Abstract
Hematopoietic stem cell transplantation (HSCT) is a rigorous therapy that carries significant risk of morbidity and mortality to individuals with hematologic malignancies undergoing this treatment. While relationships between psychosocial factors, immune function, and clinical outcomes have been documented in other cancer populations, similar studies of cancer patients undergoing HSCT have not yet been conducted. The clinical significance of these relationships may be particularly salient in this population given the critical role of a timely immune recovery and optimal immune regulation in preventing infections, mitigating risk for graft-versus-host disease, and eliminating malignant cells, thereby reducing morbidity and mortality. Evidence for the potential role of biobehavioral processes following HSCT is reviewed, mechanisms by which psychosocial factors may influence immune processes relevant to post-transplant outcomes are discussed, and a framework to ground future psychoneuroimmunology (PNI) research in this area is provided. The review suggests that the recovery period following HSCT may provide a “window of opportunity” during which interventions targeting stress-related behavioral factors can influence the survival, health, and well-being of HSCT recipients.
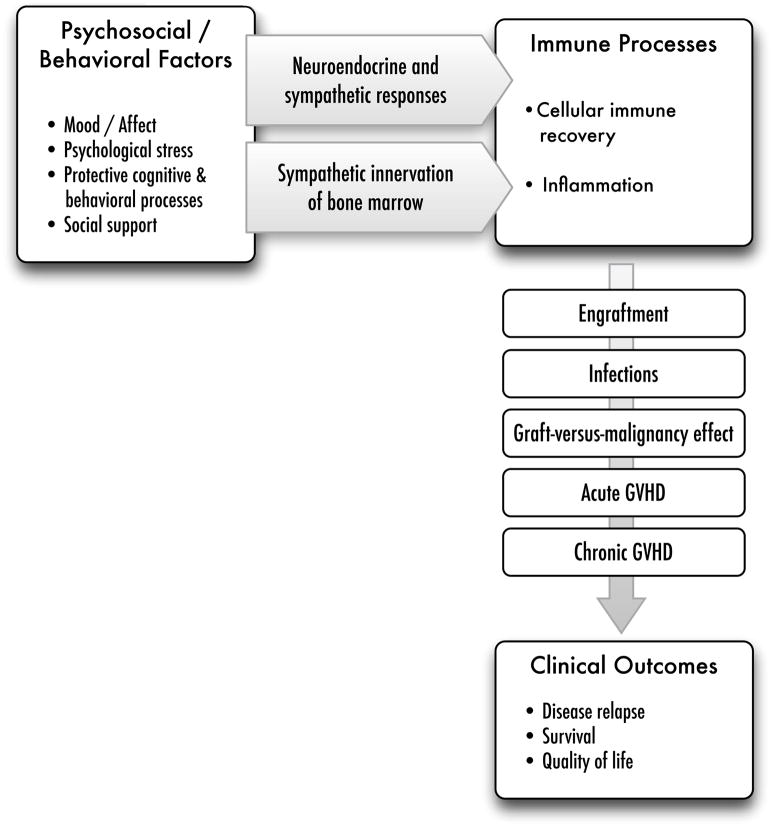
Hematopoietic stem cell transplantation (HSCT) is a rigorous therapy that carries significant risk of morbidity and mortality to individuals with hematologic malignancies undergoing this treatment. While there are a growing number of psychoneuroimmunology (PNI) studies of other cancer populations, there is a dearth of research on patients undergoing HSCT. The clinical significance of PNI relationships may be particularly salient in this population given the critical role of a timely immune recovery and optimal immune regulation in reducing morbidity and complications and preventing recurrence. This review focuses on evidence suggesting the importance of biobehavioral processes in the recovery following HSCT and potential mechanisms by which psychosocial factors influence immune processes relevant to critical post-transplant outcomes. Figure 1 outlines the biobehavioral pathways discussed.
Figure 1.
This biobehavioral model of HSCT illustrates the contributions of psychosocial processes and behavioral factors to recovery following HSCT. Stress-related behavioral factors can activate the HPA and SNS axes. The secreted products of these pathways (glucocorticoids, catecholamines) and the direct sympathetic innervation of the bone marrow microenvironment can modulate cell recovery following transplant and promote (or moderate) an inflammatory environment that predisposes the HSCT recipient to moderate or severe GVHD. Immune recovery plays a critical role in several different clinical events following HSCT, including preventing infections, promoting the activity of effector cells on residual disease, and mitigating acute and chronic GVHD, thereby reducing the risk for disease recurrence, ensuring survival, and facilitating a more optimal quality of life.
Hematopoietic Stem Cell Transplantation (HSCT)
HSCT is performed by administering intense chemotherapy with or without radiation therapy followed by infusion of autologous or allogeneic hematopoietic stem cells. See Copelan (2006) for an excellent overview. In brief, autologous transplantation involves the infusion of the patients’ own hematopoietic stem cells and allows patients to receive high-dose chemotherapy that can eradicate disease at the cost of ablating the bone marrow. It is most frequently used for multiple myeloma, other plasma cell disorders, and aggressive lymphomas. In allogeneic transplantation, patients receive hematopoietic stem cells or bone marrow product from an HLA-matched donor or umbilical cord. It is used to treat acute and chronic leukemias, myelodysplastic and myeloproliferative disorders, lymphomas, and aplastic anemias and other bone marrow failure disorders. While high-dose therapy provides short-term disease control, the therapeutic potential of allogeneic transplant derives from a “graft-versus-malignancy” effect in which donor-derived immune cells detect and eliminate remaining circulating malignant cells. Consequently, reduced intensity, nonmyeloablative regimens have increasingly been used to take advantage of this effect while reducing the toxicity and morbidity.
HSCT is a risky procedure with a high rate of treatment-related morbidity and mortality. Common side effects include nausea, diarrhea, anorexia, and fatigue. Significant early complications include gastrointestinal mucosal injury (mucositis), veno-occlusive disease of the liver, pulmonary failure, and infections. Allogeneic transplantation also carries a high risk of acute graft-versus-host disease (aGVHD). Two-year allogeneic transplant-related mortality ranges from 6–51%, depending on pre-transplant disease status and comorbidities (Bacigalupo et al., 2004). Later complications include increased risk for cardiovascular disease, diabetes, chronic fatigue, secondary malignancies, and chronic graft-versus-host disease (cGVHD), a cause of significant long-term disability. While autologous transplantation is not associated with GVHD or most of the long-term risks seen following allogeneic transplant, there is a high rate of relapse, approximately 40–70% (e.g., Porrata and Markovec, 2004). While many patients are discharged from the hospital within a month, functional recovery occurs slowly over 6 to 12 months and the burden of ongoing medical care remains high with the need for many medications, frequent clinical visits, and common subsequent complications often related to GVHD and infection.
It is therefore not surprising that many HSCT patients report significant emotional distress. While elevated distress typically subsides over the first year, some degree of emotional distress persists among a significant subset of patients (Andrykowski et al., 2005; Syrjala et al., 2004), and depression is one of the most commonly reported concerns following HSCT. A prospective, longitudinal study found that the psychological recovery lagged behind the physical recovery after transplant, with 33% of HSCT recipients reporting clinically significant depressive symptoms at 90 days post-transplant and 79% continuing to report significant general psychological distress at 1 year post-transplant (Syrjala et al., 2004). A study of 662 long-term HSCT survivors who were an average of 7 years post-transplant reported continued decrements in several quality of life domains, including physical and functional abilities along with social and psychological adjustment, with prevalent problems including depression, fatigue, and cognitive dysfunction (Andrykowski et al., 2005). There is growing interest in understanding the psychological sequelae after HSCT, particularly given emerging evidence that depression and other emotional concerns are associated with poorer quality of life (e.g., Syrjala et al., 2004) and may affect the post-transplant course and outcome.
Stress-Related Psychosocial Factors and Post-Transplant Outcomes
A growing literature suggests that psychosocial factors may serve as risk or protective factors for HSCT outcomes. A review by Hoodin and colleagues (2006) summarized considerable evidence that emotional status predicts mortality following HSCT, with 10 of 15 relevant studies included in the review documenting significant links. The adverse influence of depression was the most frequently identified risk factor and appeared to exert the most salient effects for early post-transplant mortality. Earlier studies reviewed were often limited by retrospective designs and small sample sizes and/or failed to account for medical and demographic factors known to contribute to mortality following HSCT. However, Hoodin et al. observed that more recent, methodologically rigorous studies were more likely to find significant effects of emotional states on HSCT outcomes. Following this review, additional evidence for the negative prognostic significance of psychological distress and depression has emerged. For example, a large prospective study followed 138 allogeneic transplant recipients for at least 2 years post-transplant. The authors examined a comprehensive panel of medical and sociodemographic characteristics as potential predictors of survival. Depressive symptoms assessed at the time of hospital admission for HSCT emerged as an independent predictor of mortality after accounting for other relevant factors. Moreover, depression was not correlated with other predictors, suggesting it was not simply a marker of an unfavorable health status (Grulke et al., 2008). In another large study, the presence of depression or anxiety was a significant risk factor for mortality following HSCT; in fact, these factors predicted mortality more strongly than cardiac conditions, diabetes, cerebrovascular disease, and other common comorbidities (Sorror et al, 2005). Although not all studies have found significant links between depression and HSCT outcomes (e.g., Perreira et al., 2010), taken together with the prior findings, these studies suggest depression should be considered a risk factor for a poorer outcome following HSCT.
Potentially protective psychological factors (or their absence) have been investigated as predictors of mortality, including social support, optimism, and spirituality. In a study of patients undergoing autologous transplantation, those who perceived their social support to be “problematic” showed increased risk of mortality; however, perceptions of “positive” social support were not associated with mortality (Frick et al., 2005). Another large study found that optimistic expectations about HSCT outcomes predicted better survival at 2 months post-transplant after accounting for relevant medical and demographic predictors (Lee et al., 2003). However, there were no significant effects beyond 2 months post-transplant, and the study was limited by the heterogeneous sample of both autologous and allogeneic transplant recipients. A recent study of allogeneic recipients found that those who reported “spiritual absence” (a lack of spiritual or religious coping resources) had a higher mortality rate by 1 year post-transplant (Pereira et al., 2010). The authors note that the findings are limited by a modest sample size, precluding the ability to control for a larger number of medical factors that may be associated with mortality.
In sum, while the evidence for other psychosocial factors is still preliminary and has been limited by design issues and sample size, there is stronger evidence that depression is a risk factor for greater mortality and reduced survival time following HSCT. There are several possible pathways by which depression may influence HSCT outcomes. Depressed patients may be more likely to engage in health-impairing behaviors such as tobacco and alcohol use, both of which are associated with poorer outcomes and increased mortality following HSCT (e.g., Ehlers et al., 2011; Stagno et al., 2008). Depressed patients may also be less likely to comply with the rigorous requirements of post-transplant care, including taking multiple medications properly and attending frequent clinic appointments. Depression may also adversely influence immune processes important to recovery following HSCT. While systematic research linking mood disturbance, immune function, and HSCT outcomes is still lacking, we will discuss potential biobehavioral pathways for these effects.
Although hematologic cancers have not been the focus of most biobehavioral cancer research, it has been hypothesized that immune-mediated diseases such as leukemias and lymphomas are particularly susceptible to biobehavioral influences (e.g., Andersen et al., 1994; Costanzo et al., 2011). In addition, the early recovery following successful treatment of a malignancy can be viewed as a “window of opportunity,” a time period when psychosocial factors are more likely to have an influence on immune processes and disease course. These interactions may be of particular significance for HSCT patients because any modulating influence on immune processes could have a salient effect on relapse and survival. Specifically, the speed and success of immune reconstitution following transplant are directly associated with overall and progression-free survival (e.g, Porrata and Markovic, 2004) and the most prevalent and serious clinical complications of HSCT are mediated by immune processes. As such, understanding PNI relationships may be particularly fruitful for improving the outcomes of patients undergoing HSCT. Figure 1 outlines a biobehavioral framework for the contributions of behavioral factors to immune-mediated clinical outcomes following HSCT. The specific immune processes, clinical outcomes, and potential biobehavioral mechanisms are elaborated in the next section.
Biobehavioral Research Targets: Immune Processes Critical to HSCT Outcomes
Cellular Immune Recovery / Engraftment
Engraftment is monitored clinically by absolute neutrophil counts, white blood cell counts and platelet counts. Time-to-engraftment varies based on stem cell source and graft type, but commonly occurs around 10–20 days post-transplant. Recovery of other immune cell populations can take considerably longer. NK cell kinetic and functional recovery occurs early, beginning 2–3 weeks after transplant and returning to normal levels within 1–2 months (Auletta and Lazarus, 2005; Porrata and Markovic, 2004). An early recovery of innate immunity confers greater protection against infection and occurs sooner than the restoration of B and T cell functions, which may take many months to longer than a year to be fully reconstituted. Monocyte (Mo) and associated dendritic cell (DC) subpopulations are the next to reemerge, providing a critical bridge between innate and adaptive immunity. Mature Mo expressing CD16+ receptors are thought to promote leukocyte recovery, activate cytokines, and facilitate the immune response to minimal residual disease (MRD) following HSCT (Tanaka et al., 1999). B cell numbers begin to recover by 3 months post-transplant, but B cell function and immunoglobulin production can be impaired for several months or even years (Auletta and Lazarus, 2005; Porrata and Markovec, 2004). T cells are among the last to recover. Re-emergence of CD4+ and T regulatory (Treg) subpopulations lag substantially behind the more quickly recovered CD8+ cells, leading to an inverted CD4/CD8 ratio and a resulting dysregulation that can facilitate the development of opportunistic infections, GVHD, and other complications. In many patients, there is a long-term or even permanent reduction in the diversity of the T cell repertoire.
Infections
The recovery of immune competence is critical for minimizing tissue damage and providing protection against bacterial and viral pathogens. Damage to mucocutaneous barriers and delayed immune recovery contribute to the high incidence of infections in the acute post-transplant period, with 75% of patients developing infections during the initial month following transplant (Majhail and Weisdorf, 2008), particularly bacterial infections. In the months following transplantation, ongoing T cell dysfunction and hypogammaglobulinemia place patients at continued risk for infection, including reactivation of Varicella zoster virus and Cytomegalovirus (CMV).
Graft-versus-malignancy effect
A timely immune recovery reduces risk for disease relapse and improves survival via a “graft-versus-malignancy” effect. In the allogeneic setting, lower relapse rates are due to the activity of effector cells on MRD, with a substantial literature indicating links between the recovery of lymphocyte subsets and reduced risk for recurrence and mortality (e.g., Kim et al., 2006; Thoma et al., 2011). While less well-characterized, a similar effect may occur after autologous transplantation. Specifically, there is evidence for the prognostic significance of absolute lymphocyte count (ALC) at 15 days post-transplant, with findings indicating that an ALC above a threshold of 500 cells/mL is associated with reduced relapse risk and better overall and progression-free survival (Porrata et al., 2008; Porrata and Markovic, 2004). Examination of lymphocyte subsets suggests that CD16+CD56+CD3- NK cells may be the primary population driving these beneficial effects (Porrata et al., 2008). As NK cell kinetic and functional recovery occurs early, NK cell subsets are likely to be important in early surveillance and containment of MRD. The role of Mo has not been as thoroughly investigated as lymphocyte populations. However, a recent study found that higher absolute Mo counts at 30 days post-transplant predicted better overall and progression-free survival following allogeneic transplantation (Thoma et al., 2011).
Potential biobehavioral mechanisms
Figure 1 outlines pathways by which psychosocial factors may modulate immune recovery and related clinical complications, thereby influencing HSCT outcomes. It is now widely accepted that many of the host- or recipient-derived cells essential to the recovery of hematopoiesis and immunity also express receptors for factors that are responsive to the extensive crosstalk between psychological state and the neuroendocrine and immune systems. These effects are mediated by the sympathetic nervous system (SNS), hypothalamic-pituitary-adrenal (HPA) axis, and a number of other hormones and peptides. Effects may occur via glucocorticoid and beta-adrenergic receptors on mononuclear leukocytes and/or neuroendocrine modification of lymphocyte trafficking (Dhabhar and McEwen, 1997; Elenkov et al., 2000; Khan et al., 1986). Beta-adrenergic signaling pathways relevant to cancer control have been well-characterized (e.g., Cole and Sood, 2012), including influences on cellular immune function. While much of the prior research has focused on lymphocyte subsets, Mo may also be sensitive to these influences due to the high density of beta-adrenergic receptors (Kavelaars et al., 1997).
Also relevant to immune recovery following HSCT is the sympathetic innervation of the bone marrow hematopoietic environment. There is evidence of particularly extensive innervation in the sinusoidal and parenchymal areas that influence hematopoiesis (e.g., Elenkov et al., 2000). Mouse models of bone marrow transplantation have shown that early hematopoietic progenitors are especially sensitive to catecholamines and that adrenergic influences play a role in triggering stem cells into cycle (e.g., Byron, 1972) and their migration out of the bone marrow (e.g., Katayama et al., 2006). Another series of studies demonstrated that adrenergic antagonists can stimulate platelet and granulocyte formation but inhibit lymphocyte proliferation and NK cell activation, suggesting that adrenergic stimulation may have different effects on myelopoiesis and lymphopoiesis (Maestroni and Conti, 1994). However, social stress appeared to have the opposite effect; mice repeatedly exposed to a social stressor showed increased numbers of neutrophils and monocytes but a reduction of B and T cells in the bone marrow, suggesting that stress favors myelopoiesis at the expense of lymphopoiesis (Engler et al., 2004). Similarly, corticosteroid administration in a murine model depleted pre-B cells, did not affect erythrocytes or monocytes, and increased granulocytes in the bone marrow (Laakko and Fraker, 2002). Taken together, the findings suggest that stress-related physiological pathways can modulate stem cell proliferation and differentiation in the bone marrow, providing a plausible pathway by which stress-related behavioral factors can influence the reconstitution of innate and adaptive immunity following HSCT.
Cellular processes and secreted soluble mediators essential to the initial expansion and differentiation of the progenitor cells and then their continued proliferation may also be influenced by stress-related behavioral factors. Among the cytokines that have traditionally been studied in the field of PNI, IL-6 and the larger IL-6 family (e.g., G-CSF leptin, LIF, OSM, IL-11) are especially important. Additional cytokines to target that may also be sensitive to biobheavioral influences and can be distinguished by their involvement in the restoration of key cell subsets, including neutrophils (IL-8), NK cells (IFNalpha, IL-2, IL-7, IL-12), and dendritic cell/Mo lineages (TNFalpha, IL-4, GM-CSF). Beyond the essential functional actions of these cytokines, many chemokines have been shown to have stimulatory or inhibitory roles, affecting cell proliferation, migration, and activation. A full summary of the many other relevant biological pathways that influence the cell recovery after HCST goes beyond the scope of this paper but can be found in excellent reviews (e.g., Auletta and Lazarus, 2005).
Inflammation
Inflammation in the post-transplant period is common due to tissue damage from conditioning therapy and the high occurrence of infections and plays a key role in the pathogenesis of GVHD in the allogeneic transplant setting. GVHD occurs when donor T cells attack recipient tissues including the liver, skin, and intestinal mucosa. A “cytokine storm” of inflammatory factors ensues, with elevated levels of cytokines such as TNFα and IL-1 serving as reliable indicators of risk for the development of more severe GVHD (Ferrara and Reddy, 2006). GVHD occurs in acute and chronic varieties, each with a distinct but related underlying pathophysiology and clinical implications.
Acute graft-versus-host disease (aGVHD)
Acute GVHD typically occurs within the first 60 days post-transplant and is mediated by alloreactive donor T cells that have been activated by recipient antigen-presenting cells. Severe aGVHD is associated with significant morbidity and mortality and is the single most important factor affecting survival other than relapse (Ferrara and Reddy, 2006). Most patients receiving an allogeneic transplant (approximately 60–80%) experience some degree of aGVHD (Majhail and Weisdorf, 2008).
Chronic graft-versus-host disease (aGVHD)
Chronic GVHD is mediated by alloreactive donor T-cells that are activated by donor-derived antigen-presenting cells and usually appears later, generally 50–200 days post-transplant. Approximately 50% of patients will develop cGVHD, with less than 5% of those who develop severe forms surviving long-term (Horowitz and Sullivan, 2006). Moreover, cGVHD can substantially undermine the recovery following transplant and is a significant cause of long-term disability for HSCT survivors.
Potential biobehavioral mechanisms
The biobehavioral model depicted in Figure 1 also incorporates the effects of inflammation on GVHD, and there is preliminary evidence for a relationship between psychosocial factors and GVHD. A small study found that patients who reported greater anxiety prior to HSCT were at elevated risk for developing severe aGVHD (Gregurek et al., 1996). In a study of spirituality and mortality following HSCT, those reporting greater spiritual absence were particularly likely to succumb to complications of GVHD (Pereira et al., 2010). Both studies were limited in their ability to account for other predictors of GVHD, and neither study examined potential mechanisms for these relationships.
An augmented inflammatory response may play a critical role in meditating these psychobiological relationships. Specifically, an accepted clinical dogma about GVHD is that alloreactive donor immune cells recognize host antigens; however, it is only in the setting of other “danger” signals that the alloreactivity will lead to disease. These “danger” signals derive from higher levels of inflammatory mediators, including TNFα, IL-1, and IFNα released under a variety of conditions such as tissue injury from treatment and infection (Ferrara and Reddy, 2006). Links between distress or depression and inflammatory responses have been well documented in the PNI field. These relationships may be mediated by nonimmune processes, including HPA dysregulation, or more directly by the activation of lymphoid cells and proinflammatory cytokines (Black, 2002; Miller et al., 2002). It has been shown that distress or depression can sensitize the body’s defense mechanisms to engage in a prolonged state of readiness, resulting in a heightened inflammatory response when challenged (Johnson et al., 2002). Consequently, distress or depression will likely tilt the balance of the internal milieu in a way that would contribute to the initiation and perpetuation of GVHD. Interactions between stress responses and the fundamental danger signals of the body, including the release of heat shock proteins into circulation, has been of interest in the larger PNI field and may be of particular relevance in the context of HSCT.
Bidirectional Pathways
While this review has focused on the downstream effects of behavioral factors on immune recovery and regulation, the relationship is likely to be bidirectional. It is already known that proinflammatory cytokines can activate central nervous system circuitry associated with the withdrawal and conservation of energy, evoking adverse neurobehavioral and affective responses including depressed mood, fatigue, enhanced pain sensitivity, sleep disturbance, decreased activity, anorexia, and neurocognitive dysfunction. Such mechanisms have been of interest in understanding behavioral comorbidities and quality of life concerns of cancer patients (Miller et al., 2008; Seruga et al., 2008). HSCT recipients are at particularly high risk for inflammation-related impairments due to cytokines released in response to tissue damage associated with conditioning therapy, infection, and the “cytokine storm” associated with GVHD. A recent study of allogeneic transplant recipients tracked both inflammatory cytokines and several symptoms during the initial 30 days post-transplant (Wang et al., 2008). It found that increases in IL-6 and sTNF-R1 were associated with worsening of fatigue, poor appetite, pain, drowsiness, dry mouth, and disturbed sleep. While the study had a small sample, the frequent assessments and a sophisticated analytical approach that allowed the authors to model dynamic relationships and account for numerous potential covariates were strengths of this study. While still preliminary, findings suggest that assessment of inflammatory mediators can contribute to understanding of the factors that undermine quality of life following HSCT.
PNI Research Directions for HSCT: Predictors, Mediators, and Clinical Outcomes
Psychosocial / Behavioral Factors
It will be critical for biobehavioral HSCT studies to delineate the behavioral constructs that are most likely to have a salient clinical influence. Evidence from studies of psychosocial factors and HSCT outcomes clearly points to depression as a significant risk factor. However, some degree of depressed mood is a very common response following a treatment regimen as intense and difficult as HSCT. The changes in mood may have to be more severe or prolonged to affect downstream physiology. Prior research suggests that chronically depressed cancer patients are at risk for poorer outcomes, while those who experience only acute depression following diagnosis are at no greater risk (Stommel et al., 2002). Ascertaining the severity and duration of mood changes necessary to affect clinical outcomes will be an important challenge. In addition, related indicators of distress and mood disturbance, such as worry and intrusive thoughts, posttraumatic stress symptomatology, and generalized negative affect will be valuable to assess in developing a fuller clinical profile of HSCT recipients at risk for poorer outcomes.
Considering measures of cognitive and behavioral processes that promote well-being and alleviate distress will also be critical to understanding the full range of psychological experience. It is already known that protective influences such social support and optimism can mitigate the adverse effects of stress-related behavioral factors on immune function and cancer outcomes (reviewed in Costanzo et al., 2011), and we have reviewed preliminary evidence linking these contstructs to HSCT outcomes (e.g., Frick et al., 2005; Lee et al., 2003). In addition, recent studies suggest that many HSCT survivors are able to find meaning or perceive some benefit from their experience, reporting closer intimate relationships, an enhanced sense of personal strength, or an increased appreciation of life (Andrykowski et al., 2005; Widows et al., 2005). This type of psychological growth has been shown to have a beneficial influence on immune function and health in other cancer populations (Dunigan et al., 2007; McGregor et al., 2004), and therefore is likely to be a fruitful area for future inquiry alongside more traditional distress measures. It will be important to elucidate the summative influence of psychosocial risk and protective profiles that have the most salient influence on downstream physiology and HSCT outcomes in order to discern which factors should be targeted in behavioral interventions.
Immune Processes
While neutrophil counts have been a primary diagnostic endpoint used in evaluating early post-transplant recovery, the recovery of other leukocyte subsets are critical in mitigating risk for infection and enhancing the graft-versus-malignancy effect, as described previously. Good candidates to consider for investigating the mechanisms linking stress-related behavioral factors to HSCT outcomes include NK cells, which appear be primary players in eradicating MRD and reducing risk for recurrence (Porrata et al., 2008) and CD16+ Mo, which may also play a critical role in promoting the overall leukocyte recovery and an appropriate immune response to MRD (Tanaka et al., 1999). Both NK cells and Mo are also known to be extremely responsive to soluble neuroendocrine mediators and have been shown to be sensitive to psychological factors. The protracted recovery of T cell profiles is an important problem in the HSCT setting. A quicker T cell recovery and restoration of healthier ratios of T cell subsets can reduce morbidity and mortality related to infections, and regulatory T cells (Tregs) can mitigate the undesirable aspects of GVHD. Within the PNI literature, there has been considerable research on the influence of stress-related factors on T cell differentiation and proliferation, and this could be applied to the HSCT setting. Assessments will require sophisticated immunophenotyping and the use of state-of-the-art multicolor flow cytometry approaches to discern the diversity and profiles of the returning leukocytes.
Cytokines are another obvious biomarker to employ in assessing soluble mediators in systemic circulation due to the central role of inflammatory cytokines in promoting and maintaining GVHD as well as the involvement of cytokines in cell differentiation and proliferation. Attention to the less commonly studied soluble mediators and cell growth promoters is also warranted, such as GM-CSF and the cytokines that promote NK cell differentiation and function such as IL-12 and IL-17. A pragmatic challenge for this line of research, however, is that assessing the levels circulating in plasma may not be sufficient. Studies of cell propensity to produce and secrete cytokines (e.g., intracellular RNA), the cytokine response following in vitro stimulation in culture, and the more sophisticated use of advanced flow cytometry methods will be needed to better examine the production of these ligands and expression of their receptors.
Clinical Complications and Outcomes
Addressing issues of clinical significance of PNI relationships in the HSCT setting is critical in translating findings to meaningful interventions. While following patients for extended periods of time to determine relapse and survival rates is ideal, the time commitment can pose challenges. More proximal HSCT outcomes that can be examined in a shorter-term study include the development of infections, the occurrence and resolution of aGVHD, and the initial emergence of cGVHD. As previously discussed, these complications are the primary causes of morbidity and early mortality and have a clinically meaningful impact on quality of life. Additional indices of recovery such as days of hospitalization, number of re-hospitalizations, time to return to work/school, and performance status can also be assessed as milestones with a medical relevance and can serve as a functional metric of the patient’s quality of life.
Confounds and Challenges
There are a number of logistical and conceptual challenges when conducting clinical PNI research in the context of HSCT. There is significant heterogeneity with respect to diagnosis, graft type, stem cell source, conditioning regimen, and supportive care. The pre-transplant treatment and disease course can differ significantly based on diagnosis, responses to initial treatment approaches, and other risk characteristics, with some patients undergoing HSCT as part of first-line therapy, whereas others receive transplants in second or later remissions. This disease and treatment history heterogeneity needs to be carefully considered when choosing an appropriate and sufficiently homogeneous target population and/or otherwise addressed in the study design and data analysis strategy.
In addition, many aspects of post-transplant care can affect the immunological measures and mediators of interest. For example, routine GVHD prophylaxis during the first few months post-transplant includes calcineurin-inhibitors, corticosteroids, and other immunosuppressive medications. Antivirals, antibiotics, and antifungals are also commonly administered, sometimes in a pre-emptive manner and also upon early signs of infection. Cytokines may be used as biological response modifiers to stimulate a faster and stronger recovery, including the common use of GM-CSF following HCST. In addition, monoclonal antibodies against certain immunomodulatory proteins are employed in experimental protocols to reduce the inflammatory components of GVHD as well as to selectively activate or inactivate certain effector cells. Because some of these interventions are routine and standardized, selection of a homogeneous target population receiving the same supportive care protocol is a useful approach and can even be creatively incorporated into the study design and aims as one means to evaluate the influence of psychosocial variables on patients’ responsiveness to immunomodulatory agents. Where selective inclusion/exclusion criteria are not practical, sophisticated statistical modeling strategies that can account for differences in medications and treatment approaches are critical.
Another challenge is the pace of change and evolution in the field of HSCT medicine. In the allogeneic setting, a combination of factors including the increased use of reduced-intensity conditioning regimens, better GVHD prophylaxis, and early treatment of viral and fungal infections, has decreased treatment-related mortality for patients in first or second remission from 34–37% prior to 1990 to 6–25% (Bacigalupo et al., 2004). Given the rapid pace of change, research findings on psychosocial influences and clinical outcomes can quickly become outdated. In addition, the researcher must endeavor to keep up with the technical innovations taking place in fields such as multicolor flow cytometry and the multiplexing strategies for cytokines and other analytics, along with the advances in molecular biology and genomics. The ability to examine physiological pathways in more exquisite detail will facilitate discoveries about the mechanisms that mediate better clinical outcomes of HSCT and hopefully allow us to tailor interventions to stimulate these pathways.
Conclusions and Translational Directions: Improving Health, Well-Being, and Survival
Although there are many conceptual and practical challenges, the clinical significance of a timely immune recovery and immune-induced complications, along with the psychological challenges of a risky and difficult treatment regimen, make HSCT an especially important context for understanding biobehavioral influences on immunity, cancer progression, and survival. We have described the critical role of immune function for successful recovery following HSCT, focusing on the importance of a timely recovery of leukocyte subsets and optimal regulation of inflammatory processes in order to prevent infections, mitigate risk for GVHD, and eliminate malignant cells, thereby reducing risk for disease relapse and ensuring survival (see Figure 1). Many of the traditional neuroendocrine mechanisms known to be of importance in other PNI contexts are likely to be highly relevant in HSCT, including both HPA and SNS function. Sympathetic innervation of the bone marrow and adrenergic modulation of hematopoiesis are likely to have a particularly critical role in this setting. The high potential for translational relevance makes this an especially appealing area to apply the PNI perspective.
Well-designed studies with carefully selected target populations, appropriate behavioral and immune targets and assessment strategies, and attention to clinical outcomes are needed to identify characteristics of patients who are most likely to be benefit from interventions that target stress-related behavioral factors, help to determine the optimal timing for these interventions, and provide guidance regarding the types of treatments that may be most effective. We have already outlined the psychosocial and immune processes that appear most promising and have suggested that the early recovery period may be a promising window in which to intervene. Because HSCT requires substantial preparative therapies and procedures (e.g., stem cell mobilization and collection for autologous transplantation, induction therapy and procurement of donor product in the allogeneic setting), there is a unique opportunity to intervene during the weeks or even months prior to HSCT. There is also ample opportunity to implement interventions during the typically long hospital stay and/or frequent follow-up clinic visits during the acute recovery. Psychological interventions utilizing cognitive-behavioral approaches, stress management strategies, and newer approaches that incorporate acceptance and mindfulness-based modalities have shown promise in other cancer populations (see Costanzo et al., 2011). Interventions targeting health practices relevant to stress-related processes, such as treatment of sleep and circadian disturbances, may also be beneficial. Pharmacological approaches, including emerging research on beta blockers, and more effective use of psychotropic medications such as SSRIs and SNRIs may have additional therapeutic value in the HSCT setting.
The advent of HSCT has extended the lifespan of patients and in many cases permits the chance for full remission and long-term survival. However, this treatment does not come without costs, including a protracted immune and clinical recovery and the related physical and psychological sequelae that can undermine quality of life for many months or even years. The challenge for behavioral scientists is to understand the biobehavioral processes that may deter immune restoration or engender a better recovery, thereby influencing the clinical course and outcome. This is a critical step in developing novel therapeutic strategies tailored to the unique needs of this population with the potential to contribute to improved health, quality of life, and longer survival following HSCT.
Acknowledgments
This work was supported by grants K07 CA136966 and R21 CA133343 from the National Cancer Institute and an award from the Forward Lymphoma Foundation.
Footnotes
Conflict of Interest Statement:
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, Horowitz MM, Sobocinski KA, Rizzo JD, Wingard JR. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35:835–857. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Sormani MP, Lamparelli T, Gualandi F, Occhini D, Bregante S, Raiola AM, di Grazia C, Dominietto A, Tedone E, Piaggio G, Podesta M, Bruno B, Oneto R, Lombardi A, Frassoni F, Rolla D, Rollandi G, Viscoli C, Ferro C, Garbarino L, Van Lint MT. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1238–1247. [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Byron JW. Evidence for a -adrenergic receptor initiating DNA synthesis in haemopoietic stem cells. Exp Cell Res. 1972;71:228–232. doi: 10.1016/0014-4827(72)90283-2. [DOI] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunol Allergy Clin North Am. 2011;31:109–132. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dunigan JT, Carr BI, Steel JL. Posttraumatic growth, immunity and survival in patients with hepatoma. Dig Dis Sci. 2007;52:2452–2459. doi: 10.1007/s10620-006-9477-6. [DOI] [PubMed] [Google Scholar]
- Ehlers SL, Gastineau DA, Patten CA, Decker PA, Rausch SM, Cerhan JR, Hogan WJ, Ebbert JO, Porrata LF. The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant. 2011;46:285–290. doi: 10.1038/bmt.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ferrara JLM, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Frick E, Motzke C, Fischer N, Busch R, Bumeder I. Is perceived social support a predictor of survival for patients undergoing autologous peripheral blood stem cell transplantation? Psychooncology. 2005;14:759–770. doi: 10.1002/pon.908. [DOI] [PubMed] [Google Scholar]
- Gregurek R, Labar B, Mrsic M, Batinic D, Ladika I, Bogdanic V, Nemet D, Skerlev M, Jakic-Razumovic J, Klain E. Anxiety as a possible predictor of acute GVHD. Bone Marrow Transplant. 1996;18:585–589. [PubMed] [Google Scholar]
- Grulke N, Larbig W, Kachele H, Bailer H. Pre-transplant depression as risk factor for survival of patients undergoing allogeneic haematopoietic stem cell transplantation. Psychooncology. 2008;17:480–487. doi: 10.1002/pon.1261. [DOI] [PubMed] [Google Scholar]
- Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplantation. 2006;38:255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- Horowitz ME, Sullivan KM. Chronic graft-versus-host disease. Blood Reviews. 2006;20:15–27. doi: 10.1016/j.blre.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao W-M, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. Journal of Neuroimmunology. 1997;77:211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Khan MM, Sansoni P, Silverman ED, Engleman EG, Melmon KL. Beta-adrenergic receptors on human suppressor, helper, and cytolytic lymphocytes. Biochem Pharmacol. 1986;35:1137–1142. doi: 10.1016/0006-2952(86)90150-4. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:1119–1128. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- Laakko T, Fraker P. Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology. 2002;105:111–119. doi: 10.1046/j.1365-2567.2002.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Loberiza FR, Rizzo JD, Soiffer RJ, Antin JH, Weeks JC. Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9:389–396. doi: 10.1016/s1083-8791(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Noradrenergic modulation of lymphohematopoiesis. Int J Immunopharmacol. 1994;16:117–122. doi: 10.1016/0192-0561(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Majhail N, Weisdorf D. Complications after hematopoietic stem cell transplantation. In: Hoffman R, editor. Hematology: Basic Principles and Practice. 5. Churchill Livingstone; 2008. [Google Scholar]
- McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increased benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Christian LM, Patidar S, Bishop MM, Dodd SM, Athanason R, Wingard JR, Reddy VS. Spiritual absence and 1-year mortality after hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2010;16:1171–1179. doi: 10.1016/j.bbmt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Litzow MR, Winters JL, Markovic SN. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant. 2008;14:807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrata LF, Markovic SN. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clin Exp Med. 2004;4:78–85. doi: 10.1007/s10238-004-0041-4. [DOI] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S, Busby K, Shapiro A, Kotz M. Patients at risk: addressing addiction in patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2008;42:221–226. doi: 10.1038/bmt.2008.211. [DOI] [PubMed] [Google Scholar]
- Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94:2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, Martin PJ. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Honda J, Imamura Y, Shiraishi K, Tanaka K, Oizumi K. Surface phenotype analysis of CD16+ monocytes from leukapheresis collections for peripheral blood progenitors. Clin Exp Immunol. 1999;116:57–61. doi: 10.1046/j.1365-2249.1999.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma MD, Huneke TJ, Decook LJ, Johnson ND, Wiegand RA, Litzow MR, Hogan WJ, Porrata LF, Holtan SG. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia post myeloablative allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, Lee B, Giralt SA. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widows MR, Jacobsen PB, Booth-Jones M, Fields KK. Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychol. 2005;24:266–273. doi: 10.1037/0278-6133.24.3.266. [DOI] [PubMed] [Google Scholar]