Abstract
AIM:
Reproductive toxicity is a major challenge associated with aluminum (Al) exposure. Studies that associated Al with reproductive dysfunction did not account for the possible influence of Allium cepa extract. This study, therefore, investigates the influence of A. cepa on aluminum-induced reproductive dysfunction.
MATERIALS AND METHODS:
Six male rats per group were assigned to one of the following four treatment groups: The control animals were on control diet. A. cepa-treated rats received 1 ml of the extract/100 g body weight (BW), Al-treated rats received 100 mg AlCl3 /kg BW, and A.cepa+Al received 1 ml of the extract/100 g BW plus 100 mg AlCl3 /kg BW. Rats were orally administered their respective doses. A. cepa treatment was for 8 weeks, while Al treatment was for the last 3 days of the experimental period.
RESULTS:
Results obtained showed that Al significantly decreased (P < 0.05) plasma testosterone, follicular stimulating hormone (FSH), luteinizing hormone (LH), sperm count, motility, morphology and viability, superoxide dismutase (SOD) and catalase (CAT) activities, while lipid peroxidation index [malondialdehyde (MDA)] was significantly (P < 0.05) increased. Reproductive hormones (except testosterone), sperm qualities, and enzymatic antioxidants were significantly (P < 0.05) increased in A. cepa-treated rats and A. cepa plus Al-treated rats, while MDA was significantly (P < 0.05) improved. Weights of testes were comparable in all groups.
CONCLUSION:
It is thus suggested that Al exerts reproductive dysfunction by oxidative damage. A. cepa antagonizes the toxic effects of AlCl3 and improves the antioxidant status and sperm quality of male rat. However, testosterone level did not increase with A. cepa treatment.
KEY WORDS: Allium cepa, aluminum toxicity, fertility, lipid peroxidation, reproductive hormones, sperm
INTRODUCTION
Aluminum (Al) has been reported to be an environmental factor that may contribute to some diseases, affect several enzymes and other biomolecules, and induce free radical-mediated cytotoxicity. Studies on laboratory animals have shown that Al induces reproductive toxicity and exerts a significant adverse effect on the steroidogenesis.[1–3] Al accumulation has been associated with necrosis of the sperm cells and infertility.[4,5] Alessio et al.[6] reported that high serum level of Al in mine workers caused a decrease in their thyroid stimulating hormone and prolactin. Renal failure patients on dialysis with high serum Al also showed low reproductive potentials.[7]
Mechanisms associated with Al-induced infertility have been reported. Studies have reported Al to block voltage-gated calcium channels,[8–10] thereby impairing gonadotrophin secretion in the hypophysis[11,12] with resultant low sperm counts.[13,14] Al has also been reported to cause testicular toxicity by increasing testicular nitric oxide.[5,15] Some studies have shown that Al reduces antioxidants[3] and increases lipid peroxidation,[16–18] though none has reported its effect on testicular lipid peroxidation.
Allium cepa has been reported to have medicinal potentials.[19–21] Studies have also documented the antioxidant value of A. cepa.[22–25] The antioxidant effect of A. cepa has been associated with reduced lipid peroxidation index [malondialdehyde (MDA)] and increased superoxide dismutase (SOD).[25] The present study was therefore designed to investigate the effect of Al on reproductive hormones, sperm quality, and lipid peroxidation profile, and the possible role of A. cepa against Al-induced changes on reproduction profile.
MATERIALS AND METHODS
Animals
Male white rats of Wistar strain weighing between 150 and 200 g were used for the experiment. They were housed in standard rat cages under laboratory conditions with 12:12 h light/dark cycle at 25 ± 2°C. The animals were allowed to acclimatize for 2 weeks.[26] The experiment was conducted in accordance with the guidelines of the US National Institute of Health (NIH) on the care and use of laboratory animals.
Treatment
Six rats per group were assigned to one of the following four treatment groups:
Control: 0 ml A. cepa/100 g and 0 mg AlCl3 /kg body weight (BW)
AcE-treated rats: 1 ml A. cepa/100 g BW
Al-treated rats: 100 mg AlCl3 /kg BW
AcE + Al-treated rats: 1 ml A. cepa/100 g BW plus 100 mg AlCl3 /kg BW
The doses were similar to those used in previous studies.[24,25,27] Rats were orally administered their respective doses once daily. A. cepa treatment was for 8 weeks, while Al treatment was for the last 3 days of the experimental period. All rats, both control and test groups, were fed on standard rat chow and water ad libitum. Blood samples were collected 24 h after the treatment period.
Preparation of aluminum chloride (AlCl3)
Four grams of aluminum chloride was dissolved in 100 ml distilled water to prepare a stock solution (40 mg/ml). The solution was prepared weekly and kept in a plane bottle at 4°C.
AlCl3 was daily administrated to rats orally at a sub-lethal dose level of 100 mg/kg BW.
Extraction of A. cepa
AcE was prepared following the procedures from previous studies.[21,25] Fresh A. cepa (common onion) bulbs were rinsed thoroughly in distilled water, air-dried, and 200 g was then blended. Juice was then filtered and squeezed out of it using a tight sieve. The juice was prepared on weekly basis following the same procedure and kept at 4°C to prevent it from losing its potency.[24,25]
Collection of blood samples and animal sacrifice
Each rat was sacrificed by cervical dislocation and blood samples were obtained by cardiac puncture. Serum was obtained by centrifugation at 300 rpm for 10 mins. Testes were excised, rinsed in potassium chloride (KCl), homogenized, and preserved in buffer solution for biochemical investigation.
Testes weight and reproductive hormones
Testes were excised and weighed. Testosterone, follicular stimulating hormone (FSH), and luteinizing hormone (LH) were analyzed using standard enzyme immunosorbent assay (EIA) test kit.
Sperm characteristics analysis
Epididymis sperm was obtained by mincing the epididymis in normal saline and filtering through a nylon mesh (80-μm pore size). The sperms were counted using a hemocytometer. The numbers of sperm in five squares (four corners and the center) in the center grid of both sides were counted based on the dilution factor and averaged following previous methods.[28,29]
The caudal epididymis was dissected and minced in pre-warmed normal saline (37°C). One drop of sperm suspension was placed on a glass slide to analyze 200 motile sperms in four different fields. The motility of the epididymal sperms was evaluated microscopically within 2–4 mins of their isolation from the epididymis and data were expressed as percent motility.[29,30]
Sperm morphology was done by staining the sperm smears on the microscope slides with two drops of Walls and Ewas stain and air-dried. The slides were examined under the microscope using ×100 objectives under oil immersion. The normal sperm cells were counted and the percentage was calculated.[29]
A viability study (percentage of live spermatozoa) was done using eosin/nigrosin stain. Semen was squeezed onto a microscope slide and two drops of the stain were added. The motile (live) sperm cells were unstained, while the non-motile (dead) sperm absorbed the stain. The stained and the unstained sperm cells were counted using ×40 objectives of the microscope and an average for each was taken from which percentage viability was calculated.[29]
Lipid peroxidation profile
Estimation of lipid peroxidation based on the reaction of MDA with thiobarbituric acid (TBA) forming MDA–TBA2 that absorbs strongly at 532 nm was followed according to the method of Varshney and Kale.[31] The level of SOD activity in the supernatant was determined by the method of Fridovich and Misra.[32] Catalase activity was determined by following the consumption of exogenous H2O2, measured according to the previous study, at 560 nm.[33]
Histological study
Testicular tissues were fixed in Bouin's fluid for 6 h and transferred into 10% formalin. They were dehydrated with varying percentage of ethanol. Sections were cleared in xylene and embedded in molten wax. Thin sections were cut (5 μm), stained with hematoxylin and eosin, and microscopically analyzed.
Statistical analysis
All results are expressed as mean ± SEM. The differences between the mean values were evaluated by analysis of variance (ANOVA) followed by unpaired Student's t-test (two-tailed P value). Values of P < 0.05 were considered statistically significant.[34]
RESULTS
Effect of A. cepa and aluminum on reproductive hormones
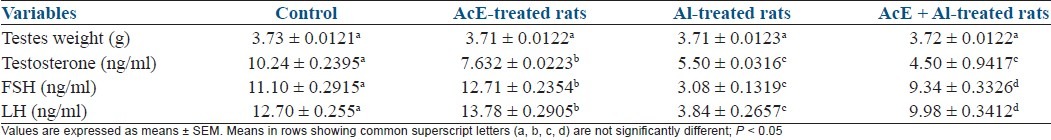
Table 1 shows that the weights of testes were similar in all groups (P > 0.05). Testosterone was significantly (P < 0.05) reduced in all treated rats. FSH and LH were significantly (P < 0.05) increased in A. cepa-treated group, but significantly (P < 0.05) reduced in Al-treated rats when compared with all groups. FSH and LH in AcE + Al rats were significantly (P < 0.05) higher than in the Al-treated rats, but significantly (P < 0.05) lower than in the control and A. cepa-treated rats.
Table 1.
Effect of Allium cepa (AcE) on testes weight and reproductive hormones in aluminum (Al)-treated rats
Effect of A. cepa and aluminum on sperm characteristics
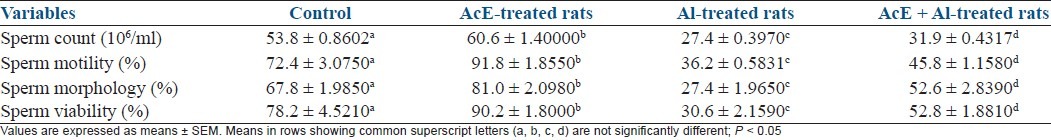
In comparison with all groups, sperm quality was significantly (P < 0.05) improved in A. cepa-treated rats. Al treatment caused significant (P < 0.05) impairment of sperm quality when compared with all groups. Sperm quality was significantly (P < 0.05) enhanced in A. cepa + Al rats when compared with Al-treated rats [Table 2].
Table 2.
Effect of Allium cepa (AcE) on sperm quality in aluminum (Al)-treated rats
Effect of A. cepa and aluminum on lipid peroxidation status
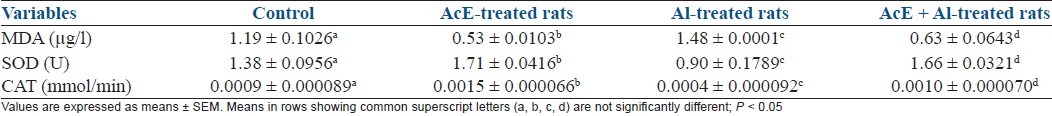
A. cepa treatment led to significant (P < 0.05) enhancement of lipid peroxidation status when compared with all groups. In comparison with other groups, rats treated with Al showed significantly (P < 0.05) increased lipid peroxidation and reduced antioxidant levels. A. cepa + Al treatment showed significant (P < 0.05) improvement of lipid peroxidation status when compared with Al-treated rats [Table 3].
Table 3.
Effect of Allium cepa (AcE) on lipid peroxidation profile in aluminum (Al)-treated rats
Effect of A. cepa and luminum on testicular cytoarchitecture
Al treatment led to degenerative necrosis with degeneration of spermatogenic cells. This effect was milder in A. cepa + Al treatment. A. cepa treatment showed testicular cytoarchitecture similar to that of control [Figure 1].
Figure 1.
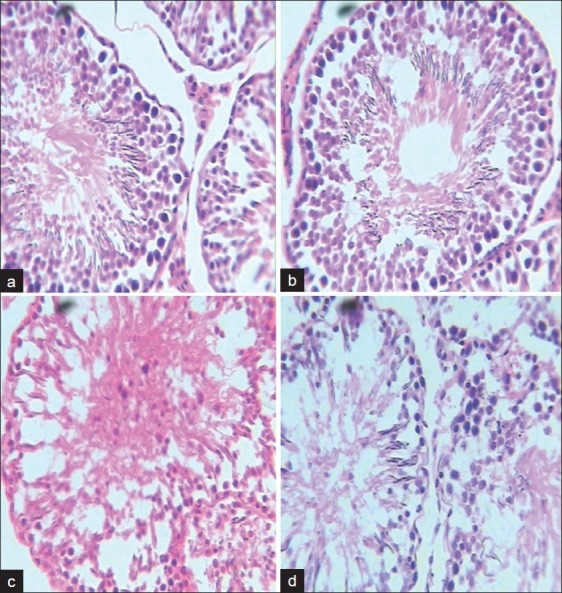
Histograph of the testes showing the seminiferous tubules. Al treatment led to degenerative necrosis with degeneration of spermatogenic cells. This effect was milder in AcE+Al treatment. AcE treatment showed similar testicular cytoarchitecture to the control
DISCUSSION
The result of this study shows that Al did not affect the weight of the testes. This is inconsistent with previous studies[1,35] that reported reduced body weight gain and testes weight on Al treatment. Dissimilarity observed in this study might be due to the duration of Al treatment. This implies that short-term Al treatment is unlikely to affect the weight of testes, while its long-term treatment would cause reduced testicular weight. Similarly, A. cepa treatment in both A. cepa rats and A. cepa + Al rats showed comparable weight of the testes with the control and Al-treated rats. This is in consonance with a previous study.[25] This confirms the low caloric and protein content of A. cepa.[36,37]
The study results show that testosterone, FSH, and LH were significantly reduced in Al-treated rats. This is in agreement with previous studies.[3,15,38] This might be associated with calcium channel blocking effect of Al,[8,9] which led to impaired secretion of gonadotrophins in the hypophysis,[11,12] and thus low testosterone level. It might also be due to high testicular nitric oxide levels and low cAMP associated with Al, which suppressed steroidogenesis.[15] On the other hand, A. cepa treatment improved LH and FSH levels, probably by inhibiting these mechanisms. However, A. cepa reduced testosterone level also.
Al treatment led to impaired sperm quality. This is in agreement with previous studies[1,39,40] that documented reduced sperm count, motility, and viability on Al treatment. The reduced sperm count observed in Al-treated rats could be associated with reduced gonadotrophins and testosterone seen in rats, since these hormones are essential for spermatogenesis. LH stimulates the interstitial cells of the Leydig to secrete testosterone, which in association with FSH is necessary for stimulation of spermatogenesis.[41] Reduction of these hormones in Al-treated rats led to low sperm count. The reduced motility and viability with increased morphological abnormality seen in Al-treated rats could be associated with Al-induced increase in nitric oxide, which has been reported to cause reduced rate and motility, as well as increased morphological abnormalities of sperm cells.[42]
In a healthy body, reactive oxygen species (ROS) and antioxidants remain in balance. When the balance is disrupted toward an overabundance of ROS, oxidative stress occurs, which influences reproductive lifespan. Oxidative stress results from an imbalance between prooxidants (free radical species) and the body's scavenging ability (antioxidants).[43] ROS not only serve as key signal molecules in physiological processes, but also have a role in pathological processes involving reproductive fecundity. Results from this study show that Al increased the lipid peroxidation index (MDA) and reduced testicular antioxidants. This is in tandem with previous studies.[3,17,18] This observation could be responsible for the reduced reproductive hormones and poor sperm quality seen in Al-treated rats, since ROS have been proposed to have a role in steroidogenesis and gametogenesis.[43] However, A. cepa, which has been reported to have antioxidant potential,[25] led to improved oxidative status in experimental animals. A. cepa-induced impairment of lipid peroxidation and enhancement of antioxidant levels could be associated with the raised FSH and LH levels as well as the improved sperm quality seen with A. cepa treatment when compared to Al-treated rats.
Histopathologic study reveals altered testicular architecture with area of degenerative necrosis of germ cells lining the seminiferous tubules in Al-treated rats when compared with other groups. This agrees with previous study.[44] This observation could be linked to Al-induced oxidative damage and the ability of Al to cross the blood–testis barrier after inducing oxidative stress and lipid peroxidation that damages the biological membrane of the testes. This could also be attributed to the low sperm count, motility, viability, and morphological abnormality seen in Al-treated rats. Penetration of Al through the blood–testis barrier could cause degeneration and alteration of spermatogenic cells. Observation in this study shows that A. cepa treatment considerably increased the formation of antioxidant products and reduced lipid peroxidation, thus maintaining the cytoarchitecture of the testes.
Phytochemical screening of A. cepa showed that it contains abundant flavonoids, and weak saponins, tannins, glycosides, sterols, and triterpenoids.[45] The effects of A. cepa observed in this study could be attributed to the activities of the flavonoid constituent. Flavonoids are known antioxidants and enhance the oxidation status as seen in this study. However, they have been implicated as antifertility agents.[46,47] Studies have reported that flavonoids cause antispermatogenic effects with reduced sperm qualities.[48–50] However, observations from this study showed that though A. cepa flavonoids reduced testosterone level, they improved the sperm quality by preventing lipid peroxidation. Furthermore, the increased LH and FSH seen with reduced testosterone could suggest that A. cepa inhibited testosterone synthesis at the testicular level probably by inhibiting cholesterol conversion.
CONCLUSION
The results of this study show that Al has harmful effects on male reproductive profile in experimental rat model enough to cause infertility via oxidative damage. A. cepa antagonizes Al-induced damage by alleviating oxidative stress, thus enhancing sperm quality. However, caution should be taken because A. cepa resulted in reduced testosterone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yousef MI, El-Morsy AM, Hassan MS. Aluminium-induced deterioration in reproductive performance and seminal plasma biochemistry of male rabbits: Protective role of ascorbic acid. Toxicology. 2005;215:97–107. doi: 10.1016/j.tox.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Yousef MI, Kamel KL, ElGuendi MI, El-Demerdash FM. An in vitro study on reproductive toxicity of aluminium chloride on rabbit sperm: The protective role of some antioxidants. Toxicology. 2007;239:213–23. doi: 10.1016/j.tox.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food and Chem Toxicology. 2009;47:1168–75. doi: 10.1016/j.fct.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Guo CH, Lu YF, Hsu GS. The influence of aluminum exposure on male reproduction and offspring in mice. Environ Toxicol Pharmacol. 2005;20:135–41. doi: 10.1016/j.etap.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Guo CH, Lin CY, Yeh MS, Hsu GS. Aluminum induced suppression of testosterone through nitric oxide production in male mice. Environ Toxicol Pharmacol. 2005;19:33–40. doi: 10.1016/j.etap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Alessio L, Apostoli P, Ferioli A, Di Sipio I, Mussi I, Rigosa C, et al. Behaviour of biological indicaators of internal dose and some neuro-endocrine test in aluminium workers. Med Lav. 1989;80:290–300. [PubMed] [Google Scholar]
- 7.Yamamoto Y, Sofikitis N, Miagawa I. Effects of chronic renal failure on hypothalamo-pituitary-testicular axis functions and fertility in rats. Intl J Uorol. 1996;3:484–90. doi: 10.1111/j.1442-2042.1996.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 8.Platt B, Busselberg D. Actions of aluminium on voltage-activated calcium channels currents. Cell Mol Neurobiol. 1994;14:819–29. doi: 10.1007/BF02088687. [DOI] [PubMed] [Google Scholar]
- 9.Büsselberg D, Platt B, Michael D, Carpenter DO, Haas HL. Mammalian voltage-activated-calcium channels currents are blocked by Pb2+, Zn2+, and Al3+ J Neurophysiol. 1994;71:1491–7. doi: 10.1152/jn.1994.71.4.1491. [DOI] [PubMed] [Google Scholar]
- 10.Busselberg D. Calcium channels as target sites of heavy metals. Toxicol Lett. 1995;82-83:255–61. doi: 10.1016/0378-4274(95)03559-1. [DOI] [PubMed] [Google Scholar]
- 11.Tse A, Tse FW, Almers W, Hill B. Rhythmic exocytosis stimulated GnRH-induced calcium oscillations in ratsgonadotropes. Science. 1993;260:82–4. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- 12.Mills LR, Niesen CE, So AP, Carlen PL, Spigelman I, Jones OT. N-type Ca2+ channels are located on somatadendrites a subpopulation of dendrite spines on live hippocampal pyramidal neurons. J Neurosci. 1994;14:6815–24. doi: 10.1523/JNEUROSCI.14-11-06815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingo JL, Paternian JL, Liobet JM, Corbella J. The effects ofaluminium ingestion on reproduction and postnatal survival in rats. Life Sci. 1987;41:1127–31. doi: 10.1016/0024-3205(87)90631-x. [DOI] [PubMed] [Google Scholar]
- 14.Llobet JM, Colomina MT, Sirvent JJ, Domingo JL, Corbella J. Reproductive toxicology of aluminium in male mice. Fundam Appl Toxicol. 1994;29:45–51. doi: 10.1006/faat.1995.1038. [DOI] [PubMed] [Google Scholar]
- 15.Guo C, Huang C, Chen S, Wang Hsu G. Serum and testicular testosterone and nitric oxide products in aluminum-treated mice. Environ Toxicol Pharmacol. 2001;10:53–60. doi: 10.1016/s1382-6689(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 16.Gutteridge JM, Quinlan GJ, Clark I, Halliwell B. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim Biophys Acta. 1985;835:441–7. doi: 10.1016/0005-2760(85)90113-4. [DOI] [PubMed] [Google Scholar]
- 17.Dua R, Gill KD. Aluminium phosphide exposure: Implications on rat brain lipid peroxidation and antioxidant defence system. Pharmacol Toxicol. 2001;89:315–9. doi: 10.1034/j.1600-0773.2001.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 18.Anane R, Creppy EE. Lipid peroxidation as a pathway of aluminium cytotoxicity in human skin fibroblast cultures: Prevention by superoxide dismutase + catalase and vitamin E and C. Hum Exp Toxicol. 2001;20:477–81. doi: 10.1191/096032701682693053. [DOI] [PubMed] [Google Scholar]
- 19.Dorant E, van den Brandt PA, Goldbohm RA. Prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477–84. doi: 10.1093/carcin/17.3.477. [DOI] [PubMed] [Google Scholar]
- 20.Cavagnaro PF, Sance MM, Galmarini CR. Effect of heating on Onion (Allium cepa L.) antiplatelet activity and pungency sensory perception. Food science and Technology International. 2007;13:447–53. [Google Scholar]
- 21.Azu NC, Onyeagba RA, Nworie O, Kalu J. Antibacterial activity of Allium cepa (Onions) and Zingiber officinale (Ginger) on Staphylococcus aureus and Pseudomonas aeruginosaisolated from high vaginal swab. Internet J Trop Med. 2007;3:2. [Google Scholar]
- 22.Helen A, Krishnakumar K, Vijayammal PL, Augusti KT. Antioxidant effect of onion oil (Allium cepa Linn) on the damages induced by nicotine in rat as compared to alpha-tocopherol. Toxicol Lett. 2000;116:61–8. doi: 10.1016/s0378-4274(00)00208-3. [DOI] [PubMed] [Google Scholar]
- 23.Prakash D, Singh BN, Upadhyay G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa) Food chemistry. 2007;102:1389–93. [Google Scholar]
- 24.Ige SF, Salawu EO, Olaleye SB, Adeeyo OA, Badmus J, Adeteke AA. Onion (Alluim cepa) extract prevents cadmium-induced renal dysfunction. Indian J Nephrol. 2009;19:140–4. doi: 10.4103/0971-4065.59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ige SF, Akhigbe RE, Adewale AA, Badmus JA, Olaleye SB, Ajao FO, et al. Effect of Allium cepa (Onion) extract on Cadmium – induced nephrotoxicity in rats. Kidney Res J. 2011;1:41–7. [Google Scholar]
- 26.Akhigbe RE, Olatunji LA, Soladoye AO, Oyeyipo IP. Effect of angiotensin 1-converting enzyme inhibitor, captopril, on body weight, and food and water consumption in oral contraceptive-treated rats. Am J Biochem Mol Biol. 2011;1:95–100. [Google Scholar]
- 27.Sun H, Hu C, Jia L, Zhu Y, Zhao H, Shao B, et al. Effects of Aluminum Exposure on Serum Sex Hormones and Androgen Receptor Expression in Male Rats. Biol Trace Elem Res. 2011;144:1050–8. doi: 10.1007/s12011-011-9098-6. [DOI] [PubMed] [Google Scholar]
- 28.Freund M, Carol B. Factors affecting haemocytometer count of sperm concentration in human semen. J Reprod Fertil. 1964;8:149–55. doi: 10.1530/jrf.0.0080149. [DOI] [PubMed] [Google Scholar]
- 29.Raji Y, Oloyo AK, Morakinyo AO. Studies in the reproduction activities of method and extract of Ricinuscommunis seed in male albino rats. Asian J Androl. 2006;8:115–21. doi: 10.1111/j.1745-7262.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 30.Morrisey RE, Schwctz BA, James C, Monica DR, Teague JL, Moris RW. Evaluation of rodent sperm, vagina cytology and Reproductive organ weight from National Toxicology Programme-13 weeks studies. Fundam Appl Toxicol. 1988;11:343–58. doi: 10.1016/0272-0590(88)90159-5. [DOI] [PubMed] [Google Scholar]
- 31.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–43. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 32.Fridovich I, Misra HP. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 33.Jiang ZY, Woolland ACS, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1972;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- 34.Akhigbe RE, Ige SF, Afolabi AO, Oyeyipo PI, Ajao FO, Ajayi FA. Water balance and serum levels of some electrolytes in oral contraceptive-treated female wistar rats. J Med Sci. 2008;8:591–4. [Google Scholar]
- 35.Khattab Hala AH, AbdallaInas ZA, Kamel Gehan M. Grape seed extract alleviate reproductive toxicity caused by aluminium chloride in male rats. J Am Sci. 2010;6:1200–9. [Google Scholar]
- 36.Haimmouril MK, Ereife JK. Chemical analysis of onion cell wall biopolymers. Quim Anal (Barcelona) 1997;16:141–5. [Google Scholar]
- 37.Zeier J, Schreiber L. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledonous species: Chemical composition in relation to fine structure. Planta. 1998;206:349–61. [Google Scholar]
- 38.Mohammad SR, Palan MJ. Effect of aluminium on testosterone hormones in male rats. J Med Sci. 2006;6:296–9. [Google Scholar]
- 39.Singh HP, Singh CK, Singh RR. Effect of potash alum (aluminium potassium sulphate) on human semen and sperm. Indian J Physiol Pharmacol. 1998;42:311–4. [PubMed] [Google Scholar]
- 40.Hovatta O, Venäläinen ER, Kuusimäki L, Heikkilä J, Hirvi T, Reima I. Aluminium, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum Reprod. 1998;13:115–9. doi: 10.1093/humrep/13.1.115. [DOI] [PubMed] [Google Scholar]
- 41.Sembulingam K, Sembulingam P. Essentials of Medical Physiology. 3rd ed. New Delhi: Jaypee Brothers Ltd; 2004. Male reproductive system; pp. 371–418. [Google Scholar]
- 42.Latchoumycandane C, Chitra KC, Mathur PP. The effect of methoxychlor on the epididymal antioxidant system of adult rats. Reprod Toxicol. 2002;16:161–72. doi: 10.1016/s0890-6238(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khattab IK. Histological and ultrastructural studies on the testis of rat after treatment with aluminium chloride. Aust J Basic Appl Sci. 2007;1:63–72. [Google Scholar]
- 45.Ugwoke CEC, Ezugwe CO. Phytochemical screening and promiximate composition and onion bulb (Allium cepa L) J Pharm Allied Sci. 2010;7 ISSN: 1596-8499. [Google Scholar]
- 46.Iranloye B, Owokunle B. Effect of Garcinia kola seed extract on female reproductive functions in rats. Int J Pharmacol. 2008;4:276–81. [Google Scholar]
- 47.Thakare VN, Kothavade PS, Dhote VV, Deshpande AD. Antifertility activity of ethanolic extract of Allium cepa Linn in rats. Int J Pharm Tech Res. 2009;1:73–8. [Google Scholar]
- 48.Aravindakshan M, Chauhan PS, Sundaram K. Studies on germinal effects of quercetin, a naturally occurring flavonoid. Mutat Res. 1985;144:99–106. doi: 10.1016/0165-7992(85)90010-7. [DOI] [PubMed] [Google Scholar]
- 49.Srivastav A, Chandra A, Singh M, Jamal F, Rastogi P, Mani RS, et al. Inhibition of hyaluronidase activity of human and rat spermatozoa in vitro and antispermatogenic activity in rats in vivo by Terminaliachebula, a flavonoid rich plant. Reprod Toxicol. 2010;29:214–24. doi: 10.1016/j.reprotox.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Das S, Parveen S, Kundra CP, Pereira BMJ. Reproduction in male rats is vulnerable to treatment with the flavonoid-rich seed extracts of Vitexnegundo. Phytother Res. 2004;18:8–13. doi: 10.1002/ptr.1352. [DOI] [PubMed] [Google Scholar]


