Abstract
Early pregnancy complications are more common in women who conceive after infertility treatment. Most of these occur before 12 weeks of gestation and include miscarriage, vaginal bleeding, intrauterine hematoma, vanishing twin, and ectopic pregnancy (EP). The incidence of EPs following infertility treatment is much higher compared with that in spontaneous pregnancies. The occurrence of an EP is very distressing to an infertile couple, who has lots of hopes pinned on the treatment outcome, especially because of the cost incurred and the physical and mental trauma both have gone through during the treatment process. The association between infertility and EP is complex, as it can be a consequence of infertility as well as a cause. The two principal risk factors for an EP are genital tract infections and tubal surgeries. Though several etiologies are proposed, but patients with tubal factor infertility are at an increased risk of an EP. Earlier diagnosis of EP helps to improve prognosis and optimize subsequent fertility. It is pivotal to evaluate the likelihood of subsequent occurrence of an EP and be too vigilant when treating. The correct choice of the treatment modality should be made to prevent the recurrence. The early prediction of the pregnancy outcome therefore has great importance for both the couple and clinician. Today with the help of sensitive beta human chorionic gonadotropin (β-hCG) assays and transvaginal sonography, one can diagnose an EP prior to symptoms, and conservative treatment for the preservation of the fallopian tube is possible. Conservative management in the form of expectant and medical management should be considered as a first-line treatment modality, provided that the overall clinical picture suggests that it is safe to do so. If not, laparoscopic management of EPs appears to be the favored approach of management as compared to laparotomy.
KEY WORDS: β-hCG, conservative management, ectopic pregnancy, laparoscopy, laparotomy, tubal factor infertility
INTRODUCTION
Early pregnancy complications are more common in women who conceive after infertility treatment. Most of these occur before 12 weeks of gestation and include miscarriage, vaginal bleeding, intrauterine hematoma, vanishing twin, and ectopic pregnancy (EP). The incidence of EPs following infertility treatment is much higher compared with that in spontaneous pregnancies. The occurrence of an EP is very distressing to an infertile couple, who has lots of hopes pinned on the treatment outcome, especially because of the cost incurred and the physical and emotional trauma both have gone through during the treatment process. An EP leads the couple into the universe of tubal factor infertility and probably assisted reproductive technology (ART) treatment in future to conceive. Today there is no consensus that ART is indicated in those women who had an EP in the past. One has to elicit the history and determine the cause and mode of treatment to plan the future line of management. When an EP occurs during infertility treatment, it marks a reproductive failure, which always requires psychological counselling before further treatment. Regardless of the treatment strategy, a successful outcome requires a subsequent ongoing intrauterine pregnancy, which is the ultimate goal of fertility treatment. An EP resulting from fertility treatment is a specific entity, and better knowledge of it should help to improve diagnosis and prognosis, simplify treatment, and optimize subsequent fertility. We must remember when choosing the modality of treatment that this group of women especially with a history of previous distal tubal disease are at a higher risk of developing recurrent EPs. So we need to make the right choice between conservative, medical, or surgical treatment and nonconservative tubal surgery to improve the subsequent outcome of fertility treatment.
EPIDEMIOLOGY
The knowledge of risk factors such as history of genital infections, tubal surgery, smoking, woman's age, and her history of spontaneous or elective abortion, use of an intrauterine device (IUD), or infertility increases the risk of EP. The incidence is higher among women whose pregnancies result from ovulation induction (OI), especially with clomiphene citrate. In the USA in 1999, EPs accounted for 2.2% of the clinical pregnancies from all IVF cycles, regardless of the ART technique used, and 1.9% of those from ICSI (Anonymous, 2002). In the same year, in France, the rates were 3.4% for IVF and 1.9% for ICSI.[1]
ETIOLOGY
Multiple factors contribute to the relative risk of EP in women with infertility. Anything that hampers the migration of the embryo to the endometrial cavity could predispose women to ectopic gestation. The most logical explanation for the increasing frequency of EPs is previous pelvic infection; however, most patients presenting with an EP have no identifiable risk factor.
The following risk factors have been linked with EPs.
Pelvic inflammatory disease
The most common cause is antecedent infection with Chlamydia trachomatis. Chlamydial infection has a range of clinical presentations, from asymptomatic cervicitis to salpingitis and florid pelvic inflammatory disease (PID). More than 50% of women who have been infected are unaware of the exposure. Other organisms causing PID, such as Neisseria gonorrhea, increase the risk of an EP. A history of salpingitis increases the risk of an EP by fourfold. The incidence of tubal damage increases after successive episodes of PID (i.e., 13% after 1 episode, 35% after 2, and 75% after 3 episodes).
History of a prior ectopic pregnancy
After one EP, a patient incurs a 7- to 13-fold increase in the likelihood of another EP. Overall, a patient with prior pregnancy has a 50%–80% chance of having a subsequent intrauterine gestation, and a 10%–25% chance of a future tubal pregnancy.
History of tubal surgery and conception
Prior tubal surgery (salpingostomy, neosalpingostomy, fimbrioplasty, tubal reanastomosis, and lysis of peritubal or periovarian adhesions) has an increased risk for developing EP. This in turn depends on the degree of damage and the extent of anatomic alteration.
Use of fertility drugs or assisted reproductive technology
OI with clomiphene citrate or injectable gonadotrophins therapy has been linked with a fourfold increase in the risk of an EP in a case–control study. This finding suggests that multiple oocytes and high levels of circulating estrogens may be significant factors.
Increasing age
The highest rate of EP is found in women aged 35–44 years. A 3- to 4-fold increase in the risk for developing an EP exists in this group as compared to women aged 15–24 years. The probable cause for this high incidence is the progressive loss of the myoelectrical activity in the fallopian tube with age, which is responsible for tubal motility.
Salpingitis isthmica nodosum
Salpingitis isthimica nodosum is defined as the microscopic presence of tubal epithelium in the myosalpinx or beneath the tubal serosa. This tubal epithelium protrudes trough the tube, like small diverticula. Histopathological sections of the fallopian tube from women with EP have revealed that approximately 50% of patients treated with salpingectomy have evidence of salpingitis isthmica nodosum. Salpingitis isthmica nodosum could be either congenital or as a result of inflammation or endometriosis resulting in tubal changes.
Smoking
Cigarette smoking has been shown to be a risk factor for developing an EP. The elevated risk ranges from 1.6 to 3.5 times as compared to nonsmokers. On the basis of laboratory studies in humans and animals, researchers have postulated several mechanisms by which cigarette smoking might play a role in EPs. These mechanisms include one or more of the following: delayed ovulation, altered tubal and uterine motility, or altered immunity.
Others
Other risk factors associated with an increased incidence of EP include previous diethylstilbestrol (DES) exposure, a T-shaped uterus, prior abdominal surgery, failure with progestin-only contraception, and ruptured appendix.
PATHOPHYSIOLOGY
Most EPs are located in the fallopian tube. The most common site is the ampullary portion of the tube, where over 80% occur. The next most common sites are the isthmic segment of the tube (12%), the fimbria (5%), and then the cornual and interstitial region of the tube (2%). The nontubal site of EPs are abdominal pregnancies accounting for 1.4% of EPs and ovarian and cervical sites accounting for 0.2% each. At times pregnancy can also occur in the rudimentary horn of a bicornuate uterus.
CLINICAL PRESENTATION
The classical clinical presentation of an EP is amenorrhea, abdominal pain, and vaginal bleeding. Usually only 50% of patients will present typically. The clinician should have a high index of suspicion if a woman presents with symptoms of early pregnancy, lower abdominal pain, or dyspareunia, and there is a presence of tenderness in the fornices and on cervical movement, and presence of adenexal mass or bogginess in the posterior fornix. At times, if there is blood in the peritoneal cavity, there could be abdominal tenderness, rigidity, and guarding. It has been observed that only 40%–50% of patients with an EP will present with vaginal bleeding; 50% will have a palpable adnexal mass and 75% may have abdominal tenderness. In the olden days, about 20% of patients with EPs would be hemodynamically compromised at the initial presentation, suggesting a rupture. But today, the diagnosis is made at an early stage of pregnancy with the availability of modern diagnostic techniques. Moreover, the patients especially those who have undergone infertility treatment present early for beta human chorionic gonadotrophin (β-hCG) and transvaginal sonography (TVS) for diagnosis of pregnancy so that the luteal phase support could be continued.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis included the following:
Appendicitis
Salpingitis
Ruptured corpus luteum, cyst, or ovarian follicle
Spontaneous abortion or threatened abortion
Ovarian torsion
Urinary tract/renal disease
Intrauterine pregnancies with other abdominal or pelvic problems such as degenerating fibroids.
DIAGNOSIS
With the advent of TVS, serum β-hCG measurements, and improved clinical algorithms, an EP can be diagnosed early without the use of invasive measures like culdocentesis or laparoscopy.
Transvaginal ultrasound
When the clinician suspects EP, with the help of TVS, he looks at the deciduas, adenexa for the presence of single or multiple corpora lutea, hematosalpinx, or gestational sac close to the ovaries or in the pouch of Douglas (POD). The sonologist should remember that the stimulated ovaries could misguide one in the diagnosis as EP, as there could be probe tenderness, or may mask the ectopic implantation due to its large size. Peritoneal fluid is seen frequently in women with ovarian hyperstimulation syndrome (OHSS) though generally anechoic, and the adnexa are commonly pathologic in ART (hydrosalpinx, endometriosis, etc.), and it is difficult to identify a hematosalpinx or ectopic sac in this pelvic context.
One needs to look for the presence of heterotopic pregnancy [Figure 1], the incidence of which is higher especially in this group of women who have undergone treatment for infertility as compared to cases with spontaneous conception (1%–3 %). Most of the times as more than one embryo is transferred, a wide variety of possible forms of ectopic and heterotopic pregnancies have been described: bilateral tubal ectopic,[2] interstitial[3] or ovarian heterotopic,[4] triple heterotopic,[4] and bilateral interstitial sextuplet ectopic after bilateral salpingectomy.[5] Therefore, the clinician has to look at the adenexa despite the presence of intrauterine gestational sac with yolk sac and fetal pole, especially if the β-hCG is not corresponding to the weeks of gestation or (higher).
Figure 1.
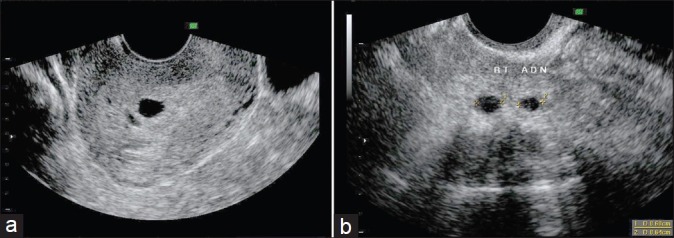
Heterotopic pregnancy. (a) Two intrauterine sacs, (b) Two right adenexal sacs
It is therefore recommended that an early (6–8 weeks) transvaginal [Figure 2] and systematic ultrasound examination be done by a specialist.[6–8] Most infertility specialists perform the scan at 5 weeks of pregnancy when the β-hCG level is 1000 mIU/ml or more.
Figure 2.
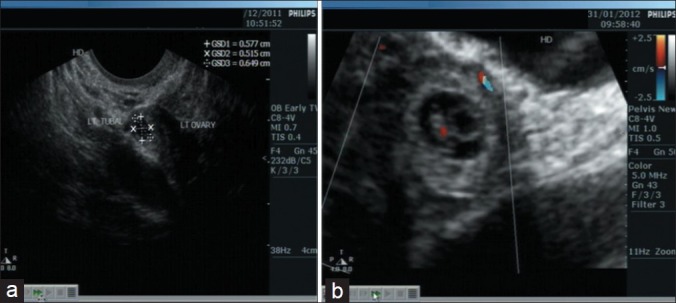
Tubal pregnancy (a) Sac in left adenexa, (b) Presence of yolk sac, fetal pole and heart
Beta human chorionic gonadotropin
At an early stage, it is difficult to distinguish an intrauterine pregnancy and spontaneous abortion from EP using β-hCG levels. Though with infertility treatment, knowledge of the date of fertilization is more precise, but the risk of multiple pregnancies is higher making it difficult to interpret the β-hCG levels. A single plasma hCG assay, as early as 11–12 days after embryo transfer, can accurately differentiate viable and nonviable pregnancies. Nonetheless, the threshold values vary between studies, depending especially on the exact day of the assay in relation to the date of embryo transfer or ovulation.[9–14]
After ART, an early level of hCG on day 12 after a day 3 transfer or on day 10 after a blastocyst transfer should be between 50 and 150 mIU/ml. This level should double every 24–36 h and the estimation of β-hCG levels 1 week later should have values between 1000 and 1500 mIU/ml. Thus, serial hCG measurements are needed to document either a growing, potentially viable, or a nonviable pregnancy and this improves the positive predictive value of using β-hCG to diagnose early pregnancy or ectopic gestation. The absence of an intrauterine gestational sac when the hCG concentration is above the discriminatory zone implies an abnormal gestation. It is better to have a more conservative discriminatory zone, that is, higher hCG level, so as to minimize the risk of terminating a viable pregnancy.[15]
Chen et al.[16] have proposed doing two assays, 15 (D15) and 22 (D22) days after embryo transfer. Their study included 198 treatment cycles. They found that a single plasma hCG assay >150 mIU/ml on D15 indicates a normally developing pregnancy with a positive predictive value (PPV) of 89% but a negative predictive values (NPV) of only 51%. When this assay is 150 mIU/ml on D15 but the ratio of hCG on D22 to hCG on D15 (hCG D22:hCG D15 ratio) >15, the PPV, i.e., the likelihood of a normally developing pregnancy, remained 90%. If hCG on D15 is <150 mIU/ml and the hCG D22:hCG D15 ratio <15, the NPV, i.e., the risk that the pregnancy will not continue normally, is 84%. They therefore concluded that the ability to identify abnormal pregnancies increases with this double assay, which enables physicians to monitor these cases more closely. If an intrauterine sac is not visualised at a β-hCG level of 1000 mIU/ml, it is diagnostic of an EP.
Progesterone
If progesterone (P4) values on day 10 after ET are low, it is diagnostic of an EP. But in patients who have had infertility treatment, it is difficult to define a particular threshold value for P4 due to the wide variations observed in progesterone levels because of the presence of multiple corpora lutea (in intrauterine insemination [IUI] and ART cycles due to controlled ovarian stimulation) as against a single corpus luteum secreting progesterone in spontaneous conception.
MOLECULAR BIOLOGY IN EP DIAGNOSIS
Some teams have looked into molecular biology to improve diagnosis of EP.[2] They suggested the use of some protein profiles from a maternal blood sample to differentiate an intrauterine from an EP much earlier. This method, if confirmed, would revolutionize the diagnosis and management of EP avoiding unnecessary laparoscopy and allowing medical treatment of asymptomatic EP at the very beginning of the pregnancy.
Treatment
Earlier diagnosis of EP enables a successful medical treatment and avoids conservative or radical surgical treatment involving either laparoscopy or laparotomy. The treatment modality is determined by the presence or absence of hemodynamic stability. Hemodynamically unstable patients are usually best treated by prompt laparotomy and these patients require salpingectomy because of extensive tubal damage, but occasionally can be treated by salpingotomy. Hemodynamically stable patients with an unruptured EP can be treated either medically or by laparoscopic techniques. Success rates reported in the literature vary from 65% to 95% for medical treatment and from 72% to 95% for conservative surgical treatment.[17–29] The great variation seen is due to the heterogeneity of the patient inclusion criteria and of the definition of treatment failure. The conservative management of EP includes expectant management and medical management. As a general principle, especially in patients with infertility, conservative management should be considered as first-line treatment, provided that the overall clinical picture suggests that it is safe to do so. There have been several excellent reviews on the expectant and medical management of EP,[30] and there seems to be a well-accepted consensus on these aspects of management. In contrast, till date, there are a number of controversies regarding the surgical management of EPs. If surgical treatment is necessary, one needs to decide whether the approach should be laparoscopic or open laparotomy. Again an important decision needs to be taken if surgery is undertaken – should salpingectomy or salpingotomy to be performed?
Medical
Today the most commonly used form of medical treatment is methotrexate (MTX) administration either intramuscularly or in the ectopic sac. MTX is a chemotherapeutic drug given at a dose of 1 mg/kg or based on the surface area calculation of 50 mg/m2. It may be given in a single or multiple doses. There are currently no randomized controlled trials comparing medical treatment versus salpingectomy. Appropriate patient selection is the crux of successful treatment with MTX. β-hCG levels, size of ectopic mass, and the presence or absence of yolk sac on ultrasound determines the success of medical therapy.
Though it has been seen that the failure rates are lower with lower β-hCG levels, till date no consensus has been reached regarding an absolute hCG level that serves as a relative contraindication to MTX therapy. Failure of treatment was defined as the requirement of surgical therapy after MTX therapy was begun.
Studies have used various criteria as guidance as to the suitability of patients to undertake medical management. These include serum hCG not more than 5000 mIU/l[29] or 10,000 IU;[31] no fetal heart seen on the ultrasound scan;[25,28–29,31] EP of relatively small size no more than 3.5 cm[25,28,29] or 4 cm;[31] no signs of active bleeding; and inability to come for regular follow-up.
It is important that the patient does not have hepatic or renal dysfunction. In the presence of contraindication to general anesthesia, multiple previous pelvic surgeries, and morbid obesity, medical treatment is still indicated even if the hCG level is ≥ 5000 mIU/l and/or the adnexal mass is >4 cm.
Prior to the first dose of MTX, women should be screened with a complete blood count, liver function tests, serum creatinine, and blood type and Rh. Women having a history of pulmonary disease should also have a chest X-ray done because of the risk of interstitial pneumonitis in patients with underlying lung disease.
Today guidelines published by American Society for Reproductive Medicine (ASRM) and/or American College of Obstetricians and Gynecologists (ACOG) help direct appropriate patient selection.[32] The absolute indications and contraindications largely focus on patient safety.
MTX may be administered intramuscularly in either a single dose[33] or multiple doses on alternate days.[20,23,24,26,34,35]
In the single-dose regimen, MTX is given in a dose of 1 mg/kg body weight after the β-hCG level is documented. β-hCG is repeated on days 4 and 7 after the MTX dose, and if β-hCG decreases by <15% between days 4 and 7, the MTX dose is repeated. Thus, the single-dose regimen includes provision for additional doses of MTX when the response is inadequate.[33,36,37]
In the multiple-dose regimen, injections of MTX at a dose of 1 mg/kg (on D0, D2, D4, D6) are alternated with four doses of 0.1 mg/kg of leucovorin IM (on D1, D3, D5, D7), as Tanaka (1982) initially proposed. β-hCG levels are done on the day of MTX administration before the dose is administered. MTX is continued until hCG falls by 15% from its peak concentration.
However, the optimal dose and time of MTX is yet to be determined. In both single and multiple-dose MTX treatment protocols, once hCG levels have met the criteria for the initial decline, hCG levels are followed serially at weekly intervals to ensure that concentrations decline steadily and become undetectable. The complete resolution of an EP usually takes 2–3 weeks but can also take as long as 6–8 weeks when pretreatment hCG levels are in higher ranges.[34,37,38]
When declining hCG levels rise again, the diagnosis of a persistent EP is made. The failure rate increases sharply with higher baseline β-hCG levels – between 2000 to 4999 mIU/ml and 5000 to 9999 mIU/ml: 3.77% (95% confidence interval [CI]: 1.04%, 9.38%) vs. 14.29% (95% confidence interval: 5.94%, 27.24%), respectively. Odds ratios comparing failed treatments between these two hCG ranges were also the only statistically significant findings noted after comparisons were made between the narrow hCG ranges: 3.79 (95% confidence interval: 1.16, 12.33; P = 0.018).[39]
There have been no randomized trials directly comparing the two different MTX treatment protocols. In a meta-analysis including data from 26 articles and 1327 cases, the overall success rate for MTX treatment was 89%.[37] The success rate of the multiple-dose regimen was 92.7% (95% CI 89–96), which was statistically significantly higher than that achieved with the single-dose regimen (88.1%; 95% CI 86–90).[37] After controlling for initial hCG values and the presence of embryonic cardiac activity, the failure rate for the single-dose therapy was higher than that for multiple-dose treatment (odds ratio 4.75; 95% CI 1.77–12.62).[33] A small randomized clinical trial also noted that the single-dose therapy has a higher failure rate, but the difference was smaller (RR ¼ 1.50; 95% CI 0.44–5.01).[40]
MTX-induced embryopathy is a serious and avoidable complication that may arise when a viable pregnancy is misdiagnosed as an EP, and therefore a correct diagnosis is of great importance before any treatment is initiated.
Side effects of the MTX therapy
MTX may cause decreased platelet, red blood cell, and white blood cell count; liver enzyme elevation; stomatitis; gastrointestinal colicky pain; chest pain; and increased sensitivity to the sun and so an increased risk of sunburn.
SURGICAL THERAPY
Surgical therapy is recommended if the patient is hemodynamicaly unstable, the hCG level is > 10,000, adnexal mass > 4 cm, contraindication to medical therapy exists, and if regular outpatient follow-up is not possible. Surgery may offer many advantages in the management of EPs as a correct diagnosis of the presence or absence of an EP can be made. Moreover, follow-up is less demanding and the patient can attempt to conceive immediately after she recovers from the surgery in contrast to a waiting period of 3 months after medical therapy due to the potential teratogenic effects.
Laparoscopy or laparotomy
Laparoscopic surgery [Figure 3] is better than laparotomy, as it results in less blood loss, a shorter hospital stay, shorter operating time, less analgesia, and a shorter convalescence period. In India, especially in rural areas, laparotomy is still performed for EP due to lack of facilities for laparoscopic surgery and expertise required for the same.
Figure 3.
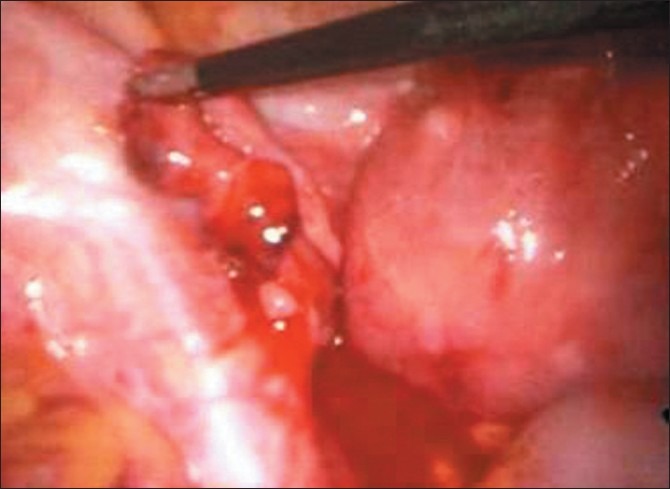
Ampulary ectopic pregnancy leaking from fimbrial end
The Cochrane review[29] reported that laparoscopic salpingotomy is less successful than laparotomy salpingotomy in the elimination of tubal pregnancy (RR 0.90; 95% CI 0.83–0.97). This was due to the higher persistence of trophoblastic tissue in the laparoscopic surgery group (RR 3.6, 95% CI 0.63–21). However, there was no difference in the future conception (RR 1.2, 95% CI 0.88–1.5) or the repeat EP rate (RR 0.43, 95% CI 0.15–1.2) between the laparoscopic group and the laparotomy group.
Should the laparoscopic treatment be conservative or radical?
The chances of a subsequent intrauterine pregnancy are similar for all EP patients, regardless of whether their treatment was conservative or radical though some studies have shown a higher pregnancy rate for salpingotomy as compared to salpingectomy.
Till date, there are no randomized controlled trials comparing the two surgical techniques.
Bouyer et al.[41] reported an intrauterine pregnancy rate of 68% for conservative surgery as compared to 48% for radical surgery in a patient population of 835 EPs.
The Royal College of Obstetricians and Gynaecologists[42] recommends that salpingotomy should be considered as the primary treatment in the presence of normal tube or contralateral tubal disease unless there is evidence of gross hydrosalpinx or severely damaged tube in the ipsilateral tube. Otherwise a salpingotomy may compromise the success of IVF.[43] One more problem with salpingectomy is the impairment of blood supply to the ovary and thus a decreasing ovarian reserve.[44–46] The other problem with surgical treatment is persistent trophoblastic disease (PTD). It is exceedingly rare in women who have salpingectomy, but can occur in up to 8% of women who undergo salpingotomy. Hence, it is important to follow up patients who have undergone salpingotomy with β-hCG measurement so that treatment can be instituted if the β-hCG concentrations have not fallen as desired. In such cases, MTX treatment appears to be effective in a single dose of 50 mg/m2 or 1 mg/kg.
But one needs to keep in mind that in case of bleeding if extensive diathermy is used, the tubal mucosa may be damaged resulting in adhesions, hydrosalpinx, and also an increased incidence of repeat EP. Tubal rupture should no longer be considered as a strict indication for salpingectomy. If the tubal damage is not too big and the rupture is linear and limited, the rupture site can be used to evacuate the trophoblast and save the tube.[47–49]
Repeat ectopic pregnancy rate
Initially it was thought that by performing a salpingectomy instead of salpingotomy, one eliminates the likelihood of a repeat EP. However, Yao and Tulandi's[50] meta-analysis of nine studies reported that the recurrent EP rate after salpingectomy was 9.9%, which was only slightly lower than 14.8% after salpingotomy. This was comparable with a study by Clausen et al.[51] that reported a recurrent EP rate of 15% after salpingotomy compared with 10% after salpingectomy.
Only when the surgeon is meticulous in his or her surgical technique, after an accurate case selection and precise diagnosis, can the risk of recurrent EP on the same side be reduced.[52]
Medical versus surgical treatment
Both conservative surgery and medical therapy may be viewed as the appropriate first-line therapy. Success rates and fertility after treatment are comparable for medical therapy and conservative surgical treatment but there are many advantages of medical treatment over surgery. It is relatively noninvasive, possibly cheaper, and relatively “skill free” to administer. Multiple-dose MTX treatment has a lower failure rate than the single-dose therapy.
The Cochrane review combined four randomized controlled trials involving 265 women with a small unruptured tubal EP.[21,24,30,31] It showed that one single dose of systemic MTX intramuscularly was significantly less successful than laparoscopic salpingotomy in the elimination of tubal EP (OR 0.38, 95% CI 0.20–0.71). However, with the multiple-dose regimen, the treatment success rate rose, and the combined results showed no evidence of a difference with laparoscopic salpingotomy (OR 1.1, 95% CI 0.52–2.3).
Three studies examined tubal patency in a total of 115 women and found no significant differences between single-/multiple-dose MTX treatment versus laparoscopic salpingotomy.[24,30,31] There was no significant difference in the subsequent intrauterine pregnancy rate. There were no adverse effects in the laparoscopy group although in the MTX group, there were two cases of minor mouth ulcerations, two cases of dry eyes, and a further case of vaginal dryness.
Decision in ART management after an index EP
ART management should be guided not by the EP history but by the etiology, management, and presence or absence of other factors affecting fertility. This enables us to counsel the patient either for spontaneous conception, IUI, or ART. Women more than 35 years of age having infertility for more than 4 years in the presence of tubal disease are at an increased risk of recurrence of an EP. We also know that after ART management, fertility after EP cannot be considered without the risk of recurrence.
CASE REPORTS
Going through these case studies will tell us how to assess and treat patients with EP who have undergone infertility treatment.
Case 1
Mrs. TA, 28 year old and married since 1 year, was anxious to conceive due to her irregular cycles as a result of PCOS. Her age at menarche was 12 years, with menstrual cycles being irregular for 1–2 days every 35–45 days. She also had a history of premenstrual pain, and tension and spotting with dysmenorrhea. She had undergone ureteroscopy for renal calculi twice. She also had a family history of hemophilia with two of her brothers being affected and her mother was a carrier. Her three maternal uncles also had hemophilia.
She was obese with moderate hirsutism. Clinically, her uterus was normal sized, firm, and mobile with all fornices being clear. At her baseline ultrasound, the uterine size was 70.6/ 33.2/42.2 mm; endometrial thickness was 6.1 mm; both ovaries were enlarged with multiple follicles and increased stroma.
Her baseline hormone levels on day 2 of the cycle were as follows: follicle stimulating hormone (FSH) – 5.6 mIU/ml, luteinizing hormone (LH) – 5.3 mIU/ml, dehydroepiandro sulfate (DHEAS) – 780 ng/ml, androstenidione – 2.7 ng/ml, estradiol (E2) – 20.73 pg/ml, progesterone (P4) – 3.68 ng/ml, thyroid stimulating hormone (TSH) – 9.4 uIU/ml, free T4 (FT4) – 1.25, fasting insulin (FI) – 15.8 uU/ml. Fasting Insulin levels were repeated after 3 months of Metformin (500 mg twice a day) which were 3.1 uU/ml. Semen analysis done was normal.
In view of the family history of haemophilia, she was evaluated to rule out a carrier state. Her prothrombin time (PT) was 13.2 s (control 12 s), activated partialthromboplastin time (aPTT) 33 s (control 30 s), factor VIII c activity 86% (60–150), factor IX c assay – 60% (60–150), and factor XIII screening showed stable clot. Her results for PCR for carrier state revealed that she was not a carrier for hemophilia. Her hysterosalphingography (HSG) showed a normal uterine cavity with a good filling of both tubes with the bilateral spill. The right tube was morphologically normal with mild dilatation and clumping of the distal end of the left tube. Tubal pressures were low.
She was started on 50 μg of tablet eltroxin and tablet metformin 500 mg BD. Metformin was discontinued after 2 months due to gastrointestinal side effects.
The first cycle was monitored without OI drugs, with no ovulation documented after monitoring till day 21 of the cycle. In the second cycle, clomiphene citrate (CC) 100 mg was given from days 2 to 6, and ovulation was documented on day 22. In the next two cycles, the dose of CC was increased to 150 mg. Ovulation was documented on days 15 and 17. In the next three cycles, letrazole 5 mg was given from days 3 to 7, and ovulation was documented on day 14 or 15 of the cycle. As there was no pregnancy even with letrazole, CC was given again in the seventh cycle. She had three mature follicles on day 12, when hCG 5000 IU was given intramuscular, and ovulation was documented on days 14 and 15. Micronized progesterone vaginal pessaries were given for luteal support. A total of 20 days after ovulation, β-hCG done on November 19, 2007, was 410 mIU/ml. β-hCG was repeated again on November 22, 2007, which had increased to 1085 mIU/ml. USG revealed a single intrauterine (IU) sac with a gestational sac diameter (GSD) of 4.4 mm (5 weeks 1 day).
On November 26, 2007, a repeat TVS did not show any increase in GSD (5.8 mm = 5 weeks 2 days). No yolk sac or fetal pole was seen. Repeat TVS after a week on December 3, 2007, documented a gestational sac of 8.4 mm (5 weeks 4 days) with no yolk sac or fetal pole seen. There was minimal tenderness in the right fornix, but no ectopic gestational sac (GS) seen in either fornix. The β-hCG level on that day was 9515 mIU/ml, which was too high for an anembryonic pregnancy. β-hCG was repeated on December 6, 2007, and was 11342 mIU/ml; TVS documented a GS of 10.5 mm (5 weeks 6 days) with no fetal pole or yolk sac seen. There was another small GS seen measuring 4 mm . There were also two small sacs measuring 4 and 6 mm, seen in the right adenexa, the left adenexa appearing normal with no free fluid in POD. There was no abdominal tenderness or guarding or tenderness in all fornices. On December 8, 2007, the β-hCG was 12947 mIU/ml with IU GS of 10.5 mm (5 weeks 6 days) with no fetal pole or yolk sac . The two sacs in the right adenexa had increased in size to 8 and 6 mm, respectively. There was no free fluid in POD. The patient was clinically asymptomatic and stable so a decision to start medical therapy was taken. Inj MTX 1 mg/kg was given IM with inj. leucovorine 0.1/kg given IM on alternate days.
The patient was followed up on a regular basis clinically and by TVS. She was also closely monitored for β-hCG, hemoglobin (HB), complete blood count (CBC), liver function test (LFT), and peripheral smears.
The β-hCG levels after MTX injection were as follows:
December 12, 2007, 11,967 mIU/ml
December 14, 2007, 10,164 mIU/ml
December 17, 2007, 6292 mIU/ml
December 20, 2007, 3232 mIU/ml
December 22, 2007, 1088 mIU/ml
December 24, 2007, 791 mIU/ml
December 28, 2007, 90 mIU/ml
January 2, 2008, 9 mIU/ml.
Serial TVS done showed regression of intrauterine and right adenexal sacs. Once the β-hCG levels were less than 1000 mIU/ml, the patient had per vaginal bleeding. TVS showed the products in the cervical canal which were then removed under aseptic precautions.
A total of six injections of MTX were given. All her blood parameters were normal throughout the treatment.
Once β-hCG was below 10 mIU/ml, she was advised not to conceive for 6 months, with follow-up of β-hCG after 1 and 2 months which was normal.
After 6 months, she was again given CC for OI, and ovulation was documented on day 15. She conceived in the second treatment cycle with timed intercourse. Her -hCG 20 days after ovulation was 3571 mIU/ml with an IU GS of 9 mm corresponding to 5 weeks 3 days seen on TVS. Corpus luteum was seen on the right side with no other adenexal pathology. Repeat β-hCG after 1 week was 15,000 mIU/ml. A gestational sac with yolk sac and fetal pole corresponding to 6 weeks and 5 days was seen. Fetal heart was also documented. She had a normal vaginal delivery at 39 weeks of gestation. She delivered a female baby weighing 3.4 kg.
Case 2
Mrs SS presented with secondary infertility for 6 months. She had a full-term normal delivery female baby aged 4.5 years alive and healthy, after which she used barrier contraception for 3 years and IUCD for 1.5 years for spacing. HSG done outside on February 7, 2007, showed right tubal block with the left tube filling poorly. Cannulation was done under fluoroscopic guidance, after which both the tubes showed free spill. Endometrium was positive for tuberculosis PCR. She had undergone four cycles of ovulation induction, two with clomiphene citrate and two with gonadotrophins. She ovulated in all the cycles but her endometrium was thin and lesser than 7.5 mm in all four cycles.
Her first scan done at our clinic was on day 19 of the menstrual cycle. The uterus was normal, but the endometrium was only 7.8 mm thick. Both ovaries were normal with a volume of 4.96 and 4.20 cm3 and an antral follicle count (AFC) of seven. With this history and investigation report, the patient was planned for OI with IUI. Two cycles of IUI were performed with tablet Letrazole 5 mg given from days 3–7. The dermal application of EstroGel was done once the follicles were >16 mm for the treatment of thin endometrium. The ovulation occurred on days 13 and 15. The endometrial thickness on the day of IUI was 12.8 and 12.3 mm. We did two more cycles of IUI with gonadotrophins. Ovulation was documented on days 11 and 14 of the cycle and the endometrial thickness was 11.6 and 11.1 mm. As there was no pregnancy, the patient was counselled for in vitro fertilization (IVF). Hysteroscopy was done prior to IVF, which revealed adhesions at the fundus and both cornu. The endometrium was fibrotic and pearly white in color except for a small portion on the anterior wall. The cavity was smaller than normal in size. Adhesiolysis was done and the patient was put on conjugated estrogen (Premarin) 1.25 mg twice a day for 25 days with medroxyprogesterone 10 mg twice a day for the last 10 days. This hormone replacement therapy (HRT) was given for 3 months. A repeat hysteroscopy done after 3 months revealed reformation of adhesions at the fundus which were cut using scissors and HRT given again for 3 months. Before IVF, hysteroscopy did not show any adhesions but the endometrium was thin and fibrotic on the posterior wall.
The protocol used for IVF was long luteal down-regulation from day 21 with gonadotrophins started on day 3 of the cycle when the estradiol was 18 pg/ml and progesterone was 1.15 ng/ml. She was started on urinary FSH 300 IU and as the estradiol level on day 8 of the cycle was 6 pg/ml and the follicular size was 8–12 mm, the dose was increased by 75 IU and continued till day 13. On day 14, Rec. hCG 250 mcg was given subcutaneously when there were six follicles 16–18 mm in diameter. On the day of hCG, estradiol was 3265 pg/ml and progesterone was 2.8 ng/ml. Oocyte retrieval was done 35 h later. Luteal phase support was given with intramuscular inj. Gestone 100 mg (Ferring Pharmaceuticals, India). The luteal phase was monitored for ovarian hyperstimulation syndrome (OHSS). Ten days after ET, β-hCG was 53 mIU/ml and a repeat level after 7 days was 1030 mIU/ml. At that time, a single gestational sac was seen with a mean gestational sac diameter (MGSD) of 4.5 mm corresponding to 5 weeks and 1 day. Two days later, the patient complained of minimal bleeding per vaginum. The β-hCG level was 1258 mIU/ml, the MGSD was 4.6 mm and both adenexa showed multiple corpora lutea. No gestational sac was seen in the adenexa. One week later, the MGSD had increased to 8.5 mm which corresponded to 5 weeks and 5 days, but no fetal pole was seen and the bleeding had stopped. Despite the increase in the MGSD, there was no corresponding increase in the β-HCG levels which were 1567 mIU/ml. Two days later, the patient had three syncopal attacks. On examination, the vitals were normal but there was tenderness in the right iliac fossa and fornix. On TVS, the IU sac with yolk sac was seen, but there was also a complex mass of 90 × 70 mm in the right adenexa with free fluid in POD, which was turbid. Diagnosis of right EP with coexisting IU pregnancy was made. In view of an IU pregnancy and right adenexal mass, the decision for laparoscopy was taken. At laproscopy, right partial salpingectomy for ruptured EP was done. One week later, the TVS showed a irregular gestational sac with MGSD of 5.8 mm and the β-hCG level was 820 mIU/ml. The repeat hCG level after 5 days was 418 mIU/ml and the gestational sac had not increased in size. Decision for a curettage was taken. One week later, the β-hCG level was 5 mIU/ml. A frozen embryo transfer was done in a natural cycle, and a biochemical pregnancy was documented with the β-hCG level going to 378 mIU/ml.
Case 3
VM, a 34-year-old woman married since 14 years, presented to us in 2004 with primary infertility. Her cycles were of 7–15 days every 45–60 days. Her HSG done in 1994 was normal. Her husband's past semen analysis showed severe oligoasthenospermia with a count of 6 million and motility of 22%. Pelvic examination was normal and at TVS, the uterus was 78 × 43 × 58 mm in size; both ovaries were normal with an antral follicle count of 5. Right adenexa revealed a hydrosalpinx. The decision for hysterolaparoscopy was taken. At hysteroscopy, there was a polyp on the left lateral wall which was cut. Ostia, cornu, fundus, cavity, and endometrium were normal. At laparoscopy, uterus, tubes, and ovaries were normal. The right tube had a large paratubal cyst which was simulating a hydrosalpinx at TVS. On day 2, hormone tests were done with results as follows: FSH – 3.5 mIU/ml; LH – 2.2 mIU/ml; prolactin – 21.4 ng/ ml; TSH – 3.3 uIU/ml. The patient desired two cycles of IUI, which were done with CC 150 mg from days 2 to 6 of MC. She developed one dominant follicle, on the right in the first cycle, and on the left in the second cycle. As she failed to conceive, ICSI was planned. On day 2, estradiol (E2) was 36.6 pg/ml and progesterone 1.4 ng/ml. Controlled ovarian stimulation (COS) was started with FSH 150 IU and hMG 75 IU. On day 5, the E2 level was 33.6 and only one follicle of size 8.3 mm was seen on the left side. The hMG dose was increased to 150 IU. On day 10 of COS, there was only one follicle of 10.5 mm size and the E2 level was 34 pg/ml. The decision to cancel the cycle was taken and blood for AMH sent. The AMH values were 0.04 ng/ml. The decision for oocyte donation was taken as the ovarian reserve was poor. Oocyte donation was done in a GnRH agonist long protocol HRT cycle. Three grade 1 embryos – two 10-cell and one 8-cell – were transferred. First β-hCG level 14 days after ET was 68 mIU/ml. Eight days later, the β-hCG level was 9781 mIU/ml and a gestational sac measuring 4 × 3.3 × 3.8 mm corresponding to 5 weeks 2 days was seen, but the sac was placed in the lower part of the uterine cavity (cervical pregnancy). Five days later, she came with spotting; the MGSD was 6.6 mm, with the yolk sac seen but no fetal pole. The β-hCG level was 11,875 mIU/ml which reduced to 9781 after 2 days, and on TVS, the sac in the lower part of the uterus extending into the cervix was irregular in size. In view of a cervical pregnancy, we decided to treat the patient with inj. MTX 60 mg IM. A total of five doses of MTX alternating with leucovorin were given. It took almost 2 months for the β-hCG levels to reach 10 mIU/ml. The second cycle of oocyte donation was again done 10 months later in an HRT cycle as the patient was menstruating. This time we did a day 5 transfer and two expanded blastocysts of grade 4AA were transferred. The first β-hCG level after 10 days was 134 mIU/ml and a second one 7 days later was 1168 mIU/ml. Despite the beta hCG being > 1000 mIU/ml, no intrauterine sac was seen. Five days later, she came with pain in the lower abdomen, giddiness, and vomiting. Her vital parameters were normal, and there was no abdominal tenderness and rigidity. At TVS, the endometrial thickness was 20 mm with no gestational sac; a small sac was visualised in the right adenexa, though there was no probe tenderness. The β-hCG level was was 5654 mIU/ml. The decision for medical therapy was taken and she was started on inj. MTX 1 mg/kg alternating with leucovorin. A total of four doses were given. This time also, it took 50 days for the β-hCG level to become negative. Five months later, another cycle of oocyte donation was done in an HRT cycle. Two grade 1 eight-cell embryos were transferred. This time also, her first β-hCG level was positive, and 1 week later, it increased to 1359, and an IU gestational sac corresponding to 5 weeks and 3 days was visualised. Unfortunately, she had a missed abortion at 7 weeks of gestation with fetal heart first becoming slow for 2 days, and which later stopped. A medical abortion was done. In June 2011, her FSH level on day 2 was 0.44 and LH was 0.01; one more cycle of COH with FSH150 IU+ hMG 300 IU was tried. No follicular growth was seen after 10 days with an E2 level of 24 pg/ml; so the cycle was cancelled. After a month, one more cycle of oocyte donation was done. It was a day 3 transfer where one 10-cell grade A and one 8-cell grade B blastocyst were transferred. LP support was given with both progesterone and estrogen. The first β-hCG level was 503 and the second after 1 week was 6148 mIU/ml. Despite a β-hCG level of 6148 mIU/ml, no gestational sac in the uterine cavity or adenexal region was seen, and so a diagnosis of pregnancy of unknown location was made. Again she was treated with inj. MTX when the levels did not come down.
CONCLUSION
Ectopic pregnancy is a major emotional and medical problem in a woman's reproductive life. It often complicates an infertility treatment or results in infertility, therefore, management strategy must always be directed towards optimizing subsequent fertility. We know that infection, previous surgeries, and drugs used for controlled ovarian stimulation are important etiological factors for EP to occur. This is evident from Cases illustrated above. In Case 2, it was tuberculous infection of the tubes and endometrium which resulted in EP. At times, there is no etiological factor but the patient has repeated EPs. This is evident from Case 3, who had normal tubes and the endometrium was also normal at hysteroscopy. Despite this, in both IVF cycles with oocyte donation she had an EP.
A combination of TVS and hCG assay is sensitive and a more specific and less operator-dependent method of diagnosing EP. In a population that has received infertility treatment, combining TVS and hCG may reduce the number of patients with ruptured EP but at substantial additional costs. But this will reduce the number of surgeries done for EP, as these patients could be treated medically, and the subsequent chance of pregnancy in the following cycles increases. This is evident from Case 1 which is discussed above. This patient conceived after an EP when ovulation occurred from the same side as the previous EP which was treated medically.
Thus, there is a place for medical treatment in women with low hCG concentrations and a small gestational sac without the fetal pole seen in the adenexa. A variable-dose MTX regimen is more effective compared with the single-dose regimen, but the fixed multiple-dose regimen is associated with a high rate of side effects.
The surgical treatment of choice is salpingotomy instead of salpingectomy as it is associated with a higher future pregnancy rate; though it has a slightly higher recurrent EP rate and a higher persistent trophoblastic disease rate, it is less likely to compromise ovarian blood supply and ovarian function. Careful cleaning of the abdomen and pelvis at laparoscopic treatment prevents the risk of postoperative adhesion formation and trophoblastic implantation. Therefore, it is essential to strictly monitor the hCG level until it is totally undetectable after conservative laparoscopic treatment. Postoperative, prophylactic, single-dose systemic MTX may reduce the incidence of persistent EP after salpingotomy.
If salpingectomy is considered, total salpingectomy should be preferred in women who may in future require ARTs. This will reduce the incidence of recurrent tubal pregnancy though an interstitial pregnancy can still occur.
Whether a patient has salpingectomy or salpingotomy, she should be counselled appropriately for the risk of recurrent EP in future pregnancies. Prognosis for fertility is significantly correlated with the patient's history and age, regardless of the type of surgical treatment and tubal disease increasing the risk.
Thus, in all patients who are being treated for infertility, performing the β-hCG levels and a TVS 20 days after ovulation will help us in diagnosing EP early, making it possible to treat them medically enabling them to have a higher conception rate in the subsequent cycles with or without treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Society for Assisted Reproductive Technology and American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 1999 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2002;78:918–93. doi: 10.1016/s0015-0282(02)04198-5. [DOI] [PubMed] [Google Scholar]
- 2.Abusheikha N, Salha O, Brinsden P. Extra-uterine pregnancy following assisted conception treatment. Hum Reprod Update. 2002;6:80–92. doi: 10.1093/humupd/6.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Atri M, Leduc C, Gillett P, Bret PM, Reinhold C, Kintzen G, et al. Role of endovaginal sonography in the diagnosis and management of ectopic pregnancy. Radiographics. 1996;16:755–75. doi: 10.1148/radiographics.16.4.8835969. [DOI] [PubMed] [Google Scholar]
- 4.Bonatz G, Lehmann-Willenbrock E, Kunstmann P, Semm I, Hedderich J, Semm K. Management of patients with persistent beta-hCG values following laparoscopic surgical and local drug treatment for ectopic pregnancy. Int J Gynaecol Obstet. 1994;47:33–8. doi: 10.1016/0020-7292(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 5.Adoni A, Milwidsky A, Hurwitz A, Palti Z. Declining beta-HCG levels: An indicator for expectant approach in ectopic pregnancy. Int J Fertil. 1986;31:40–2. [PubMed] [Google Scholar]
- 6.Johnson N, McComb P, Gudex G. Heterotopic pregnancy complicating in vitro fertilization. Aust N Z J Obstet Gynaecol. 1998;38:151–5. doi: 10.1111/j.1479-828x.1998.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 7.Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril. 1999;71:282–6. doi: 10.1016/s0015-0282(98)00441-5. [DOI] [PubMed] [Google Scholar]
- 8.Vourtsi A, Antoniou A, Stefanopoulos T, Kapetanakis E, Vlahos L. Endovaginal color doppler sonographic evaluation of ectopic pregnancy in women after in vitro fertilization and embryo transfer. Eur Radiol. 1999;9:1208–13. doi: 10.1007/s003300050819. [DOI] [PubMed] [Google Scholar]
- 9.Heiner JS, Kerin JF, Schmidt LL, Wu TC. Can a single, early quantitative human chorionic gonadotropin measurement in an in vitro fertilization-gamete intrafallopian transfer program predict pregnancy outcome? Fertil Steril. 1992;58:373–7. doi: 10.1016/s0015-0282(16)55232-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt LL, Asch RH, Frederick JL, Rojas FJ, Stone SC, Balmaceda JP. The predictive value of a single beta human chorionic gonadotropin in pregnancies achieved by assisted reproductive technology. Fertil Steril. 1994;62:333–8. doi: 10.1016/s0015-0282(16)56887-3. [DOI] [PubMed] [Google Scholar]
- 11.Fridstrom M, Garoff L, Sjoblom P, Hillensjo T. Human chorionic gonadotropin patterns in early pregnancy after assisted reproduction. Acta Obstet Gynecol Scand. 1995;74:534–8. doi: 10.3109/00016349509024385. [DOI] [PubMed] [Google Scholar]
- 12.Qasim SM, Callan C, Choe JK. The predictive value of an initial serum beta human chorionic gonadotropin level for pregnancy outcome following in vitro fertilization. J Assist Reprod Genet. 1996;13:705–8. doi: 10.1007/BF02066422. [DOI] [PubMed] [Google Scholar]
- 13.Bjercke S, Tanbo T, Dale PO, Morkrid L, Abyholm T. Human chorionic gonadotrophin concentrations in early pregnancy after in-vitro fertilization. Hum Reprod. 1999;14:1642–6. doi: 10.1093/humrep/14.6.1642. [DOI] [PubMed] [Google Scholar]
- 14.Poikkeus P, Hiilesmaa V, Tiitinen A. Serum HCG 12 days after embryo transfer in predicting pregnancy outcome. Hum Reprod. 2002;17:1901–5. doi: 10.1093/humrep/17.7.1901. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol. 2004;104:50–5. doi: 10.1097/01.AOG.0000128174.48843.12. [DOI] [PubMed] [Google Scholar]
- 16.Chen CD, Ho HN, Wu MY, Chao KH, Chen SU, Yang YS. Paired human chorionic gonadotrophin determinations for the prediction of pregnancy outcome in assisted reproduction. Hum Reprod. 1997;12:2538–41. doi: 10.1093/humrep/12.11.2538. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez H, Pauthier S, Doumerc S, Lelaidier C, Olivennes F, Ville Y, et al. Ultrasound-guided injection of methotrexate versus laparoscopic salpingotomy in ectopic pregnancy. Fertil Steril. 1995;63:25–9. [PubMed] [Google Scholar]
- 18.Fernandez H, Pauthier S, Sitbon D, Vincent Y, Doumerc S. Role of conservative therapy and medical treatment in ectopic pregnancy: Literature review and clinical trial comparing medical treatment and conservative laparoscopic treatment. Contracept Fertil Sex. 1996;24:297–302. [PubMed] [Google Scholar]
- 19.Maymon R, Shulman A. Controversies and problems in the current management of tubal pregnancy. Hum Reprod Update. 1996;2:541–51. doi: 10.1093/humupd/2.6.541. [DOI] [PubMed] [Google Scholar]
- 20.Hajenius PJ, Engelsbel S, Mol BW, Van der Veen F, Ankum WM, Bossuyt PM, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet. 1997;350:774–9. doi: 10.1016/s0140-6736(97)05487-1. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez H, Marchal L, Vincent Y. Fertility after radical surgery for tubal pregnancy. Fertil Steril. 1998;70:680–6. doi: 10.1016/s0015-0282(98)00251-9. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez H, Yves Vincent SC, Pauthier S, Audibert F, Frydman R. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239–43. doi: 10.1093/humrep/13.11.3239. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwkerk PT, Hajenius PJ, Ankum WM, Van der Veen F, Wijker W, Bossuyt PM. Systemic methotrexate therapy versus laparoscopic salpingostomy in patients with tubal pregnancy. Part I. Impact on patients’ health-related quality of life. Fertil Steril. 1998;70:511–7. doi: 10.1016/s0015-0282(98)00212-x. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwkerk PT, Hajenius PJ, Van der Veen F, Ankum WM, Wijker W, Bossuyt PM. Systemic methotrexate therapy versus laparoscopic salpingostomy in tubal pregnancy Part II Patient preferences for systemic methotrexate. Fertil Steril. 1998;70:518–22. doi: 10.1016/s0015-0282(98)00213-1. [DOI] [PubMed] [Google Scholar]
- 25.Saraj AJ, Wilcox JG, Najmabadi S, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: A randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989–94. doi: 10.1016/s0029-7844(98)00324-x. [DOI] [PubMed] [Google Scholar]
- 26.Dias Pereira G, Hajenius PJ, Mol BW, Ankum WM, Hemrika DJ, Bossuy PM, et al. Fertility outcome after systemic methotrexate and laparoscopic salpingostomy for tubal pregnancy. Lancet. 1999;353:724–5. doi: 10.1016/s0140-6736(98)02250-8. [DOI] [PubMed] [Google Scholar]
- 27.Mol BW, Van der Veen F, Bossuyt PM. Implementation of probabilistic decision rules improves the predictive values of algorithms in the diagnostic management of ectopic pregnancy. Hum Reprod. 1999;14:2855–62. doi: 10.1093/humrep/14.11.2855. [DOI] [PubMed] [Google Scholar]
- 28.Sowter MC, Farquhar CM, Gudex G. An economic evaluation of single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured ectopic pregnancy. Br J Obstet Gynecol. 2001;108:204–12. doi: 10.1111/j.1471-0528.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 29.Sowter MC, Farquhar CM, Petrie KJ, Gudex G. A randomised trial comparing single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured tubal pregnancy. Br J Obstet Gynecol. 2001;108:192–203. doi: 10.1111/j.1471-0528.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Hajenius PJ, Mol BW, Bossuyt PM, Ankum WM, Van Der Veen F. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000324. doi: 10.1002/14651858.CD000324. [DOI] [PubMed] [Google Scholar]
- 31.El-Sherbiny M, El-Gharieb I, Mera I. Methotrexate versus laparoscopic surgery for the management of unruptured tubal pregnancy. Middle East Fertil Soc J. 2003;8:256–62. [Google Scholar]
- 32.Bulletin - Medical management of tubal pregnancy. No. 3, Dec 1998. Clinical management guidelines for obstetrician–gynecologists. Int J Gynaecol Obstet. 1999;65:97–103. [PubMed] [Google Scholar]
- 33.Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754–7. [PubMed] [Google Scholar]
- 34.Stovall TG, Ling FW, Buster JE. Outpatient chemotherapy of unruptured ectopic pregnancy. Fertil Steril. 1989;51:435–8. [PubMed] [Google Scholar]
- 35.Mol BW, Hajenius PJ, Engelsbel S, Ankum WM, Hemrika DJ, Van der Veen F, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol. 1999;181:945–51. doi: 10.1016/s0002-9378(99)70330-3. [DOI] [PubMed] [Google Scholar]
- 36.Lipscomb GH, Bran D, McCord ML, Portera JC, Ling FW. Analysis of three hundred fifteen ectopic pregnancies treated with single-dose methotrexate. Am J Obstet Gynecol. 1998;178:1354–8. doi: 10.1016/s0002-9378(98)70343-6. [DOI] [PubMed] [Google Scholar]
- 37.Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: A meta-analysis comparing “single dose and multidose” regimens. Obstet Gynecol. 2003;101:778–84. doi: 10.1016/s0029-7844(02)03158-7. [DOI] [PubMed] [Google Scholar]
- 38.Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115–20. doi: 10.1016/S0140-6736(97)11476-3. [DOI] [PubMed] [Google Scholar]
- 39.Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cut off to guide methotrexate treatment of ectopic pregnancy: A systematic review. Fertil Steril. 2007;87:481–4. doi: 10.1016/j.fertnstert.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Alleyassin A, Khademi A, Aghahosseini M, Safdarian L, Badenoosh B, Hamed EA. Comparison of success rates in the medical management of ectopic pregnancy with single-dose and multiple-dose administration of methotrexate: A prospective, randomized clinical trial. Fertil Steril. 2006;85:1661–6. doi: 10.1016/j.fertnstert.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 41.Bouyer J, Job-Spira N, Pouly JL, Coste J, Germain E, Fernandez H. Fertility following radical, conservative-surgical or medical treatment for tubal pregnancy: A population-based study. BJOG. 2000;107:714–21. doi: 10.1111/j.1471-0528.2000.tb13330.x. [DOI] [PubMed] [Google Scholar]
- 42.The management of tubal pregnancies. In: ‘Green top’ Guideline No.21. London: Royal College of Obstetricians and Gynaecologists; 1999. Royal College of Obstetricians and Gynaecologists. [Google Scholar]
- 43.Ozmen B, Diedrich K, Al-Hasani S. Hydrosalpinx and IVF: Assessment of treatments implemented prior to IVF. Reprod Biomed Online. 2007;14:235–41. doi: 10.1016/s1472-6483(10)60792-4. [DOI] [PubMed] [Google Scholar]
- 44.Lass A, Ellenbogen A, Croucher C, Trew G, Margara R, Becattini C, et al. Effect of salpingectomy on ovarian response to superovulation in an in vitro fertilization–embryo transfer program. Fertil Steril. 1998;70:1035–8. doi: 10.1016/s0015-0282(98)00357-4. [DOI] [PubMed] [Google Scholar]
- 45.Chan CC, Ng EH, Li CF, Ho PC. Impaired ovarian blood flow and reduced antral follicle count following laparoscopic salpingectomy for ectopic pregnancy. Hum Reprod. 2003;18:2175–80. doi: 10.1093/humrep/deg411. [DOI] [PubMed] [Google Scholar]
- 46.Meng XH, Zhu YM. Effect of salpingectomy on ovarian function. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35:555–9. doi: 10.3785/j.issn.1008-9292.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Pouly JL, Chapron C, Manhes H, Canis M, Wattiez A, Bruhat M. Multifactorial analysis of fertility after conservative laparoscopic treatment of ectopic pregnancy in a series of 223 patients. Fertil Steril. 1991;56:453–60. doi: 10.1016/s0015-0282(16)54539-7. [DOI] [PubMed] [Google Scholar]
- 48.Dubuisson JB, Morice PC, De Gayffier A, Mouelhi T. Salpingectomy—the laparoscopic surgical choice for ectopic pregnancy. Hum Reprod. 1996;11:1199–203. doi: 10.1093/oxfordjournals.humrep.a019355. [DOI] [PubMed] [Google Scholar]
- 49.Job-Spira N, Fernandez H, Bouyer J, Pouly JL, Germain E, Coste J. Ruptured tubal ectopic pregnancy: Risk factors and reproductive outcome: Results of a population-based study in France. Am J Obstet Gynecol. 1999;180:938–44. doi: 10.1016/s0002-9378(99)70665-4. [DOI] [PubMed] [Google Scholar]
- 50.Yao M, Tulandi T. Current status of surgical and nonsurgical management of ectopic pregnancy. Fertil Steril. 1997;67:421–33. doi: 10.1016/s0015-0282(97)80064-7. [DOI] [PubMed] [Google Scholar]
- 51.Clausen I. Conservative versus radical surgery for tubal pregnancy. A review. Acta Obstet Gynecol Scand. 1996;75:8–12. doi: 10.3109/00016349609033276. [DOI] [PubMed] [Google Scholar]
- 52.Nardo LG. The dilemma of recurrent ectopic pregnancy after tubal surgery and in-vitro fertilization. What to do? Reprod Biomed Online. 2005;10:300. doi: 10.1016/s1472-6483(10)61786-5. [DOI] [PubMed] [Google Scholar]


