Abstract
Background:
High intake of omega-6 polyunsaturated fatty acids (PUFA) has been associated with clinical progression in prostate cancer (CaP). This study investigates the signalling mechanism by which the omega-6 PUFA arachidonic acid (AA) induces prostatic cellular migration to bone marrow stroma.
Methods:
Western blot analysis of the PC-3, PC3-GFP, DU 145 and LNCaP cells or their lipid raft (LR) components post AA stimulation was conducted in association with assays for adhesion and invasion through the bone marrow endothelial monolayers.
Results:
Arachidonic acid increased transendothelial migration of PC3-GFP cells (adhesion 37%±0.08, P=0.0124; transmigration 270%±0.145, P=0.0008). Akt, Src and focal adhesion kinase (FAK) pathways were induced by AA and integrally involved in transendothelial migration. LR were critical in AA uptake and induced Akt activity. Ephrin receptor A2 (EphA2), localised in LR, is expressed in DU 145 and PC-3 cells. Arachidonic acid induced a rapid increase of EphA2 Akt-dependent/ligand-independent activation, while knockdown of the EphrinA1 ligand decreased AA induced transendothelial migration, with an associated decrease in Src and FAK activity. Arachidonic acid activated Akt in EphA2− LNCaP cells but failed to induce BMEC transendothelial invasion.
Conclusion:
Arachidonic acid induced stimulation of EphA2 in vitro is associated fundamentally with CaP epithelial migration across the endothelial barrier.
Keywords: Ephrin A2, omega-6, bone marrow endothelial cells, bone marrow stroma, prostate cancer, metastasis
Prostate cancer (CaP) has a predilection to metastasise to the bone marrow stroma (BMS) of the axial skeleton; this causes major morbidity and mortality (Loberg et al, 2005).
The clinical incidence of CaP, and its risk of progression to metastasis and death varies internationally, notwithstanding studies showing a universally high incidence of microscopic CaP in humans at a young age (Franks, 1954; Thompson et al, 2007). In western societies, clinical CaP is more prevalent compared with developed non-western countries such as Japan (Parkin et al, 2005) but the adoption of ‘western’ lifestyles in ‘non-western’ men has been associated with increased disease prevalence in migratory populations (Shimizu et al, 1991). This has led to the proposition that diet may influence biological aggression in this disease. Using human primary BMS, we have shown that malignant prostate epithelial cell (PEC) invasion towards BMS without adipocytes is significantly decreased. Prostate epithelial cells migrate towards bone marrow adipocytes and take up lipids from them. The omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid (AA) is of particular importance, inducing adoption of an aggressive, migratory phenotype (Brown et al, 2006; Gazi et al, 2007; Brown et al, 2010). Arachidonic acid intake has been associated with poorer CaP prognosis (Wynder et al, 1994), and increased risk of developing CaP of high grade (Chavarro et al, 2007; Szymanski et al, 2010). Intra-prostatic AA levels are decreased within cancer tissue compared with adjacent normal tissue, suggesting that AA is being metabolised preferentially by CaP cells into pro-tumoural eicosanoids (Schumacher et al, 2011). However, the mechanism by which malignant PEC detect, migrate towards and take up AA in the metastatic environment is still unknown.
The Eph receptor family is the largest of the tyrosine kinase receptor families found within LR. Downstream signalling from the Eph receptors affects control of cell morphology, adhesion, migration and invasion by cytoskeletal rearrangement (Pasquale, 2008). Ephrin receptor A2 (EphA2) is overexpressed in CaP compared with benign PEC and, in vitro, it is highly expressed in the bone metastatic cell line, PC-3, but not in the lymph node metastatic cell line, LNCaP (Walker-Daniels et al, 1999). Ephrin receptor A2 has both ligand-dependent and -independent activity. Studies have demonstrated phosphorylation of EphA2 S897 by Akt induced a ligand-independent activation of EphA2, which was associated with promotion of cell migration and invasion. Binding of the ephrin ligand to EphA2 lead to de-phosphorylation of S897 and negative regulation of ligand-independent EphA2 signalling (Miao et al, 2009).
Intracellular Akt signalling has been shown in preclinical and clinical studies to be associated with CaP metastasis. Akt-1 phosphorylation is associated with higher Gleason grade, advanced stage and poor prognosis (Ayala et al, 2004) and is a predictor of disease recurrence after primary treatment (Kreisberg et al, 2004). Additional to cell survival and growth, Akt activation regulates cellular migration (Kim et al, 2001) by activation and recycling of integrins (Li et al, 2005).
Alongside Akt, both the non-receptor tyrosine kinase Src and focal adhesion kinase (FAK) signalling pathways have been implicated in CaP progression. Src is overactive in castrate-resistant CaP (Tatarov et al, 2009) and is involved in multiple signalling pathways that are central to tumour development, including invasion, adhesion and survival (Summy and Gallick, 2003). Focal adhesion kinase is involved in adhesion-dependent cell signalling and has been implicated in mediating steps in cancer progression and metastasis in many human cancers, including CaP (Schaller, 2010).
Herein, we demonstrate that the omega-6 PUFA AA is able to increase malignant PEC adhesion to and invasion through bone marrow endothelial cells (BMEC) via an EphA2-mediated signalling pathway and that this process is linked closely to activation of key signalling pathways including Akt.
Materials and methods
Materials
All tissue culture, inhibitors and general reagents were purchased from Sigma-Aldrich (Poole, UK), except AA, purchased from MP Biomedicals (London, UK) and made up in methyl-β-cyclodextrin to produce a 10 mg ml−1 emulsion. Hygromycin B and foetal calf serum were obtained from PAA Laboratories (Yeovil, UK). Matrigel and 8 μm FluoroBlok cell culture inserts were from BD Biosciences (Oxford, UK).
Antibodies were purchased from Cell Signalling Technology (New England BioLabs (UK) Ltd, Hertfordshire, UK) Akt, P-Akts473, FAK, Src, P-Akt Substrate (RXXS/T) and Dynabeads protein G; from Invitrogen (Paisley, UK), P-Srcy418 and P-FAKy576; from Millipore (Watford, UK), EphA2 clone D7, P-Tyr 4G10, Actin clone C4 and recombinant mouse EphrinA1Fc Chimera; and from Sigma-Aldrich, SAB4500696 EFNA1
Ephrin receptor A2 and non-targeting siRNA (On-Target Plus Smartpool) were purchased from Dharmacon, Thermo Fisher Scientific (Abgene, Epsom, UK). Protease and phosphatase inhibitor cocktail were purchased from Roche (Sussex, UK).
Cell culture
All cell lines were verified by the Paterson Institute for Cancer Research tissue typing service and cultured as previously described (Hart et al, 2005; Brown et al, 2006). All PECs were serum starved in RPMI 1640, 0.1% BSA-FAF overnight before use. Bone marrow endothelial cells were cultured in long-term bone conditioned media, conditioned by BMS, on fibronectin (50 mg ml−1 in PBS) treated flasks.
Primary PEC culture
With informed consent prostatic tissue was obtained from male subjects undergoing trans-urethral resection for bladder outflow obstruction arising from castrate-resistant CaP or BPH. Prostate chips were bisected for histology and tissue culture. Prostate epithelial cell and fibroblasts were isolated by collagenase digestion followed by differential centrifugation and cultured as previously described (Hart et al, 2005) and used at passage 1–3.
Lipid raft isolation
Isolation of LR was performed as described previously (Macdonald and Pike, 2005). Cells were scraped into cold solution B (0.25 ℳ Sucrose, 20 mℳ Tris, 1 mℳ MgCl2, 1 mℳ CaCl2, anti-proteases and anti-phosphatase). After centrifugation (250 g, 2 min and 4 °C), pellets were resuspended in solution B and cells were broken using a 25 g needle. After centrifugation (1000 g, 10 min and 4 °C), supernatants were mixed with Optiprep (35% Optiprep final concentration) and overlaid with 25% and 5% Optiprep layers. After ultracentrifugation (52 000 g, 90 min and 4 °C) a band at the junction of the 5 and 25% gradient was collected (LR fraction) along with a fraction at the bottom of the tube (non-LR fraction).
Uptake of AA in PC-3 cells using flow cytometry
Serum starved (24 h) PC-3 cells were trypsinised and 2 × 105 cells ml−1 placed in RPMI 1640 supplemented with filipin III (1 μg ml−1) or chlorpromazine (1 μg ml−1) 30 min before addition of AA (45 min). Cells were fixed in 4% formalin (20 min) and staining with 5 μℳ Nile Red (10 min). Analysis was carried out using a BD FACSArray running at 15 mW with 532 nm, 780/60 nm and 585/42 nm bandpass filters, using linear amplification.
Transfection
PC3-GFP cells were transfected with 600 ng of siRNA using Dharmafect 2 (Thermo Fisher Scientific) in antibiotic free media according to manufacturers’ instructions. siRNA knockdown of target protein was confirmed by western blot.
Ephrin receptor A2 immunoprecipitation
1.5 × 106 PC3-GFP cells were rinsed once with cold PBS/MgCl2 (500 μℳ)/CaCl2 (1 mℳ), lysed in FISH buffer (10% glycerol, 50 mℳ Tris pH7.4, 100 mℳ NaCl, 1% NP-40, 2 mℳ MgCl2 and anti-phosphatase). Lysates were cleared (14 000 g, 4 min, 4 °C) before incubation with EphA2 antibody (1.5 μg) for 2 h. Samples were then incubated with 600 μg of Dynabeads Protein G for 30 min before washing in FISH buffer (3 × ).
Western blot
Cell lysates for western blot were prepared using SDS-buffer (62.5 mℳ Tris-EDTA, pH6.8, 5% SDS) and total protein content was measured using the BCA protein assay kit (Bio-Rad Laboratories, Hertfordshire, UK). Lysates were resolved on a polyacrylamide gel and transferred to a Hybond ECl nitrocellulose membrane (Amersham, GE Healthcare, Buckinghamshire, UK). Membranes were blotted overnight at 4 °C with the indicated primary antibodies and developed using the HRP substrate western blot detection system (Millipore). Blots were visualised with a BioSpectrumAC Imaging System (UVP, Cambridge, UK) and band intensities analysed using Macbiophotonics ImageJ software.
Invasion assay
Invasion assays with PC3-GFP, DU 145 and LNCaP cells were performed according to Hart et al (2005); Brown et al (2006), respectively.
Adhesion assay
Adhesion assays were performed using a variation of the protocol described by Scott et al (2001): 4 × 104 serum starved (20 h) PC3-GFP cells were added to a confluent BMEC layer with or without AA (10 μℳ). After incubation at 37 °C for 10 min non-adherent cells were removed by 2 × 200 μl PBS washes and adherent cells quantified using a BMG Labtech FLUOstar OPTIMA plate reader at 485/530 nm. Background was defined as the value of BMEC cells alone.
Statistics
All values are presented as mean±s.e.m. All assays were compared using Student’s two-tailed t-test with significance set at P<0.05.
Results
Arachidonic acid stimulates prostate cellular adhesion to and migration through bone marrow endothelium
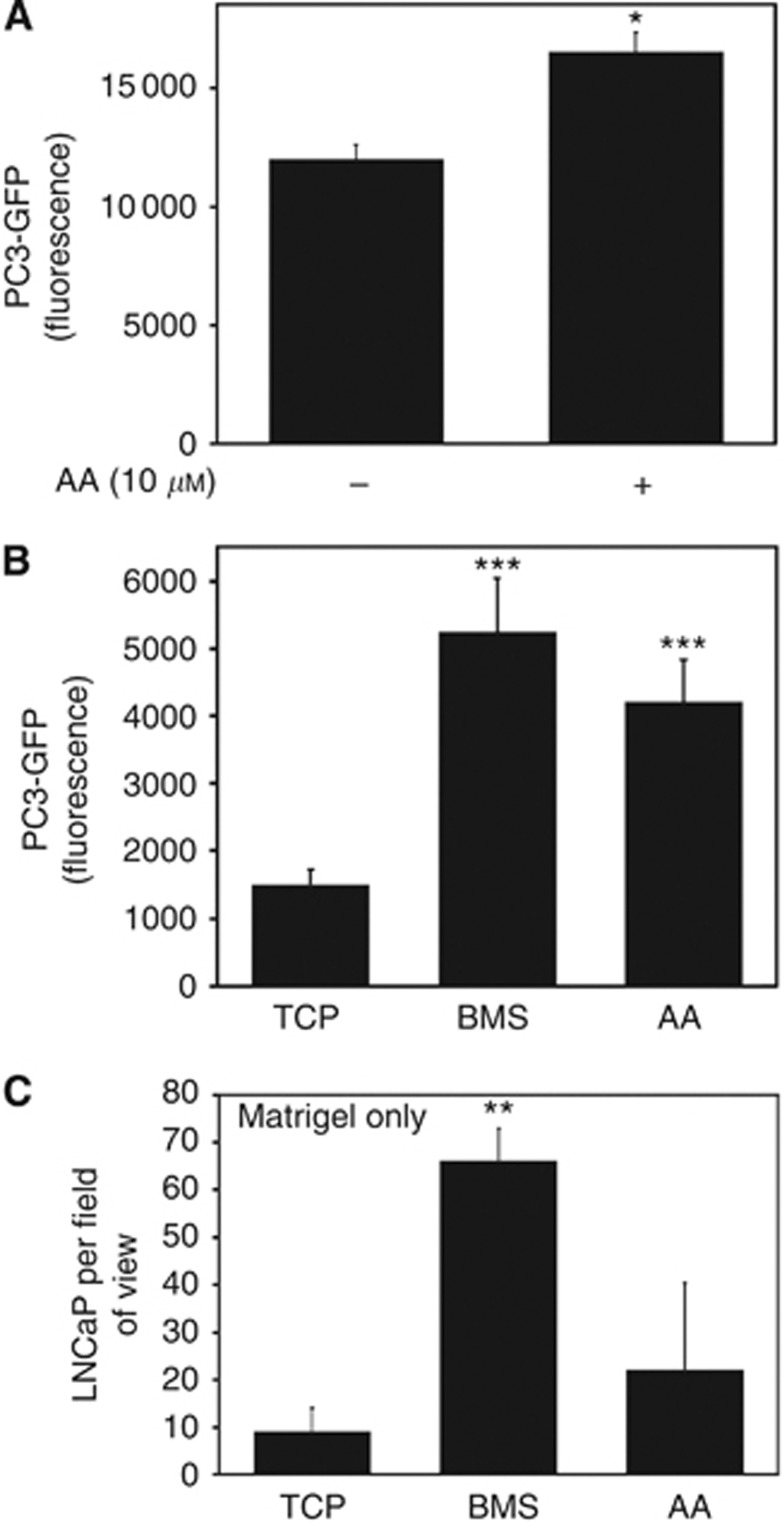
Metastatic PEC invading the axial skeletal BMS have to bind to bone marrow endothelium (BME) and invade through the endothelial and basement membrane layers. Previously, we have shown that AA is a potent inducer of invasion in a simplified invasion chamber using a Matrigel basement membrane (Brown et al, 2006). Utilising a co-culture variant of the Boyden chamber assay previously described in Hart et al (2005) (GFP intensity correlates to the number of epithelial cells (Supplementary Figure 1) our results show that AA has multiple effects on the metastatic behaviour of malignant PEC. Treatment of PC3-GFP cells with AA significantly increased adhesion to BMEC (37%±0.08, P=0.0124) (Figure 1A). Moreover, AA was a potent stimulator of invasion through a BMEC/Matrigel barrier, with 10 μℳ AA inducing similar levels to BMS (Figure 1B). This stimulatory ability was restricted to PC3-GFP: AA did not stimulate LNCaP cells to invade through Matrigel (10±5 vs 22±18; P=0.89 for TCP and AA, respectively) (Figure 1C). LNCaP cells are not able to invade through a BMEC/Matrigel barrier (Figure 5A).
Figure 1.
Arachidonic acid increases PC3-GFP adhesion to and invasion through BMEC. (A) Adhesion assays were performed using PC3-GFP cells with or without AA (10 μℳ) added just before the assay. 4 × 104 PC3-GFP cells were then incubated for 10 min with a confluent layer of BMEC plated in 96 wells. Wells were washed twice in PBS. Levels of adhesion are proportional to fluorescence detected by a bottom reading BMG FLUOstar OPTIMA plate reader at 488/520 nm (excitation/emission filter). Values represent the mean of three separate experiments. *P⩽0.05. (B) Invasion assays were performed using cell culture inserts (8 μℳ pore size) coated by a confluent layer of BMEC above a synthetic basement membrane (Matrigel). 2 × 105 PC3-GFP cells were added to the top of the inserts and allowed to invade for 18 h towards tissue culture plastic (TCP), BMS or AA (10 μℳ). Levels of invasion are proportional to fluorescence detected by a bottom reading BMG FLUOstar OPTIMA plate reader at 488/520 nm (excitation/emission filter). Values represent the mean of three separate experiments made in duplicates. ***P⩽0.001. (C) Invasion assays with LNCaP cells were performed using cell culture inserts (8 μℳ pore size) coated by a synthetic basement membrane (Matrigel). 1 × 105 LNCaP cells were added to the top of the inserts and allowed to invade for 18 h towards TCP, BMS or AA (10 μℳ). Inserts were rinsed in PBS, fixed, stained in 2% crystal violet and counted. Values represent the mean of three separate experiments made in duplicates. **P⩽0.01.
Stimulation of bone marrow endothelial adhesion and invasion by AA-induced Akt, Src and FAK phosphorylation
Arachidonic acid differentially stimulated PC3-GFP but not LNCaP cells to invade (Figure 1). Preclinical and clinical studies suggest that Akt, Src and FAK intracellular signalling pathways could be important for CaP metastasis (Ayala et al, 2004; Tatarov et al, 2009; Schaller, 2010). Therefore we aimed to determine the effect of AA on Akt, Src and FAK signalling in PC3-GFP, DU 145 and LNCaP cell lines.
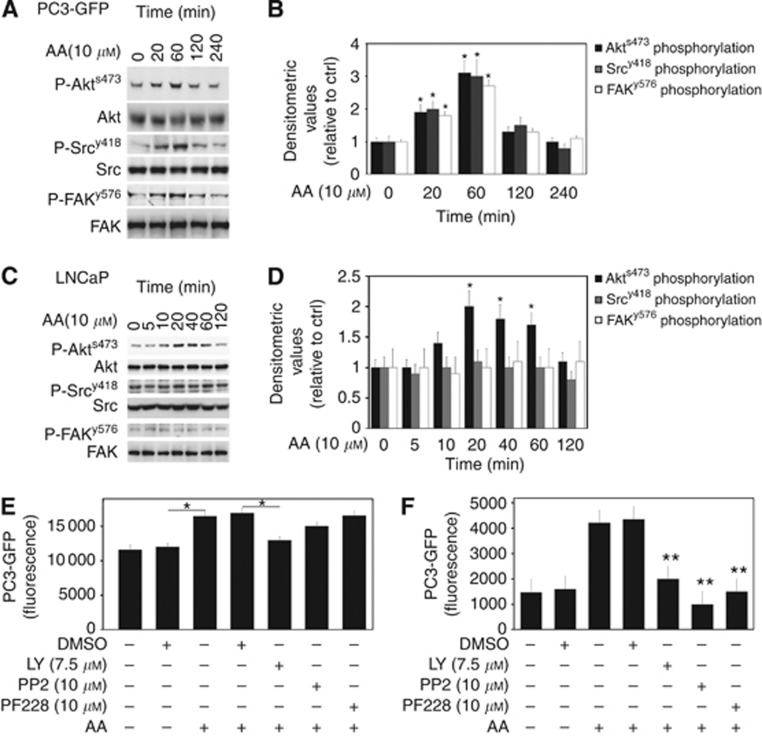
Addition of 10 μℳ AA (optimal concentration for invasion (Supplementary Figure 2)) to PC3-GFP induced rapid phosphorylation of Akt (P-Akts473), Src (P-Srcy418) and FAK (P-FAKy576), with phosphorylation being detected 20 min post AA addition and reaching a peak by 60 min (Figures 2A and B). This change in phosphorylation was confirmed in the wild-type PC-3 cells (data not shown) but was not seen in LNCaP cells where AA induced a rapid phosphorylation of Akt alone, an effect that peaked at 20 min and returned to baseline after 2 h (Figures 2C and D).
Figure 2.
Arachidonic acid increases Akt, Src and FAK activity in PC3-GFP. (A) Lysates of PC3-GFP cells incubated with AA (10 μℳ) at different times were subjected to western blotting. Phosphorylation levels of Akts473, FAKy576 and Srcy418 were compared with total levels of Akt, FAK and Src, respectively. Figures represent results of at least four separate experiments done in duplicate. (B) Quantification of densitometric values of phosphorylated Akts473, FAKy576 and Srcy418 in AA-stimulated PC3-GFP samples compared with total levels of Akt, FAK and Src, respectively. Controls without incubation with AA were set at one. *P⩽0.05. (C) Lysates of LNCaP cells incubated with AA (10 μℳ) at different times were subjected to western blotting. Phosphorylation levels of Akts473, FAKy576 and Srcy418 were compared with total levels of Akt, FAK and Src, respectively. The pictures represent the results of three separate experiments done in duplicate. (D) Quantification of densitometric values of phosphorylated Akts473, FAKy576 and Srcy418 in AA stimulated LNCaP samples and compared with the total levels of Akt, FAK and Src, respectively. Controls without incubation with AA were set at one. *P⩽0.05. (E) PC3-GFP adhesion assays to BMEC were done with addition of the Pi3K inhibitor LY29004 (7.5 μℳ), Src inhibitor PP2 (10 μℳ) and FAK inhibitors PF-228 (10 μℳ), respectively, to PC3-GFP for 30 min before the assay. Controls represent cells treated only with DMSO. Values represented at least four separate experiments. *P⩽0.05. (F) Invasion assays were performed using cell culture inserts (8 μℳ pore size) coated by a confluent layer of BMEC above a synthetic basement membrane (Matrigel). PC3-GFP cells were incubated with LY29004 (7.5 μℳ), PP2 (10 μℳ) and PF-228 (10 μℳ) for 30 min before adding cells to the inserts. Controls represent cells treated only with DMSO. Values represented at least four separate experiments. **P⩽0.01.
The addition of the PI3K inhibitor LY29004 (7.5 μℳ) inhibited both the AA-stimulated adhesion of PC3-GFP cells to the BMEC and AA-induced invasion, reducing both to unstimulated levels (Figures 2E and F, respectively). Inhibition of Src (10 μℳ PP2) or FAK (10 μℳ PF228) had no significant effect on the increased adhesion induced by AA but inhibition of both FAK and Src did significantly reduce invasion towards AA, reducing invasion to control levels (Figures 2E and F, respectively).
Arachidonic acid-induced Akt activation is LR dependent and lies ‘up stream’ of FAK and Src
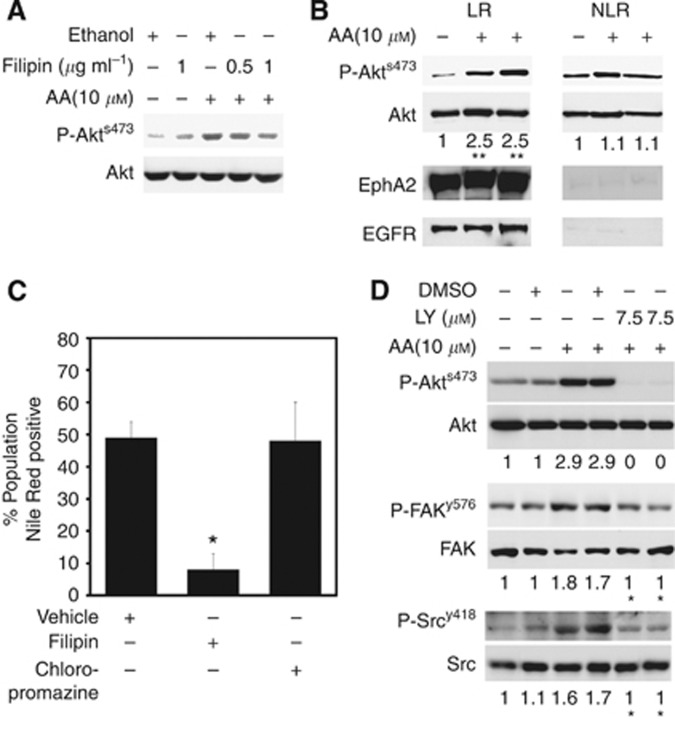
Phosphorylation of Akt relies on the presence of LR within the membrane; disruption of these by agents such as statins (Zhuang et al, 2005) results in a block in Akt phosphorylation. We sought to determine whether LR were involved in Akt phosphorylation in response to AA. Increasing doses of filipin, used to remove cholesterol from the PC3-GFP cells and hence disrupt LR within the cell membrane reduced the level of P-Akts473 in response to 10 μℳ AA (Figure 3A). We demonstrated that AA selectively induces the level of P-Akts473 in LR compared with the non-LR (Figure 3B). Moreover, AA uptake, as determined by Nile Red staining, indicative of intra-cellular lipid uptake, is decreased by filipin in PC-3 (Figure 3C), but is not affected by chlorpromazine, which is known to block clathrin-dependent endocytosis. The addition of a PI3K inhibitor before AA stimulation significantly reduced the phosphorylation of FAKy576 and Srcy418, suggesting phosphorylation of Akt is upstream of both FAK and Src in response to AA stimulation (Figure 3D).
Figure 3.
Arachidonic acid induced Akt activity is dependent on the LR and regulates FAK and Src activity. (A) PC3-GFP cells incubated with filipin (0.5 μg ml−1 and 1 μg ml−1) for 45 min before the addition of AA (10 μℳ, 1 h) were lysed and analysed by western blots. Phosphorylation levels of Akts473 were compared with the total levels of Akt. Controls cells were treated with the vehicle (ethanol) only. Figure is representative of three separate experiments. (B) PC3-GFP cells incubated with or without AA (10 μℳ, 1 h) were lysed and a detergent-free LR were isolated. Western blot analysis was performed on samples from the LR and non-LR fractions. Phosphorylation levels of Akts473 were compared with the total levels of Akt. Ephrin receptor A2 and EGFR, known to be localised in LR, were used to access quality of LR and non-LR fractions. The figure represents the results of two separate experiments. Quantification of densitometric values of phosphorylated Akts473 compared with the total levels of Akt. Controls without incubation with AA were set at one. **P⩽0.01 (ctrl vs AA). (C) FACS analysis of AA uptake by serum-starved PC-3 cells, incubated with AA (10 μℳ, 30 min) after Nile Red staining with or without pre-incubation with filipin III (1 μg ml−1) or chlorpromazine (1 μg ml−1). Results represent two separate experiments. *P⩽0.05. (D) Western blot experiment of PC3-GFP samples pre-incubated for 30 min with LY29004 (7.5 μℳ), before incubation with AA (10 μℳ) for 1 h. Phosphorylation levels of Akts473, FAKy576 and Srcy418 were compared with the total levels of Akt, FAK and Src, respectively. Densitometric values represent the results of two separate experiments, *P⩽0.05 (AA treated, DMSO vs LY29004).
Arachidonic acid stimulates PC3-GFP adhesion and invasion via EphA2
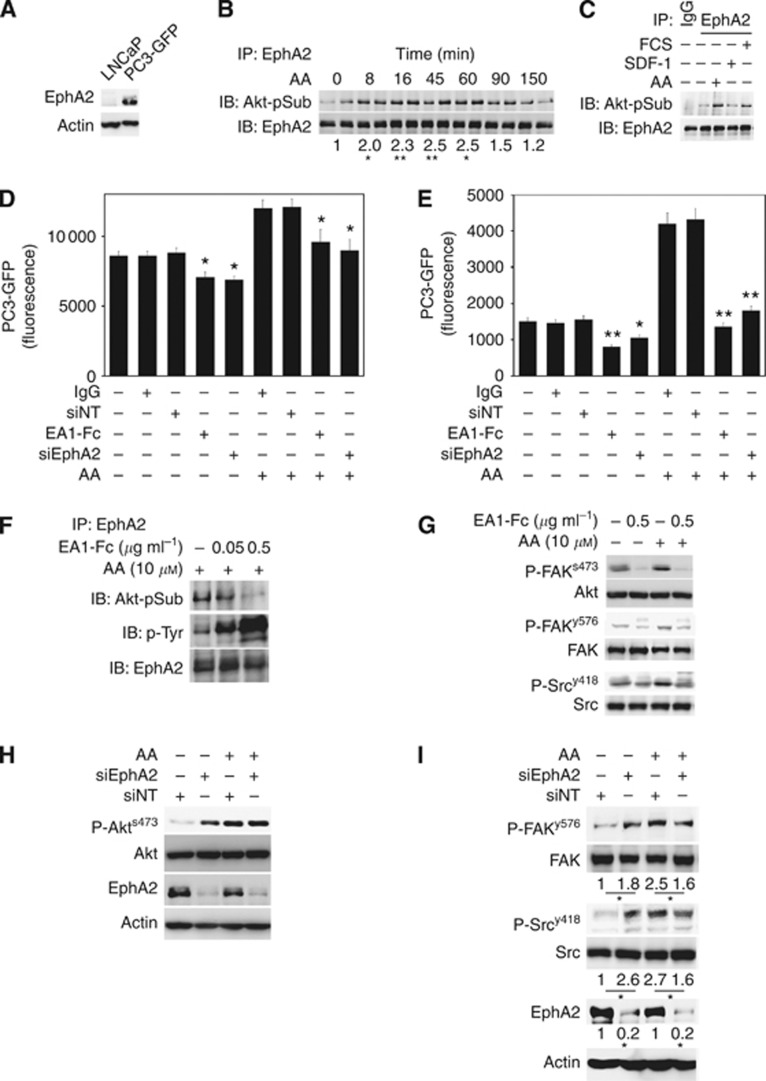
Ephrin receptor A2 activation has been shown to act either directly through its ligand, EphrinA1 or EphrinA5, or in a ligand-independent process as a P-Akt substrate (Miao et al, 2009). Moreover, FAK and Src have been shown to interact with EphA2 in several models (Miao et al, 2000; Fang et al, 2008). Figure 2 demonstrates the requirement of P-Akt for AA-induced adhesion and invasion. Therefore, we assessed the potential of AA to induce ligand-independent EphA2 signalling. Ephrin receptor A2, not expressed in LNCaP (Figure 4A), was precipitated from PC3-GFP cells stimulated with AA and assessed for phosphorylation of the Akt consensus substrate site by western blot. AA stimulation induced rapid P-Akt phosphorylation of EphA2 with increased levels being detected 8 min post stimulation. Phosphorylation peaked between 45 and 60 min, returning to near basal levels 150 min post stimulation (Figure 4B). Both foetal calf serum and SDF-1 have been shown previously to induce invasion (Hart et al, 2005; Miao et al, 2009). We assessed whether the phosphorylation of EphA2 by P-Akt was restricted specifically to AA stimulation or whether it was a general pathway. SDF-1 did not induce EphA2 phosphorylation (Figure 4C), unlike 5%foetal calf serum, which induced phosphorylation at levels similar to 10 μℳ AA. This suggests that ligand-independent activation of EphA2 is not associated with all stimulants of PEC invasion, such as SDF-1.
Figure 4.
Arachidonic acid induced ligand-independent activity of EphA2 in PC3-GFP stimulates BMEC adhesion and invasion by decreasing Akt, Src and FAK activity. (A) Western blot analysis showing EphA2 content in LNCaP and PC3-GFP samples. Loading was checked by immunoblotting the same samples with an anti-Actin antibody. Figure is representative of two separate experiments. (B) Ephrin receptor A2 immunoprecipitates from PC3-GFP lysates incubated at different times with AA (10 μℳ) were immunoblotted with antibody recognising the consensus Akt substrate sites. Loading was checked by immunoblotting the same membrane stripped with an anti-EphA2 antibody. Bands represent the mean of four experiments done in duplicate. (C) Ephrin receptor A2 immunoprecipitates from PC3-GFP lysates incubated with AA for 1 h, with foetal calf serum 5% for 15 min or with SDF-1 (15 ng ml−1) for 15 min were immunoblotted with an antibody recognising the consensus Akt substrate sites. Loading was checked by immunoblotting the same membrane stripped with an anti-EphA2 antibody. Bands represent the mean of two experiments done in duplicate. (D) PC3-GFP adhesion assays to BMEC in presence or absence of AA (10 μℳ) were done with addition of 0.5 μg ml−1 EphrinA1-Fc (EA1-Fc) or with the control IgG at the same concentration for 45 min before the assay. PC3-GFP cells were also transfected with a specific EphA2 siRNA pool (siEphA2) or with non-targeted siRNA (siNT) 2 days before the adhesion assays. Values represent the mean of three separate experiments. *P⩽0.05 between IgG or siNT controls±AA and EA1-Fc or siEphA2, respectively. (E) Invasion assays using cell culture inserts (8 μℳ pores) coated by a layer of BMEC above a synthetic basement membrane (Matrigel). PC3-GFP cells were incubated for 30 min with EA1-Fc (0.5 μg ml−1), with the control IgG at the same concentration or PC3-GFP cells were transfected with EphA2 siRNA pool (siEphA2) or with non-targeted siRNA (siNT) 2 days before the invasion assays. Values represent the mean of three separate experiments. *P⩽0.05 and **P⩽0.01 between IgG or siNT controls±AA and EA1-Fc or siEphA2, respectively. (F) Ephrin receptor A2 immunoprecipitates from PC3-GFP cells incubated with EA1-Fc or IgG at the same concentration with AA were immunoblotted with an antibody recognising the consensus Akt substrate sites. Loading was checked by immunoblotting the same membrane stripped, with an anti-EphA2 antibody. Bands represent the mean of at least four experiments done in duplicate. (G) PC3-GFP cells were incubated with EA1-Fc or IgG at the same concentration with or without AA (10 μℳ) for 1 h. P-Akt, P-FAK and P-Src levels were compared with total levels of Akt, FAK and Src, respectively. Bands represent the mean of two separate experiments done in duplicates. (H) P-Akts473 and EphA2 content were analysed (western blotting) in lysates of PC3-GFP cells transfected for 48 h with a specific EphA2 siRNA pool (siEphA2), or transfected with non-targeted siRNA (siNT) and incubated for 1 h with AA (10 μℳ). Figure represents the mean of three separate experiments. (I) Lysates from PC3-GFP cells transfected for 48 h with EphA2 siRNA pool (siEphA2) or non-targeted siRNA (NT) before incubation with 10 μℳ AA for 1 h were analysed by western blot to access P-FAKy576, P-Srcy418 and EphA2 content compared with non-targeted siRNA (siNT) samples. Densitometric values represent the mean of three separate experiments. *P⩽0.05.
To confirm that stimulation of EphA2 ligand-independent signalling by AA induced both adhesion to and invasion through BMEC, AA-induced adhesion and invasion assays were conducted in the presence of the ligand. EphrinA1-Fc ligand inhibited both AA-induced adhesion to and invasion through BMEC (Figures 4D and E). Activity of the Ephrin A1-Fc was confirmed by phospho-tyrosine western blot analysis, which showed ligand-dependent activation of EphA2 (Figure 4F). Ligand-dependent activation of EphA2 downregulated phosphorylation of Akts473, FAKy576 and Srcy418 and this downregulation was not reversed or inhibited by addition of 10 μℳ AA (Figure 4G). To further evaluate EphA2 function, a siRNA knockdown approach was used. Decreased EphA2 expression in PC3-GFP inhibited AA-induced adhesion to BMEC cells (Figure 4D) and inhibited invasion through BMEC towards 10 μℳ AA (Figure 4E). Western blot analysis of the putative members of the AA-induced signalling pathway P-Akts473, P-FAKy576 and P-Srcy418 showed that knockdown of EphA2 did not affect AA-induced phosphorylation of Akt (Figure 4H) but it did inhibit the phosphorylation of both FAK and Src (Figure 4I).
Ephrin receptor A2 expression correlates with invasive behaviour in metastatic CaP cell lines
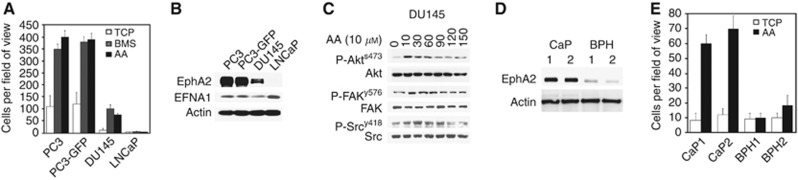
In Figure 4, we showed that EphA2 is more highly expressed in the invasive cell line PC3-GFP than in the weakly invasive LNCaP cells, suggesting that EphA2 may be a key arbiter in CaP migration/invasion. We investigated transendothelial invasion in another metastatic cell line DU 145 (Figure 5A). DU 145 cells are less invasive compared with PC-3 and PC3-GFP cells. LNCaP cells are unable to invade through a BMEC/Matrigel barrier. We sought also to determine levels of EphA2 and its ligand EphrinA1 (EFNA1) expression within these different cell lines (Figure 5B). EFNA1 is expressed in all cell lines studied and EphA2 expression correlates with the ability of AA to induce transendothelial transmigration. Using DU 145, we confirmed that AA induced Akt Src and FAK activity (Figure 5C). Finally, EphA2 levels were evaluated in primary PEC isolated from patients with BPH or high-grade CaP. Figures 5D and E show that high levels of EphA2 receptor expression correlated with malignancy and the ability to invade towards AA.
Figure 5.
Ephrin receptor A2 is increased in human prostatic metastatic tissue compared with primary CaP. (A) Invasion assays were performed using cell culture inserts (8 μℳ pore size) coated by a confluent layer of BMEC above a synthetic basement membrane (Matrigel). 1 × 105 PC-3, PC3-GFP, DU 145 and LNCaP cells were added to the top of the inserts and allowed to invade for 18 h towards tissue culture plastic (TCP), BMS or AA (10 μℳ). (B) Western blot analysis showing EphA2 and EphrinA1 (EFNA1) content in PC-3, PC3-GFP, DU 145 and LNCaP cells. Loading was checked by immunoblotting the same samples with an anti-Actin antibody. Figure is representative of two separate experiments. (C) Lysates of DU 145 cells incubated with AA (10 μℳ) at different times were subjected to western blotting. Phosphorylation levels of Akts473, FAKy576 and Srcy418 were compared with total levels of Akt, FAK and Src, respectively. Figures represent results of two separate experiments done in duplicate. (D) Lysates of primary cells from two separate patients with CaP and two patients with BPH were analysed by western blotting. Levels of EphA2 were compared with actin. Bands are representative of two separate experiments. (E) Invasion assays were performed using cell culture inserts (8 μm pore size) coated by a synthetic basement membrane (Matrigel). 1 × 105 cells from CaP samples (n=2) and from BPH samples (n=2) were added to the top of the inserts and allowed to invade for 18 h towards AA (10 μℳ). Inserts were rinsed in PBS, fixed, stained in 2% crystal violet and counted. Results are the mean of two separate experiments.
Discussion
There is increasing evidence supporting the role of lipids in cancer progression (Crawford, 2003; Chavarro et al, 2007; Aronson et al, 2010). How and why some cancer cells seek out and take up the specific lipids is unclear but this may relate to the cancer cells switching to a more glycolytic metabolism: the Warburg effect (Warburg et al, 1927). CaP serves as an excellent model for studying this lipid tropism in metastasis due to its tendency to co-localise with the BMS adipocytes (Bubendorf et al, 2000). We have shown previously that AA directly induces invasion and stimulates adipocyte formation within the BMS, increasing the stimulatory capacity of the BMS for CaP invasion and early survival of the metastasising cell (Brown et al, 2006; Gazi et al, 2007; Brown et al, 2010).
In these experiments, we have studied the differential effects of AA on the CaP cell lines LNCaP, DU 145 and PC-3. Lymph node-derived LNCaP cell line, unlike the bone metastases-derived PC-3 cells, have minimal migrational capacity and do not express EphA2. Preclinical and clinical studies suggest that Akt, Src and FAK pathways are involved in the bony metastatic process in CaP (Ayala et al, 2004; Tatarov et al, 2009; Schaller, 2010). Stimulation of PC3-GFP and LNCaP cells with AA lead to increased Akt activity in both cell lines but P-Srcy418 and P-FAKy576 were only increased in PC3-GFP. Therefore, we hypothesised that AA-induced P-Akt was upstream of a signalling molecule expressed differentially between PC-3 and LNCaP cells. Ephrin receptor A2 has been associated positively with PC-3 motility/migration (Pasquale, 2008) and we can confirm this positive association in cell lines (PC-3 and DU 145) and primary malignant PECs isolated from men with high-grade disease with invasive phenotypes. Ephrin receptor A2 is not expressed in either LNCaP cells and is significantly reduced in primary BPH cells, which do not display an invasive phenotype (Figure 5).
The role of ephrins and the Eph receptor in CaP has recently been described (Astin et al, 2010). Different combinations of Eph receptors and ligands switch contact inhibition of locomotion on and off. Activation of EphA receptors by their ligands, co-expressed by PEC, induced contact inhibition of locomotion (Astin et al, 2010). Ephrin receptor A2 is activated in a ligand-independent manner through phosphorylation of serine897 by P-Akt, thereby promoting cell migration and invasion (Miao et al, 2009). Herein, we show that another regulator of EphA2 signalling, AA, induces EphA2 phosphorylation by P-Akt to stimulate cellular motility and transmigration. Ephrin receptor A2 ligand-independent activation induced by AA was also confirmed by the abrogation of activity following addition of the ligand EphrinA1-Fc. Ephrin receptor A2 promotion of migration has been shown to be kinase independent and EphrinA1-Fc induces a decrease in Akt activity (Miao et al, 2009) in PC-3 cells. EphrinA1 has also been shown to decrease FAK activity in pancreatic cancer cells (Duxbury et al, 2004) and in PC-3 cells (Miao et al, 2000).
Arachidonic acid increased the adhesion to the BMEC layer. Tumour cell adhesion to endothelium involves two distinct steps, an initial docking step mediated via lectin–carbohydrate interactions followed by an integrin-mediated locking step (Honn and Tang, 1992). Herein, we have shown that increased BMEC adhesion induced by AA is mediated by ligand-independent EphA2 signalling and that reducing ligand-independent signalling via siRNA knockdown of EphA2, Akt inhibition or stimulation of the ligand-dependent EphA2 activity by EphrinA1, restores adhesion to original levels. Activation of EphA2 by the ligand EphrinA1-Fc decreases PC-3 adhesion to fibronectin by inactivation of integrins and FAK dephosphorylation (Miao et al, 2000). Furthermore, downregulation of Akt in MDCK cells led to a decrease in the levels of activated integrins (Ihara et al, 2011). Preliminary data gathered in our series of experiments (not shown), in which AA induces an increase in integrin β1 expression suggests that AA may increase the activity of the β1 integrin component by ligand-independent activation of EphA2.
We have shown that AA stimulates a rapid increase in Akt activity and that EphA2 phosphorylation by P-Akt is increased. At the same time we observed FAK and Src activation: this activation induced invasion through BMEC but not BMEC adhesion. FAK and Src are frequently overexpressed and active in CaP (Tatarov et al, 2009; Schaller, 2010) and our data suggest that both are downstream of EphA2, which, in turn, is downstream of Akt. We demonstrated that inhibition of Pi3Kinase decreased AA-induced Src and FAK phosphorylation and that EphA2 knockdown did not affect the levels of AA-induced P-Akt but did affect both FAK and Src phosphorylation, reducing PC-3 invasion. Ephrin receptor A2 knockdown induced an increase in Akt, Src and FAK activity in resting cells (Figures 4H and I). This may be due to the expression of EphrinA1 in the metastatic cells, such as PC-3 and DU 145 (Figure 5B). Taddei et al (2009) showed that decreased EphA2 expression could block the inhibition of Akt, Src and FAK activity induced by EphrinA1. The data presented here suggest that in the absence of AA, ligand-independent EphA2 activation of FAK and Src is suppressed by EphA2 ligand (EphrinA1) dependent activity. Addition of AA stimulates EphA2 ligand-independent activity via its phosphorylation by P-Akt, with downstream phosphorylation of FAK and Src. How EphA2 interacts with Src and FAK is unclear, but FAK and Src have been shown to bind to EphA2 (Miao et al, 2000; Fang et al, 2008), which may stabilise Src and FAK or alternatively, act as a scaffold that could help the relay of Src and FAK activation induced by AA.
Studies using a PC-3 cell line with low EphA2 expression have shown that transendothelial migration through HUVECs is not dependent on EphA2 expression (Taddei et al, 2011), a contrast to our results. This may be the result of the choice of endothelia. The HUVEC model, which comprises endothelial cells from large arteries, does not reflect the capillary endothelial barrier between blood and red bone marrow. Previously, we have shown that PEC binds poorly to HUVEC as compared with BMEC, and the migrational characteristics across HUVEC and BM endothelia are quite different (Scott et al, 2001). The chemotactic agent used is also of importance. Serum is a mixture of cytokines, growth factor and lipids, which can stimulate several different signalling pathways, whereas in this study, we are focusing on a single chemotactic agent.
The mechanism of Akt activation by AA needs further elucidation. This phenomenon is likely to occur within the LR of the cell membrane where AA is present in significant quantities (Pike et al, 2002). PC3-GFP depletion of LR by filipin decreased AA-induced Akt activity and AA uptake. Our results show that AA induces Akt activity specifically in the LR, where EphA2 is localised (Pasquale, 2008). Lipid composition of the LR has an important role in determining their efficiency in supporting cellular signalling; increasing the levels of AA in the LR may lead to increased recruitment of growth factor receptors and signalling mediators such as Akt and EphA2, increasing cellular signalling in response to external factors. Modulation of the LR composition through the incorporation of long-chain omega-3 PUFA has been shown to modulate lipid and protein composition of LR (Stulnig et al, 2001). It may be possible therefore to modulate the signal to the metastasising cell by interfering with its LR composition, in addition to targeting key receptors through dietary intervention. This would support our previous findings that omega-3 PUFA block the metastatic stimulatory ability of AA in vitro (Brown et al, 2006).
In summary, we have demonstrated further evidence of AA’s effects in promoting metastasis-linked behaviour in CaP. Key to this is the ligand-independent activity of EphA2, highlighting its potential importance as a future prognostic and therapeutic target in CaP.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation: ‘Bourse chercheur debutant’ (PBLAP3-129433/1) and ‘Bourse pour chercheur avance aupres FSBMB/Novartis’ (PASMP3_134378/1) to TT; and by supporting funds from the GU Cancer Research Group endowments, including the Christie Hospital based ‘Men Matter’ Charity.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, Cohen P, Heber D, Kobayashi N (2010) Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol 183: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD (2010) Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol 12: 1194–1204 [DOI] [PubMed] [Google Scholar]
- Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, Ohori M, Wheeler T, Harper W (2004) High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res 10: 6572–6578 [DOI] [PubMed] [Google Scholar]
- Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N (2010) Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer 102: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW (2006) Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br J Cancer 94: 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum Pathol 31: 578–583 [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J (2007) A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 16: 1364–1370 [DOI] [PubMed] [Google Scholar]
- Crawford ED (2003) Epidemiology of prostate cancer. Urology 62: 3–12 [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene 23: 1448–1456 [DOI] [PubMed] [Google Scholar]
- Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J (2008) Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 121: 358–368 [DOI] [PubMed] [Google Scholar]
- Franks LM (1954) Latent carcinoma of the prostate. J Pathol Bacteriol 68: 603–616 [DOI] [PubMed] [Google Scholar]
- Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW (2007) Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res 48: 1846–1856 [DOI] [PubMed] [Google Scholar]
- Hart CA, Brown M, Bagley S, Sharrard M, Clarke NW (2005) Invasive characteristics of human prostatic epithelial cells: understanding the metastatic process. Br J Cancer 92: 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn KV, Tang DG (1992) Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev 11: 353–375 [DOI] [PubMed] [Google Scholar]
- Ihara Y, Inai Y, Ikezaki M (2011) Alteration of integrin-dependent adhesion and signaling in EMT-like MDCK cells established through overexpression of calreticulin. J Cell Biochem 112: 2518–2528 [DOI] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 15: 1953–1962 [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM (2004) Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res 64: 5232–5236 [DOI] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW (2005) Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell 9: 663–673 [DOI] [PubMed] [Google Scholar]
- Loberg RD, Logothetis CJ, Keller ET, Pienta KJ (2005) Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol 23: 8232–8241 [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ (2005) A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res 46: 1061–1067 [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B (2000) Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol 2: 62–69 [DOI] [PubMed] [Google Scholar]
- Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B (2009) EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 16: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52 [DOI] [PubMed] [Google Scholar]
- Pike LJ, Han X, Chung KN, Gross RW (2002) Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088 [DOI] [PubMed] [Google Scholar]
- Schaller MD (2010) Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci 123: 1007–1013 [DOI] [PubMed] [Google Scholar]
- Schumacher MC, Laven B, Petersson F, Cederholm T, Onelov E, Ekman P, Brendler C (2011) A comparative study of tissue omega-6 and omega-3 polyunsaturated fatty acids (PUFA) in benign and malignant pathologic stage pT2a radical prostatectomy specimens. Urol Oncol e-pub ahead of print 31 March 2011; doi:10.1016/j.urolonc.2011.01.014 [DOI] [PubMed]
- Scott LJ, Clarke NW, George NJ, Shanks JH, Testa NG, Lang SH (2001) Interactions of human prostatic epithelial cells with bone marrow endothelium: binding and invasion. Br J Cancer 84: 1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63: 963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W (2001) Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem 276: 37335–37340 [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22: 337–358 [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Wheeler DC, Mucci LA (2010) Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr 92: 1223–1233 [DOI] [PubMed] [Google Scholar]
- Taddei ML, Parri M, Angelucci A, Bianchini F, Marconi C, Giannoni E, Raugei G, Bologna M, Calorini L, Chiarugi P (2011) EphA2 induces metastatic growth regulating amoeboid motility and clonogenic potential in prostate carcinoma cells. Mol Cancer Res 9: 149–160 [DOI] [PubMed] [Google Scholar]
- Taddei ML, Parri M, Angelucci A, Onnis B, Bianchini F, Giannoni E, Raugei G, Calorini L, Rucci N, Teti A, Bologna M, Chiarugi P (2009) Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol 174: 1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J (2009) SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res 15: 3540–3549 [DOI] [PubMed] [Google Scholar]
- Thompson IM, Pauler Ankerst D, Chi C, Goodman PJ, Tangen CM, Lippman SM, Lucia MS, Parnes HL, Coltman CA (2007) Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J Clin Oncol 25: 3076–3081 [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS (1999) Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate 41: 275–280 [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E (1927) The Metabolism of Tumors in the Body. J Gen Physiol 8: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynder EL, Rose DP, Cohen LA (1994) Nutrition and prostate cancer: a proposal for dietary intervention. Nutr Cancer 22: 1–10 [DOI] [PubMed] [Google Scholar]
- Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR (2005) Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest 115: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.