Abstract
BACKGROUND
Alcohol dependence (ALC) is a disorder with an impulsive and compulsive “drive” towards alcohol consumption and an inability to inhibit alcohol consumption. Neuroimaging studies suggest that these behavioral components correspond to an increased involvement of regions that mediate appetitive drive and reduced involvement of regions that mediate executive control within top-down networks. Little is known, however, about whether these characteristics are present after long periods of abstinence.
METHODS
Resting state functional magnetic resonance imaging data were collected to examine resting state synchrony (RSS) differences between 23 long-term abstinent alcoholics (LTAA; 8 females, age: M=48.46, SD=7.10), and 23 non-substance abusing controls (NSAC; 8 females, age: M=47.99, SD=6.70). Using seed-based measures, we examined resting-state synchrony with the nucleus accumbens (NAcc) and the subgenual anterior cingulate cortex (ACC). All participants were assessed with the intra/extradimensional set shift task outside of the scanner to explore the relationship between RSS and cognitive flexibility.
RESULTS
Compared to NSAC, LTAA showed (a) decreased synchrony of limbic reward regions (e.g., caudate and thalamus) with both the ACC seed and the NAcc seed and (b) increased synchrony of executive control regions (e.g., DLPFC) with both the NAcc seed and the subgenual ACC seed. RSS differences were significantly correlated with task performance.
CONCLUSIONS
The results are consistent with an interpretation of an ongoing compensatory mechanism in long-term abstinent alcoholics evident during rest, in which decision making networks show reduced synchrony with appetitive drive regions and increased synchrony with inhibitory control regions. In addition, RSS differences were associated with cognitive flexibility. These resting state findings indicate an adaptive mechanism present in long-term abstinence that may facilitate the behavioral control required for to maintain abstinence.
Keywords: alcohol, abstinence, fMRI, resting state, anterior cingulate
INTRODUCTION
Alcohol dependence is characterized by continued alcohol use despite negative biological, psychological, legal, and social consequences (American Psychiatric Association 1994). It is thought of as a disorder with two components: a) an overwhelming impulsive and compulsive “drive” towards alcohol consumption (Kamarajan et al. 2005), and b) an inability to “hit the brakes”, or inhibit alcohol consumption. Functional neuroimaging studies suggest that these aspects of alcohol dependence correspond to a) increased involvement of regions that mediate appetitive drive and b) reduced involvement of regions that mediate executive control, within both bottom-up and top-down networks (Akine et al. 2007; Li et al. 2009; Park et al. 2011). The characteristics of bottom-up and top-down networks in active alcohol dependence and in abstinence may help identify brain mechanisms that underlie these states.
Enhanced bottom-up reward drive in active alcohol dependence
Alcohol consumption is first sought for its reinforcing effects. After chronic use, however, alcohol consumption becomes a compulsion, and its rewarding effects (positive reinforcement) diminish along with a shift toward alcohol consumption primarily to avoid withdrawal effects (negative reinforcement). Positive and negative reinforcement are largely mediated by the bottom-up reward network. The reward network is comprised of mesocorticolimbic regions that mediate aspects of drug addiction such as responses to rewarding stimuli (i.e., ventral tegmental area and nucleus accumbens), memory of rewarding stimuli (i.e., amygdala and hippocampus), and regulation of emotion and executive function (i.e., prefrontal and anterior cingulate cortices)(Everitt and Robbins 2005).
The nucleus accumbens (NAcc) is an important reward processing region that mediates appetitive drive and behavior towards rewards (Everitt and Robbins 2005; Taha and Fields 2006). It has been hypothesized that dopaminergic dysfunction (i.e., abnormally increased levels of dopamine, a neurotransmitter that regulates reward) in the NAcc in alcohol dependence is involved in the lack of behavioral flexibility necessary to generate new adaptive behavior and the inability to extinguish alcohol-related habits (Chen et al. 2011). Given the importance of the NAcc, one focus of the present study is to examine how NAcc fluctuations during rest are synchronized with other brain regions.
Compromised top-down processes in current alcohol dependence
Top-down processes include executive functions involved in planning, regulating, or reversing complex goal-directed behavior. A core aspect of alcohol dependence is poor regulation of behavior and emotion. Alcohol dependent individuals show an inability to manage the appropriate experience and expression of emotion (e.g., extremes in emotional responsiveness to social situations, negative affect, mood swings) (Berking et al. 2011; Fox et al. 2008). Dysfunctional emotion regulation has been considered a primary trigger for relapse (Berking et al. 2011; Cooper et al. 1995) and has been associated with prefrontal dysfunction (Lyvers 2000), particularly in the subgenual anterior cingulate cortex (sgACC) (Salloum et al. 2007). The sgACC has a central role within the limbic and paralimbic system involving emotional responsiveness and regulation of behavior in the context of rewarding and punishing outcomes (Drevets et al. 1997; AM Kelly et al. 2009; Phan et al. 2005). Given the sgACC’s role in the regulation of emotion and reward processes, the present study also examines the pattern of synchrony between resting fluctuations of the sgACC and other brain regions.
Differences within the bottom-up and top-down networks during abstinence
While the above section suggests that current alcohol dependence is associated with exaggerated bottom-up and compromised top-down neural network functioning, there is evidence suggesting that abstinent individuals may have overcome these dysfunctional patterns of network functioning. There seems to be compensatory mechanisms in long-term abstinence that increases inhibition of reward seeking and attenuates the reward seeking drive. A recent study by Nestor et al. (2011) found that when performing a task that measures inhibitory control (i.e., go/no-go task), long-term abstinent smokers compared to current smokers exhibit increased activity in frontally mediated executive control networks and decreased NAcc activity. Nestor et al. (2011) suggest that long-term abstinent smokers regulate their responses more than current smokers (by slowing down responses) to achieve performance comparable to healthy controls.
Neuroimaging studies in alcohol dependence have suggested that there are brain activation changes within the reward circuitry during abstinence. An fMRI study using the monetary incentive delay task (MIDT) in 5–37 day abstinent alcoholics found reduced NAcc activation when processing monetary reward and increased NAcc activation when viewing alcohol related cues in alcoholics compared to healthy controls (Wrase et al. 2007). Another fMRI study in 7–14 day abstinent alcoholics found that reduced NAcc activation during anticipation of reward was correlated with higher impulsivity and alcohol craving, suggesting that alterations in NAcc activity may influence the maintenance of abstinence (Beck et al. 2009).
Neuroimaging studies have reported inconsistent results regarding brain activity in frontal executive control regions during extended abstinence. For example, a study by Akine et al. (2007) reported that alcoholics abstinent: 39.8 ± 12.1 months show lower activity during a recognition task in right dorsolateral prefrontal cortex and anterior cingulate cortex when compared to healthy controls. Because task performance showed no impairment, the authors suggested that reduced frontal activity may represent “latent lesions” that are still present after long periods of abstinence (Akine et al. 2007). Grüsser et al. (2004), however, found no significant differences in frontal task-evoked brain activity during a verbal fluency task between 264.75 ± 198.24 day abstinent alcoholics and controls. In addition, because short-term abstinent alcoholics in the same study had lower frontal brain activity compared to controls, the authors proposed that there is an increase in frontal brain activity with continued abstinence such that long-term abstinent alcoholics are comparable to healthy controls.
The examination of the brain synchrony patterns during rest allows us to investigate the organization of neural networks in clinical populations irrespective of cognitive processes related to task performance (Biswal et al. 1997). Because there is evidence that the strength of resting state synchrony (RSS) and amplitude are correlated to task-related BOLD signal fluctuations (Mennes et al. 2010; Mennes et al. 2011) and to behavior outside the scanning session (Koyama et al. 2011), it has been proposed that resting neural fluctuations provide a “blueprint to be used as a repertoire for responses to the external world” (Mennes et al. 2010).
Purpose of present study
Our main research goal was to examine the functional organization of bottom-up and top-down neural networks in alcohol dependent individuals that have been successful in maintaining abstinence for extended periods of time. We hypothesized that such individuals would exhibit either normal (i.e., not different from control subjects) bottom-up and top-down neural network RSS or would exhibit compensatory patterns of RSS relative to controls, with enhanced synchrony of prefrontal (i.e., inhibitory or executive control) and reduced synchrony of appetitive drive reward regions in the bottom-up and top-down neural networks. Secondarily, we sought to explore the relationship between RSS patterns and clinically relevant behaviors.
Based on previous reports of compensatory mechanisms in long-term abstinence (Chanraud et al. 2011; Nestor et al. 2010), we hypothesized that, compared to non-substance abusing controls (NSAC), long-term abstinent alcoholics (LTAA) will show either a) a similar (i.e., normal) pattern of synchrony, or b) an enhanced synchrony of prefrontal regions and reduced synchrony of mesolimbic regions in the bottom-up and top-down networks. In addition, given the relationship between RSS and behavior (Mennes et al. 2010; Mennes et al. 2011; Zhu et al. 2011), we hypothesized that increased RSS involving prefrontal executive control regions, will be positively correlated with performance on a task assessing the ability to properly adapt previously learned responses in accord with changing contingencies (i.e., measuring cognitive flexibility).
METHODS
Participants
Twenty-three LTAA (abstinent 7.91 ± 7.80 years) were compared to 23 gender and age comparable NSAC. All subjects were recruited from the island of Oahu (Table 1). LTAA were recruited through advertisements and fliers posted in various treatment programs including Alcoholic Anonymous meetings. LTAA met DSM-IV lifetime criteria for alcohol dependence (American Psychiatric Association 1994), but not for lifetime abuse or dependence on any other drugs of abuse (other than nicotine or caffeine). In addition, subjects completed the computerized Diagnostic Interview Schedule (C-DIS)(Levitan et al. 1991) to ascertain externalizing, anxiety or mood disorder symptoms and diagnoses (Table 1A and 1B). All subjects’ substance use history was gathered using the Lifetime Drinking History instrument designed by Skinner and Sheu (1982), administered separately for alcohol and for each other substance used (including nicotine).
Table 1A.
Demographics, Symptom Counts, and Alcohol Use Measures of Non-Substance Abusing Controls (NSAC) and Long-Term Abstinent Alcoholic (LTAA) Participants
| Characteristic | NSAC (n = 23)
|
LTAA (n = 23)
|
Effect Size Partial Eta2 (% variance) | Odds Ratio | ||
|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | |||
| Age, (yrs) | 47.99 | 6.70 | 48.46 | 7.10 | 0.05 | -- |
| Education, (yrs) | ||||||
| Subject | 14.74 | 2.38 | 13.22 | 2.33 | 9.8* | -- |
| Mother | 13.52 | 4.29 | 14.52 | 4.47 | 1.3 | -- |
| Father | 14.04 | 4.88 | 14.95 | 5.13 | 0.9 | -- |
| Female, n (%) | 8 | 34.78% | 8 | 34.78% | -- | 1 |
| Nicotine Use | ||||||
| History, n (%) | 3 | 13.04% | 11 | 47.83% | -- | 0.16* |
| Current, n (%) | 2 | 8.70% | 7 | 30.43% | -- | 0.22 |
| Symptom Counts (Lifetime) | ||||||
| Externalizing | 9.13 | 12.19 | 27.17 | 16.96 | 28.1*** | -- |
| Anxiety | 5.13 | 7.17 | 11.73 | 13.09 | 9.3* | -- |
| Mood | 5.57 | 9.28 | 17.00 | 15.10 | 17.9** | -- |
| Symptom Counts (Current) | ||||||
| Externalizing | 2.30 | 2.96 | 8.13 | 9.54 | 15.1** | -- |
| Anxiety | 1.87 | 4.79 | 6.04 | 9.57 | 7.0 | -- |
| Mood | 0.61 | 1.53 | 2.09 | 3.29 | 8.0 | -- |
| Average Dose (Lifetime; standard number of drinks per month) | 10.48 | 8.19 | 188.51 | 163.03 | 38.3a | -- |
| Dose During Peak Use (number of drinks per month) | 16.83 | 14.64 | 328.65 | 258.78 | 43.1a | -- |
| Length of Abstinence (number of days) | -- | -- | 2888.78 | 2848.14 | -- | -- |
| Additional substances used | ||||||
| Cannabis, n | 1b | 4.35% | 4b | 17.39% | ||
| Cocaine, n | 1b | 4.35% | ||||
p<0.05,
p<0.01,
p<0.001
statistical comparisons are inappropriate since the variable is related to selection criteria
recreational substance use only, no Ss met criteria for abuse or dependence; subjects had been abstinent from these substances for an average of 18 years at the time of assessments.
Table 1B.
Current and lifetime psychiatric diagnoses in NSAC and LTAA. NSAC, non-substance abusing controls; LTAA, long-term abstinent alcoholics; SD, standard deviation
| Current Diagnoses | Lifetime Diagnoses | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Psychiatric Diagnoses | LTAA (n = 23) | NSAC (n = 23) | Odds Ratio | LTAA (n = 23) | NSAC (n = 23) | Odds Ratio |
| Count (n) | Count (n) | Count (n) | Count (n) | |||
| Internalizing disorders | 3 | 2 | 1.5 | 16 | 6 | 2.67** |
| Mood | 3 | 2 | 1.5 | 13 | 6 | 2.16* |
| Dysthymia | 0 | 0 | N/A | 1 | 0 | ∞ |
| Manic Episode | 0 | 0 | N/A | 2 | 0 | ∞ |
| Hypomanic | 0 | 0 | N/A | 0 | 0 | N/A |
| Major Depressive Disorder | 2 | 1 | 2 | 10 | 5 | 2 |
| Bipolar | 1 | 1 | 1 | 3 | 1 | 3 |
| Anxiety | 1 | 1 | 1 | 7 | 2 | 3.5 |
| Agoraphobia | 0 | 0 | N/A | 2 | 0 | ∞ |
| Obsessive Compulsive | 0 | 0 | N/A | 0 | 0 | N/A |
| Panic Disorder | 0 | 0 | N/A | 1 | 0 | ∞ |
| Social Phobia | 0 | 0 | N/A | 0 | 0 | N/A |
| Post-Traumatic Stress Disorder | 1 | 1 | 1 | 6 | 2 | 3 |
| Externalizing Disorders | 3 | 0 | ∞ | 7 | 1 | 7* |
| Attention Deficit Hyperactivity Disorder | 2 | 0 | ∞ | 4 | 1 | 4 |
| Antisocial Personality Disorder | 2 | 0 | ∞ | 37 | 15 | 2.47** |
| Conduct Disorder | 0 | 0 | N/A | 0 | 0 | N/A |
Significant Chi Square (1-sided):
p<0.05,
p<0.01,
p<0.001
A breathalyzer test to screen for alcohol (Alco-Sensor IV, Intoximeters, Inc., Saint Louis, MO) and a saliva screen for drugs (Oral Fluid Drug Screen Device, Innovacon, Inc., San Diego, CA) was performed for all subjects on each testing day, with negative findings required for participation (no subjects failed the screens). Participants received monetary compensation for their participation. Exclusion criteria for both groups included: a) significant history of head trauma or cranial surgery; b) current or lifetime history of diabetes, stroke, or hypertension that required medical intervention; c) current or lifetime history of a significant neurological disorder; d) clinical or laboratory evidence of active hepatic disease; e) clinical evidence for Wernicke-Korsakoff syndrome, and f) lifetime diagnosis of schizophrenia or schizophreniform disorder (as assessed by the C-DIS).
Variables in Table 1A that showed significant differences between groups (years of education, smoking history, symptom counts) were used as covariates in a second level group analysis of covariance (ANCOVA) as presented below.
Imaging data acquisition
Resting functional magnetic resonance (fMRI) data were collected using a twelve-channel head coil on a Siemens Tim Trio 3.0 T scanner (Siemens Medical Solutions, Erlangen, Germany) located at Queen’s Medical Center in Honolulu. Subjects were instructed to lay motionless in the scanner with their eyes closed. The imaging sequence was a gradient-echo spiral in/out sequence with parameters of TE=30 ms, TR=2000 ms, flip angle=60°, 28 interleaved axial 5 mm thick contiguous slices, FOV=22 cm, and a 3.44×3.44 mm in-plane resolution (64×64 matrix size)(Glover and Law 2001; Noll et al. 1995). Images were reconstructed using a custom gridding reconstruction program with a field map based off resonance correction (Jackson et al. 1991; Noll et al. 1991). Spiral-in images and spiral-out images were magnitude squared summed to improve signal-to-noise and to recover signal loss caused by susceptibility variations in the brain. FMRI session included a total 123 volumes for a total scan time of 4:06. The first two volumes were discarded from data analysis to ensure magnetization reached steady state. The last volume was acquired with a 31 ms TE for the field map measurement and was also excluded from fMRI analysis.
A high-resolution T1-weighted structural image was also acquired using an MPRAGE sequence with parameters of TE=4.11 ms TR=2200 ms, flip angle=12°, 160 sagittal slices, slice thickness=1 mm, slice gap=0.5 mm, FOV=256 mm. The T1-weighted image was used in the data analysis for image registration purposes.
FMRI data preprocessing
All imaging data was preprocessed using the AFNI (Analysis of Functional NeuroImages (Ward 2000)) and FMRIB Software Libraries (FSL; FMRIB, Oxford, United Kingdom). Preprocessing consisted of: slice time correction; three-dimensional motion correction; temporal despiking; spatial smoothing (full-width at half maximum=6mm); mean-based intensity normalization; temporal band-pass filtering (0.009–0.1 Hz); and linear and quadratic detrending. Probabilistic independent component analysis (PICA) was conducted for each individual to denoise individual data by removing components that represented noise such as head motion (“rim-like” artifacts around the brain), scanner artifacts (e.g. slice dropouts, high-frequency noise, and field inhomogeneities), and physiological noise (e.g. respiration, cardiac frequencies, white matter or cerebrospinal fluid fluctuations). Noise components were selected by spatial and temporal characteristics detailed in the MELODIC (FSL) manual (http://fmrib.ox.ac.uk/fslcourse/lectures/melodic.pdf) and based on (RE Kelly, Jr. et al. 2010) for selection criteria of noise components. A between-groups t-test conducted to look for differences in the sum of total percent variance accounted for in components removed showed no significant group differences (t(1,44)=0.89, p=0.38).
Region of interest selection and seed generation
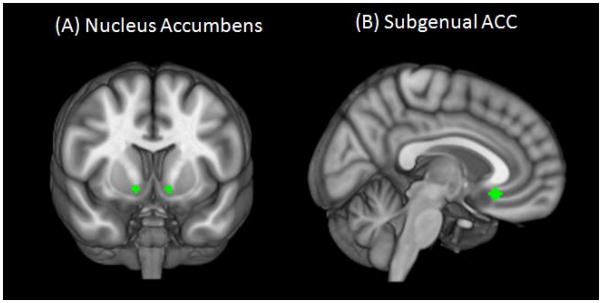
Two regions of interest (ROIs) were selected in order to examine RSS within the bottom-up and top-down networks. To examine RSS within the bottom-up network, a spherical seed with 3.5 mm radius was placed at left and right NAcc based on the Talaraich Daemon atlas from AFNI (Lancaster et al. 2000)(MNI coordinates: x=±12, y=10, z=−9)(Figure 1A). Left and right NAcc were first analyzed separately, but because results were highly similar (e.g. r=0.81, p=7.47E-12), left and right seeds were combined. To examine RSS within the top-down network, a spherical seed with 3.5 mm radius was placed at the sgACC region with the same coordinates as previously described in AM Kelly et al.(2009); MNI coordinates: x=5, y=25, z=−10)(Figure 1B).
Figure 1.
(A) Nucleus accumbens and (B) subgenual anterior cingulate cortex seeds used to examine strength of resting state synchrony overlaid on Montreal Neurological Institute brain in neurological orientation (right is right).
Resting state individual-level analysis
For each participant and for each seed (sgACC and NAcc), a multiple regression analysis (FSL-FEAT (Smith et al. 2004)) on the denoised data was performed. This analysis generated a map of statistical parameter estimates (PEs) for each voxel, for each individual, for each seed. All voxels in the PEs maps showed the degree of positive or negative correlations with the corresponding seed time-series for each seed for each participant.
Resting state group-level analysis
To compare RSS between groups, we first used a whole-brain approach to create statistical parametric maps of the z statistics (FSL-FLAME (Smith et al. 2004)). First-level group analyses were carried out by feeding the PEs maps of each individual into a mixed-effects model. Group-level analyses produced z-score maps showing between group differences in RSS for each seed at each voxel in the brain. To control for false positive findings, a threshold/cluster method derived from Monte Carlo simulations (AlphaSim, AFNI (Ward 2000)) was applied to all maps generated in the group-level analysis. Monte Carlo simulations (1000 iterations) accounted for the 6-mm full-width half-maximum Gaussian filter with a connectivity radius of 7.1mm. On the basis of these simulations, the family-wise ± of 0.05 was preserved with an a priori voxel-wise probability of 0.001 and three-dimensional clusters with a minimum volume of 1204 ±L (151 voxels). Resulting clustered and thresholded z-maps corrected for multiple comparisons showed regions with differences in RSS between the NSAC and LTAA groups (see Table 2).
Table 2.
Identification of anatomy, BA, hemispheric location (L/R), and coordinates (x,y,z) in MNI space for regions in which LTAA showed significantly different RSS (manifested as significantly different z-scores that survived thresholding [p<0.05 familywise and p<0.001 voxelwise] and clustering to correct for multiple comparisons, see methods section for specifics) than NSAC within (A) the NAcc map and (B) the subgenual ACC map after first-level group analysis. Regions in which LTAA showed higher strength of RSS are in italics, regions in which LTAA showed lower strength of RSS have normal font. Regions with asterisks (* or **) are regions that remained to be significantly different between groups after second-level group analysis (ANCOVA). F-value, significance and effect size presented show ANCOVA analysis results. BA, Brodmann Area; L, left; R, right; MNI, Montreal neurological institute; LTAA, long-term abstinent alcoholics; RSS, resting state synchrony; NSAC, non-substance abusing controls; NAcc, nucleus accumbens; ACC, anterior cingulate cortex; ANCOVA, analysis of covariance.
| (A) NAcc
| |||||||
|---|---|---|---|---|---|---|---|
| Anatomy of region | L/R | x | y | z | F | Significance | Effect size -partial eta2 |
| Dorsolateral prefrontal cortex, BA 10* | L | −27 | 61 | 26 | 5.66 | 0.022* | 0.13* |
| Superior frontal gyrus, BA 6 | R | 23 | 29 | 59 | 3.90 | 0.056 | 0.09 |
| Caudate* | L | −12 | −1 | 15 | 4.29 | 0.045* | 0.10 |
| R | 11 | 3 | 11 | ||||
| Anterior nucleus of thalamus** | L | −10 | −14 | 16 | 8.30 | 0.006* | 0.18** |
| R | 10 | 13 | 14 | ||||
| Medial dorsal thalamus* | L | −6 | −25 | 5 | 5.00 | 0.031 | 0.12* |
| R | 8 | −21 | 7 | ||||
| Postcentral gyrus, BA 2 | L | −46 | −30 | 44 | 2.84 | 0.100 | 0.07 |
| R | 46 | −31 | 40 | ||||
| Inferior parietal lobule, BA 40* | L | −36 | −54 | 59 | 4.45 | 0.041* | 0.11* |
| (B) Subgenual ACC
| |||||||
|---|---|---|---|---|---|---|---|
| Anatomy of region | L/R | x | y | z | F | Significance | Effect size -partial eta2 |
| Dorsolateral prefrontal cortex, BA 8** | R | 26 | 28 | 54 | 7.99 | 0.007** | 0.17** |
| Dorsolateral prefrontal cortex, BA 46** | R | 52 | 34 | 20 | |||
| Caudate | L | −12 | 8 | 12 | 3.01 | 0.091 | 0.07 |
| R | 12 | 4 | 11 | ||||
| Medial dorsal thalamus** | L | −12 | 2 | 12 | 8.44 | 0.006** | 0.18** |
| R | 6 | −2 | 10 | ||||
p<0.05,
p<0.01; Regions in which z-scores remained to be significantly different between groups after second level analysis (ANCOVA).
A second level analysis was performed to investigate whether demographic and symptom variables found to be significantly different between groups such as history of nicotine use, years of education, lifetime externalizing, anxiety and mood symptom counts and current externalizing symptom counts (Table 1A) had an effect on RSS differences found between groups. Mean z-scores from regions where significant differences were found between groups identified above (Table 2) were extracted for each subject. An ANCOVA was conducted with mean z-scores (representing strength of RSS) within these regions as dependent variables, demographic and symptom variables found to be significantly different between groups as covariates, and group membership as the fixed factor. Results focused on regions that still showed significant group differences at p<0.05.
Behavioral task
Because LTAAs have successfully maintained abstinence for long periods of time, an outcome that requires inhibiting the habit of alcohol consumption as well as learning new behaviors to cope with craving, it is important to assess cognitive flexibility in LTAAs. In a separate session before the scan, participants completed an Intradimensional/extradimensional set shift task (IED, (Cambridge Cognition 2006)). IED performance requires cognitive flexibility an important behavioral construct in LTAAs’ social behavior. IED assesses an individual’s ability to change a learned behavior when response contingencies change. IED measures the ability to a) learn associations between a stimulus and response and b) switch to new association after stimulus contingencies are reversed, which requires inhibiting a previously learned stimulus-response association.
Because fMRI studies that examined set shifting performance have found increased DLPFC activity related to cognitive flexibility (Freyer et al. 2009; Ghahremani et al. 2010; Remijnse et al. 2005), clusters involving DLPFC in which LTAA showed significant RSS differences when compared to NSAC were used for correlation analyses.
Correlates of RSS
Partial correlations were conducted for both groups separately to examine the association between DLPFC’s RSS strength within both the reward and executive control networks and IED task performance. Partial correlations controlled for variables that showed significant group differences in Table 1A (history of nicotine use, years of education, lifetime externalizing, anxiety and mood symptom counts and current externalizing symptom counts).
We focused the correlation analysis on DLPFC for the following reasons: (1) DLPFC is known to mediate a variety of executive control aspects such as inhibition, control of emotion, delaying of reward, and working memory, (Aron and Paulus 2007; MacDonald et al. 2000), all aspects supporting long-term abstinence, (2) we found group differences in DLPFC’s RSS synchrony within both the reward and executive control networks (Figures 4 and 6) and wished to explore how these differences could be related to task performance; and (3) IED task measures set shifting or cognitive flexibility, an individual’s ability to adapt and change previously learned behavior, this specific aspect of executive control has been consistently found to be mediated by DLPFC (Cole et al. 2010; Provost et al. 2011; Ravizza and Carter 2008).
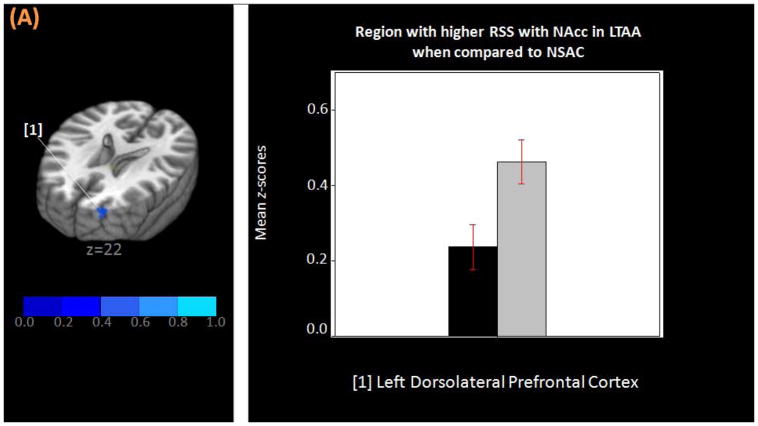
Figure 4.
(A) Three-dimensional MNI brain in neurological orientation with slices cut at z=22 showing region (in blue) in which LTAA have higher strength of resting state synchrony than NSAC in the NAcc network. (B) Bar graph showing higher strength of resting state synchrony between NAcc and left DLPFC in LTAA (gray bars) when compared to NSAC (black bars). MNI, Montreal neurological institute; LTAA, long-term abstinent alcoholics; NSAC, non-substance abusing controls; NAcc, nucleus accumbens; DLPFC, dorsolateral prefrontal cortex.
Figure 6.
(A) Three-dimensional MNI brain in neurological orientation with slices cut at z=21 and z=43 showing regions (in blue) in which LTAA have higher strength of resting state synchrony than NSAC in the subgenual ACC network. (B) Bar graph showing higher strength of resting state synchrony between subgenual ACC and right DLPFC in LTAA (gray bars) when compared to NSAC (black bars). MNI, Montreal neurological institute; LTAA, long-term abstinent alcoholics; NSAC, non-substance abusing controls; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex.
For clusters within the DLPFC RSS strength consisted of extracted mean Z scores from voxels defined by the DLPFC clusters that showed significant group differences in RSS within the reward and executive control networks separately. Values were extracted from each individual subject’s reward and executive control network maps.
Similar partial correlations were computed within the LTAA group only, to examine the association between strength RSS involving DLPFC and length of abstinence, while controlling for variables that showed significant group differences in Table 1A.
RESULTS
RSS in the reward network
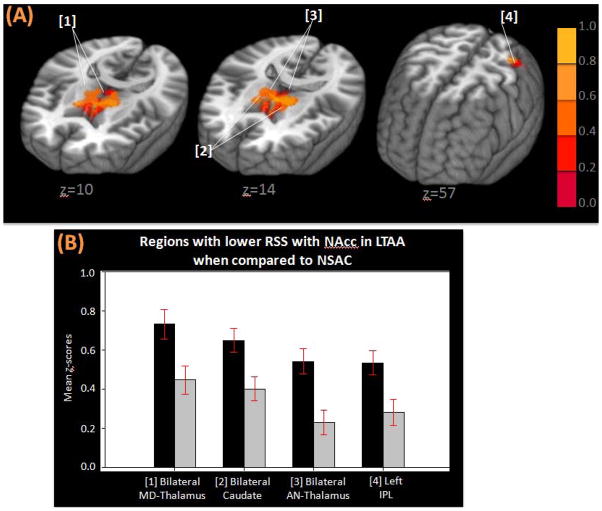
First-level group analysis (FSL-FLAME) results showed that LTAA had lower RSS than NSAC between the bilateral NAcc seeds and medial dorsal thalamus, bilateral caudate, the anterior nucleus of the thalamus, bilateral postcentral gyri and left inferior parietal lobule. Lower RSS in LTAA means that LTAA, when compared to NSAC, had lower z-scores representing correlations (temporal synchrony) of BOLD signal fluctuations between NAcc and regions mentioned above. LTAA had higher RSS than NSAC between bilateral NAcc seeds and the left DLPFC and the right superior frontal gyrus (SFG).
Second-level group analysis (ANCOVA) results showed that, after controlling for covariates, LTAA continued to exhibit lower RSS than NSAC between the NAcc seed and bilateral caudate, the anterior nucleus of the thalamus, medial dorsal thalamus, and left inferior parietal lobule (Figure 3 and Table 2A). Similarly, after controlling for covariates, LTAA exhibited higher RSS than NSAC between the NAcc seed and left DLPFC (Figure 4 and Table 2B).
Figure 3.
(A) Three-dimensional MNI brain in neurological orientation with slices cut at z=10, z=14, z=57 showing regions (in orange) in which LTAA have lower strength of resting state synchrony than NSAC in the NAcc network. (B) Bar graphs showing lower strength of resting state synchrony with NAcc LTAA (gray bars) when compared to NSAC (black bars). MNI, Montreal neurological institute; LTAA, long-term abstinent alcoholics; NSAC, non-substance abusing controls; NAcc, nucleus accumbens; MD-thalamus, medial dorsal thalamus; AN-thalamus, anterior nucleus of the thalamus; IPL, inferior parietal lobule
RSS differences in the executive control network
First-level group analysis (FSL-FLAME) results showed that LTAA had lower RSS than NSAC between the subgenual anterior cingulate cortex (sgACC) seed and bilateral caudate and bilateral anterior nucleus of thalamus. LTAA had higher RSS than NSAC between the ACC seed and two clusters within the right dorsolateral prefrontal cortex (DLPFC; Brodmann areas 8 and 46).
Second-level group analysis (ANCOVA) results showed that, after controlling for covariates, LTAA still had lower RSS strength than NSAC between the sgACC seed and bilateral anterior nucleus of the thalamus (Figure 5 and Table 2B). ANCOVA results also showed that, after controlling for covariates, LTAA still had higher RSS strength than NSAC between the sgACC seed and right DLPFC (Figure 6 and Table 2B).
Figure 5.
(A) Three-dimensional MNI brain in neurological orientation with slice cut at z=12 showing region (in orange) in which LTAA have lower strength of resting state synchrony than NSAC in the subgenual ACC network. (B) Bar graph showing lower strength of resting state synchrony between subgenual ACC and the anterior nucleus of the thalamus in LTAA (gray bars) when compared to NSAC (black bars). MNI, Montreal neurological institute; LTAA, long-term abstinent alcoholics; NSAC, non-substance abusing controls; ACC, anterior cingulate cortex; AN-thalamus, anterior nucleus of the thalamus.
IED task performance
There were no group differences in any of the metrics measured by the IED task (Table 3).
Table 3.
Independent sample t-test results showing no group difference in Intradimensional/Extradimensional Set Shift task performance.
| Behavioral Measure | NSAC (n = 23)
|
LTAA (n = 23)
|
t-value | p-value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Total number of Blocks completed successfully | 0.07 | 0.67 | −0.16 | 0.95 | 0.94 | 0.35 |
| Total number of trials completed on all attempted Blocks | −0.46 | 1.07 | −0.41 | 0.90 | −0.16 | 0.87 |
| Adjusted total number of errors | 0.05 | 0.60 | −0.11 | 0.84 | 0.73 | 0.47 |
Correlates of RSS
Partial correlations results (controlling for history of nicotine use, years of education, lifetime externalizing, anxiety and mood symptom counts and current externalizing symptom counts) in the LTAA group showed a significant positive correlation between total number of IED trials completed in all blocks (higher number of trials means poorer cognitive control) and RSS strength (higher z-score means stronger RSS) between NAcc and DLPFC (r=0.58, p=0.014) (significance survived Bonferroni correction for multiple comparisons, p-value needed to be < 0.017 = 0.05/3 tests) (Figure 7A). NSAC did not show any significant correlations between IED performance and RSS strength.
Figure 7.
Partial regression plots showing relationship between strength of resting state synchrony in LTAA and (A) number of trials needed to switch sets during intradimensional/extradimensional set-shift task performance and (B) length of abstinence in days. Values in both regression plots are the residuals after both variables presented (in the x and y axes) have been adjusted for all other variables in the model (history of nicotine use, years of education, lifetime externalizing, anxiety and mood symptom counts and current externalizing symptom). Individual LTAA participants are represented by circles. LTAA, long-term abstinent alcoholics; NAcc, nucleus accumbens; MD-Thalamus, medial dorsal thalamus; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex.
Partial correlation results (controlling for the same variables as above) in the LTAA group showed a negative correlation between duration of abstinence (number of days) and RSS strength between sgACC and DLPFC (r=−0.51, p=0.04, uncorrected; Figure 7B).
DISCUSSION
The aim of the present study was to investigate RSS patterns in the reward and executive control networks in LTAA compared to NSAC. We found group differences in RSS within these networks which were related in LTAA to cognitive flexibility and length of abstinence. LTAA RSS differed from NSAC with (1) lower RSS with appetitive drive regions and (2) higher RSS with executive control regions. Measures of these RSS differences between LTAA and NSAC were associated with cognitive flexibility and length of abstinence in LTAA. Because LTAA showed normal cognitive flexibility outside the scanner, and because this measurable behavior was correlated with the RSS measures, we interpret the RSS differences found in LTAA as being consistent with a compensatory mechanism in LTAA which supports the behavioral adaptations needed to achieve and maintain abstinence.
Reduced RSS of reward processing regions in LTAA when compared to NSAC
Within the bottom-up reward network, LTAA compared to NSAC showed lower RSS between the NAcc seed and bilateral caudate and thalamus (Figure 3). These regions have specific but related roles in reward-processing. The thalamus relays information regarding anticipation of reward and orienting attention toward rewards (Roiser et al. 2010) by receiving afferent inputs from NAcc and sending efferent projections to prefrontal cortex (Alexander et al. 1986). A recent study, by Vollstädt-Klein and colleagues (2010) found that when participants are exposed to alcohol related cues, heavy social drinkers without alcohol dependence recruited NAcc while alcohol dependent individuals recruited caudate to process alcohol cues. A simplified but illustrative view of the substance dependence process is that NAcc first mediates the establishment and definition of a reward, thalamus relays this information to cortex to orient attention and goal-directed behavior towards the reward, and, if the reward is processed numerous times, the caudate mediates habit formation and maintenance (Everitt and Robbins 2005; Vollstädt-Klein et al. 2010). Hence, the lower RSS between NAcc and both thalamus and caudate found in LTAA may reflect an ongoing compensatory predisposition for not facilitating synchronization of brain activity in regions known to be involved in reward processing. An alternative explanation to reduced RSS found in LTAA is that chronic alcohol consumption may have caused a decrease in neuronal density in nucleus accumbens (Lebedev et al. 2008) affecting communication with other regions in the reward network (i.e. thalamus and caudate). This alternative needs to be further explored in future volumetric studies.
Enhanced RSS of executive control regions in LTAA
Within the bottom-up reward network, RSS between NAcc seed and dorsolateral prefrontal cortex (DLPFC) was increased in LTAA compared to controls (Figure 4). While NAcc has been associated with impulsive behavior towards reward (McClure et al. 2004), DLPFC has been associated with executive control of behavior (Delgado et al. 2008; Hare et al. 2009; McClure et al. 2004). It has been hypothesized that the interaction between these two regions is associated with a subject’s choices and in the initiation of motivated behavior (Ballard et al. 2011; McClure et al. 2004), such that DLPFC dominance results in inhibition and NAcc dominance results in impulsive behavior. While LTAA in the present study did not show significant differences in executive control of behavior during task performance compared to NSAC, our findings of RSS differences may reflect a compensatory mechanism in which LTAA have an ongoing increased level of DLPFC-NAcc synchrony (during rest) in an attempt to increase executive control (inhibition) to maintain normal (i.e., non-addictive) behavior.
Within the top-down executive control network, there was higher RSS between the sgACC seed and DLPFC in LTAA compared to NSAC (Figure 6). A study by Hare et al. (2009) examining neural substrates of emotion regulation in dieters found that dieters who were rated as having more self-control had increased coherence between sgACC and DLFPC (when choosing healthy over unhealthy food). Authors suggested that emotional regulation is mediated by increased involvement of frontal regions within the top-down executive control network (Hare et al. 2009). This hypothesis is related to the present findings of an association between length of abstinence and RSS strength between sgACC and DLPFC (Figure 7B). LTAAs with a shorter length of abstinence had higher RSS strength between sgACC-DLPFC. Individuals with shorter abstinence are more vulnerable to relapse than individuals with longer abstinence. Individuals with shorter abstinence may need more constant emotional regulation (reflected here by increased RSS between sgACC and DLPFC) to successfully manage emotional situations and avoid relapse. On the other hand, individuals with longer abstinence, who are in lower risk for relapse, may have a lower need for regulating emotion, hence the lower RSS between sgACC and DLPFC.
There are important limitations of the present study. First, because the current study is cross sectional, it does not demonstrate changes in RSS during abstinence. In this regard, cross-sectional studies comparing active alcoholics to controls may reflect differences between groups in predisposing factors as well as the effects of active alcoholism. Carefully designed longitudinal studies are essential to determining the role of RSS in alcohol dependence and its treatment. Second, because task performance was not measured in the scanner, a direct relationship between IED task performance and brain activity cannot be established based on the present study’s data. Given present and previous correlation results (Mennes et al. 2010; Zhu et al. 2011), however, there is evidence that synchrony of neural networks during rest is related to measurable behavior. Third, given previous reports from volumetric studies, future directions should include an investigation on brain regional volume differences within areas that showed significant RSS differences between groups in the present study. Finally, there is evidence that exposure to stress increases the chance of relapse (Sinha 2007). Because LTAA in the current study have successfully maintained abstinence, further research to examine whether the resting state differences presented here are associated with better responses to stressful situations is warranted.
In conclusion, we believe that present results are consistent with an interpretation of an ongoing compensatory mechanism in LTAA during rest. Within circuits involved in appetitive drive and inhibitory control, LTAA with multi-year abstinence showed a) an attenuated synchrony of resting state fluctuations with striatal regions that mediate appetitive drive and b) an accentuated synchrony of resting state fluctuations with frontal regions that mediate executive control. These RSS measures were related to cognitive flexibility and length of abstinence. We believe these findings reflect adaptive mechanisms that support the long-term abstinent alcoholic being ready to successfully inhibit or stop behaviors that may lead to substance use. Current results are line with research that highlights the key role of executive control networks that mediate impulsive drive in addiction.
Figure 2.
One-sample t-test within LTAA and NSAC within the (A) nucleus accumbens and the (B) subgenual anterior cingulate networks. Maps have been clustered and thresholded with a method derived from Monte Carlo simulations (1000 iterations, AlphaSim, AFNI) accounted for full-width half-maximum Gaussian filter with a connectivity radius of 7.1 mm (family-wise of 0.05 as preserved with an a priori voxel-wise probability of 0.001 and three dimensional clusters with a minimum volume of 6848 μL (856 voxels). Each colored voxel shows standardized z-scores representing the correlation between the voxel and the corresponding seed timeseries.
Acknowledgments
This work was supported by the National Institutes for Health Grants #5R01AA016944 and #5R01AA013659.
References
- Akine Y, Kato M, Muramatsu T, Umeda S, Mimura M, Asai Y, Tanada S, Obata T, Ikehira H, Kashima H, Suhara T. Altered brain activation by a false recognition task in young abstinent patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1589–1597. doi: 10.1111/j.1530-0277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. (dsm-iv) [Google Scholar]
- Aron JL, Paulus MP. Location, location: Using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102(Suppl 1):33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31:10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS. Simultaneous assessment of flow and bold signals in resting-state functional connectivity maps. NMR in Biomedicine. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition. Cantabeclipse: Test administration guide. Version 3.0.0. Cambridge, U.K: 2006. [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bagic A, Kass R, Schneider W. Prefrontal dynamics underlying rapid instructed task learning reverse with practice. J Neurosci. 2010;30:14245–14254. doi: 10.1523/JNEUROSCI.1662-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Alcohol Clin Exp Res. 2008;33:388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Freyer T, Valerius G, Kuelz AK, Speck O, Glauche V, Hull M, Voderholzer U. Test-retest reliability of event-related functional mri in a probabilistic reversal learning task. Psychiatry Res. 2009;174:40–46. doi: 10.1016/j.pscychresns.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cerebral Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out bold fmri for increased snr and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmpfc valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for fourier inversion using gridding [computerised tomography application] Medical Imaging, IEEE Transactions on. 1991;10:473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a go/no-go task. Biol Psychol. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: Defining a procedure for artifact removal from fmri data. J Neurosci Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AA, Droblenkov AV, Shabanov PD. Structural changes in mesocorticolimbic dopaminergic system of the brain during long-term alcoholization in rats. Bull Exp Biol Med. 2008;146:816–819. doi: 10.1007/s10517-009-0414-5. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Blouin AG, Navarro JR, Hill J. Validity of the computerized dis for diagnosing psychiatric inpatients. Can J Psychiatry. 1991;36:728–731. [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. “ Loss of control” In alcoholism and drug addiction: A neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced bold activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal bold activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in” Bottom-up” And” Top-down” Neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Noll DC, Meyer CH, Pauly JM, Nishimura DG, Macovski A. A homogeneity correction method for magnetic resonance imaging with time-varying gradients. IEEE Trans Med Imaging. 1991;10:629–637. doi: 10.1109/42.108599. [DOI] [PubMed] [Google Scholar]
- Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral k-space mr imaging of cortical activation. J Magn Reson Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn S, Park JE, Kim SH, Yu IK, Sohn JH. Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scand J Psychol. 2011;52:1–7. doi: 10.1111/j.1467-9450.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Provost JS, Petrides M, Simard F, Monchi O. Investigating the long-lasting residual effect of a set shift on frontostriatal activity. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr358. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. Shifting set about task switching: Behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 2008;46:2924–2935. doi: 10.1016/j.neuropsychologia.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: An event-related fmri study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Stephan KE, den Ouden HE, Friston KJ, Joyce EM. Adaptive and aberrant reward prediction signals in the human brain. Neuroimage. 2010;50:657–664. doi: 10.1016/j.neuroimage.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the mast. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural mr image analysis and implementation as fsl. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Hermann D, Rabinstein J, Wichert S, Klein O, Ende G, Mann K. Increased activation of the acc during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res. 2010;34:771–776. doi: 10.1111/j.1530-0277.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fmri data. Alcohol Clin Exp Res 2000 [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, Luo YL, Dilks DD, Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J Neurosci. 2011;31:10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]