Abstract
Background:
miRNAs are small noncoding RNA molecules that can be released into body fluids. Germ cell tumours (GCTs) overexpress miRNAs of the miR-371-3 cluster. Thus, serum levels of these miRNAs may correlate with tumour load.
Methods:
miRNAs of the miR-371-3 cluster were quantified in cubital vein blood samples of 20 GCT patients with clinical stage 1, and of 4 patients with advanced stages before and after treatment. In six patients testicular vein blood (TVB) was examined additionally. Seventeen healthy males served as controls. Likewise, expression of miRNAs in 15 matching tumour specimens was measured.
Results:
In all patients, serum levels of miRNAs 371-3 were much higher than in controls. In stage 1, levels decreased postoperatively 336.7-fold, 7.4-fold, and 7.7-fold for miRNAs 371a-3p, 372, and 373-3p, respectively (P<0.01). Also, in those cases with advanced disease levels dropped to the normal range after completion of treatment. miR-371-3 levels in TVB exceeded those in peripheral blood in all cases. Expression of miR-371a-3p was also documented in tumour tissue. However, no correlation was found regarding the extent of miRNA expression in tissue and the values measured in matching serum.
Conclusion:
Thus, miR-371a-3p serum level appears to be a useful biomarker in GCTs.
Keywords: testicular germ cell tumour, microRNA, seminoma, biomarker
Testicular germ cell tumours (GCTs) represent the most frequent malignancy among men aged 20–35 years (Bosetti et al, 2011). Today, GCT is a paradigm of a curable cancer. Clinical management is greatly based on serum biomarker monitoring (Albers et al, 2011). Markers currently used involve α-fetoprotein (AFP), β-human chorionic gonadotropin (bHCG), and lactate dehydrogenase (LDH) (Barlow et al, 2010), but only 60% of all GCT patients have elevations of these markers and, particularly, LDH shows a quite low specificity (Barlow et al, 2010).
Thus, there is still an ongoing need for new biomarkers in the field. MicroRNAs (miRNAs) are small RNA molecules involved in several biological processes that cover embryogenic development, cell differentiation, apoptosis, and tumorigenesis (Bartel, 2004; Esquela-Kerscher and Slack, 2006; Carthew and Sontheimer, 2009; Farazi et al, 2011). They have gained a lot of interest because of their potential use as biomarkers in various cancer types because some of these molecules are abundantly expressed in cancer tissues (Catto et al, 2011) and most of them reveal high stability in body fluids (Reis et al, 2010).
The miR-371-3 cluster encodes of seven mature miRNAs and apparently belongs to a larger imprinted region of the long arm of chromosome 19. It can be speculated that akin to C19MC, that is, a juxta-posed cluster (Noguer-Dance et al, 2010; Wei et al, 2011) miR-371-3 underlies epigenetic silencing in adult somatic cells.
In human testicular GCTs, miR-372 and miR-373-3p have an oncogenic potential allowing the cells to display tumorigenic growth in the presence of wild-type p53 (Voorhoeve et al, 2006; Gillis et al, 2007).
Recently, Palmer et al (2010) have profiled and analysed the miRNA expression in malignant GCTs. As a rule, the clusters miR-371-3 and miR-302 were found to be overexpressed in this malignancy. Interestingly, the corresponding miRNAs can also be detected in serum: high expression of miRNAs of these clusters was demonstrated in the serum of a 4-year-old boy with a yolk sac tumour. After chemotherapy the level strongly declined (Belge et al, 2012). In a recent report on eleven GCT- cases the average expression of the miR-371-3 declined after surgery (Belge et al, 2012). These reports indicate that assessing miR-371-3 in serum might be clinically valuable.
We looked to the levels of miR-371-3 in serum and to the expression of these miRNAs in tumour tissue in a consecutive series of GCT patients. Our aim was to evaluate the potential use of miRNAs 371-3 as new biomarkers in this disease.
Materials and methods
Patients, blood, and tumour samples
From 1 June 2011 to 21 May 2012, a total of 24 patients with histologically proven GCT (13 pure seminoma, 11 nonseminoma) were enrolled in this prospective study. Median age was 37 (18–60) years. Cubital vein blood samples were obtained during routine blood examinations usually 3 days before orchiectomy and 5–6 days postoperatively. Eleven of these patients have been briefly documented previously (Belge et al, 2012). In six patients, testicular vein blood (TVB) samples were analysed too.
All patients had given informed consent. The study had been approved by the local ethics committee. Twenty patients had clinical stage 1 disease (Table 1). Serum aliquots were frozen within 2 h of receipt and stored at −80°C before further processing. Seventeen male patients, aged 23–51 years (median 37), with non-malignant scrotal diseases served as controls.
Table 1. Clinical features of 20 patients with clinical stage 1 disease (cases 1–20) and of four patients with clinical stage 2a (case 23), 2b (case 24), 3 (case 21 and 22) (Lugano classification).
|
bHCG (IU l
−1)
|
AFP (ng ml
−1)
|
LDH (U l
−1)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Histology | Age (years) | Tumour diameter (cm) | Tumour volume (cm 3 ) | Pre surgery | Post surgery | Pre surgery | Post surgery | Pre surgery | Post surgery |
| 1 | NS | 21 | 2.6 | 9.20 | 3.5 | <1.20 | NA | 0.91 | n.a. | 172 |
| 2 | S | 55 | 1.5 | 1.77 | <1.20 | <1.20 | 4.69 | 4.49 | 160 | 160 |
| 3 | S | 39 | 1.5 | 1.77 | <1.20 | <1.20 | 1.82 | 1.66 | 145 | 159 |
| 4 | NS | 28 | 2.5 | 8.18 | 1495.9 | 62.3 | 113.92 | 37.92 | 173 | 169 |
| 5 | S | 41 | 5.5 | 87.11 | <1.20 | <1.20 | 4.48 | 4.39 | 187 | NA |
| 6 | NS | 18 | 2.0 | 4.19 | <1.20 | <1.20 | 2.26 | 1.86 | 240 | 180 |
| 7 | NS | 23 | 1.8 | 3.05 | <1.20 | <1.20 | 1.31 | 1.31 | 166 | 195 |
| 8 | S | 38 | 2.8 | 11.49 | <1.20 | <1.20 | 3.26 | 2.39 | NA | 154 |
| 9 | NS | 39 | 3.5 | 22.45 | <5 | <1.20 | 183.35 | 8.15 | 245 | 192 |
| 10 | S | 45 | 1.1 | 0.70 | <1.20 | <1.20 | 4.25 | 4.26 | 186 | 208 |
| 11 | S | 50 | 1.5 | 1.77 | <1.20 | <1.20 | 0.41 | 0.51 | 185 | 222 |
| 12 | S | 60 | 1.6 | 2.15 | <1.20 | <1.20 | 19.09 | 19.85 | 291 | 248 |
| 13 | S | 39 | 6.5 | 143.79 | 2.6 | <1.20 | 2.56 | 1.72 | 224 | 180 |
| 14 | NS | 35 | 3.5 | 22.45 | <1.20 | <1.20 | 102.73 | 71.91 | 183 | 171 |
| 15 | S | 26 | 3.3 | 18.82 | 44 | 1.4 | 1.26 | 0.79 | 186 | 216 |
| 16 | S | 46 | 2.4 | 7.24 | <1.20 | <1.20 | 4.85 | 4.05 | 192 | 291 |
| 17 | NS | 28 | 0.9 | 0.38 | <1.20 | <1.20 | 1.3 | 1.1 | 153 | 140 |
| 18 | S | 48 | 1.5 | 1.77 | <1.20 | <1.20 | 1.62 | 1.79 | 170 | 220 |
| 19 | S | 41 | 2.5 | 8.18 | <1.20 | <1.20 | 2.07 | 4.98 | 225 | 185 |
| 20 | S | 41 | 5.0 | 65.45 | <1.20 | 1.20 | 5.07 | 3.80 | 331 | 235 |
| 21 | NS | 23 | 6.0 | 113.10 | >200 000 | 4829.7 | 7.31 | 3.01 | 3149 | 376 |
| 22 | NS | 46 | 6.5 | 143.79 | 18.09 | <1.20 | 737.60 | 78.66 | 2098 | 386 |
| 23 | NS | 27 | 2.0 | 4.19 | 67.10 | 73.80 | 2.18 | 2.32 | 202 | 202 |
| 24 | NS | 29 | 1.9 | 3.59 | 2.20 | 2.20 | 1.77 | 1.56 | 220 | 181 |
Abbreviations: AFP=α-fetoprotein; NA=not available; NS=non-seminoma; LDH=lactate dehydrogenase; S=seminoma.
Histology of tumours, patient age, and classical serum tumour markers (normal ranges bHCG: 0–6 IU l−1; AFP: 0–10.5 ng ml−1; LDH: 120–240 U l−1).
Cases 21 and 22: post-surgery measurements refer to analysis after first chemotherapy cycle. The median follow-up period is 6 months. None of the patients has relapsed so far.
To study miRNAs serum levels during the course of chemotherapy in metastasised disease, four patients with nonseminomatous GCT with stages IIa–III (Lugano classification) were examined (cases 21–24, Table 1). In these cases, blood samples were drawn before the start of treatment and repeatedly during the three or four chemotherapy cycles and finally after completion of therapy. Chemotherapy consisted of cisplatin, etoposide, and bleomycin according to current guidelines (Krege et al, 2008).
Formalin-fixed and paraffin-embedded (FFPE) tumour tissue samples of 15 patients were analysed additionally.
RNA Isolation
For RNA isolation from serum samples 200 μl frozen serum was thawed on ice, total RNA extracted using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) and RNA was quantified by spectrophotometry (Eppendorf, Hamburg, Germany).
Total RNA from six 5-μm sections of each FFPE tumour specimen was prepared using the innuPREP Micro RNA Kit (Analytik Jena AG, Jena, Germany) according to the manufacturer’s instructions with a few modifications. Lysis of the paraffin sections preceding RNA isolation was conducted using TLS-lysis solution and Proteinase K from the innuPREP DNA Micro Kit (Analytik Jena AG) without prior deparaffinisation. Sections were incubated for 1 h at 60°C and 15 min at 80°C (Flor et al, 2012).
cDNA synthesis
For serum samples reverse transcription (RT) was carried out using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany). Fifty-five nanograms of total RNA from each sample was used.
Reverse transcription primers represented an equal mixture of three miRNA (miR-371a-3p, miR-372, miR-373-3p)-specific stem-loop-primers from the relevant miRNA assays (Applied Biosystems). The reactions with a final volume of 15 μl were incubated in the GeneAmp PCR-System 2700 (Applied Biosystems) at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min, respectively.
For the 18S rRNA assay, RNA of each sample was reverse transcribed with 200 U μl−1 of M-MLV reverse transcriptase (Invitrogen, Karlsruhe, Germany), RNase-Out, 150 ng random hexamers, 2 μl 0.1 M DTT, 4 μl 5 × first strand buffer and 100 mℳ dNTPs according to the manufacturer’s instructions. RNA was initially denatured at 65°C for 5 min and subsequently kept on ice for 1 min. Reverse transcription was performed at 37°C for 50 min followed by inactivation of the reverse transcriptase at 70°C for 15 min.
On FFPE tumour specimens RT was carried out for miR-371a-3p and RNU6B as endogenous control using the TaqMan MicroRNA Reverse Transcription Kit and stem-loop-primers from the relevant TaqMan microRNA assays (Applied Biosystems) with 200 ng of RNA per sample as described earlier (Rippe et al, 2010).
Preamplification of RT products
Preamplification has been carried out by Murray et al (2011) and we have thus also performed preamplification for serum samples. One microgram of each miRNA assay was diluted in 12 μl nuclease-free water. The PCR with a final volume of 50 μl (12.5 μl of this solution, 12.5 μl of RT product, 25 μl TaqMan Universal PCR Master Mix (Applied Biosystems)) was performed at 95°C for 10 min, followed by 14 cycles of 95°C for 15 s and 60°C for 4 min using the GeneAmp PCR-System 2700 (Applied Biosystems). Endogenous controls were processed in identical manner. The preamplification product was diluted 1 : 5 in nuclease-free water.
Quantitative real-time PCR
For the quantitative real-time PCR, 9 μl of the preamplification product was added to 10 μl TaqMan Universal PCR Master Mix and 1 μl of 20 × TaqMan microRNA assay using the Applied Biosystems 7300 real-time PCR System (Applied Biosystems). The relative quantification was performed with 18S rRNA as endogenous control (Li et al, 2010; Kong et al, 2012). For each sample the reactions were performed in triplicate. A negative control of amplification was performed for each sample without reverse transcriptase. Non-template negative controls for each miRNA and 18S rRNA were included in every plate. PCR conditions were 10 min at 95°C, followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. Data were analysed using the 7300 system software (Applied Biosystems). Cycle threshold (Ct) values were normalised to the internal control, 18S rRNA (Livak and Schmittgen, 2001; Antonov et al, 2005).
The quantitative real-time PCR for FFPE tumour tissue samples was performed without prior preamplification using the TaqMan Universal PCR Master Mix and TaqMan microRNA assay as described earlier (Rippe et al, 2010).
Measurements of serum tumour markers
The serum tumour markers bHCG, AFP and LDH were measured according to laboratory medicine practice guidelines (Sturgeon et al, 2008).
Statistical analysis
Descriptive statistical analysis was performed using Excel software (Microsoft Corp., Redmond, WA, USA). Statistical comparisons of preoperative with postoperative serum levels were performed by using the two-sided Mann–Whitney U-test. We also performed statistical comparisons of serum levels of GCT patients with controls. A P-value of less than 0.05 was considered significant.
Results
miRNAs serum levels in comparison with controls
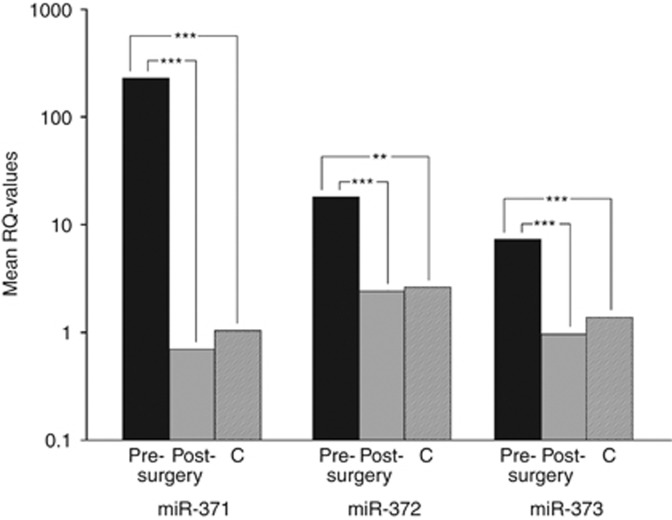
Preoperative serum levels of all three miRNAs were much higher in GCT patients than levels found in controls (Figure 1). These differences were statistically significant with P-values of P<0.0001, P<0.01, and P<0.001 regarding miR-371a-3p, miR-372, and miR-373-3p, respectively. By looking closer, it became clear that individual values varied considerably but values of patients exceeded the mean value of controls in 17, 15, and 16 cases, respectively, for miR-371a-3p, miR-372, and miR-373-3p. Likewise, 16, 8, and 7 cases had serum levels higher than the highest value determined among controls for miR-371a-3p, miR-372, and miR-373-3p. Interestingly, the two teratomas included in this study had serum levels in the range of controls for all three miRNAs whereas 11out of 13 seminomas showed serum levels clearly exceeding those of controls. With regard to classical biomarkers, AFP was increased in four patients and bHCG in two (Table 1).
Figure 1.
Results of the relative miRNA quantifications in GCTs and controls. The mean values of the three miRNAs, miR-371a-3p, miR-372, and miR-373-3p, in serum of 20 patients with GCTs, pre- and postoperatively. C: mean value of 17 controls; **highly significant, ***extremely significant. The y axis is plotted on a log10 scale. 18S rRNA was used as endogenous control and post-surgery sample of case number 1 served as calibrator.
miRNA levels after surgery
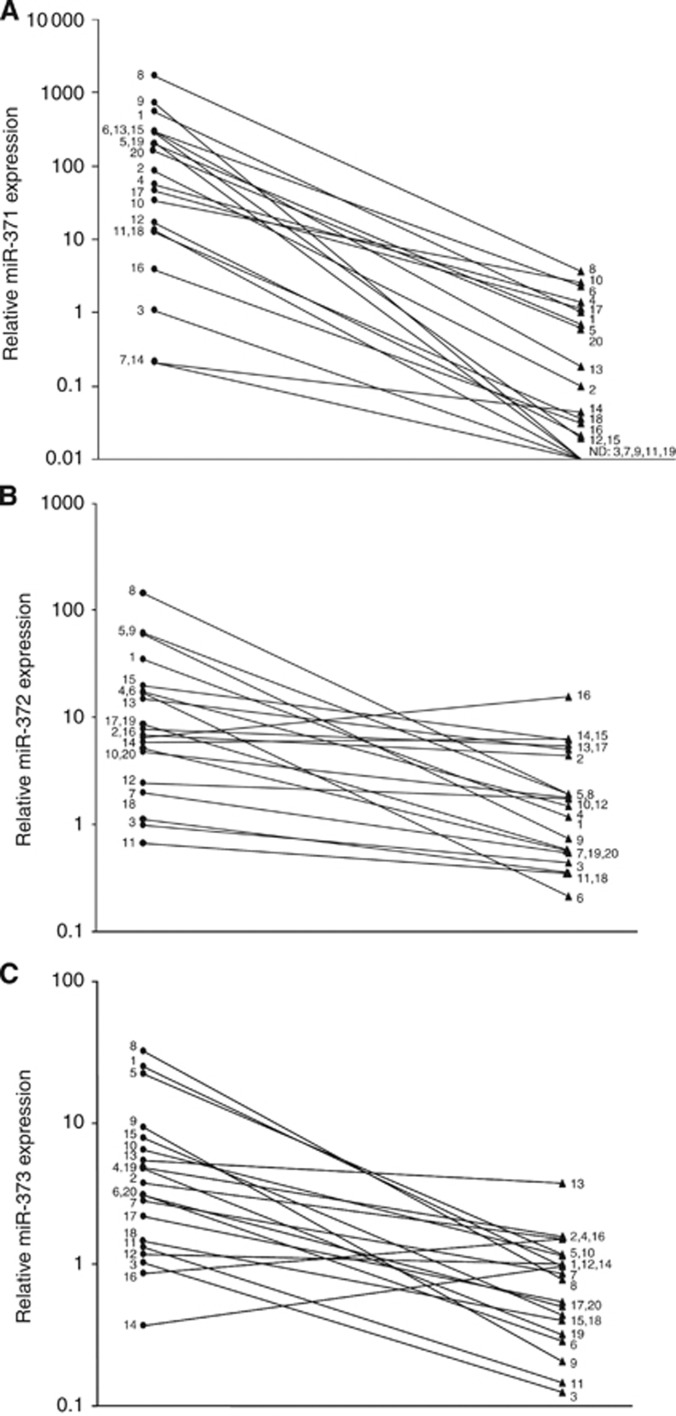
In the stage 1 cases, serum levels of each of the three miRNAs decreased significantly after orchiectomy (Figure 1) with a 336.7-fold (P<0.0001), 7.4-fold (P<0.001), and 7.7-fold (P<0.0001) level reduction for miR-371a-3p, miR-372, and miR-373-3p, respectively. Nevertheless, changes of individual serum levels varied considerably across the sample. The greatest change subsequent to surgery was noted with miR-371a-3p. It is Noteworthy that all of the patients had a decline of this miRNA (Figure 2A). With regard to miR-372 and miR-373-3p, two patients did not show postoperative decline (Figure 2B and C).
Figure 2.
(A–C) Results of miR-371-3 quantification in 20 individual GCT patients with clinical stage 1. Comparison of preoperative ( ) and postoperative (
) and postoperative ( ) miRNA serum levels. (A) miR-371a-3p. (B) miR-372. (C) miR-373-3p. The y axis is plotted on a log10 scale. Case numbers are identical with those in Table 1.
) miRNA serum levels. (A) miR-371a-3p. (B) miR-372. (C) miR-373-3p. The y axis is plotted on a log10 scale. Case numbers are identical with those in Table 1.
miRNA serum levels in TVB
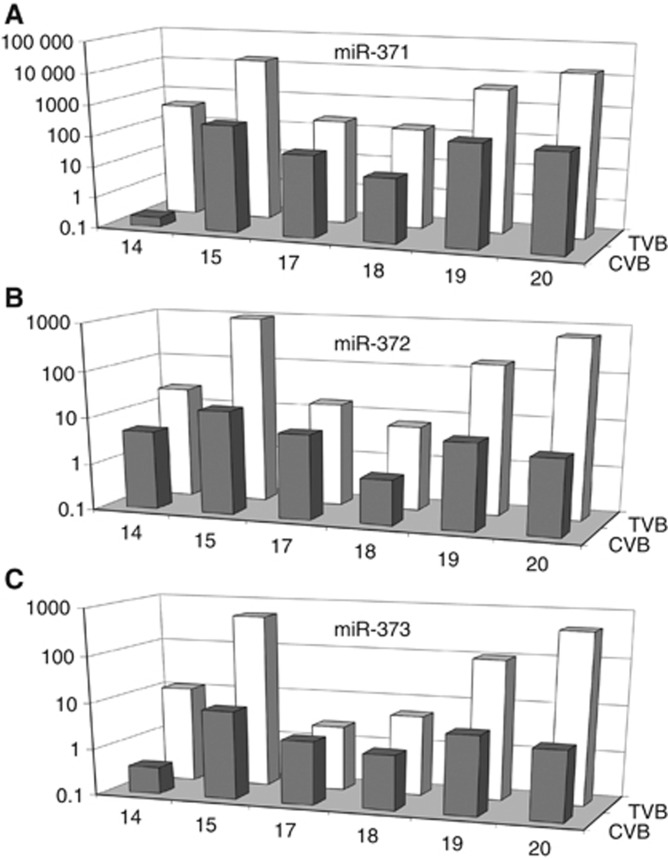
If the elevated miRNA levels as determined in the GCT patients result from their secretion by the tumour one would expect their local concentration in serum of TVB to be higher than in serum of the cubital vein blood. Serum levels of all three miRNAs in TVB clearly exceeded the levels in peripheral blood in all of the six patients evaluated (Figure 3A–C). This result suggests that the level of miRNA expression decreases along a gradient surrounding the tumour.
Figure 3.
Levels of miRNAs 371-3 in TVB in comparison with levels in preoperative cubital vein blood (CVB) in six GCT patients. The y axis is plotted on a log10 scale.
miRNA serum levels during chemotherapy
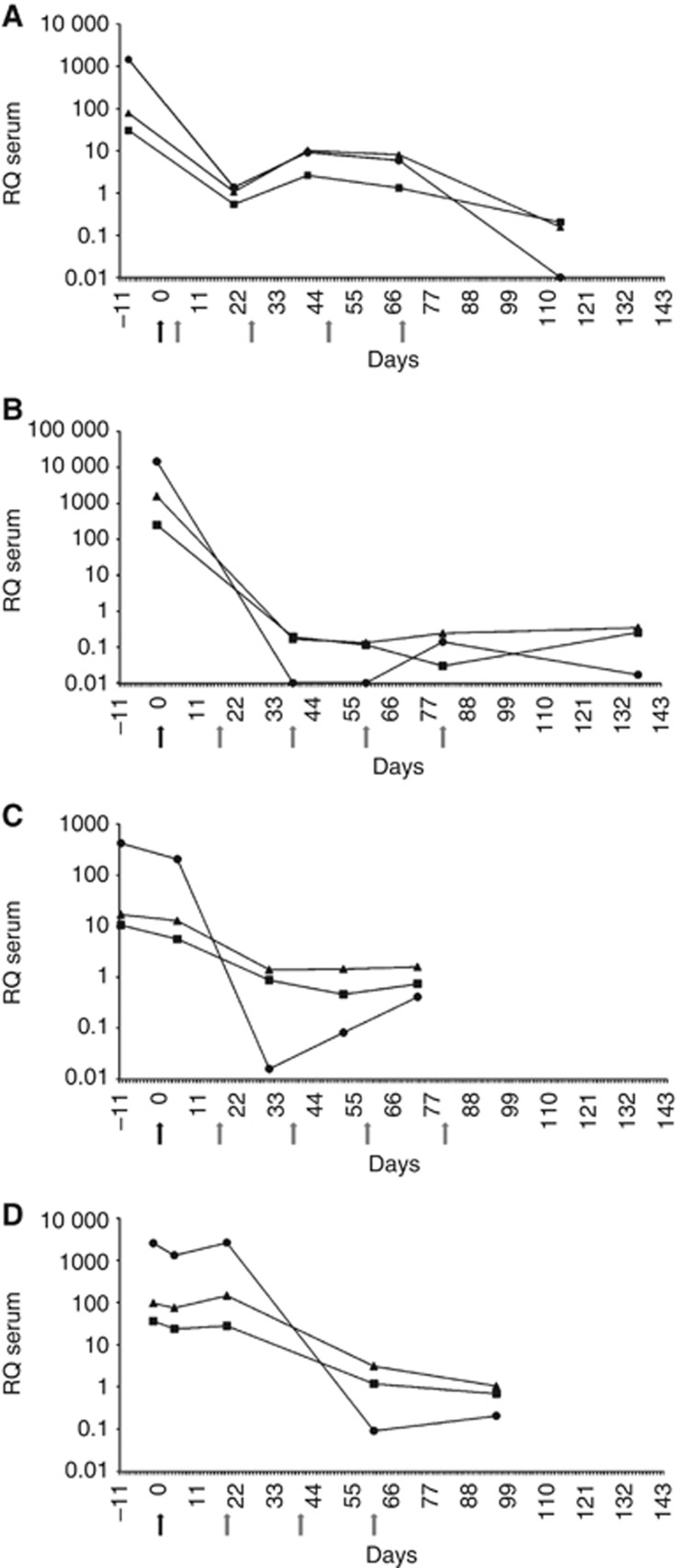
In the four cases with advanced disease, levels decreased to the normal range after the completion of treatment. Two of the cases had the second serum test taken after surgery and the first cycle of chemotherapy (Figure 4A and B), while the other two cases had the second analysis done right after surgery but before initiation of chemotherapy (Figure 4C and D). In these latter cases only a slight decrease of the postoperative levels of miRNAs miR-371a-3p, miR-372, and miR-373-3p was noted (Figure 4C and D). However, a marked reduction occurred after the first cycle of chemotherapy, leading to miRNA levels within the range of controls after completion of therapy akin to the former two cases. Obviously, miRNAs circulating in the serum are not only derived from the primary tumour but also from metastatic tissue.
Figure 4.
(A–D) Results of miR-371-3 quantification in four GCT patients with nonseminoma clinical stage 3 (A, B), stage 2a (C), and stage 2b (D), respectively. Serial measurements of miR-371-3 expression in serum from time of diagnosis to completion of chemotherapy.  : miR-371a-3p.
: miR-371a-3p.  : miR-372.
: miR-372.  : miR-373-3p. The y axis is plotted on a log10 scale. Black arrow indicates the timing of surgery of the primary tumour and grey arrows indicate the timing of chemotherapy cycles.
: miR-373-3p. The y axis is plotted on a log10 scale. Black arrow indicates the timing of surgery of the primary tumour and grey arrows indicate the timing of chemotherapy cycles.
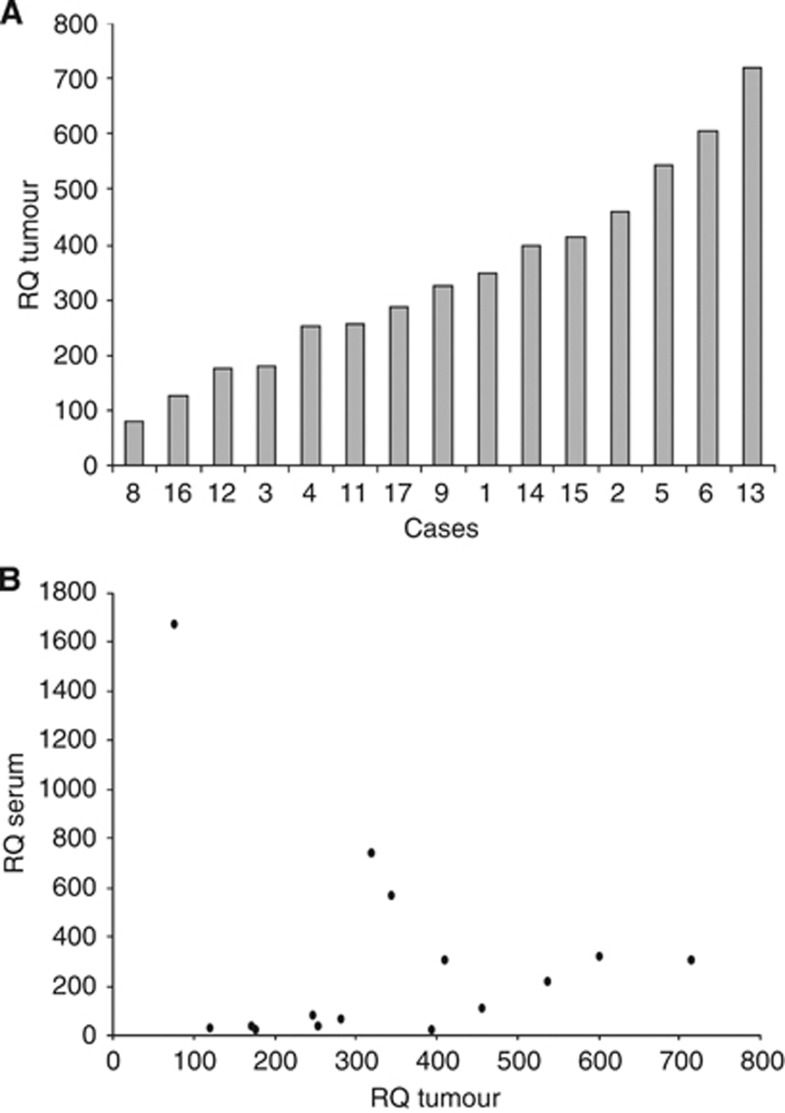
Matching of miRNA expression in tumour tissue with miRNA serum levels
We restricted this analysis to miR-371a-3p because this miRNA had shown the greatest pre-/postoperative changes in serum. The expression of miR-371a-3p varied considerably among the 15 tumour samples (Figure 5A). In matching the miRNA expression level of the tumour tissue with the corresponding serum levels, no obvious correlation was found (Figure 5B). Also, tumour volume was not associated with serum levels of miRNA-371a-3p (data not shown). Thus, the extent of miRNA serum levels does not mirror the expression of the corresponding miRNAs in tumour tissue. So, other unknown factors seem to contribute largely to the release of miRNAs into the patient’s blood.
Figure 5.
Results of the relative level of miR-371a-3p in tumour tissue and in serum of 15 GCT patients, preoperatively. (A) Expression of miRNA-371a-3p in tumour samples. (B) Diagram showing the expression of miR-371a-3p in tumour specimens and its presence in serum, preoperatively, revealing no obvious correlation.
Discussion
The central result of this study is the documentation of increased serum levels of miRNAs miR-371-3 in patients with testicular GCTs followed by their significant decline after the completion of treatment. Of the three miRNAs quantified, miR-371a-3p appears to be the most promising marker because it revealed a considerably high expression, particularly in seminomas and also the most intense postoperative decrease. Therapy-related changes of serum levels of miRNA-371-3 were first demonstrated by Murray et al (2011) in a patient with paediatric GCT. The present results are fully in line with that initial report as all of the stage 1 patients uniformly demonstrated a decline of serum miRNA levels into the range of controls thus being compatible with the presumably tumour-free status after orchiectomy. Moreover, four patients with disseminated disease showed gradual decline of serum levels entering the normal range after completion of treatment. Obviously, the dropping of miRNA serum levels mirrors the decreasing amount of vital tumour tissue during the course of chemotherapy.
The post-surgery measurements had usually been performed within 1 week after orchiectomy. The return of elevated miRNA levels into normal range within that time-span suggests a rapid clearance rate of only few days or even less. This assumption is corroborated by nearly identical findings in most of the patients examined. In fact, the cancer-related elevation of miR-371a-3p serum levels and their rapid clearance subsequent to cancer treatment constitute attributes of a useful biomarker of testicular GCTs. Generally, miRNAs released into body fluids seem to have a great potential as biomarkers of various diseases (Mitchell et al, 2008; Reis et al, 2010; Wittmann and Jäck, 2010; Catto et al, 2011; Li et al, 2011; Murray and Coleman, 2012). In particular, miRNA-141 and miRNA-375 have been suggested to represent robust serum biomarkers of prostatic cancer. With regard to GCTs, members of the miRNA-302 and miRNA-371-3 clusters have been found to be overexpressed in tumour cells (Looijenga et al, 2007; Murray et al, 2010; Palmer et al, 2010; Li et al, 2011). The tumour tissue examinations reported here are in accordance with that experience. miRNA-371-3 serum levels can be measured by widely standardised quantitative real-time PCR techniques. Yet, elevated miRNA serum levels have been reported in no more than a dozen GCT patients so far (Murray et al, 2011; Belge et al, 2012). Currently the finding of divergent expression of miRNA levels in tumour tissue and in corresponding serum remains unresolved. However, much higher miRNA levels in TVB serum than in peripheral blood serum may point to the tumour-bearing testis as the source of blood-level elevation of miR-371a-3p. As pointed out by Murray et al (2011) these features make this molecule a promising biomarker for testicular GCTs.
Nonetheless, still many issues need to be resolved. So far, it is unknown as to how specific the miRNA-371-3 cluster is in regard to GCTs. Actually, these miRNAs have been found overexpressed in thyroid neoplasms, too (Rippe et al, 2010), as a result of chromosomal rearrangements targeting the cluster (Belge et al, 1997). Thus, the presence of thyroid adenomas might instigate false-positive results and should be subject of further studies. Nevertheless, although limited by the still low number of patients evaluated so far, miR-371a-3p apparently involves high sensitivity matching or even exceeding that of the existing markers. Clearly, the three biomarkers currently in use (AFP, bHCG, and LDH) are extremely valuable tools with respect to clinical management of GCT patients (Krege et al, 2008). But, shortcomings result from insufficient specificity with a lack of expression of these biomarkers in major sub-populations of GCT patients. Accordingly, only 20% of seminoma patients have increased bHCG whereas AFP is never elevated in seminomas (Weissbach et al, 1997). In only 60–70% of the patients with nonseminomatous GCTs, one of the classic markers, AFP and bHCG, is elevated (Doherty et al, 1997; Trigo et al, 2000; Barlow et al, 2010). It is noteworthy that only one-quarter of our stage 1 patients (n=5) had elevations of the classical markers whereas all three miRNAs were elevated in three quarters or more of the patients (15–17 of 20 cases).
In GCT new biomarkers are particularly appreciated as the traditional imaging techniques used for clinical follow-up are increasingly disdained because of the untoward radiation exposure to young individuals. In addition, new markers would be useful because international patterns of care are shifting by favouring expectant strategies with no immediate treatment after surgery (Albers et al, 2011).
Perspectives
Ever since the introduction of bHCG and AFP into clinical use, a large variety of new biomarkers have been suggested to aid monitoring GCT patients. But, none has made its way into clinical practice. The use of miRNA serum levels may open up a new dimension of biomarkers combining the prospects of possibly high specificity and sensitivity in GCT patients. The data reported herein are promising, but clearly, much is yet to be done to refine the method, technically, and to define, clinically, what kind of patients might best benefit from it.
Acknowledgments
This study was supported by the Bremer Krebsgesellschaft, the regional branch of the Deutsche Krebsgesellschaft. We acknowledge the technical assistance of Dirk Mumm and laboratory staff of Albertinen-Krankenhaus Zentrallabor in processing serum samples of patients and the helpful advice given by Dr Reinhard Hübotter, Bremen.
Footnotes
The University of Bremen has filed a patent application for the use of miR-371-3 for the diagnosis of tumours with 19q13 rearrangements.
References
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP European Association of Urology (2011) EAU guidelines on testicular cancer: 2011 update. Eur Urol 60: 304–319 [DOI] [PubMed] [Google Scholar]
- Antonov J, Goldstein DR, Oberli A, Baltzer A, Pirotta M, Fleischmann A, Altermatt HJ, Jaggi R (2005) Reliable gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab Invest 85: 1040–1050 [DOI] [PubMed] [Google Scholar]
- Barlow LJ, Badalato GM, McKiernan JM (2010) Serum tumor markers in the evaluation of male germ cell tumors. Nat Rev Urol 7: 610–617 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Belge G, Dieckmann KP, Spiekermann M, Balks T, Bullerdiek J (2012) Serum levels of microRNAs miR-371-3: A novel class of serum biomarkers for testicular germ cell tumors? Eur Urol 61: 1068–1069 [DOI] [PubMed] [Google Scholar]
- Belge G, Garcia E, Rippe V, Fusco A, Bartnitzke S, Bullerdiek J (1997) Breakpoints of 19q13 translocations of benign thyroid tumors map within a 400 kilobase region. Genes Chromosomes Cancer 20: 201–203 [PubMed] [Google Scholar]
- Bosetti C, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Levi F (2011) Trends in mortality from urologic cancers in Europe, 1970-2008. Eur Urol 60: 1–15 [DOI] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catto JW, Alcaraz A, Bjartell AS, De Vere White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L, Schlomm T, Visakorpi T (2011) MicroRNA in prostate, bladder, and kidney cancer: a systematic review. Eur Urol 59: 671–681 [DOI] [PubMed] [Google Scholar]
- Doherty AP, Bower M, Christmas TJ (1997) The role of tumour markers in the diagnosis and treatment of testicular germ cell cancers. Br J Urol 79: 247–252 [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ (2006) Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 6: 259–269 [DOI] [PubMed] [Google Scholar]
- Farazi TA, Spitzer JI, Morozov P, Tuschl T (2011) miRNAs in human cancer. J Pathol 223: 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor I, Neumann A, Freter C, Helmke BM, Langenbuch M, Rippe V, Bullerdiek J (2012) Abundant expression and hemimethylation of C19MC in cell cultures from placenta-derived stromal cells. Biochem Biophys Res Commun 422: 411–416 [DOI] [PubMed] [Google Scholar]
- Gillis A, Stopp H, Hermus R, Oosterhuis J, Sun Y, Chen C, Guenther S, Sherlock J, Veltma I, B, aeten J (2007) High throughput microRNAome analysis in human germ cell tumors. J Pathol 213: 319–328 [DOI] [PubMed] [Google Scholar]
- Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, Qin YR, Guan XY (2012) MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut 61: 33–42 [DOI] [PubMed] [Google Scholar]
- Krege S, Beyer J, Souchon R, Albers P, Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C, Cavallin-Stahl E, Classen J, Clemm C, Cohn-Cedermark G, Culine S, Daugaard G, De Mulder PH, De Santis M, de Wit M, de Wit R, Derigs HG, Dieckmann KP, Dieing A, Droz JP, Fenner M, Fizazi K, Flechon A, Fossa SD, del Muro XG, Gauler T, Geczi L, Gerl A, Germa-Lluch JR, Gillessen S, Hartmann JT, Hartmann M, Heidenreich A, Hoeltl W, Horwich A, Huddart R, Jewett M, Joffe J, Jones WG, Kisbenedek L, Klepp O, Kliesch S, Koehrmann KU, Kollmannsberger C, Kuczyk M, Laguna P, Galvis OL, Loy V, Mason MD, Mead GM, Mueller R, Nichols C, Nicolai N, Oliver T, Ondrus D, Oosterhof GO, Ares LP, Pizzocaro G, Pont J, Pottek T, Powles T, Rick O, Rosti G, Salvioni R, Scheiderbauer J, Schmelz HU, Schmidberger H, Schmoll HJ, Schrader M, Sedlmayer F, Skakkebaek NE, Sohaib A, Tjulandin S, Warde P, Weinknecht S, Weissbach L, Wittekind C, Winter E, Wood L, von der Maase H (2008) European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): part I. Eur Urol 53: 478–496 [DOI] [PubMed] [Google Scholar]
- Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, Borges M, Goggins M (2010) Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res 70: 5226–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen J, Hu X, Huang Y, Li Z, Zhou L, Tian Z, Ma H, Wu Z, Chen M, Han Z, Peng Z, Zhao X, Liang C, Wang Y, Sun L, Chen J, Zhao J, Jiang B, Yang H, Gui Y, Cai Z, Zhang X (2011) Comparative mRNA and microRNA expression profiling of three genitourinary cancers reveals common hallmarks and cancer-specific molecular events. PLoS One 6: e22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Gillis AJ, Stoop H, Hersmus R, Oosterhuis JW (2007) Relevance of microRNAs in normal and malignant development, including human testicular germ cell tumours. Int J Androl 30: 304–314 [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Coleman N (2012) Testicular cancer: a new generation of biomarkers for malignant germ cell tumours. Nat Rev Urol 9: 298–300 [DOI] [PubMed] [Google Scholar]
- Murray MJ, Halsall DJ, Hook CE, Williams DM, Nicholson JC, Coleman N (2011) Identification of microRNAs from the miR-371∼373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am J Clin Pathol 135: 119–125 [DOI] [PubMed] [Google Scholar]
- Murray MJ, Saini HK, van Dongen S, Palmer RD, Muralidhar B, Pett MR, Piipari M, Thornton CM, Nicholson JC, Enright AJ, Coleman N (2010) The two most common histological subtypes of malignant germ cell tumour are distinguished by global microRNA profiles, associated with differential transcription factor expression. Mol Cancer 9: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefevre A, Coullin P, Moore GE, Cavaille J (2010) The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet 19: 3566–3582 [DOI] [PubMed] [Google Scholar]
- Palmer RD, Murray MJ, Saini HK, van Dongen S, Abreu-Goodger C, Muralidhar B, Pett MR, Thornton CM, Nicholson JC, Enright AJ, Coleman N, Children's C, Leukaemia G (2010) Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res 70: 2911–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LO, Pereira TC, Lopes-Cendes I, Ferreira U (2010) MicroRNAs: a new paradigm on molecular urological oncology. Urology 76: 521–527 [DOI] [PubMed] [Google Scholar]
- Rippe V, Dittberner L, Lorenz VN, Drieschner N, Nimzyk R, Sendt W, Junker K, Belge G, Bullerdiek J (2010) The two stem cell microRNA gene clusters C19MC and miR-371-3 are activated by specific chromosomal rearrangements in a subgroup of thyroid adenomas. PLoS One 5: e9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, Babaian R, Bast RC, Dowell B, Esteva FJ, Haglund C, Harbeck N, Hayes DF, Holten-Andersen M, Klee GG, Lamerz R, Looijenga LH, Molina R, Nielsen HJ, Rittenhouse H, Semjonow A, Shih Ie M, Sibley P, Soletormos G, Stephan C, Sokoll L, Hoffman BR, Diamandis EP National Academy of Clinical B (2008) National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem 54: e11–e79 [DOI] [PubMed] [Google Scholar]
- Trigo JM, Tabernero JM, Paz-Ares L, Garcia-Llano JL, Mora J, Lianes P, Esteban E, Salazar R, Lopez-Lopez JJ, Cortes-Funes H (2000) Tumor markers at the time of recurrence in patients with germ cell tumors. Cancer 88: 162–168 [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R (2006) A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124: 1169–1181 [DOI] [PubMed] [Google Scholar]
- Wei Q, Sun Z, He X, Tan T, Lu B, Guo X, Su B, Ji W (2011) Derivation of rhesus monkey parthenogenetic embryonic stem cells and its microRNA signature. PLoS One 6: e25052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach L, Bussar-Maatz R, Mann K (1997) The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur Urol 32: 16–22 [PubMed] [Google Scholar]
- Wittmann J, Jack HM (2010) Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta 1806: 200–207 [DOI] [PubMed] [Google Scholar]