Abstract
Objective
Personality disorders (PDs) have been shown to be modestly heritable. Accurate heritability estimates are, however, dependent on reliable measurement methods, as measurement error deflates heritability. The aim of this study was to estimate the heritability of DSM-IV avoidant and dependent personality disorder, by including two measures of the PDs at two time points.
Method
Data were obtained from a population-based cohort of young adult Norwegian twins, of whom 8045 had completed a self-report questionnaire assessing PD traits. 2794 of these twins subsequently underwent a structured diagnostic interview for DSM-IV PDs. Questionnaire items predicting interview results were selected by multiple regression, and measurement models of the PDs were fitted in Mx.
Results
The heritabilities of the PD factors were 0.64 for avoidant PD and 0.66 for dependent PD. No evidence of common environment, that is, environmental factors that are shared between twins and make them similar, was found. Genetic and environmental contributions to avoidant and dependent PD seemed to be the same across sexes.
Conclusion
The combination of both a questionnaire- and an interview assessment of avoidant and dependent PD results in substantially higher heritabilities than previously found using single-occasion interviews only.
Keywords: twin studies, personality disorders, genetics
Introduction
Avoidant and dependent personality disorders (PDs) (subsequently referred to as AVPD and DEPD) are included in the DSM-IV cluster C, along with obsessive–compulsive PD. Individuals with these disorders often appear anxious or fearful (1). AVPD and DEPD have been found to be associated with normal personality traits, especially neuroticism (2–6), as well as internalizing Axis I disorders (7).
The term ‘heritability’ refers to the extent to which genetic factors influence variation in a trait in a population and is defined as the proportion of the total variance in a trait that is accounted for by genetic factors. Results from a vast amount of twin and adoption studies the last decades have established that there is a substantial genetic contribution to most psychiatric (and somatic-) disorders, as well as a range of normal traits and behaviors (8–10).
We have previously estimated the heritability of all the cluster C PDs using single-occasion structured interview data from a population-based sample of young adult twins and found that they ranged from 0.27 to 0.35 (11). Our heritability estimates appeared to be in the low end compared with the previous findings on the heritability of cluster C PDs in clinical samples (12) in children (13) and for normative personality traits resembling cluster C PDs (14–16). The estimates were also lower than what has been found for symptoms of anxiety and depression (17–20).
One possible explanation for low heritability estimates is measurement error, which may occur for instance as a result of low reliability of the method of assessment. In twin analyses, measurement errors will inflate estimates of environmental influences not shared by the twins, and deflate estimates of genetic effects or heritability. In our previous study on the heritability of cluster C PDs, we used data from single-occasion interviews (11). We demonstrated high inter-rater reliability and internal consistency, but we could not estimate test–retest reliability. However, previous studies have shown that the 2-year test–retest reliability for AVPD and OCPD is relatively low (21). The low reliability for interviews may be due to increased error resulting from a small number of items (22), underreporting (23), and rater bias (24). Therefore, the low heritability estimates obtained in the previous investigation of cluster C PDs might be due to the suboptimal test–retest reliability of the interview used.
The effect of measurement error due to imperfection in the assessment method can be diminished by including two different measures of the PDs, for instance both interview and self-report questionnaire, and/or by measuring PDs on at least two time points. It is then possible to model the PDs as latent liability factors. In contrast to observable, measured traits, a latent factor is an abstract construct that cannot be observed directly. The value of the latent factor is inferred from the measured traits. If there are multiple measures of the latent factor and these measures covary, the latent factor will only consist of the variance that is shared between the measures (25). Unreliability because of time- and instrument-specific errors is therefore not included in the latent factors, resulting in more reliable heritability estimates.
Both questionnaire and interview assessment of PDs was applied in two publications from the present sample investigating genetic and environmental contributions to cluster A and B PDs (26, 27). Using this method, the heritability estimates for the PDs varied from 0.55 to 0.72 and were substantially higher than what was found using single-occasion interviews only (0.21–0.38; 28, 29). To our knowledge, no such study has been conducted for AVPD and DEPD.
Material and methods
Sample
Data for the current analyses come from the Norwegian Institute of Public Health Twin Panel (NIPHTP). The twins are identified through information contained in the national Medical Birth Registry, which was established January 1, 1967. Two questionnaires have been distributed, the first in 1992 (all twins born between 1967 and 1975), and the second in 1998 (all twins born from 1967 to 1979). In this study, we use data from the second questionnaire, which was sent to 12 700 twins. Responses were received from 8,045 twins (63%) after one reminder (3334 pairs and 1377 twins whose co-twin did not respond). The 3334 pairs included 1052 monozygotic (MZ) males, 794 dizygotic (DZ) males, 1554 MZ females, 1310 DZ females, and 1958 opposite-sex twins. The 1377 single responders included 188 MZ males, 274 DZ males, 159 MZ females, 207 DZ females, and 549 opposite-sex twins. Age in this sample spanned from 18 to 31 years (mean, 25.6). Of the 8,045 twins that responded, 7980 had valid responses for AVPD, 7,979 for DEPD and 7822 for OCPD.
The present study also use data from a diagnostic interview of Axis I and Axis II psychiatric disorders, conducted between June 1999 and May 2004. Participants were recruited from a sample of 3153 complete twin pairs from the second questionnaire study who had given consent to be contacted again later, and 68 twin pairs drawn directly from the NIPHTP. 2801 twins were assessed with structured interviews for Axis I and Axis II disorders. Of these, 2794 responses were valid. The sample consisted of 221 MZ male twin pairs, 116 DZ male twin pairs, 448 MZ female twin pairs, 261 DZ female twin pairs, 340 opposite-sex twin pairs and 22 single responders, and the mean age was 28.1 (19–36). The response rate was 43.5% (2801 of 6442) of the eligible twins. Non-participants consisted of 0.8% pairs not willing or able to participate, 16.8% pairs in which only one twin agreed to participate, and 38.9% pairs in which none responded after reminders. In 22 pairs, where both twins initially agreed to be interviewed, one of the twins was later unable or unwilling to participate in the interview. The high rate of attrition from the questionnaire studies to the interview study has been investigated (30) and found not to affect twin analyses of mental health–related variables. The majority of the interviews were conducted face to face, but for practical reasons, 231 were interviewed over the phone. The interviews were mainly conducted by psychology students late in their training and psychiatric nurses who received a standardized training program by teachers certified by the WHO. Members of a pair were assessed by different interviewers that were blind to the information obtained from the co-twin.
Zygosity was initially determined using questionnaire items previously shown to classify correctly more than 97% of the twin pairs (31) and molecular markers for a subgroup of the sample, based on genotyping 24 microsatellite markers. The discrepancy between classifications based on the questionnaire and DNA markers implied an expected misclassification rate of 0.67% for the whole sample. The NIPHTP is described thoroughly elsewhere (32). Approval was received from the Regional Ethical Committee and the Norwegian Data Inspectorate, and written informed consent was obtained from the participants after complete description of the study.
Measures
AVPD and DEPD were assessed by a Norwegian version of the SIDP-IV (33). The SIDP-IV is a comprehensive semistructured diagnostic interview for the assessment of all DSM-IV Axis II diagnoses. The instrument includes non-pejorative questions organized into topical sections to produce a natural flow in the interview. The questions address behaviors, cognitions and feelings that have been predominant for most of the past 5 years and thus are considered to be representative for the individual’s long-term personality functioning. This 5-year assumption is supported by empirical evidence of high stability of normal personality traits during adulthood (34). Each DSM-IV criterion is scored as 0 = absent, 1 = subthreshold, 2 = present or 3 = strongly present.
In the analyses of the SIDP-IV data, we used a dimensional approach by constructing the two PDs as ordinal variables. The number of criteria scored ≥1 was used, assuming that the liability for each trait is continuous and normally distributed. The same approach has also been used in previous publications on the same sample (11, 26, 28, 29). Thus, for convenience, we refer to PDs, but we are in fact assessing a dimensional representation of PDs.
Inter-rater reliability was assessed by two raters scoring 70 audiotaped interviews. The intraclass (and polychoric) correlations for the number of endorsed criteria at the subthreshold level were high: AVPD, 0.96 and 0.97; DEPD, 0.96 and 0.99. Reliability measured by Cronbach’s α based on polychoric correlations showed good internal consistencies: AVPD, 0.89; DEPD, 0.82. The SIDP-IV has been used previously in major Norwegian studies (35, 36).
In the questionnaire, the PDs were also assessed by the Dysfunctional Personality Questionnaire (DPQ). This instrument contains 91 items developed to assess PD traits. Some of these items were developed and validated by Svenn Torgersen (e.g. 37), and the rest were selected from three established instruments (38–40). The DPQ has been used in previous publications on the present sample (26, 27).
Statistical analyses
Regression analyses
Separately for AVPD and DEPD, we conducted backward stepwise ordinal regression analyses. Ordinal regression was used instead of linear regression, as the PD variables were ordinal, rather than continuous. The analyses were first conducted in twin 1 in each pair, where we attempted to predict from the responses to the questionnaire the number of endorsed criteria in the interview. The questionnaire items that were selected were retained only if they were significantly associated with the DSM-criteria in the second sample, consisting of the second twin in each pair. This procedure was chosen to avoid selecting items that were specific to our sample and thus to increase the generalizability of the results.
Subsequently, when calculating sum scores based on the resulting questionnaire items for AVPD and DEPD, we included only the twins that had less than 4 missing items. This procedure produced a sample size of 7980 for AVPD and 7979 for DEPD. The polychoric correlations between the selected DPQ items and the number of endorsed SIPD criteria ≥1 for each of the two PDs were subsequently calculated in R (41).
Model fitting
In classical twin modeling, the total variation in traits is assumed to arise from three sources: additive genetic (A), common environmental (C), and individual-specific environmental (E) factors. As MZ twins share all their genetic material, and DZ twins share on average half of their segregating genes, A would tend to make MZ twins correlate twice as high as DZ twins. C is defined as environmental factors that contribute to similarity between twins (such as childhood socioeconomic conditions) and is further assumed to have an equal effect on MZ and DZ twins. E is per definition not shared between twins in a pair and hence does not contribute to twin similarity. E also contains the measurement error that is inherent in the total variance of the specified model. The influence on each of these variance components on the variables can be estimated using structural equation modeling (SEM; 42).
Model fitting in general involves construction of one or several models that attempt to describe the observed data as closely as possible, while also striving to keep the model simple. The models can be simplified by dropping one or more of the parameters. If the model fit to the data is not significantly worse than the full model, the simplified (or nested submodel) is retained.
To estimate the genetic and environmental influences on the PDs, we use a liability-threshold model which assumes that liability for a trait is continuous and normally distributed in the population (43). This implies that for the questionnaire assessment, the number of items responded to in the positive direction indexes the liability, whereas for the interview assessment, the number of endorsed criteria greater than 1 indexes the liability. Using so-called liability-threshold tests, we have previously shown that the number of endorsed interview criteria reflects differences of severity for both AVPD and DEPD (11).
The liability-threshold models were fitted using maximum likelihood (ML) as estimation procedure on raw data in the twin model fitting software Mx (44). The raw data option has several advantages, such as including the variance of single responders and thus maximizing power. ML expresses the likelihood of a model as a function of observed data and model parameters, measured as a log likelihood (LL). The difference in −2 times the log likelihood (Δ−2LL) is asymptotically chi-square distributed, which allows a check for significant deterioration in χ2 in nested submodels. If the difference in χ2 is non-significant, the model has an acceptable fit. However, as the likelihood ratio test has shown to not always hold under conditions like these, giving P-values that are often too high (45), we also used the Akaike Information Criterion (AIC) calculated as χ2 − 2df (46), to select the best model. AIC penalizes models that are not parsimonious (25). The preferred model is reflected by having the lowest AIC value.
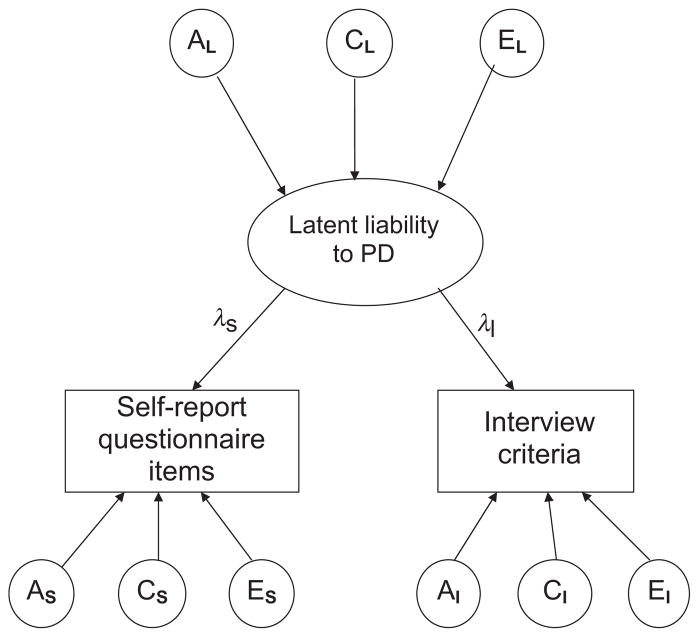
We first fitted a full ACE measurement model to the data, allowing for both qualitative and quantitative sex differences. Qualitative sex differences involve different genetic and/or environmental effects for males and females on the same trait, while quantitative sex effects involve the same genetic and environmental effects, but in different quantity for the sexes. It is possible to test for qualitative sex differences by allowing the parameter that specifies the correlation between the DZ-twin groups to be lower than 0.5. The genes shared between DZ twins are per definition 50%. Thus, if the DZ correlation is <0.5, it implies that different genes influence the trait in males and females. Testing for quantitative sex differences is carried out by allowing the A, C and E parameters to vary between the male and female twins. Each PD was modeled as a latent factor with loadings onto the two measures of PDs, the questionnaire (DPQ) and the interview (SIDP-IV) (Fig. 1). This method allows us to estimate genetic and environmental contributions to the latent PD factors and also the genetic and environmental contributions that were unique to the two PD assessment methods. The paths connecting the observed traits with the latent PD factors (labeled λS and λI, for questionnaire and interview, respectively, as showed in Fig. 1) reflect how much of the variance in the observed traits that is captured by the latent PD factors.
Fig. 1.
The measurement model used in the twin model fitting. PD = personality disorders; AL = additive genetic contributions to the latent factor; CL = common environmental contributions to the latent factor; EL = unique environmental contributions to the latent factor; λS = factor loading from latent liability to self-report questionnaire; λI = factor loading from latent liability to PD to the interview; AS = additive genetic contributions to the self-report questionnaire; CS = common environmental contributions to the self-report questionnaire; ES = unique environmental contributions to the self-report questionnaire; AI = additive genetic contributions to the interview; CI = common environmental contributions to the interview; EI = unique environmental contributions to the interview.
Results
Regression analyses
The DPQ questionnaire items that were selected from the multiple regression analyses are listed in Table 1. For AVPD, nine items were selected. Items 100, 135, 148 (reversed), 156 and 170 reflect beliefs of social ineptness, personal unappealing-ness and inferiority, as well as preoccupation with being criticized or rejected in social situations. Items 89 and 177 reflect tension and apprehension, and item 122, unwillingness to become involved with people unless certain of being liked. The polychoric correlation between the sum of these DPQ items and the number of endorsed SIDP-IV interview criteria ≥1 for AVPD was +0.57.
Table 1.
Items from the Dysfunctional Personality Questionnaire selected to assess liability to avoidant and dependent personality disorder
| Item number | Item content |
|---|---|
| AVPD | |
| 89 | I easily become discouraged when things go wrong |
| 100 | When in the presence of others, I usually remain silent |
| 122 | My lack of self-confidence can sometimes be a problem |
| 135 | I am afraid of close relationships |
| 138 | I have seen or heard things that have no logical explanation |
| −148 | Many people regard me as a lively person |
| 156 | I am quite reserved and withdrawn, except among close friends |
| 170 | It seems like other people manage to make money last longer than I do, even though they do not have more to spend than I |
| 177 | It does not come naturally to me to think fast and answer promptly |
| DEPD | |
| 89 | I easily become discouraged when things go wrong |
| −94 | I do not brood over other peoples remarks |
| −109 | I get all worked up when the situation justifies it |
| 126 | I easily get hurt if someone ridicules me or make derogatory remarks |
| 152 | When I am out with people and they discuss a specific topic, I normally just sit there and listen without saying anything |
| 169 | I sometimes feel that nobody wants to have anything to do with me |
| 177 | It does not come naturally to me to think fast and answer promptly |
| 178 | I tend to be easy to lead |
Eight items were selected for DEPD (Table 1). Items 89, 94 (reversed), and 177 reflect tension and apprehension. Items 109 (reversed), 126, 152, and 169 reflect difficulty expressing disagreement with others because of fear of loss of support or approval. Item 178 reflect difficulty making everyday decisions without an excessive amount of advice and reassurance from others and a need for others to assume responsibility for major areas of ones life. The polychoric correlation between the sum of these DPQ items and the number of endorsed SIDP-IV criteria ≥1 for DEPD was +0.45.
Model fitting
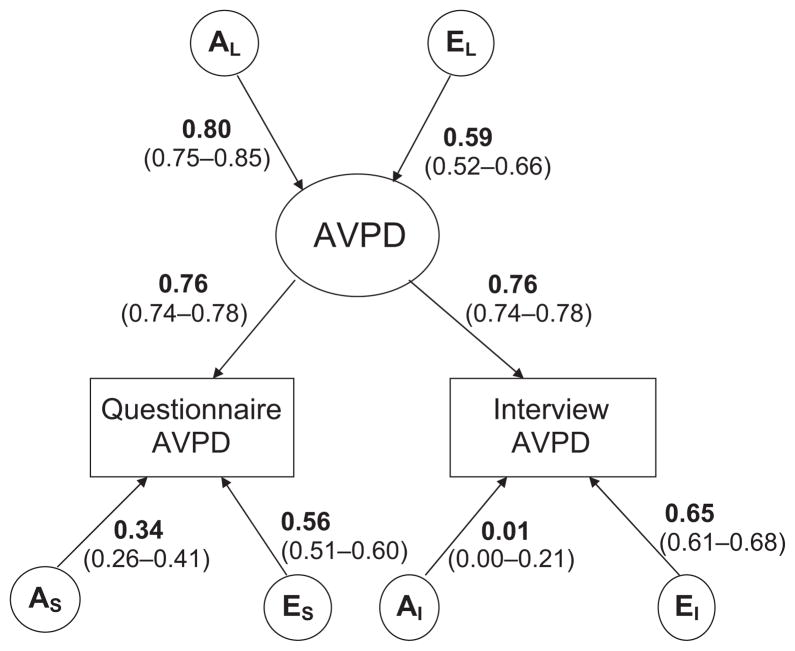
The results from the model fitting for AVPD and DEPD are shown in Table 2. For AVPD, we first fitted an ACE model (that is a model with genetic, shared environmental and unique environmental effects) for the latent PD factor as well as the measurements DPQ and SIDP-IV, allowing for both qualitative and quantitative sex differences. This full model was then compared with the reduced submodels, that is, the ACE, AE, and CE models, allowing for only quantitative and not qualitative sex differences. In this group of models, the AE model had the best fit, both according to the likelihood ratio test and AIC, whereas the CE model yielded a significantly worse fit than the full model. We then compared ACE, AE, and CE models that allowed for no sex differences with the full model. These submodels all yielded improvement in AIC, with the exception of the CE model, which both yielded a significantly worse fit according to the likelihood ratio test and also a higher AIC value. Even if the P-values should be lower than given by the likelihood ratio test, in this case, the P-values were so low that we could safely discard both the CE submodel, allowing for quantitative sex differences, and the CE model allowing for no sex differences. The AE model with no sex differences fitted the data best, as it obtained the lowest AIC value of the nested submodels (Δχ2 = 6.831, Δdf = 13, P = n.s, AIC = −19.161). Parameter estimates for the best fitting model for AVPD are shown in Fig. 2. The additive genetic and unique environmental contributions to the latent AVPD liability were estimated to be 0.80 (0.75–0.85, 95% CI) and 0.59 (0.52–0.66, 95% CI) respectively. The heritability for AVPD was thus 0.64 (0.56–0.72, 95% CI). The paths between the observed PD measures and the latent AVPD factor were estimated to be 0.76 (0.74–0.78, 95% CI), which indicates that 58% of the variance in the two measures applied could be explained by the latent AVPD construct. The genetic and unique environmental contributions that were specific for the measures were estimated to be 0.34 (0.26–0.41, 95% CI) and 0.56 (0.51–0.60, 95% CI), respectively, for the DPQ, and 0.01 (0.00–0.21, 95% CI) and 0.65 (0.61–0.68, 95% CI), respectively, for SIDP-IV.
Table 2.
Model fitting results for avoidant and dependent personality disorder
| Model | Δ−2LL | Δdf | P | ΔAIC | |
|---|---|---|---|---|---|
| AVPD GSL | |||||
| 1 | ACE | – | – | – | – |
| AVPD CSL | |||||
| 1 | ACE | 0.359 | 1 | 0.54 | −1.641 |
| 2 | AE | 6.410 | 7 | 0.49 | −7.590 |
| 3 | CE | 73.810 | 7 | 0.00* | 59.810 |
| AVPD NSL | |||||
| 1 | ACE | 6.831 | 10 | 0.74 | −13.169 |
| 2 | AE | 6.831 | 13 | 0.91 | −19.161 |
| 3 | CE | 80.094 | 13 | 0.00* | 54.094 |
| DEPD GSL | |||||
| 1 | ACE | – | – | – | – |
| DEPD CSL | |||||
| 1 | ACE | 1.304 | 1 | 0.25 | −0.696 |
| 2 | AE | 9.949 | 7 | 0.19 | −4.051 |
| 3 | CE | 49.407 | 7 | 0.00* | 35.407 |
| DEPD NSL | |||||
| 1 | ACE | 12.883 | 10 | 0.23 | −7.117 |
| 2 | AE | 12.883 | 13 | 0.46 | −13.117 |
| 3 | CE | 68.993 | 13 | 0.00* | 42.993 |
Best fitting model in bold type.
Significant at <0.001.
Fig. 2.
Parameter estimates and 95% CIs for the best fitting model for avoidant personality disorder.
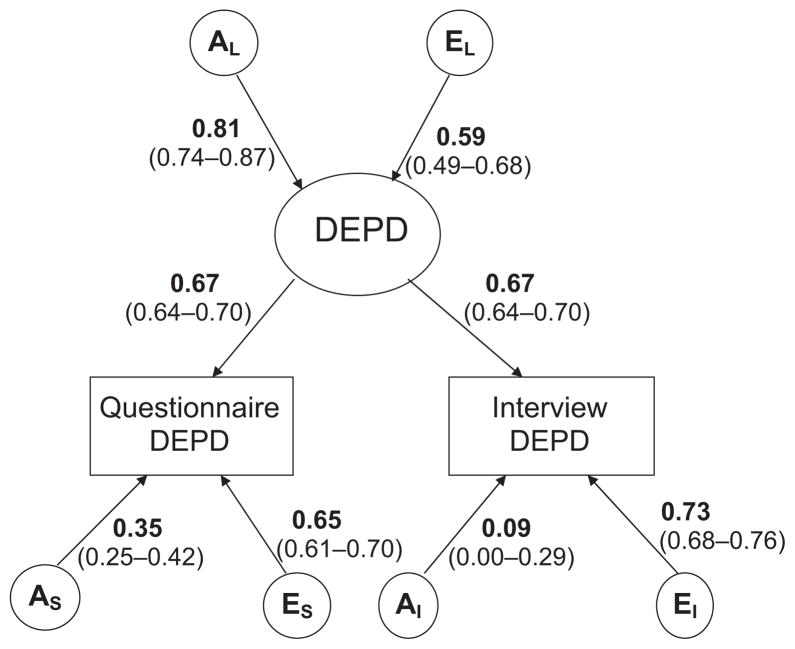
For DEPD, we followed the same procedure as for AVPD, by first fitting a full ACE model allowing for both quantitative and qualitative sex differences and subsequently testing reduced models as described above. The best fitting model was the AE model allowing for no sex differences (Δχ2 = 12.883, Δdf = 13, P = n.s, AIC = −13.117). The parameter estimates for the best fitting model for DEPD are shown in Fig. 3. The genetic and unique environmental contributions to the latent DEPD factor were 0.81 (0.74–0.87, 95% CI) and 0.59 (0.49–0.68, 95% CI) respectively. This yields a heritability estimate of 0.66 (0.55–0.76, 95% CI). The paths between the observed PD measures and the latent DEPD factor were estimated to be 0.67 (0.64–0.70, 95% CI), which suggests that 48% of the variance in the DPQ and SIDP-IV measures is explained by the latent DEPD factor. The specific genetic and unique environmental contributions to the measures were 0.35 (0.25–0.42, 95% CI) and 0.65 (0.61–0.70, 95% CI), respectively, for the DPQ and 0.09 (0.00–0.29, 95% CI) and 0.73 (0.68–0.76, 95% CI), respectively, for SIDP-IV.
Fig. 3.
Parameter estimates and 95% CIs for the best fitting model for dependent personality disorder.
Discussion
To our knowledge, this is the first study investigating the genetic and environmental contributions to the liability to AVPD and DEPD when corrected for measurement error. Our main finding was that the heritability estimates of these two PDs were substantially higher than what we previously found when using single-occasion interviews only. Using two methods of assessment for the PDs at different time points, we were able to model the PDs as latent liability factors in a measurement model. This method allowed us to control for time-and method-specific variance, which results in more reliable estimates.
The heritability estimates obtained for AVPD and DEPD in the present study were substantial, 0.64 and 0.66 respectively. These estimates are similar to what was found for cluster A and B PDs when using the same method (26, 27), and for cluster C PDs in clinical samples (12) and in children (13). Taking into account imperfections in the methods of assessment, it appears that the etiologic role of genetic factors is at least as strong for the latent factors of AVPD and DEPD as for normal personality traits and related Axis I disorders (14, 15, 17–20).
We did not find evidence of common environmental effects on the latent AVPD and DEPD factors, nor on the DPQ and SIDP-IV measures, which is in accordance with most twin studies on mental disorders (10). Neither did we find evidence of sex differences for any of the PDs, which is also in accordance with previous findings on the other DSM-IV PDs (26–29) and on cluster C PDs when assessed by single-occasion interview only (11).
We found low genetic contributions specific to the interview-based PD assessments, indicating that most of the genetic variance captured by SIDP-IV is also captured by the latent PD factors. At the same time, the unique environmental contributions to the interview-based assessment were high, which may indicate that the amount of measurement error was substantial for this assessment. The genetic contributions to the variance specific to DPQ were moderate for both PDs, and hence, a non-negligible part of the genetic variance in the DPQ was not captured by the latent PD factors. These observations indicate that SIDP-IV had greater specificity in indexing the genetic risk factors for the latent liability to AVPD and DEPD than to the DPQ. This is in accordance with previous results for cluster A and B PDs using the same method, which also found good specificity for SIDP-IV and moderate specificity for DPQ (26, 27). In the present study, the estimated paths between the observed PD measures and the latent PD factors were also moderate to high, indicating that the amount of explained variance by the latent AVPD and DEPD factors was acceptable.
The observation that the questionnaire measure (DPQ) contained more specific genetic variance than the interview measure (SIDP-IV) could be explained by something fundamental about the methods of assessment. For instance, it may be that the DPQ captures a set of genetically influenced traits that are distinct from those that impact on the SIDP-IV measure. Phenotypically, the correlations obtained between the sum of the items and the number of endorsed SIDP-IV criteria ≥1 were +0.57 and +0.45, respectively, for AVPD and DEPD. This indicates that a non-negligible amount of the variance inherent in DPQ and SIDP-IV was non-overlapping and supports the assumption that DPQ and SIDP-IV are indeed tapping different aspects of the PD traits. Poor diagnostic concordance seems, however, not to be limited to the two methods of assessments in the present sample, but a common finding when comparing different instruments designed to capture PDs (24, 47).
This study, among several others, shows the value of measurement models for obtaining reliable estimates of genetic and environmental contributions to the traits of interest. In general, an effective method to obtain reliable estimates is to use multiple indicators to measure a latent factor, which is then to a large extent unobscured by measurement error (25). A recent study demonstrated this important point by showing that heritability estimates for lifetime history of alcohol dependence increased as a function of diagnostic reliability (48). When using only one measure of the trait of interest, which is often done in psychiatric research settings (e.g., the use of a diagnostic interview only), the heritability estimates obtained are artificially low, as the genetic signal will be diminished by noise created by measurement error. This point is also made by a study of PD prevalences, which found that the cumulative prevalence of PDs, estimated from interview assessment at several time points, was substantially higher than the point prevalence, based on an interview assessment conducted at only one time point (49).
In the current study, as well as in the two previous studies on the heritability of cluster A and B PDs (26, 27), we found that when using measurement models that correct for measurement errors, the heritability estimates of the PDs increased substantially. However, a cautious note on these results seems necessary: Although our results show that PDs seem to have substantial heritability, it is worth emphasizing that high heritability does not imply genetic determinism. If an individual has a family history of PDs, this individual is not bound to develop a PD. Whether a genetic liability will lead to a trait being expressed or not is determined by a complicated interplay between both genetical and environmental factors (50).
It is reasonable to assume that the problem of measurement error is evident for Axis I psychiatric disorders as well. To be able to investigate correlates and implications of mental disorders, one should have the best possible measures of these traits. Therefore, future research could be directed toward applying measurement models whenever possible, to better understand the true heritability of psychiatric disorders.
Limitations
The results from the current study should be interpreted in light of some potentially significant limitations. First, owing to the rarity of fully syndromal diagnoses in our sample, we used dimensional representations of the three PDs instead of categorical diagnoses. However, as the twin analyses are based on the liability-threshold model (43), it should in principle make no difference if the variables studied are categorical or dimensional, as long as the dimensional variables reflect the same underlying liability as the categorical diagnoses. In addition, it has been argued by many studies that a dimensional approach captures the true nature of PDs better than a categorical approach (51–54).
Second, the findings of no common environmental contributions or sex differences could be due to limited statistical power. We therefore do not have firm grounds to conclude whether sex differences are present or not. Future studies should be conducted with sample sizes sufficiently large to detect small common environment effects and sex differences in the data.
Third, the present study did not include tests for gene–environment correlation (rGE), which reflects individual genetic differences in exposure to particular environments, or gene–environment interaction (GxE), which reflects genetic differences in susceptibility to certain environments (55). Failure to take these effects into account may result in over- or under-estimated parameter estimates (56). Future research should explore the effects of rGE and GxE on twin modeling results for AVPD and DEPD, either by including a valid and reliable measure of environment or by including adoption- or family data in the models (9).
Finally, we used a sample of young, Norwegian twins. The results may thus not be representative for other populations, such as other ethnic groups and age cohorts. However, prevalence estimates from a Norwegian epidemiological study in a community sample were within the same range as those reported from community samples from other Western countries (35). It is also not given that results from twin studies could be extrapolated to the general population. A previous study of personality addressed this concern and failed to find any systematic differences between twin and non-twin samples (57).
Significant outcomes.
The heritabilities obtained by correcting for errors of measurement by combining two assessments of avoidant and dependent PD are in the upper range of what has previously been found.
To obtain valid assessments of PDs in clinical practice, assessments should be conducted on at least two time points and preferably with both interview and questionnaire.
Although environmental factors influence the liability to personality disorders, the present study suggests that genetic factors might be equally important. This emphasizes the importance for clinicians to obtain a thorough family history from patients with symptoms of personality disorders.
Limitations.
The PD variables were constructed as dimensional representations instead of categorical diagnoses.
The sample consists of young, Norwegian twins. The results may thus not generalize to other age cohorts or ethnic groups.
It was not possible to create an index for obsessive–compulsive PD based on the questionnaire items. We were therefore only able to include two of the three PDs in DSM-IV cluster C in our analyses.
Acknowledgments
This work was supported in part by grant MH-068643 from the National Institutes of Health and by grants from The Norwegian Research Council, The Norwegian Foundation for Health and Rehabilitation, The Norwegian Council for Mental Health, and The European Commission under the program ‘Quality of Life and Management of the Living Resources’ of the 5th Framework Program (no. QLG2-CT-2002-01254).
Footnotes
Declaration of interest
None.
References
- 1.APA. Diagnostic and statistical manual of mental disorders. 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 2.Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: a meta-analytic review. Clin Psychol Rev. 2004;23:1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Widiger TA, Livesley WJ, Clark LA. An integrative dimensional classification of personality disorder. Psychol Assess. 2009;21:243–255. doi: 10.1037/a0016606. [DOI] [PubMed] [Google Scholar]
- 4.Widiger TA, Trull TJ. Plate tectonics in the classification of personality disorder - Shifting to a dimensional model. Am Psychol. 2007;62:71–83. doi: 10.1037/0003-066X.62.2.71. [DOI] [PubMed] [Google Scholar]
- 5.Saulsman LM, Page AC. Corrigendum to “The five-factor model and personality disorder empirical literature: a meta-analytic review”. Clin Psychol Rev. 2005;25:383–394. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Moran P, Coffey C, Mann A, Carlin JB, Patton GC. Dimensional characteristics of DSM-IV personality disorders in a large epidemiological sample. Acta Psychiatr Scand. 2006;113:233–236. doi: 10.1111/j.1600-0447.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 7.Røysamb E, Kendler KS, Tambs K, et al. The joint structure of DSM-IV Axis I and Axis II disorders. J Abnorm Psychol. 2011;120:198–209. doi: 10.1037/a0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard TJ. Genetic influence on human psychological traits – a survey. Curr Directions Psycholog Sci. 2004;13:148–151. [Google Scholar]
- 9.Plomin R, Defries JD, Mcclearn GE, Mcguffin P. Behavioral genetics. 4. New York: Worth Publishers; 2001. [Google Scholar]
- 10.Kendler KS, Prescott CA. Genes, environment, and psychopathology: understanding the causes of psychiatric and substance use disorders. New York: Guilford Press; 2006. [Google Scholar]
- 11.Reichborn-Kjennerud T, Czajkowski N, Neale MC, et al. Genetic and environmental influences on dimensional representations of DSM-IV cluster C personality disorders: a population-based multivariate twin study. Psychol Med. 2007;37:645–653. doi: 10.1017/S0033291706009548. [DOI] [PubMed] [Google Scholar]
- 12.Torgersen S, Lygren S, Oien PA, et al. A twin study of personality disorders. Compr Psychiatry. 2000;41:416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- 13.Coolidge FL, Thede LL, Jang KL. Heritability of personality disorders in childhood: a preliminary investigation. J Pers Disord. 2001;15:33–40. doi: 10.1521/pedi.15.1.33.18645. [DOI] [PubMed] [Google Scholar]
- 14.Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: a twin study. J Pers. 1996;64:577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang KL, Mccrae RR, Angleitner A, Riemann R, Livesley WJ. Heritability of facet-level traits in a cross-cultural twin sample: support for a hierarchical model of personality. J Pers Soc Psychol. 1998;74:1556–1565. doi: 10.1037//0022-3514.74.6.1556. [DOI] [PubMed] [Google Scholar]
- 16.Jang KL, Livesley WJ, Vernon PA, Jackson DN. Heritability of personality disorder traits: a twin study. Acta Psychiatr Scand. 1996;94:438–444. doi: 10.1111/j.1600-0447.1996.tb09887.x. [DOI] [PubMed] [Google Scholar]
- 17.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV Axis I and All Axis II disorders. Am J Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjerde LC, Røysamb E, Czajkowski N, et al. Strong genetic correlation between interview-assessed internalizing disorders and a brief self-report symptom scale. Twin Res Hum Genet. 2011;14:64–72. doi: 10.1375/twin.14.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tambs K, Czajkowsky N, Røysamb E, et al. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mcglashan TH, Grilo CM, Sanislow CA, et al. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: toward a hybrid model of Axis II disorders. Am J Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livesley WJ, Jang KL. The behavioral genetics of personality disorder. Annu Rev Clin Psychol. 2008;4:23. doi: 10.1146/annurev.clinpsy.4.022007.141203. [DOI] [PubMed] [Google Scholar]
- 23.Moum T. Mode of administration and interviewer effects in self-reported symptoms of anxiety and depression. Soc Indic Res. 1998;45:279–318. [Google Scholar]
- 24.Zimmermann M. Diagnosing personality disorders. A review of issues and research methods. Arch Gen Psychiatry. 1994;51:225–245. doi: 10.1001/archpsyc.1994.03950030061006. [DOI] [PubMed] [Google Scholar]
- 25.Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- 26.Kendler KS, Myers J, Torgersen S, Neale MC, Reichborn-Kjennerud T. The heritability of cluster A personality disorders assessed by both personal interview and questionnaire. Psychol Med. 2007;37:655–665. doi: 10.1017/S0033291706009755. [DOI] [PubMed] [Google Scholar]
- 27.Torgersen S, Myers J, Reichborn-Kjennerud T, Røysamb E, Kubarych T, Kendler KS. The heritability of Cluster B personality disorders assessed both by personal interview and questionnaire. J Pers Disord. doi: 10.1521/pedi.2012.26.6.848. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendler KS, Czajkowski N, Tambs K, et al. Dimensional representations of DSM-IV Cluster A personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2006;36:1583–1591. doi: 10.1017/S0033291706008609. [DOI] [PubMed] [Google Scholar]
- 29.Torgersen S, Czajkowski N, Jacobson K, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- 30.Tambs K, Ronning T, Prescott CA, et al. The norwegian institute of public health twin study of mental health: examining recruitment and attrition bias. Twin Res Hum Genet. 2009;12:158–168. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnus P, Berg K, Nance WE. Predicting zygosity in Norwegian twin pairs born 1915–1960. Clin Genet. 1983;24:103–112. doi: 10.1111/j.1399-0004.1983.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 32.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Res. 2002;5:415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 33.Pfohl BB, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) Iowa City: University of Iowa, Department of Psychiatry; 1995. [Google Scholar]
- 34.Mccrae RR, Costa JPT. Personality in adulthood. New York, NY: Guilford Press; 1990. [Google Scholar]
- 35.Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 36.Helgeland MI, Kjelsberg E, Torgersen S. Continuities between emotional and disruptive behavior disorders in adolescence and personality disorders in adulthood. Am J Psychiatry. 2005;162:1941–1947. doi: 10.1176/appi.ajp.162.10.1941. [DOI] [PubMed] [Google Scholar]
- 37.Torgersen S. Hereditary-environmental differentiation of general neurotic, obsessive, and impulsive hysterical personality traits. Acta Geneticae Medicae et Gemellologiae. 1980;29:193–207. doi: 10.1017/s0001566000007935. [DOI] [PubMed] [Google Scholar]
- 38.Conte RC, Plutchik R, Karasu TB, Jerret I. A self-report borderline scale. Discriminative validity and preliminary norms. J Nerv Mental Dis. 1980;168:428–435. doi: 10.1097/00005053-198007000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lazare A, Klerman GL, Armor DJ. Oral, obsessive, and hysterical personality patterns. An investigation of psychoanalytic concepts by means of factor analysis. Arch Gen Psychiatry. 1966;14:624–630. doi: 10.1001/archpsyc.1966.01730120064008. [DOI] [PubMed] [Google Scholar]
- 40.Foulds GA. Personality and personal illness. London: Tavistock; 1965. [Google Scholar]
- 41.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [updated 2005; cited]; Available from: http://www.r-project.org/ [Google Scholar]
- 42.Neale MC, Maes HH. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Akademic Publisher B.V; 2000. [Google Scholar]
- 43.Falconer DS. Inheritance of liability to certain diseases estimated from incidence among relatives. Annu Hum Genet. 1965;29:51–76. [Google Scholar]
- 44.Neale MC, Boker MS, Maes HH. Mx: statistical modelling. 6. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- 45.Dominicus A, Skrondal A, Gjessing H, Pedersen N, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet. 2006;36:331–340. doi: 10.1007/s10519-005-9034-7. [DOI] [PubMed] [Google Scholar]
- 46.Akaike H. Factor analysis and AIC. Psychometrica. 1987;52:317–332. [Google Scholar]
- 47.Widiger TA, Coker LA. Assessing personality disorders. In: Butcher JN, editor. Clinical personality assessment: practical approaches. Oxford University Press; New York: 2002. pp. 407–434. [Google Scholar]
- 48.Ystrøm E, Reichborn-Kjennerud T, Aggen SH, Kendler KS. Alcohol dependence in men: reliability and heritability. Alcoholism. 2011;35:1716–1722. doi: 10.1111/j.1530-0277.2011.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson JG, Cohen P, Kasen S, Skodol AE, Oldham JM. Cumulative prevalence of personality disorders between adolescence and adulthood. Acta Psychiatr Scand. 2008;118:410–413. doi: 10.1111/j.1600-0447.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 50.Sterelny K, Griffiths PE. Sex and death: an introduction to the philosophy of biology. Chicago: University of Chicago Press; 1999. [Google Scholar]
- 51.Oldham JM, Skodol AE. Charting the future of Axis II. J Pers Disord. 2000;14:17–29. doi: 10.1521/pedi.2000.14.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Skodol AE, Oldham JM, Bender DS, et al. Dimensional representations of DSM-IV personality disorders: relationships to functional impairment. Am J Psychiatry. 2005;162:1919–1925. doi: 10.1176/appi.ajp.162.10.1919. [DOI] [PubMed] [Google Scholar]
- 53.Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the diagnostic and statistical manual of mental disorders – fifth edition. J Abnorm Psychol. 2005;114:494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- 54.Hopwood CJ, Malone JC, Ansell EB, et al. Personality assessment in DSM-5: empirical support for rating severity, style, and traits. J Pers Disord. 2011;25:305–320. doi: 10.1521/pedi.2011.25.3.305. [DOI] [PubMed] [Google Scholar]
- 55.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatr. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plomin R, Defries JC, Loehlin JC. Genotype-environment interaction and correlation in analysis of human-behavior. Psychol Bull. 1977;84:309–322. [PubMed] [Google Scholar]
- 57.Johnson W, Krueger RF, Bouchard TJ, Mcgue M. The personalities of twins: just ordinary folks. Twin Res. 2002;5:125–131. doi: 10.1375/1369052022992. [DOI] [PubMed] [Google Scholar]