Abstract
Background
Formaldehyde is used in many occupational settings, most notably in manufacturing, health care, and embalming. Formaldehyde has been classified as a human carcinogen, but its mechanism of action remains uncertain.
Methods
We carried out a cross-sectional study of 43 formaldehyde exposed-workers and 51 unexposed age and sex-matched controls in Guangdong, China to study formaldehyde’s early biologic effects. To follow-up our previous report that the total lymphocyte count was decreased in formaldehyde-exposed workers compared to controls, we evaluated each major lymphocyte subset (i.e., CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and B cells) and T cell lymphocyte subset (CD4+ naïve and memory T cells, CD8+ naïve and memory T cells, and regulatory T cells). Linear regression of each subset was used to test for differences between exposed workers and controls, adjusting for potential confounders.
Results
Total NK cell and T cell counts were about 24% (p=0.037) and 16% (p=0.0042) lower, respectively, among exposed workers. Among certain T cell subsets, decreased counts among exposed workers were observed for CD8+ T cells (p=0.026), CD8+ effector memory T cells (p=0.018), and regulatory T cells (CD4+FoxP3+: p=0.04; CD25+FoxP3+: p=0.008).
Conclusions
Formaldehyde exposed-workers experienced decreased counts of NK cells, regulatory T cells, and CD8+ effector memory T cells; however, due to the small sample size these findings need to be confirmed in larger studies.
Keywords: formaldehyde, NK cell, B cell, T cell, T cell subset
Introduction
Formaldehyde is a colorless, flammable chemical that is used in building materials, such as particleboard, plywood, glues, adhesives, and many other household products. Formaldehyde is also commonly used as an industrial fungicide, germicide, and disinfectant, and as a preservative in mortuaries and medical laboratories (ATSDR 1999).
Given formaldehyde’s widespread use, there has been substantial interest in adverse health outcomes that might be associated with this ubiquitous exposure. Formaldehyde was classified as a nasopharyngeal carcinogen by the International Agency for Research on Cancer (IARC) in 2006 (IARC Monographs 2006) and as a leukemogen in 2009 (Baan et al. 2009); however, the classification as a leukemogen is controversial because of disagreement regarding mechanisms through which formaldehyde might affect leukemia.
We have carried out a cross-sectional study to evaluate the biologic effects associated with occupational exposure to formaldehyde in Guangdong, China (Zhang et al. 2010). We previously reported that the total lymphocyte count was decreased among the formaldehyde-exposed workers compared to controls (Zhang et al. 2010). Similar to our findings, other investigators have also found suggestions that solvents and other occupational exposures may alter lymphocyte counts (Biro et al. 2002; Tompa et al 2007). To further explore the effects that formaldehyde exposure may potentially exert on lymphocyte subsets, we evaluated the major lymphocyte subsets (natural killer (NK) cells, B cells, T cells) and T cell lymphocyte subsets including CD4+ naïve and memory T cells, CD8+ naïve and memory T cells, and regulatory T cells among formaldehyde exposed and unexposed workers.
Methods
This study has been previously described (Zhang et al. 2010). As such, this study utilizes the same biological samples collected from the same subjects reported by Zhang et al. (2010). Briefly, a cross-sectional study of 43 workers currently exposed to formaldehyde in a factory that produced formaldehyde-melamine resins and a factory that used formaldehyde-melamine resins to manufacture plastic utensils were enrolled in June and July of 2006. A control population was selected from three workplaces in the same geographic region as factories with formaldehyde exposure and enrolled workers who had comparable demographic and socioeconomic characteristics and who were engaged primarily in manufacturing. Based on a detailed inspection, control workplaces did not have occupational exposures to formaldehyde or any other hematotoxic or genotoxic chemicals in excess of exposure levels in the general population. Unexposed controls (n = 51) were frequency-matched to cases by sex and age (±5 years) and enrolled from three workplaces in the same geographic region as factories with formaldehyde exposure. For all potential exposed workers and unexposed controls, subjects were excluded if they had a history of cancer, chemotherapy, radiotherapy, or previous occupations with notable exposure to benzene, butadiene, styrene and/or ionizing radiation. The participation rates for exposed workers and controls were 92% and 95%, respectively. The study was approved by Institutional Review Boards at the U.S. National Cancer Institute and the Guangdong National Poison Control Center in China. Participation was voluntary and all subjects gave written informed consent.
Full-shift formaldehyde exposure was monitored in the factories with UMEx 100 diffusion samplers. Personal exposure to several other organic compounds was measured for the formaldehyde-exposed workers by 3M organic vapor monitors. Organic vapor monitors were analyzed for chloroform, methylene chloride, tetrachloroethylene, trichloroethylene, and benzene. No hydrocarbons were detected in any of these samples. A questionnaire-based interview, assessing demographics, lifestyle characteristics, and occupational history, was administered to all subjects.
Major lymphocyte subsets (i.e., CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and B cells) were analyzed on the same day that the peripheral blood sample was collected (Sallusto et al. 1999). For the T lymphocyte subsets, peripheral white blood cells were preincubated with Fc block for 15 min at 4°C and immunostained with Abs recognizing the following antibody panel: T cell subsets-CD3 (UCHT1)-FITC, CD4 (RPA-T4 or SK3)-PE, CD8 (SK1)-PerCP-Cy5.5, CD45 (HI30)-APC; Naïve verses memory CD4+ T cells-CD45RA (HI100)-FITC, CCR7- PE (150503, R&D systems), CD4 (RPA-T4 or SK3)-PerCP-Cy5.5, CD62L (Dreg 56)-APC; Naïve verses memory CD8+ T cells-CD45RA (HI100)-FITC, CCR7-PE (150503, R&D systems), CD8 (SK1)-PerCP-Cy5.5, CD62L (Dreg 56)-APC; B cells-CD80 (L307.4)-FITC, CD86 (2331)-PE, CD19 (HIB19)-APC; NK/NKT cells-CD16 (3G8)-FITC, CD56 (B159)-PE, CD3 (SK7)-PerCP-Cy5.5, CD45 (HI30)-APC. Mouse IgG1 (MOPC -21) was served as an isotype control. All the antibodies are purchased from BD Biosciences unless otherwise specified. FCM data were acquired using a flow cytometer BD FACSCaliburTM. For intracellular FoxP3 staining, peripheral white blood cells were first surface-stained with the following fluorescent Abs against CD4 (RPA-T4)-FITC and CD25 (M-A251)-PE. The cells were then fixed, permeabilized and stained with anti-FoxP3 antibody using the Anti-Human Foxp3-APC Staining Set kit according to the manufacturer’s instructions (eBiosciences). Cells were then acquired and analyzed by FCM as described above. Lymphocyte gate was wet based on the forward and side scatter. Measurements from blinded quality control replicates interspersed among the samples did not identify outlier batches (i.e. batch mean being one standard deviation greater than the other batch means). Assay intraclass correlation coefficients were > 89% for each lymphocyte subset.
Unadjusted means and standard deviations (cells/μl) were determined for each cell count subset. Linear regression using the natural logarithm (ln) of each subset was used to test for differences between unexposed and exposed workers. All statistical models were adjusted for age (as a continuous variable) and sex. Potential confounders, including current cigarette smoking status (yes/no), current alcohol consumption (yes/no), recent infections (flu or respiratory infections in the previous month), and body mass index (BMI), were also included in a model for a specific subset if the regression coefficient was altered by 15% or more. Models adjusting for only age, sex, and recent infections did not differ from the most parsimonious model of potential confounders. The total lymphocyte percent from the CBC was used to calculate lymphocyte subsets, and an additional calculation was carried out using the lymphocyte percent obtained by flow cytometry.
Results
Controls and workers exposed to formaldehyde were comparable with regard to age, gender, and other characteristics described in (Table 1).
Table 1.
Demographic characteristics and formaldehyde exposure level
| Controls (n=51) | Formaldehyde exposed workers (n=43) | |
|---|---|---|
|
|
||
| Age, mean (SD) | 30 (7) | 31 (6) |
| BMI, mean (SD) | 22 (3) | 21 (3) |
| Sex | ||
| Female n (%) | 7 (14) | 6 (14) |
| Male n (%) | 44 (86) | 37 (86) |
| Current Smoking | ||
| No n (%) | 28 (55) | 25 (58) |
| Yes n (%) | 23 (45) | 18 (42) |
| Current Alcohol Drinking | ||
| No n (%) | 30 (59) | 32 (74) |
| Yes n (%) | 21 (41) | 11 (26) |
| Infection | ||
| No n (%) | 36 (71) | 26 (60) |
| Yes n (%) | 15 (29) | 17 (40) |
| Formaldehyde Exposure (ppm) | ||
| Mean (10th, 90th percentile) | <0.03 | 1.28 (0.63, 2.51) |
We compared the lymphocyte and lymphocyte subset counts (mean ± standard deviation) among formaldehyde-exposed workers and unexposed controls. We previously reported that lymphocyte cell counts were about 19% lower in workers exposed to formaldehyde (1792 ± 452) compared to controls (2206 ± 589) (Figure 1) (Zhang et al. 2010).
Figure 1.
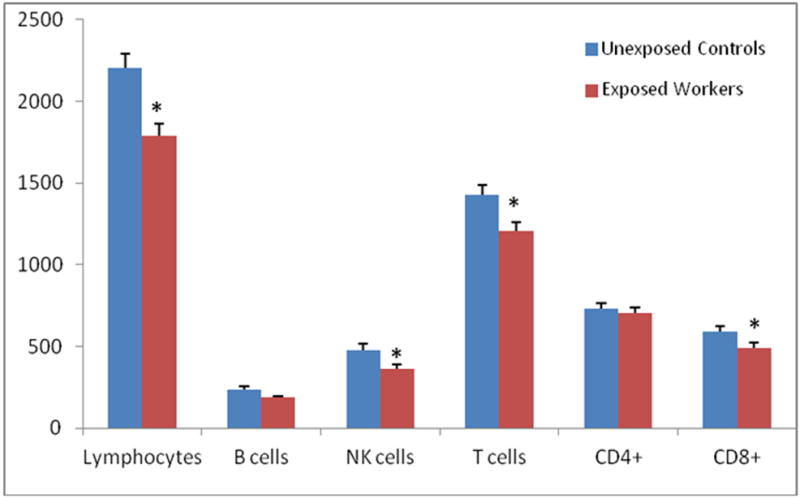
Lymphocyte and major lymphocyte subset counts (mean ± standard error) among formaldehyde exposed workers and unexposed controls†.
†Results for total lymphocyte counts previously reported in (Zhang L, 2010). Lymphocytes and lymphocyte subset counts in relation to formaldehyde exposure. Differences in cell counts were tested by linear regression analysis of ln-transformed end point, adjusting for relevant covariates as described in the methods section; *P < 0.05.
We then evaluated cell counts of the major lymphocyte subsets including NK cells (CD3-CD16+CD56+), B cells (CD19+) and T cells (CD3+). In general, all major lymphocyte subsets were decreased among exposed workers compared to controls (Figure 1). Workers exposed to formaldehyde had on average about a 24% decrease in NK cell counts (3650 ± 1570) compared to controls (4790 ± 2650) (p = 0.037) (Figure 1). We also observed decreased T cell counts among exposed workers (1203 ± 353) compared to controls (1429 ± 421) (p = 0.0042). B cell counts did not differ between exposed workers and controls.
We analyzed CD4+ and CD8+ T cells counts, and their respective subsets, to explore the associations between formaldehyde exposure and specific T cell lymphocyte subpopulations. Differences were not observed between exposed workers and controls for CD4+ T cells, CD4+ naïve T cells (CD45RA+CCR7+) and CD4+ central memory T cells (CD45RA-CCR7+) (Figures 1 and 2). Total CD8+ cell counts were decreased among exposed (488 ± 217) compared to controls (591 ± 254) (Figure 1), with the CD8+ effector memory subset (CD45RA-CCR7-) being specifically affected (Figure 2; Table 2). Intriguingly, regulatory T cells (CD4+FoxP3+ or CD25+FoxP3+) were also significantly decreased in exposed workers by about 20% compared to controls (Figure 2; Table 2).
Figure 2.
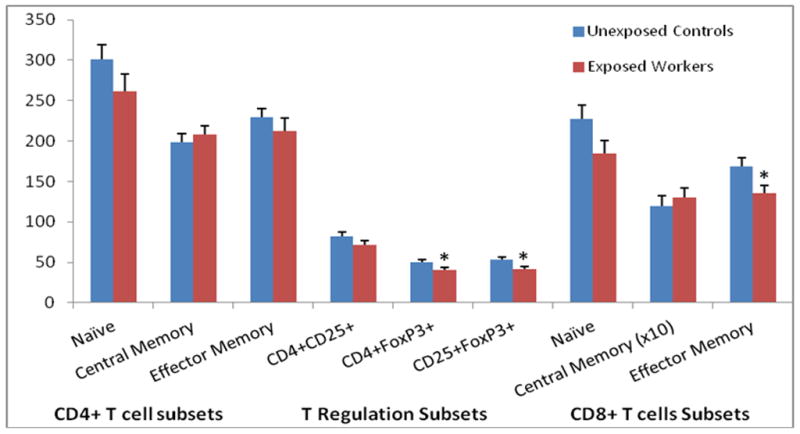
T cell subset counts (mean ± standard error) among formaldehyde exposed workers and unexposed controls†.
† T cell lymphocyte subset counts in relation to formaldehyde exposure. Differences in cell counts were tested by linear regression analysis of l n-transformed end point, adjusting for relevant covariates as described in the methods section; *P < 0.05.
Table 2.
Lymphocyte subset cell counts [mean ± standard deviation (std)] among formaldehyde (FA) exposed workers and unexposed controls.
| Controls† | FA exposed Workers† | P value†† | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | (std) | n | mean | (std) | ||
|
|
|||||||
| CD3+CD4+ | |||||||
| CD4 naïve (CD45RA+CCR7+) | 41 | 301 | 131 | 32 | 261 | 140 | 0.14‡‡ |
| CD4 memory (CD45RA−) | |||||||
| Central Memory (CD45RA-CCR7+) | 41 | 198 | 80 | 32 | 208 | 74 | 0.88¥¥ |
| Effector Memory (CD45RA-CCR7−) | 41 | 229 | 82 | 32 | 212 | 105 | 0.09 |
| T Regulation subset | |||||||
| CD4+CD25+ | 51 | 82 | 38 | 43 | 72 | 33 | 0.16 |
| CD4+FoxP3+ | 51 | 50 | 22 | 43 | 41 | 18 | 0.04 |
| CD25+FoxP3+ | 51 | 53 | 23 | 43 | 42 | 19 | 0.008 |
| CD3+CD8+ | |||||||
| CD8 naïve (CD45RA+CCR7+) | 41 | 227 | 122 | 32 | 185 | 102 | 0.15¥ |
| CD8 memory (CD45RA−) | |||||||
| Central Memory (CD45RA-CCR7+) | 41 | 12 | 9 | 32 | 13 | 8 | 0.74¥¥ |
| Effector Memory (CD45RA-CCR7−) | 41 | 169 | 76 | 32 | 135 | 64 | 0.018 |
unadjusted mean (±SD) cells/μl blood;
P value compares exposed workers to controls, adjusted for age and sex;
adjusted for age, sex, and smoking;
adjusted for age, sex, and BMI;
adjusted for age, sex, smoking, infection, BMI, and alcohol use.
Discussion
This is the first epidemiological investigation of the impact occupational formaldehyde exposure has on all major lymphocyte subsets and certain T lymphocyte subsets. Our exploration of these lymphocyte subsets found that workers exposed to formaldehyde experienced decreased cell counts of NK cells, regulatory T cells, and CD8+ effector memory T cells.
The decreased T cell lymphocyte counts among subjects exposed to formaldehyde are consistent with a previous study conducted in China (Ye et al. 2005). For example, when evaluating the two major classes that T cells differentiate into, CD4+ and CD8+ T cells, our findings and the previous study both observed a decrease in CD8+ T cells but no change in CD4+ T cells (Ye et al. 2005). This present study expands on the previous study by evaluating CD4+ and CD8+ T cells subsets and finding that CD8+ effector memory T cells were decreased among formaldehyde-exposed workers compared to unexposed controls.
Given that T cells are critical for the response to immune-related challenges, our results suggest that formaldehyde exposure alters immune function. In response to antigenic stimulation, mature CD8+ naïve T cells undergo clonal expansion and differentiate into effector cells, which either undergo apoptosis or develop into effector-memory cells (Marrack and Kappler 2004; Stockinger et al. 2004). The resulting CD8+ effector memory T cells that lack CCR7 expression do not recirculate to the lymph nodes but instead circulate to inflamed tissues where the effector cells can react to antigens (Sallusto and Lanzavecchia 2000). Therefore, decreased CD8+ effector memory T cell counts may reflect a decreased capacity to respond to antigenic-related inflammation. Through this mechanism, the decrease in CD8+ effector memory T cells among exposed workers suggests that formaldehyde exposure may result in immunosuppression by reducing capacity for individuals to respond to antigens and antigenic-related inflammation.
The decreased counts we observed in regulatory T cell counts among exposed workers suggests that formaldehyde exposure may result in defective immunosuppression. Regulatory T cell subsets make-up about 5–10% of peripheral CD4+ T cells and are highly involved in the body’s immune suppression activities (Ke et al. 2008). Regulatory T cells influence the immune response by direct immunosuppressive functions, as well as indirect regulation of components of the adaptive immune system (i.e. CD4+ T cells, CD8+ T cells, NK cells) and the innate immune system cells (i.e. macrophages, dendritic cells, neutrophils) (Ke et al. 2008). These immunosuppressive functions culminate with regulatory T cells being involved in anti-tumor immunity and autoimmunity mechanisms (Horwitz et al. 2002; Akbari et al. 2003). A lack of regulatory T cells can result in the development of autoimmune disease in humans, such as immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX), autoimmune gastritis, autoimmune hepatitis, thyroiditis, diabetes and inflammatory bowel disease (Ochs et al. 2007; Brunkow et al. 2001; Wildin et al. 2002; Buckner 2010). Beyond autoimmune diseases, T regulatory cells have been found to play a role in lymphomas through the targeting and killing of B cell lymphoma cells and the suppression of anti-tumor T cell-mediated immune responses (Banham et al. 2006). Regulatory T cells have also been found to be involved in the antileukemia response (Hus et al. 2008).
NK cells play a key regulatory role in immune response and are a critical mediator of the anti-tumor response (Dunn et al. 2004). Specifically, NK cells protect against the effects from infectious agents. For example, in mice lacking NK cells, infections have been found to be more severe (Tupin et al. 2007). In humans, viral-associated cancers, such as nasopharyngeal cancer, and possibly lymphomas, caused by Epstein-Barr virus (EBV), are increased in immune suppressed individuals and hypothesized to be due to the subjects’ decreased ability to protect against infections because of the absence of lymphocytes, such as T cells and NK cells (Boshoff and Weiss 2002; Pattle and Farrell 2006). Regulatory T cells have also been mechanistically associated with nasopharyngeal carcinoma (Li et al. 2009). Further, the association between formaldehyde and nasopharyngeal carcinoma has been reported as more pronounced in EBV positive subjects (Hildesheim et al. 2001).
In conclusion, we found that only certain lymphocyte subsets were decreased in workers exposed to formaldehyde compared to controls. Declines in NK, regulatory T, CD8+ T and CD8+ effector memory T cells were associated with formaldehyde exposure in our study. These alterations, although modest, could potentially lead to subtle alterations in immune function and anti-tumor response. These results provide new insights into potential mechanisms of action for formaldehyde’s carcinogenicity; however, due to the small sample size, along with the limited exposure range, caution should be used when interpreting these findings until confirmed in larger studies that include subjects with a wider range of formaldehyde exposures to evaluate dose-response relationships, and that possibly evaluate the functional capacity of the subsets of interest.
Acknowledgments
Funding
This work was supported by intramural funds from the National Cancer Institute, and grants from the National Institute of Environmental Health Sciences (P42ES04705 and R01ES017452), and the Northern California Center for Occupational and Environmental Health, and the Department of Science and Technology of Guangdong Province, China (2007A050100004).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Reference List
- Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15:627–633. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agenct for Toxic SUbstances and Disease Registry) Toxicological profile for formaldehyde. US Department of Health and Human Services; 1999. [PubMed] [Google Scholar]
- Baan R, Grosse Y, Straif K, Secretan B, El GF, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part F: chemical agents and related occupations. Lancet Oncol. 2009;10:1143–1144. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- Banham A, Powrie F, Suri-Payer E. FOXP3+ regulatory T cells: Current controversies and future perspectives. European Journal of Immunology. 2006;36:2832–2836. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- Biró A, Pállinger E, Major J, Jakab MG, Klupp T, Falus A, Tompa A. Lymphocyte phenotype analysis and chromosome aberration frequency of workers occupationally exposed to styrene, benzene, polycyclic aromatic hydrocarbons or mixed solvents. Immunol Lett. 2002;81(2):133–40. doi: 10.1016/s0165-2478(01)00342-x. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Weiss R. Aids-related malignancies. Nat Rev Cancer. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The Three Es of Cancer Immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Hildesheim A, Dosemeci M, Chan CC, Chen CJ, Cheng YJ, Hsu MM, Chen IH, Mittl BF, Sun B, Levine PH, Chen JY, Brinton LA, Yang CS. Occupational Exposure to Wood, Formaldehyde, and Solvents and Risk of Nasopharyngeal Carcinoma. Cancer Epidemiology Biomarkers & Prevention. 2001;10:1145–1153. [PubMed] [Google Scholar]
- Horwitz DA, Gray JD, Zheng SG. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 2002;4:241–246. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus I, Schmitt M, Tabarkiewicz J, Radej S, Wojas K, Bojarska-Junak A, Schmitt A, Giannopoulos K, Dmoszynska A, Rolinski J. Vaccination of B-CLL patients with autologous dendritic cells can change the frequency of leukemia antigen-specific CD8+ T cells as well as CD4+CD25+FoxP3+ regulatory T cells toward an antileukemia response. Leukemia. 2008;22:1007–1017. doi: 10.1038/leu.2008.29. [DOI] [PubMed] [Google Scholar]
- Formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. 2006. IARC Monographs on the Evaluation of the Carcinogenic Risks to Human. [PMC free article] [PubMed] [Google Scholar]
- Ke X, Wang J, Li L, Chen IH, Wang H, Yang XF. Roles of CD4+CD25(high) FOXP3+ Tregs in lymphomas and tumors are complex. Front Biosci. 2008;13:3986–4001. doi: 10.2741/2986. [DOI] [PubMed] [Google Scholar]
- Li J, Qian CN, Zeng YX. Regulatory T cells and EBV associated malignancies. International Immunopharmacology. 2009;9:590–592. doi: 10.1016/j.intimp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T Cell Viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38:112–121. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- Pattle SB, Farrell PJ. The role of Epstein Barr virus in cancer. Expert Opin Biol Ther. 2006;6:1193–1205. doi: 10.1517/14712598.6.11.1193. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunological Reviews. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Kassiotis G, Bourgeois C. CD4 T-cell memory. Seminars in Immunology. 2004;16:295–303. doi: 10.1016/j.smim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Tompa A, Jakab MG, Biro A, Magyar B, Major J. Health, Genotoxicology, and Immune Status of Road Pavers in Hungary. Journal of Occupational and Environmental Hygiene. 2007;4:154–162. [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Micro. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. Journal of Medical Genetics. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Yan W, Xie H, Zhao M, Ying C. Cytogenetic analysis of nasal mucosa cells and lymphocytes from high-level long-term formaldehyde exposed workers and low-level short-term exposed waiters. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2005;588:22–27. doi: 10.1016/j.mrgentox.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tang X, Rothman N, Vermeulen R, Ji Z, Shen M, Qiu C, Guo W, Liu S, Reiss B, Freeman LB, Ge Y, Hubbard AE, Hua M, Blair A, Galvan N, Ruan X, Alter BP, Xin KX, Li S, Moore LE, Kim S, Xie Y, Hayes RB, Azuma M, Hauptmann M, Xiong J, Stewart P, Li L, Rappaport SM, Huang H, Fraumeni JF, Smith MT, Lan Q. Occupational Exposure to Formaldehyde, Hematotoxicity, and Leukemia-Specific Chromosome Changes in Cultured Myeloid Progenitor Cells. Cancer Epidemiology Biomarkers & Prevention. 2010;19:80–88. doi: 10.1158/1055-9965.EPI-09-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]


