitor, R788, when given to NZB/NZW lupus prone mice, delayed onset of proteinuria and prolonged survival, suggesting Syk inhibitors could be relevant and potentially beneficial in human SLE [153].
Abl/C-Kit
Imatinib
Imatinib is a small molecule protein tyrosine kinase inhibitor that targets the Philadelphia chromosome Bcr/Abl translocation. It is FDA approved for the treatment of Bcr/Abl positive chronic myelogenous leukemia as well as c-kit expressing gastrointestinal stromal tumors (GIST) [154, 155]. Imatinib also inhibits other tyrosine kinases including c-kit and affects platelet derived growth factor (PDGF) and transforming growth factor β (TGF β) signaling [156], which are involved in the pathogenesis of autoimmune diseases.
Systemic sclerosis (SSc) is a chronic, autoimmune fibrosing disease characterized by inflammation, vasculopathy, and fibrosis of the skin and various internal organs. The pathogenesis of SSc involves overexpression and abnormal signaling of the TGFβ and PDGF pathways [157, 158]. In vitro studies of dermal fibroblasts incubated with imatinib had reduced production of extracellular matrix proteins. In vivo pre-clinical studies in bleomycin-induced dermal fibrosis mice similarly showed reduced dermal thickness and decreased production of extracellular matrix proteins [156]. Imatinib was also found to reduce dermal and hypodermal thickening in the tight skin 1 (TSK-1) mouse model [159].
Several small clinical trials have evaluated imatinib in SSc, but with limited data due to high rates of adverse events. An initial proof of concept pilot study enrolled 10 patients with diffuse cutaneous SSc who were randomized to imatinib versus placebo, but 200mg twice a day dosing was poorly tolerated due to edema, fluid retention, nausea, diarrhea, and anemia and was therefore, prematurely discontinued [160]. A follow-up phase I/II open label trial of imatinib in SSc patients at a higher dose of 600mg daily also resulted in a significant number of similar adverse events without significance in the primary endpoint of forced vital capacity (FVC) [161]. 400mg once daily dosing was studied in an open labeled phase II trial of 30 SSc patients, but there was a large number of serious adverse events including pneumonia, respiratory failure, and edema despite a 22% improvement in modified Rodnan skin thickness score (MRSS) and a 6.4% improvement in FVC [162].
Imatinib has also been studied in pre-clinical trials of RA [163–165], and a few small case reports have reported clinical benefit in the human disease [166–168]. Given the significant side effects noted in SSc trials and the promising future of other oral small molecule tyrosine kinase inhibitors, imatinib will not likely hold a significant market share for RA treatments in the future.
CONCLUSIONS
There have been many exciting developments in the therapeutic targeting of immune cells which has been largely driven by advances in our understanding of the pathophysiology of autoimmune diseases. As a field, we are moving away from indiscriminate cytotoxic therapies and toward directed antibody-based and small molecule therapeutics with the hope of improved efficacy, fewer adverse side effects, and improvement in patient outcomes. In the extracellular pathways, direct B cell depletion therapy with rituximab and B cell functional inhibition with belimumab have been most successful to date, but there is room for improvement in outcomes, particularly in treating the severe manifestation of SLE. Combination therapy of B cell depletion and functional BLyS inhibition may be one novel approach for managing these difficult SLE cases. Additionally, there are several new agents in the earlier stages of development for inflammatory arthritis including the class of CCR1 antagonists, and combination therapy with chemokine receptor antagonism and other biologics may be another potential strategy to treat difficult cases. With respect to targeting intracellular signaling pathways, several exciting developments and positive large-scale clinical trials have come to fruition this year. The success of JAK inhibition in RA has the potential to be a practice changing therapy from intravenous and injectable biologic therapies to oral therapies. In contrast, the ubiquity of tyrosine kinase signaling pathways and relative lack of true target specificity of many the earlier tyrosine kinase inhibitors has resulted in decreased utility due to untoward toxicities and adverse events. As the field of cellular immune therapies continues to expand over the next several years, we will hopefully be able to minimize toxicity and ideally tailor our patients’ treatment based on disease pathophysiology and patient specific manifestations.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
- 1.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Current opinion in immunology. 2008;20(3):332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis research & therapy. 2011;13(Suppl 1):S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorner T, Kinnman N, Tak PP. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacology & therapeutics. 2010;125(3):464–75. doi: 10.1016/j.pharmthera.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Munoz LE, et al. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008;17(5):371–5. doi: 10.1177/0961203308089990. [DOI] [PubMed] [Google Scholar]
- 5.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365(22):2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 6.Hansen A, Lipsky PE, Dorner T. B cells in Sjogren’s syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis research & therapy. 2007;9(4):218. doi: 10.1186/ar2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepse N, et al. Immune regulatory mechanisms in ANCA-associated vasculitides. Autoimmunity reviews. 2011;11(2):77–83. doi: 10.1016/j.autrev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 8.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 9.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103(7):2738–43. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 10.Buch MH, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2011;70(6):909–20. doi: 10.1136/ard.2010.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mease PJ. B cell-targeted therapy in autoimmune disease: rationale, mechanisms, and clinical application. The Journal of rheumatology. 2008;35(7):1245–55. [PubMed] [Google Scholar]
- 12.Stashenko P, et al. Characterization of a human B lymphocyte-specific antigen. Journal of immunology. 1980;125(4):1678–85. [PubMed] [Google Scholar]
- 13.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunological reviews. 2009;230(1):128–43. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 14.Dorner T, Goldenberg DM. Targeting CD22 as a strategy for treating systemic autoimmune diseases. Therapeutics and clinical risk management. 2007;3(5):953–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384(6610):634–7. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SB, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis and rheumatism. 2006;54(9):2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 17.Emery P, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis and rheumatism. 2006;54(5):1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 18•.Singh JA, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis care & research. 2012;64(5):625–39. doi: 10.1002/acr.21641. The 2012 American College of Rheumatology updated 2008 recommendations for treating rheumatoid arthritis including a treatment algorithm for using DMARDs and biologic therapies in RA as well recommendations for tuberculosis screening and vaccinations. There are new recommendations for the use of rituximab in RA including special populations such as patients with malignancy, hepatitis, and congestive heart failure patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falk RJ, et al. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. Arthritis and rheumatism. 2011;63(4):863–4. doi: 10.1002/art.30286. [DOI] [PubMed] [Google Scholar]
- 20.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368(9533):404–18. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 21.de Groot K, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Annals of internal medicine. 2009;150(10):670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 22.Mukhtyar C, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Annals of the rheumatic diseases. 2009;68(3):310–7. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 23.Bosch X, et al. Immunotherapy for antineutrophil cytoplasmic antibody-associated vasculitis: challenging the therapeutic status quo? Trends in immunology. 2008;29(6):280–9. doi: 10.1016/j.it.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Rutgers A, Kallenberg CG. Refractory disease in antineutrophil cytoplasmic antibodies associated vasculitis. Current opinion in rheumatology. 2012;24(3):245–51. doi: 10.1097/BOR.0b013e3283529756. [DOI] [PubMed] [Google Scholar]
- 25.Genentech. Rituxan Prescribing Information. 2012 [cited 2012 August 24 ]; Available from: http://www.rituxan.com/index.html.
- 26.Popa ER, et al. Differential B- and T-cell activation in Wegener’s granulomatosis. The Journal of allergy and clinical immunology. 1999;103(5 Pt 1):885–94. doi: 10.1016/s0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 27.Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. Journal of the American Society of Nephrology : JASN. 2006;17(5):1235–42. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Puerta JA, et al. B-cell depleting agents for ANCA vasculitides: a new therapeutic approach. Autoimmunity reviews. 2012;11(9):646–52. doi: 10.1016/j.autrev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 29••.Jones RB, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. The New England journal of medicine. 2010;363(3):211–20. doi: 10.1056/NEJMoa0909169. One of two practice changing studies of rituximab in severe ANCA vasculitis patients, which led to FDA approval of rituximab for treatment of ANCA vasculitis. [DOI] [PubMed] [Google Scholar]
- 30.Stone JH, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. The New England journal of medicine. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee EP, Laliberte KA, Niles JL. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(8):1394–400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roubaud-Baudron C, et al. Rituximab maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis. The Journal of rheumatology. 2012;39(1):125–30. doi: 10.3899/jrheum.110143. [DOI] [PubMed] [Google Scholar]
- 33.Lamprecht P, Gause A, Gross WL. Cryoglobulinemic vasculitis. Arthritis and rheumatism. 1999;42(12):2507–16. doi: 10.1002/1529-0131(199912)42:12<2507::AID-ANR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Casals M, et al. The cryoglobulinaemias. Lancet. 2012;379(9813):348–60. doi: 10.1016/S0140-6736(11)60242-0. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub P, et al. Treatment of hepatitis C virus-related systemic vasculitis. The Journal of rheumatology. 2005;32(11):2078–82. [PubMed] [Google Scholar]
- 36.Landau DA, et al. Relapse of hepatitis C virus-associated mixed cryoglobulinemia vasculitis in patients with sustained viral response. Arthritis and rheumatism. 2008;58(2):604–11. doi: 10.1002/art.23305. [DOI] [PubMed] [Google Scholar]
- 37.De Vita S, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis and rheumatism. 2012;64(3):843–53. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 38.Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis and rheumatism. 2012;64(3):835–42. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox RI. Sjogren’s syndrome. Lancet. 2005;366(9482):321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 40.Vitali C, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the rheumatic diseases. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meijer JM, et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2010;62(4):960–8. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 42.Seve P, et al. Successful treatment with rituximab in a patient with mental nerve neuropathy in primary Sjogren’s syndrome. Rheumatology international. 2007;28(2):175–7. doi: 10.1007/s00296-007-0396-4. [DOI] [PubMed] [Google Scholar]
- 43.Looney RJ, Anolik J, Sanz I. B lymphocytes in systemic lupus erythematosus: lessons from therapy targeting B cells. Lupus. 2004;13(5):381–90. doi: 10.1191/0961203304lu1031oa. [DOI] [PubMed] [Google Scholar]
- 44.Pons-Estel GJ, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in arthritis and rheumatism. 2010;39(4):257–68. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Casals M, et al. Rituximab in systemic lupus erythematosus: A systematic review of off-label use in 188 cases. Lupus. 2009;18(9):767–76. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- 46.Ramos-Casals M, et al. B-cell-depleting therapy in systemic lupus erythematosus. The American journal of medicine. 2012;125(4):327–36. doi: 10.1016/j.amjmed.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merrill JT, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis and rheumatism. 2010;62(1):222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rovin BH, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis and rheumatism. 2012;64(4):1215–26. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 49.Ostergaard M, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis and rheumatism. 2010;62(8):2227–38. doi: 10.1002/art.27524. [DOI] [PubMed] [Google Scholar]
- 50.Taylor PC, et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Annals of the rheumatic diseases. 2011;70(12):2119–25. doi: 10.1136/ard.2011.151522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carson KR, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. The lancet oncology. 2009;10(8):816–24. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 52.Carnahan J, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(10 Pt 2):3982S–90S. [PubMed] [Google Scholar]
- 53.Dorner T, et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis research & therapy. 2006;8(3):R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daridon C, et al. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis research & therapy. 2010;12(6):R204. doi: 10.1186/ar3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26(25):3704–13. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- 56.Traczewski P, Rudnicka L. Treatment of systemic lupus erythematosus with epratuzumab. British journal of clinical pharmacology. 2011;71(2):175–82. doi: 10.1111/j.1365-2125.2010.03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinfeld SD, et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren’s syndrome: an open-label phase I/II study. Arthritis research & therapy. 2006;8(4):R129. doi: 10.1186/ar2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. The Journal of clinical investigation. 2009;119(5):1066–73. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groom J, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. The Journal of clinical investigation. 2002;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15(9):570–6. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 61.Cheema GS, et al. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis and rheumatism. 2001;44(6):1313–9. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 62.Baker KP, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis and rheumatism. 2003;48(11):3253–65. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 63.Jacobi AM, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis and rheumatism. 2010;62(1):201–10. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31. doi: 10.1016/S0140-6736(10)61354-2. The BLISS-52 trial resulted in FDA approval of belimumab for treatment of active lupus in patients who had previously failed other standard immunosuppressive therapies. Belimumab treated patients had significantly higher systemic lupus responder index (SRI) response rate than patients receiving placebo. [DOI] [PubMed] [Google Scholar]
- 65.Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2011;63(12):3918–30. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manzi S, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stohl W. Biologic Differences Between Various Inhibitors of the BLyS/BAFF Pathway: Should We Expect Differences Between Belimumab and Other Inhibitors in Development? Current rheumatology reports. 2012 doi: 10.1007/s11926-012-0254-6. [DOI] [PubMed] [Google Scholar]
- 68.Hsu H, et al. A novel modality of BAFF-specific inhibitor AMG623 peptibody reduces B-cell number and improves outcomes in murine models of autoimmune disease. Clinical and experimental rheumatology. 2012;30(2):197–201. [PubMed] [Google Scholar]
- 69.Pharma A. Anthera Updates Phase 3 Plans Following Results from the Phase 2b PEARL-SC Dose Ranging Study of Blisibimod. 2012 [cited 2012 August 24]; Available from: http://investor.anthera.com/releasedetail.cfm?ReleaseID=687254.
- 70.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. The New England journal of medicine. 2001;344(12):907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 71.van Kuijk AW, et al. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Annals of the rheumatic diseases. 2006;65(12):1551–7. doi: 10.1136/ard.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunological reviews. 2009;229(1):307–21. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 73.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunological reviews. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 74.Moreland LW, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis and rheumatism. 2002;46(6):1470–9. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- 75.Schiff M, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the rheumatic diseases. 2008;67(8):1096–103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Genovese MC, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. The New England journal of medicine. 2005;353(11):1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 77.Westhovens R, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Annals of the rheumatic diseases. 2009;68(12):1870–7. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bathon J, et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Annals of the rheumatic diseases. 2011;70(11):1949–56. doi: 10.1136/ard.2010.145268. [DOI] [PubMed] [Google Scholar]
- 79.Emery P, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial) Annals of the rheumatic diseases. 2010;69(3):510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keystone EC, et al. Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb ATTUNE study. Annals of the rheumatic diseases. 2012;71(6):857–61. doi: 10.1136/annrheumdis-2011-200355. [DOI] [PubMed] [Google Scholar]
- 81.Weinblatt M, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Annals of the rheumatic diseases. 2007;66(2):228–34. doi: 10.1136/ard.2006.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mease P, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis and rheumatism. 2011;63(4):939–48. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 83.Schiffer L, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. Journal of immunology. 2003;171(1):489–97. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 84.Merrill JT, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2010;62(10):3077–87. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 85.Sugiyama H, et al. Alefacept in the treatment of psoriasis. Clinics in dermatology. 2008;26(5):503–8. doi: 10.1016/j.clindermatol.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 86.Miller GT, et al. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responses. The Journal of experimental medicine. 1993;178(1):211–22. doi: 10.1084/jem.178.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Majeau GR, et al. Mechanism of lymphocyte function-associated molecule 3-Ig fusion proteins inhibition of T cell responses. Structure/function analysis in vitro and in human CD2 transgenic mice. Journal of immunology. 1994;152(6):2753–67. [PubMed] [Google Scholar]
- 88.Mease PJ, Gladman DD, Keystone EC. Alefacept in combination with methotrexate for the treatment of psoriatic arthritis: results of a randomized, double-blind, placebo-controlled study. Arthritis and rheumatism. 2006;54(5):1638–45. doi: 10.1002/art.21870. [DOI] [PubMed] [Google Scholar]
- 89.Talamonti M, et al. Efalizumab. Expert opinion on drug safety. 2011;10(2):239–51. doi: 10.1517/14740338.2011.524925. [DOI] [PubMed] [Google Scholar]
- 90.Papp KA, et al. Efalizumab for the treatment of psoriatic arthritis. Journal of cutaneous medicine and surgery. 2007;11(2):57–66. doi: 10.2310/7750.2007.00006. [DOI] [PubMed] [Google Scholar]
- 91.Viguier M, et al. Onset of psoriatic arthritis in patients treated with efalizumab for moderate to severe psoriasis. Arthritis and rheumatism. 2008;58(6):1796–802. doi: 10.1002/art.23507. [DOI] [PubMed] [Google Scholar]
- 92.Amu S, et al. CD25-expressing B-lymphocytes in rheumatic diseases. Scandinavian journal of immunology. 2007;65(2):182–91. doi: 10.1111/j.1365-3083.2006.01889.x. [DOI] [PubMed] [Google Scholar]
- 93.Martin JF, et al. An IL-2 paradox: blocking CD25 on T cells induces IL-2-driven activation of CD56(bright) NK cells. Journal of immunology. 2010;185(2):1311–20. doi: 10.4049/jimmunol.0902238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vincenti F, Nashan B, Light S. Daclizumab: outcome of phase III trials and mechanism of action. Double Therapy and the Triple Therapy Study Groups. Transplantation proceedings. 1998;30(5):2155–8. doi: 10.1016/s0041-1345(98)00571-5. [DOI] [PubMed] [Google Scholar]
- 95.Nussenblatt RB, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yeh S, et al. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. Journal of autoimmunity. 2008;31(2):91–7. doi: 10.1016/j.jaut.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sen HN, et al. High-dose daclizumab for the treatment of juvenile idiopathic arthritis-associated active anterior uveitis. American journal of ophthalmology. 2009;148(5):696–703 e1. doi: 10.1016/j.ajo.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brok HP, et al. Prophylactic and therapeutic effects of a humanized monoclonal antibody against the IL-2 receptor (DACLIZUMAB) on collagen-induced arthritis (CIA) in rhesus monkeys. Clinical and experimental immunology. 2001;124(1):134–41. doi: 10.1046/j.1365-2249.2001.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin R. Anti-CD25 (daclizumab) monoclonal antibody therapy in relapsing-remitting multiple sclerosis. Clinical immunology. 2012;142(1):9–14. doi: 10.1016/j.clim.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 100.Szekanecz Z, et al. Chemokines and angiogenesis in rheumatoid arthritis. Frontiers in bioscience. 2009;1:44–51. doi: 10.2741/e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis and rheumatism. 2005;52(3):710–21. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- 102.Szekanecz Z, Koch AE, Tak PP. Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. The Netherlands journal of medicine. 2011;69(9):356–66. [PubMed] [Google Scholar]
- 103.Katschke KJ, Jr, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis and rheumatism. 2001;44(5):1022–32. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 104.Haringman JJ, et al. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Annals of the rheumatic diseases. 2006;65(3):294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. Journal of leukocyte biology. 2010;88(1):41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 106.Vierboom MP, et al. Inhibition of the development of collagen-induced arthritis in rhesus monkeys by a small molecular weight antagonist of CCR5. Arthritis and rheumatism. 2005;52(2):627–36. doi: 10.1002/art.20850. [DOI] [PubMed] [Google Scholar]
- 107.Pfizer. Selzentry Prescribing Information. 2012 [cited 2012 Sept 4]; Available from: http://www.selzentry.com/
- 108.Fleishaker DL, et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis research & therapy. 2012;14(1):R11. doi: 10.1186/ar3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gerlag DM, et al. Preclinical and clinical investigation of a CCR5 antagonist, AZD5672, in patients with rheumatoid arthritis receiving methotrexate. Arthritis and rheumatism. 2010;62(11):3154–60. doi: 10.1002/art.27652. [DOI] [PubMed] [Google Scholar]
- 110.Gladue RP, Brown MF, Zwillich SH. CCR1 antagonists: what have we learned from clinical trials. Current topics in medicinal chemistry. 2010;10(13):1268–77. doi: 10.2174/156802610791561237. [DOI] [PubMed] [Google Scholar]
- 111.Amat M, et al. Pharmacological blockade of CCR1 ameliorates murine arthritis and alters cytokine networks in vivo. British journal of pharmacology. 2006;149(6):666–75. doi: 10.1038/sj.bjp.0706912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gladue RP, et al. CP-481,715, a potent and selective CCR1 antagonist with potential therapeutic implications for inflammatory diseases. The Journal of biological chemistry. 2003;278(42):40473–80. doi: 10.1074/jbc.M306875200. [DOI] [PubMed] [Google Scholar]
- 113.Haringman JJ, et al. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2003;62(8):715–21. doi: 10.1136/ard.62.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vergunst CE, et al. MLN3897 plus methotrexate in patients with rheumatoid arthritis: safety, efficacy, pharmacokinetics, and pharmacodynamics of an oral CCR1 antagonist in a phase IIa, double-blind, placebo-controlled, randomized, proof-of-concept study. Arthritis and rheumatism. 2009;60(12):3572–81. doi: 10.1002/art.24978. [DOI] [PubMed] [Google Scholar]
- 115.Tak PP, et al. Annals of the rheumatic diseases. 2012. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. [DOI] [PubMed] [Google Scholar]
- 116.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 117.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological reviews. 2001;81(2):807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 118.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and molecular biology reviews : MMBR. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schett G, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis and rheumatism. 2000;43(11):2501–12. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 120.Cruz CD, et al. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005;116(3):411–9. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 121.Singh K, et al. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. Journal of immunology. 2009;183(12):8258–67. doi: 10.4049/jimmunol.0901784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 123.Han Z, et al. Jun N-terminal kinase in rheumatoid arthritis. The Journal of pharmacology and experimental therapeutics. 1999;291(1):124–30. [PubMed] [Google Scholar]
- 124.Guma M, et al. JNK-1 deficiency limits macrophage-mediated antigen-induced arthritis. Arthritis and rheumatism. 2011;63(6):1603–12. doi: 10.1002/art.30271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guma M, et al. JNK1 controls mast cell degranulation and IL-1{beta} production in inflammatory arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(51):22122–7. doi: 10.1073/pnas.1016401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Annals of the rheumatic diseases. 2010;69(Suppl 1):i77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Derijard B, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–5. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 128.Tournier C, et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes & development. 2001;15(11):1419–26. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fremin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. Journal of hematology & oncology. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wallace EM, et al. Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Current topics in medicinal chemistry. 2005;5(2):215–29. doi: 10.2174/1568026053507723. [DOI] [PubMed] [Google Scholar]
- 131.Thiel MJ, et al. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: potential proinflammatory mechanisms. Arthritis and rheumatism. 2007;56(10):3347–57. doi: 10.1002/art.22869. [DOI] [PubMed] [Google Scholar]
- 132.Sundarrajan M, et al. Expression of the MAPK kinases MKK-4 and MKK-7 in rheumatoid arthritis and their role as key regulators of JNK. Arthritis and rheumatism. 2003;48(9):2450–60. doi: 10.1002/art.11228. [DOI] [PubMed] [Google Scholar]
- 133.Inoue T, et al. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthritis and rheumatism. 2006;54(7):2127–35. doi: 10.1002/art.21919. [DOI] [PubMed] [Google Scholar]
- 134.Lee SI, et al. Regulation of inflammatory arthritis by the upstream kinase mitogen activated protein kinase kinase 7 in the c-Jun N-Terminal kinase pathway. Arthritis research & therapy. 2012;14(1):R38. doi: 10.1186/ar3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inoue T, et al. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5484–9. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoshizawa T, et al. Role of MAPK kinase 6 in arthritis: distinct mechanism of action in inflammation and cytokine expression. Journal of immunology. 2009;183(2):1360–7. doi: 10.4049/jimmunol.0900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nature reviews Drug discovery. 2006;5(7):549–63. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 138.Pesu M, et al. Therapeutic targeting of Janus kinases. Immunological reviews. 2008;223:132–42. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Milici AJ, et al. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis research & therapy. 2008;10(1):R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kremer JM, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis and rheumatism. 2009;60(7):1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 141.Fleischmann R, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis and rheumatism. 2012;64(3):617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 142.Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. The New England journal of medicine. 2012;367(6):495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 143•.van Vollenhoven RF, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. The New England journal of medicine. 2012;367(6):508–19. doi: 10.1056/NEJMoa1112072. A 12-month large, multicenter, international phase III trial of tofacitinib with methotrexate compared to placebo or adalimumab in active RA also found significant improvement in RA compared to placebo and similar efficacy to adalimumab. [DOI] [PubMed] [Google Scholar]
- 144.Ghosh D, Tsokos GC. Spleen tyrosine kinase: an Src family of non-receptor kinase has multiple functions and represents a valuable therapeutic target in the treatment of autoimmune and inflammatory diseases. Autoimmunity. 2010;43(1):48–55. doi: 10.3109/08916930903374717. [DOI] [PubMed] [Google Scholar]
- 145.Cha HS, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. The Journal of pharmacology and experimental therapeutics. 2006;317(2):571–8. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 146.Pine PR, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clinical immunology. 2007;124(3):244–57. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 147.Weinblatt ME, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis and rheumatism. 2008;58(11):3309–18. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 148.Weinblatt ME, et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. The New England journal of medicine. 2010;363(14):1303–12. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 149.Genovese MC, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis and rheumatism. 2011;63(2):337–45. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 150.Pamuk ON, Tsokos GC. Spleen tyrosine kinase inhibition in the treatment of autoimmune, allergic and autoinflammatory diseases. Arthritis research & therapy. 2010;12(6):222. doi: 10.1186/ar3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nature immunology. 2001;2(9):764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 152.Krishnan S, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. Journal of immunology. 2008;181(11):8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bahjat FR, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis and rheumatism. 2008;58(5):1433–44. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]
- 154.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. The New England journal of medicine. 2002;347(7):472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 155.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. The New England journal of medicine. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 156.Distler JH, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis and rheumatism. 2007;56(1):311–22. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 157.Varga J, Whitfield ML. Transforming growth factor-beta in systemic sclerosis (scleroderma) Frontiers in bioscience. 2009;1:226–35. doi: 10.2741/s22. [DOI] [PubMed] [Google Scholar]
- 158.Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology. 2008;47(Suppl 5):v2–4. doi: 10.1093/rheumatology/ken265. [DOI] [PubMed] [Google Scholar]
- 159.Akhmetshina A, et al. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis and rheumatism. 2009;60(1):219–24. doi: 10.1002/art.24186. [DOI] [PubMed] [Google Scholar]
- 160.Pope J, et al. Imatinib in active diffuse cutaneous systemic sclerosis: Results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis and rheumatism. 2011;63(11):3547–51. doi: 10.1002/art.30549. [DOI] [PubMed] [Google Scholar]
- 161.Khanna D, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis and rheumatism. 2011;63(11):3540–6. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spiera RF, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Annals of the rheumatic diseases. 2011;70(6):1003–9. doi: 10.1136/ard.2010.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Akashi N, et al. Comparative suppressive effects of tyrosine kinase inhibitors imatinib and nilotinib in models of autoimmune arthritis. Modern rheumatology/the Japan Rheumatism Association. 2011;21(3):267–75. doi: 10.1007/s10165-010-0392-5. [DOI] [PubMed] [Google Scholar]
- 164.Koyama K, et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Modern rheumatology/the Japan Rheumatism Association. 2007;17(4):306–10. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- 165.Paniagua RT, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. The Journal of clinical investigation. 2006;116(10):2633–42. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eklund KK, et al. Maintained efficacy of the tyrosine kinase inhibitor imatinib mesylate in a patient with rheumatoid arthritis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2008;14(5):294–6. doi: 10.1097/RHU.0b013e318188b1ce. [DOI] [PubMed] [Google Scholar]
- 167.Eklund KK, Joensuu H. Treatment of rheumatoid arthritis with imatinib mesylate: clinical improvement in three refractory cases. Annals of medicine. 2003;35(5):362–7. doi: 10.1080/07853890310001339. [DOI] [PubMed] [Google Scholar]
- 168.Vernon MR, Pearson L, Atallah E. Resolution of rheumatoid arthritis symptoms with imatinib mesylate. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2009;15(5):267. doi: 10.1097/RHU.0b013e3181b0d352. [DOI] [PubMed] [Google Scholar]