Background: Rim21, Dfg16, and Rim9 are considered to form a pH sensor machinery in yeast.
Results: Transient degradation of Rim21 abolished pH sensing, whereas that of Dfg16 and Rim9 did not.
Conclusion: Rim21 is the sensor protein that detects ambient pH.
Significance: Elucidating the ambient pH-sensing mechanism is essential for understanding the adaptation of fungi, including fungal pathogens, to their local environment.
Keywords: Lipids, Membrane Bilayer, Membrane Lipids, Membrane Proteins, pH Regulation, Yeast, Lipid Asymmetry, Rim101 Pathway, pH Sensing
Abstract
External alkalization activates the Rim101 pathway in Saccharomyces cerevisiae. In this pathway, three integral membrane proteins, Rim21, Dfg16, and Rim9, are considered to be the components of the pH sensor machinery. However, how these proteins are involved in pH sensing is totally unknown. In this work, we investigated the localization, physical interaction, and interrelationship of Rim21, Dfg16, and Rim9. These proteins were found to form a complex and to localize to the plasma membrane in a patchy and mutually dependent manner. Their cellular level was also mutually dependent. In particular, the Rim21 level was significantly decreased in dfg16Δ and rim9Δ cells. Upon external alkalization, the proteins were internalized and degraded. We also demonstrate that the transient degradation of Rim21 completely suppressed the Rim101 pathway but that the degradation of Dfg16 or Rim9 did not. This finding strongly suggests that Rim21 is the pH sensor protein and that Dfg16 and Rim9 play auxiliary functions through maintaining the level of Rim21 and assisting in its plasma membrane localization. Even without external alkalization, the Rim101 pathway was activated in a Rim21-dependent manner by either protonophore treatment or depletion of phosphatidylserine in the inner leaflet of the plasma membrane, both of which caused plasma membrane depolarization like the external alkalization. Therefore, plasma membrane depolarization seems to be one of the key signals for the pH sensor molecule Rim21.
Introduction
Microorganisms that grow over a wide pH range, such as the yeast Saccharomyces cerevisiae and the filamentous fungi Aspergillus nidulans, must be capable of sensing and responding properly to changes in their ambient pH (1, 2). The adaptation to ambient pH is critical not only for survival but also for the pathogenicity of both animal and plant pathogens (3). Thus, the understanding of such adaptation mechanisms is important for a broad range of sciences, including biological, medical, and agricultural sciences.
External pH regulates expression of genes encoding secreted enzymes, permeases, and proteins involved in intracellular pH homeostasis (4–7). In yeast, the Rim101 pathway is known to mediate its adaptation to an alkaline environment, leading to expression of alkaline response genes (2). Upon stimulation of this pathway, the transcription factor Rim101 undergoes proteolytic activation by the calpain-like protease Rim13. The activated Rim101 induces alkaline response genes indirectly through repressing expression of transcription repressors such as Nrg1 and Smp1 (5, 6). Besides Rim13, three integral membrane proteins, Rim21, Dfg16, and Rim9, are essential for the Rim101 pathway (8–10). Epistatic analysis suggested that these proteins act in the most upstream of this pathway, probably sensing external alkalization (11). The arrestin-like protein Rim8 is also an essential component. Arrestin-like proteins are often involved in internalization of plasma membrane receptors and transporters via endocytosis (12, 13), although the involvement of endocytosis in the Rim101 pathway is currently under debate. In addition to these proteins, several ESCRT (endosomal sorting complex required for transport) proteins are necessary for the Rim101 pathway. ESCRT proteins, originally reported as proteins required for vacuolar protein sorting, function as subunits of large complexes (ESCRT-0, -I, -II, and -III) that are recruited in a sequential manner onto the surface of the endosome (14). Of them, ESCRT-I subunits Stp22/Vps23 and Vps28; ESCRT-II subunits Snf8/Vps22, Vps25, and Vps36; and ESCRT-III subunits Snf7 and Vps20 have been shown to be required for the Rim101 pathway (15). It has been reported that the Rim101 pathway is constitutively activated in did4Δ, vps24Δ, and vps4Δ mutants (11). These mutants lack Did4, Vps24, and Vps4, which are involved in the disassembly of the ESCRT-III complex, thus accumulating the Snf7-Vps20 subcomplex of ESCRT-III on the endosome (16). Comprehensive analysis of protein interaction suggested that Snf7 interacts with Rim20, which binds to Rim101 (17, 18), as well as with Rim13 (17, 19). Based on these findings, it was proposed that Rim101 is proteolytically cleaved by Rim13 in a large complex composed of Rim20, Snf7-Vps20, and Rim13 (11, 18).
In contrast to the proteolytic activation process of Rim101, the pH-sensing mechanism is mostly unknown. The integral membrane proteins Rim21, Dfg16, and Rim9 are thought to be potential sensor molecules, although their functional information is quite limited. Their biochemical information has also not been reported, including their cellular level, intracellular localization, post-translational modification, interrelationship, and their fate upon external alkalization. In A. nidulans, the integral membrane protein PalH is considered to be a pH sensor molecule (20, 21). PalH localizes predominantly to the plasma membrane when co-overexpressed with another membrane protein, PalI, the A. nidulans counterpart of Rim9, suggesting that PalI assists in the plasma membrane localization of PalH (22). Rim21 and Dfg16 are possible candidates for the yeast counterpart of PalH. However, their sequence homologies with PalH are relatively low (27% for Rim21 and 19% for Dfg16), and hence, their functional role is still elusive.
Recently, we reported that the Rim101 pathway senses altered lipid asymmetry in the plasma membrane as well as external alkalization (23). Like the pH-sensing mechanism, the lipid asymmetry-sensing mechanism is also unknown. To gain insight into both sensing mechanisms, biochemical characterization of the putative sensor proteins Rim21, Dfg16, and Rim9 is of the utmost importance. In this work, we demonstrate that these proteins form a complex and localize to the plasma membrane in a patchy and mutually dependent manner. Using a transient protein degradation system, we identified that Rim21 is the pH sensor protein and that Dfg16 and Rim9 play auxiliary roles through maintaining the level of Rim21 and, presumably, through assisting in its plasma membrane localization. Furthermore, we demonstrate that plasma membrane depolarization stimulates the Rim101 pathway through Rim21 even without external alkalization.
EXPERIMENTAL PROCEDURES
Yeast Strains and Media
The S. cerevisiae strains used in this study are listed in Table 1. Yeast cells were grown at 30 °C to log phase either in 1% yeast extract, 2% peptone, and 2% d-glucose or in synthetic dextrose medium (2% d-glucose and 0.67% yeast nitrogen base without amino acids) with the appropriate supplements. Alkaline treatment was performed by the addition of 1 m Tris-HCl (pH 8.0) to the medium at a final concentration of 100 mm. A 500 mm stock solution of 3-indoleacetic acid (IAA2; Nacalai Tesque, Kyoto, Japan) was prepared in ethanol and added to the medium at a final concentration of 500 μm. Because the conversion of phosphatidylserine (PS) to phosphatidylethanolamine (PE) is the major pathway for PE synthesis in yeast, CHO1-deleted cells that lack PS show a significant reduction in PE and are thus inviable in synthetic dextrose medium (24). Therefore, KCY1113 (cho1Δ) cells were grown in synthetic dextrose medium with the appropriate supplements and 1 mm ethanolamine (the precursor in an alternative pathway to PE). A 5 mm stock solution of carbonyl cyanide m-chlorophenylhydrazone (CCCP) was prepared in ethanol. For the CCCP treatment, cells were grown to log phase in synthetic dextrose medium with the appropriate supplements at pH 5.5 (buffered with 50 mm sodium citrate/HCl), and CCCP was added to the medium at a final concentration of 10 μm.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα his3 leu2 ura3 trp1 lys2 suc2 | Ref. 37 |
| YOK2027 | SEY6210, rim21Δ::KanMX4 | This study |
| YOK2053 | SEY6210, rim21Δ::NatNT2 | This study |
| YOK2054 | SEY6210, dfg16Δ::NatNT2 | This study |
| YOK2055 | SEY6210, rim9Δ::NatNT2 | This study |
| YOK2095 | SEY6210, rim21Δ::NatNT2 dfg16Δ::KanMX4 | This study |
| YOK2096 | SEY6210, rim21Δ::NatNT2 rim9Δ::KanMX4 | This study |
| YOK2097 | SEY6210, dfg16Δ::NatNT2 rim9Δ::KanMX4 | This study |
| YOK3208 | SEY6210, RIM21-2×EGFP::KanMX6 | This study |
| YOK3209 | SEY6210, RIM21-2×EGFP::KanMX6 dfg16Δ::NatNT2 | This study |
| YOK3210 | SEY6210, RIM21-2×EGFP::KanMX6 rim9Δ::NatNT2 | This study |
| YOK3211 | SEY6210, RIM21-2×EGFP::KanMX6 lem3Δ::NatNT2 | This study |
| YOK2743 | SEY6210, RIM21-EGFP::NatNT2 pil1Δ::KanMX4 | This study |
| YOK2210 | SEY6210, DFG16-EGFP::NatNT2 | This study |
| YOK2211 | SEY6210, DFG16-EGFP::NatNT2 rim21Δ::KanMX4 | This study |
| YOK2212 | SEY6210, DFG16-EGFP::NatNT2 rim9Δ::KanMX4 | This study |
| YOK2214 | SEY6210, DFG16-EGFP::NatNT2 lem3Δ::KanMX4 | This study |
| YOK2744 | SEY6210, DFG16-EGFP::NatNT2 pil1Δ::KanMX4 | This study |
| YOK2729 | SEY6210, DFG16-EGFP::NatNT2 PIL1-mCherry::KanMX6 | This study |
| YOK2229 | SEY6210, RIM9-EGFP::NatNT2 | This study |
| YOK2215 | SEY6210, RIM9-EGFP::NatNT2 rim21Δ::KanMX4 | This study |
| YOK2216 | SEY6210, RIM9-EGFP::NatNT2 dfg16Δ::KanMX4 | This study |
| YOK2218 | SEY6210, RIM9-EGFP::NatNT2 lem3Δ::KanMX4 | This study |
| YOK2745 | SEY6210, RIM9-EGFP::NatNT2 pil1Δ::KanMX4 | This study |
| YOK2731 | SEY6210, RIM9-EGFP::NatNT2 PIL1-mCherry::KanMX6 | This study |
| YOK2559 | SEY6210, RIM21-HA::KanMX6 | This study |
| YOK2560 | SEY6210, DFG16-HA::KanMX6 | This study |
| YOK2561 | SEY6210, RIM9-HA::KanMX6 | This study |
| YOK2540 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 RIM21-HA::TRP1 | This study |
| YOK2546 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 RIM21-HA::TRP1 DFG16-FLAG::KanMX6 | This study |
| YOK2547 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 RIM21-HA::TRP1 RIM9-FLAG::KanMX6 | This study |
| YOK2542 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 RIM9-HA::TRP1 | This study |
| YOK2548 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 RIM9-HA::TRP1 DFG16-FLAG::KanMX6 | This study |
| KCY662 | SEY6210, RSB1-HA::TRP1 | Ref. 23 |
| YOK2098 | SEY6210, RSB1-HA::TRP1 rim21Δ::KanMX4 | This study |
| KCY692 | SEY6210, RSB1-HA::TRP1 lem3Δ::HIS3 | Ref. 23 |
| KCY1113 | SEY6210, RSB1-HA::TRP1 cho1Δ::KanMX4 | This study |
| YOK2541 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 DFG16-HA::TRP1 | This study |
| YOK2738 | SEY6210, pep4Δ::LEU2 prb1Δ::NatNT2 DFG16-HA::TRP1 RIM21-FLAG::KanMX6 | This study |
| BY25598 | MATa his3 leu2 ura3 trp1 ade2 can1 PADH-OsTIR1-Myc::URA3 | Ref. 31 |
| YOK2848 | BY25598, RIM21-FLAG-AID::KanMX6 | This study |
| YOK2891 | BY25598, RIM21-FLAG-AID::KanMX6 DFG16-HA::TRP1 | This study |
| YOK2892 | BY25598, RIM21-FLAG-AID::KanMX6 RIM9-HA::TRP1 | This study |
| YOK2849 | BY25598, DFG16-FLAG-AID::KanMX6 | This study |
| YOK2846 | BY25598, RIM9-HA-AID::KanMX6 | This study |
Genetic Manipulation
Gene disruption was performed by replacing the entire coding region of the gene with a marker gene. Chromosomal fusion of HA, FLAG, GFP, 2×GFP, mCherry, HA-auxin-inducible degron (AID), and FLAG-AID to the C terminus was performed using PCR-based gene disruption and modification (25). The sequence encoding a respective tag, the ADH1 termination sequence, and a marker sequence (KanMX6 or TRP1) was amplified by PCR from a pFA6a vector series (25) with a primer set containing the homologous region of the target gene. Amplified cassettes were inserted directly into the chromosome by homologous recombination.
Plasmid Construction
To construct plasmids for the expression of Rim21-HA in yeast cells, the promoter region of RIM21, the RIM21-coding sequence, the HA sequence, and the 3′-UTR of RIM21 were cloned in tandem into pRS313 and pRS423 (26) to generate pOK313 and pOK315, respectively. A linker sequence (two repeats of the sequence encoding GGGS) was added before the coding sequence for HA. A multicopy plasmid overexpressing Rim21-FLAG (pOK311) was constructed in the same manner, except that the FLAG sequence was cloned instead of the HA sequence. To construct a multicopy plasmid overexpressing Rim21-HA and carrying the TRP1 marker, the PvuII fragment derived from pOK313 was ligated into the PvuII site of pRS424 to yield pOK328. The plasmid encoding Dfg16-HA (pOK314) was constructed in the same manner as the pOK313 plasmid. To construct a multicopy plasmid overexpressing Dfg16-FLAG, the promoter region of DFG16, the DFG16-coding sequence, and the FLAG sequence were cloned in tandem into pRS424 to generate pOK330. To construct RIM9-HA plasmids, the promoter region of RIM9, the RIM9-coding sequence, and the HA sequence were cloned in tandem into pRS313 and pRS423 to generate pOK318 and pOK320, respectively. The SUC2-RIM21-FLAG plasmid was constructed as follows. The BamHI site was introduced after the start codon of RIM21 into pOK311 using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) to produce pOK377. The SUC2 sequence was amplified by PCR from pSH69 (27) to have BamHI sites at both the 5′- and 3′-ends, and cloned into the BamHI site of pOK377 to generate pOK387. The plasmid for the expression of Rim21-HA lacking the C-terminal region was constructed as follows. The promoter region of RIM21 with part of the RIM21-coding sequence (positions 1–1362) was amplified from yeast genomic DNA to have a linker sequence (two repeats of the sequence encoding GGGS) after position 1362. The promoter region and coding sequence of RIM21 was excised from pOK313, and the amplified PCR fragment was instead cloned into the same site to generate pOK419. The plasmid for the expression of HA-Rim101 (pFI1) (11) was a kind gift from Dr. T. Maeda (Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo, Japan).
Dephosphorylation and Deglycosylation
Cell lysates were prepared by the alkaline-TCA method as described previously (28), except in λ-protein phosphatase (λ-PPase) buffer (1× λ-PPase buffer and 1× MnCl2 solution; New England Biolabs, Beverly, MA) supplemented with 1 mm PMSF. Each lysate was dived into two halves, and λ-PPase (New England Biolabs) was added to one of them to a final concentration of 20 units/μl. The lysate with or without λ-PPase was incubated at 30 °C for 40 min with occasional mixing by tapping. Subsequently, a one-third volume of 4× SDS sample buffer was added, and the mixture was incubated for an additional 10 min at 37 °C. After dilution with a 4× volume of endoglycosidase H (Endo H) buffer (62.5 mm sodium citrate (pH 5.5) and 1.25 mm PMSF), Endo H (Endo Hf, New England Biolabs) was added to the final concentration of 20 units/μl, and the mixture was incubated at 37 °C for 1 h with occasional mixing by tapping. The lysate was then treated with an appropriate volume of 4× SDS sample buffer at 37 °C for 10 min.
Immunoblot Analysis
Proteins were separated by SDS-PAGE and transferred to ImmobilonTM polyvinylidene difluoride membrane (Millipore, Billerica, MA) as described previously (29). The membrane was incubated with anti-FLAG (M2, Stratagene), anti-HA (Y-11, Santa Cruz Biotechnology, Santa Cruz, CA; or 3F10, Roche Diagnostics), or anti-Pgk1 (Molecular Probes, Eugene, OR) antibody. Immunodetection was performed using ECL Plus (GE Healthcare) or the Western Lightning ECL Pro system (PerkinElmer Life Sciences) with a LAS4000 bioimaging analyzer (Fujifilm, Tokyo) or x-ray films. Can Get Signal® immunoreaction enhancer solution (TOYOBO, Osaka, Japan) was used for anti-HA antibody Y-11.
Co-immunoprecipitation
Cells with or without 5 min of alkaline treatment were broken by mixing vigorously with glass beads at 4 °C for 10 min using lysis buffer (50 mm Hepes-NaOH (pH 8.0 for alkali-treated cells or pH 6.8 for non-treated cells), 150 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, 1 mm PMSF, and EDTA-free protease inhibitor mixture (Complete, Roche Diagnostics)). The cell lysate was sonicated and centrifuged at 1000 × g for 5 min to remove debris prior to treatment with 1% Triton X-100 and 1% digitonin at 4 °C for 1 h. The mixture was then centrifuged at 100,000 × g for 30 min, and the supernatant was incubated with anti-FLAG M2-agarose (Sigma) while rotating at 4 °C for 2 h. The beads were washed three times with lysis buffer containing 0.1% Triton X-100 and 0.1% digitonin. The bound proteins were eluted with SDS sample buffer and separated by SDS-PAGE.
Microscopy
The intracellular localization of GFP- and mCherry-tagged proteins was observed using an inverted fluorescence microscope (IX-81, Olympus, Tokyo) equipped with an electron-multiplying CCD camera (ImageEM C9100-13, Hamamatsu Photonics, Hamamatsu, Japan). For the simultaneous observation of GFP and mCherry fusion proteins, cells were excited simultaneously with blue (20 milliwatt, Spectra-Physics, Santa Clara, CA) and yellow (25 milliwatt, Cobalt Laser, Orlando, FL) lasers. Fluorescence was filtered with a Di01-R488/561-25 dichroic mirror (Semrock, Rochester, New York) and an Em01-R488/568-25 band-pass filter (Semrock) and separated into two channels using a U-SIP splitter (Olympus) equipped with a DM565HQ dichroic mirror (Olympus). The fluorescence was further filtered with an FF02-525/50-25 band-pass filter (Semrock) to analyze GFP and an FF01-624/40-25 band-pass filter (Semrock) to analyze mCherry.
RESULTS
Rim21, Dfg16, and Rim9 Mutually Affect Their Protein Levels and Phosphorylation Status
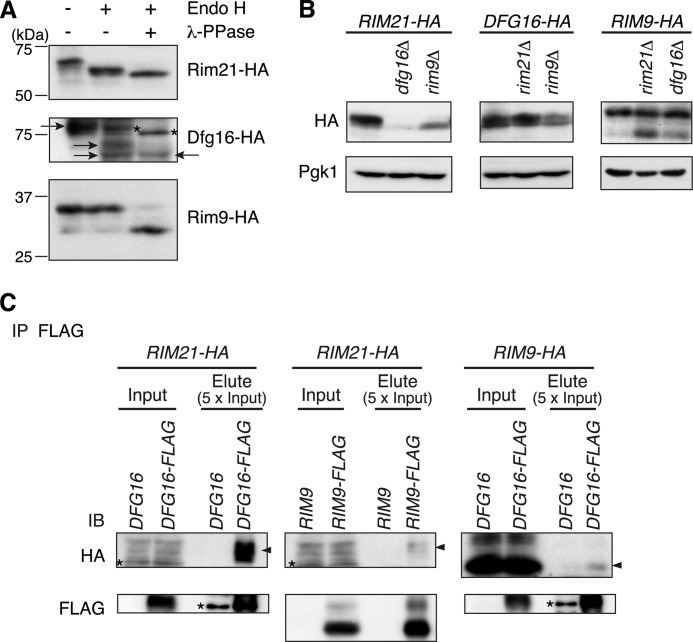
We first investigated the post-translational modification and cellular level of Rim21, Dfg16, and Rim9 by immunoblot analysis of expressed HA-tagged Rim21, Dfg16, and Rim9. Rim21-HA was detected as a 68-kDa band by SDS-PAGE, which was shifted stepwise to lower molecular masses by sequential treatment with Endo H and λ-PPase (Fig. 1A). Thus, Rim21 undergoes post-translational glycosylation and phosphorylation. Dfg16-HA was detected as an 80-kDa band. Upon incubation with Endo H, the band was shifted to two faster migrating bands, the slower of which disappeared after λ-PPase treatment. Thus, like Rim21, Dfg16 is both glycosylated and phosphorylated. Rim9-HA was detected predominantly as a 33-kDa band along with a faint 29-kDa band. Endo H treatment had no effect on these two bands, whereas incubation with λ-PPase caused a mobility shift of the 33-kDa band merging to the 29-kDa band. This suggests that Rim9 is a non-glycosylated phosphoprotein.
FIGURE 1.
Rim21, Dfg16, and Rim9 are mutually dependent in the Rim101 pathway. A, total lysates were prepared from YOK2027 (rim21Δ), YOK2054 (dfg16Δ), and YOK2055 (rim9Δ) cells harboring pOK313 (RIM21-HA), pOK314 (DFG16-HA), and pOK318 (RIM9-HA), respectively, and treated with or without Endo H and λ-PPase. Immunoblotting was performed with anti-HA antibody. Arrows indicate Dfg16-HA, and asterisks indicate nonspecific bands. B, total lysates were prepared from YOK2027, YOK2095 (rim21Δ dfg16Δ), and YOK2096 (rim21Δ rim9Δ) cells containing pOK313 (RIM21-HA); YOK2054, YOK2095, and YOK2097 (dfg16Δ rim9Δ) cells containing pOK314 (DFG16-HA); and YOK2055, YOK2096, and YOK2097 cells containing pOK318 (RIM9-HA). After Endo H treatment, immunoblotting was performed with anti-HA antibody or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. C, total lysates were prepared from YOK2540 (RIM21-HA), YOK2546 (RIM21-HA DFG16-FLAG), YOK2547 (RIM21-HA RIM9-FLAG), YOK2542 (RIM9-HA), and YOK2548 (RIM9-HA DFG16-FLAG) cells. Immunoblotting of co-precipitated proteins with anti-FLAG M2-agarose was performed with anti-HA and anti-FLAG antibodies. Arrowheads indicate co-immunoprecipitates, and asterisks indicate nonspecific bands. IB, immunoblot; IP, immunoprecipitation.
We then examined their cellular level in each deletion mutant after Endo H treatment. The Rim21-HA level was significantly reduced in dfg16Δ and rim9Δ mutant cells, particularly in dfg16Δ cells (Fig. 1B), indicating that Dfg16 and Rim9 are required for maintenance of the cellular level of Rim21. Rim9 also plays some role in maintaining Dfg16-HA because the Dfg16-HA level was slightly reduced in rim9Δ cells but not in rim21Δ cells. Both rim21Δ and dfg16Δ mutants had no effect on the phosphorylated level of Rim9-HA, but its non-phosphorylated form was increased. The results indicate that these proteins mutually influence their protein levels and phosphorylation status, implying their close interrelationship in the Rim101 pathway. The sites and functional significance of these phosphorylations are currently unknown and will be the subject of future investigations.
Given such a close interrelationship, we further investigated their possible physical interactions. The interaction of Dfg16 with Rim21 and Rim9 was first examined by co-immunoprecipitation experiments using Dfg16-FLAG and anti-FLAG antibody. Rim21-HA and Rim9-HA were co-immunoprecipitated with Dfg16-FLAG with high and low efficiency, respectively (Fig. 1C). Next, Rim9-FLAG was used to study the interaction between Rim9 and Rim21. Rim21-HA was co-immunoprecipitated with Rim9-FLAG but with low efficiency. The observed low co-immunoprecipitation efficiency involving Rim9 might be a result of its dissociation in solubilized lysates. Almost identical results were obtained with alkali-treated cells (supplemental Fig. S1). These findings suggest that Rim21, Dfg16, and Rim9 could form a pH-sensing complex in the Rim101 pathway.
Rim21 and Dfg16 Play Distinct Roles in the Rim101 Pathway
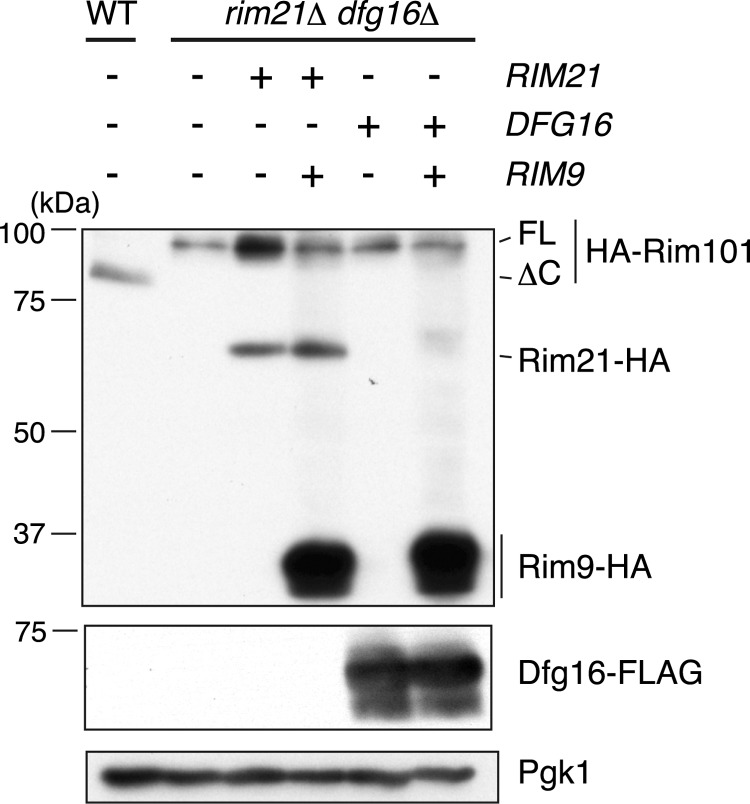
The functional relationship of Rim21 and Dfg16 is elusive at present. If one acts as the sensor subunit and the other acts as the regulatory subunit, the overexpression of the main component might bypass the requirement of the other. To test this possibility, the Rim101 pathway was monitored by the presence of proteolytically processed Rim101 in rim21Δ dfg16Δ double mutant cells after overexpression of either Rim21 or Dfg16. In alkali-treated WT cells, virtually all Rim101 was found in its processed form, indicative of the activation of the Rim101 pathway (Fig. 2). In rim21Δ dfg16Δ double mutants harboring empty vectors, the activation of the Rim101 pathway was totally abolished. Overexpression of neither Rim21 nor Dfg16 restored the activation of the Rim101 pathway. The Rim9 homolog PalI in A. nidulans is known to assist in the proper localization of the likely pH sensor PalH (22). Accordingly, Rim9 was co-overexpressed with either Rim21 or Dfg16, but the activation of the Rim101 pathway was still not restored. These results suggest that both Rim21 and Dfg16 possess distinct and indispensable roles in the Rim101 pathway.
FIGURE 2.
Functions of Rim21 and Dfg16 are irreplaceable by each other in the Rim101 pathway. After a 20-min alkaline treatment, total lysates were prepared from SEY6210 (WT) and YOK2095 (rim21Δ dfg16Δ) cells harboring pFI1 (HA-RIM101) and empty vectors (pRS423 and pRS424) with the indicated combination of plasmids for overexpression of Rim21-HA (pOK315 or pOK328), Dfg16-FLAG (pOK330), and Rim9-HA (pOK320). After Endo H treatment, immunoblotting was performed with anti-HA and anti-FLAG antibodies or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. FL, full-length; ΔC, Rim101ΔC.
Rim21, Dfg16, and Rim9 Localize to the Plasma Membrane in a Patchy and Mutually Dependent Manner
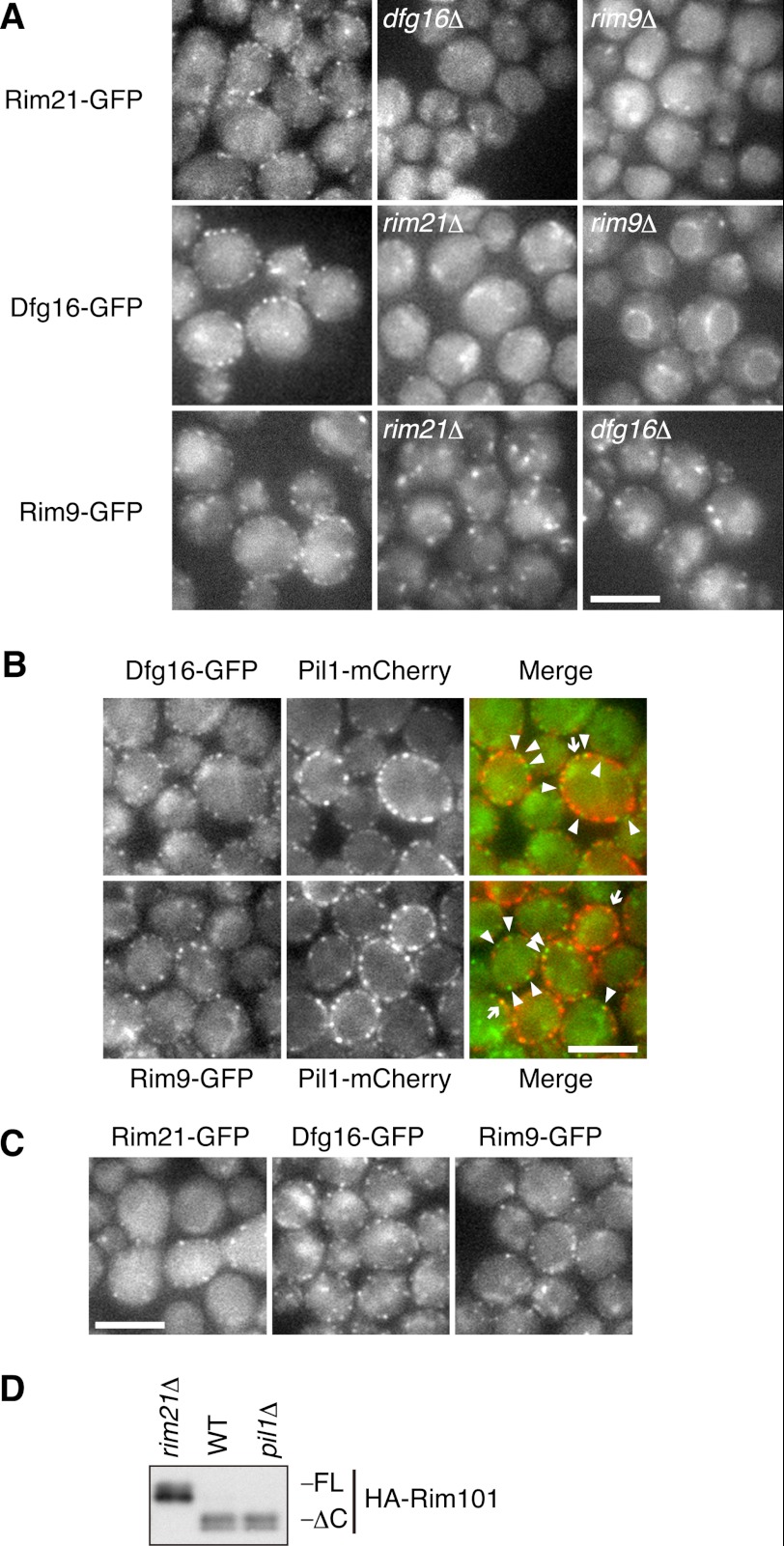
Rim21, Dfg16, and Rim9 were C-terminally tagged with GFP by chromosomal fusion, and their intracellular localization was examined at the endogenously expressed level. Because the Rim21-GFP signal was very weak, Rim21 C-terminally tagged with two tandem GFPs (2×GFP) was expressed and monitored. The GFP-tagged proteins were detected in the plasma membrane and in some internal membrane structures reminiscent of the Golgi apparatus and the endosome (Fig. 3A). In the plasma membrane, they were not dispersed evenly but were clustered in a patchy fashion. Their localization was significantly altered in each deletion mutant. In rim21Δ and rim9Δ cells, Dfg16-GFP was found in some intracellular compartments and mainly in the vacuolar membranes, respectively, but not in the plasma membranes. Likewise, Rim9-GFP accumulated mainly at intracellular punctuates reminiscent of the Golgi apparatus and the endosome, instead of in the plasma membrane, in rim21Δ and dfg16Δ cells. Rim21–2×GFP was not detected in most of the dfg16Δ and rim9Δ cells, probably due to its low protein level in these mutants (Fig. 1B). In some of the dfg16Δ and rim9Δ cells, Rim21–2×GFP was found in intracellular organelles but not in the plasma membrane. These results indicate that localization of Rim21, Dfg16, and Rim9 to the plasma membrane is mutually dependent.
FIGURE 3.
Rim21, Dfg16, and Rim9 are localized to the plasma membrane in a patchy and mutually dependent manner. A, YOK3208 (RIM21-2×EGFP), YOK3209 (RIM21-2×EGFP dfg16Δ), YOK3210 (RIM21-2×EGFP rim9Δ), YOK2210 (DFG16-EGFP), YOK2211 (DFG16-EGFP rim21Δ), YOK2212 (DFG16-EGFP rim9Δ), YOK2229 (RIM9-EGFP), YOK2215 (RIM9-EGFP rim21Δ), and YOK2216 (RIM9-EGFP dfg16Δ) cells were subjected to fluorescence microscopy. Scale bar = 5 μm. B, YOK2729 (DFG16-EGFP PIL1-mCherry) and YOK2731 (RIM9-EGFP PIL1-mCherry) cells were subjected to fluorescence microscopy. Arrows indicate Dfg16-EGFP or Rim9-EGFP that co-localized with Pil1-mCherry, and arrowheads indicate Dfg16-EGFP or Rim9-EGFP that did not co-localize with Pil1-mCherry. Scale bar = 5 μm. C, YOK2743 (RIM21-EGFP pil1Δ), YOK2744 (DFG16-EGFP pil1Δ), and YOK2745 (RIM9-EGFP pil1Δ) cells were subjected to fluorescence microscopy. Scale bar = 5 μm. D, YOK2027 (rim21Δ), YOK2210 (DFG16-EGFP), and YOK2744 (DFG16-EGFP pil1Δ) cells harboring pFI1 (HA-RIM101) were collected after a 20-min alkaline treatment. Immunoblotting was performed with anti-HA antibody. FL, full-length; ΔC, Rim101ΔC.
Given the patchy distribution of Rim21-, Dfg16-, and Rim9-GFP in the plasma membrane, one could imagine that these proteins localize to eisosomes on the plasma membrane. The eisosome is a recently discovered structure attached to the cytosolic face of the plasma membrane and distributed in a patchy manner (30). Pil1 is the main component of the eisosome and is required for the eisosome formation (30). Therefore, we tested the possibility by monitoring their co-localization with Pil1-mCherry. Only a small fraction of Dfg16- and Rim9-GFP co-localized with Pil1-mCherry (Fig. 3B). Alkaline treatment did not facilitate these co-localizations (supplemental Fig. S2). We then studied the distribution of Rim21-, Dfg16-, and Rim9-GFP in pil1Δ cells, in which eisosome proteins cannot localize in a patchy manner but accumulate in abnormal structures called eisosome remnants on the plasma membrane (30). All of the proteins were detected at the plasma membrane in a patchy fashion as observed in WT cells (Fig. 3C). In addition, alkalization of pil1Δ cells activated the Rim101 pathway (Fig. 3D). These findings suggest that Rim21, Dfg16, and Rim9 are not constitutively localized to eisosomes and that the activation of the Rim101 pathway is independent of eisosomes.
Rim21, Dfg16, and Rim9 Are Transported into the Vacuole and Degraded upon External Alkalization
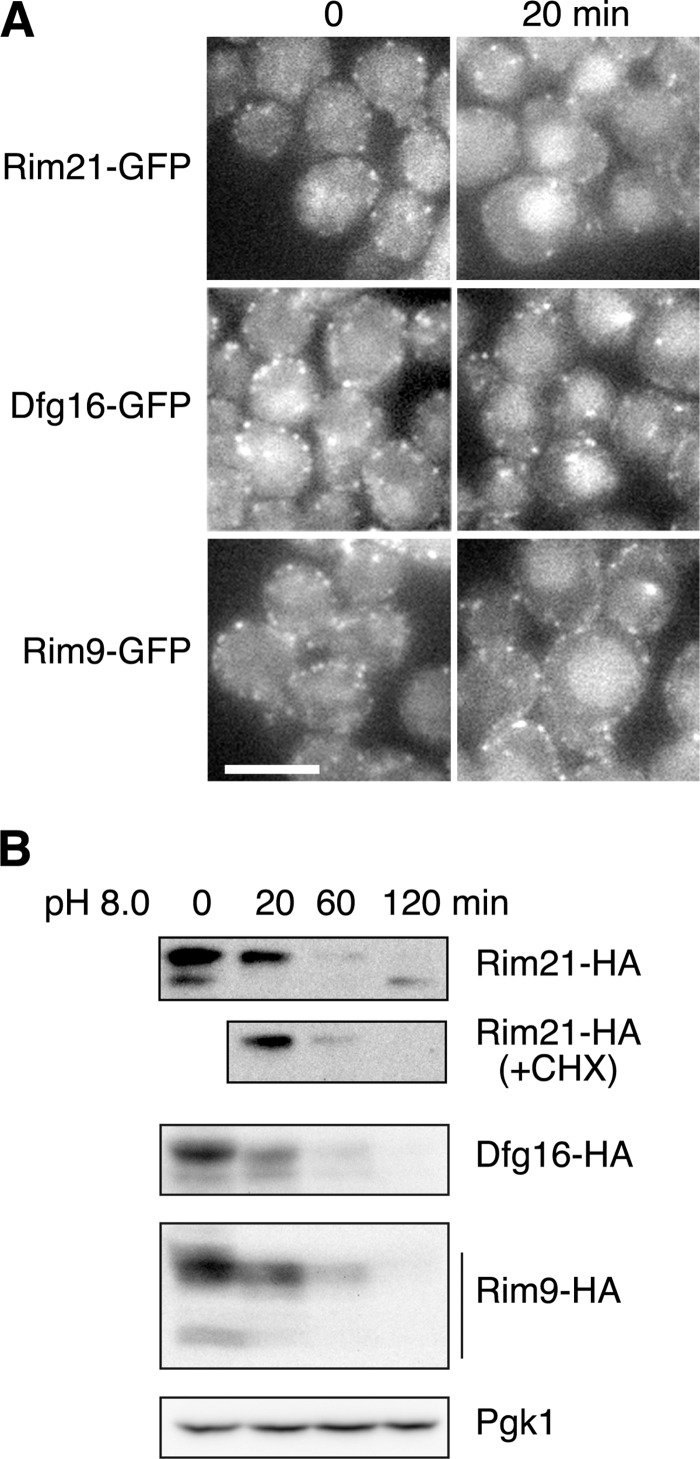
The fate of Rim21, Dfg16, and Rim9 upon external alkalization (e.g. their translocation into the cell and turnover) must be understood. We first investigated their translocation. After 20 min of alkaline treatment, significant fractions of Rim21–2×GFP and Dfg16/Rim9-GFP moved to the vacuolar lumen and intracellular punctuates reminiscent of the endosome (Fig. 4A). This observation suggests that they are internalized and transported to the vacuole upon alkalization.
FIGURE 4.
Rim21, Dfg16, and Rim9 are degraded upon external alkalization. A, before and after a 20-min alkaline treatment, YOK3208 (RIM21-2×EGFP), YOK2210 (DFG16-EGFP), and YOK2229 (RIM9-EGFP) cells were subjected to fluorescence microscopy. Scale bar = 5 μm. B, total lysates were prepared from YOK2559 (RIM21-HA), YOK2560 (DFG16-HA), and YOK2561 (RIM9-HA) cells. After Endo H treatment, immunoblotting was performed with anti-HA antibody or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. CHX, cycloheximide.
We next examined the changes in the cellular levels of Rim21, Dfg16, and Rim9 by immunoblot analysis. Upon alkalization, the levels of Rim21-, Dfg16-, and Rim9-HA were decreased significantly (Fig. 4B). This finding, together with the above results, indicates that, upon alkalization, these proteins are internalized, transported into the vacuole, and degraded. After 120 min, a small amount of non-phosphorylated Rim21-HA was detected, which was probably formed by de novo synthesis. This was confirmed by the absence of the corresponding band after alkaline treatment of Rim21-HA cells in the presence of cycloheximide, an inhibitor of de novo protein synthesis (Fig. 4B).
Rim21 Plays a Central Role in Sensing Alkaline pH
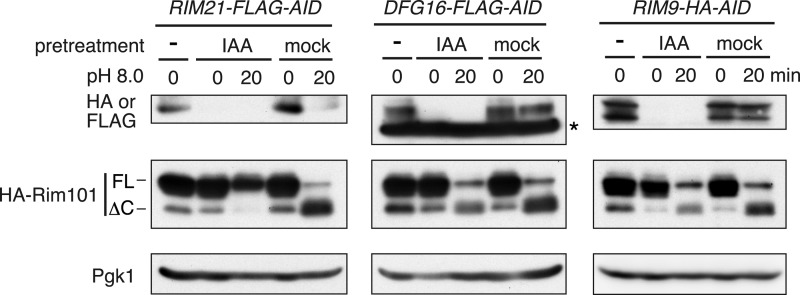
Because Rim21, Dfg16, and Rim9 have a mutual influence on their cellular levels and localization, it is difficult to examine the direct involvement of Rim21, Dfg16, and Rim9 in pH sensing using their respective deletion mutants. Consequently, we employed a transient protein degradation system called the AID system (31) to selectively deplete each protein in the plasma membrane and examined its effect on the Rim101 pathway. In the AID system, proteins tagged with an AID tag derived from a plant transcription factor are specifically degraded by a ubiquitin-proteasome system upon treatment with the phytohormone auxin (IAA). The C-terminally tagged fusion proteins Rim21-FLAG-AID, Dfg16-FLAG-AID, and Rim9-HA-AID were rapidly degraded to undetectable levels within 30 min after the addition of IAA (Fig. 5). Accordingly, RIM21-FLAG-AID, DFG16-FLAG-AID, and RIM9-HA-AID cells were subjected to alkalization after pretreatment with IAA for 30 min. The activation of the Rim101 pathway was completely abolished in RIM21-FLAG-AID cells. In contrast, activation was not altered in either DFG16-FLAG-AID or RIM9-HA-AID cells. In RIM21-FLAG-AID cells without IAA treatment (mock), the Rim101 pathway was activated upon alkalization. Furthermore, Dfg16-HA and Rim9-HA in RIM21-FLAG-AID cells were not degraded by IAA treatment (supplemental Fig. S3). On the basis of these findings, we concluded that Rim21 is the pH sensor molecule. Dfg16 and Rim9 seem to be involved indirectly in pH sensing through maintaining the Rim21 level and, presumably, through assisting in its plasma membrane localization.
FIGURE 5.
Rim21 is the sensor protein in the Rim101 pathway. YOK2848 (RIM21-FLAG-AID), YOK2849 (DFG16-FLAG-AID), and YOK2846 (RIM9-HA-AID) cells harboring pFI1 (HA-RIM101) were pretreated with 500 μm IAA or ethanol (mock) for 30 min. Before or after a 20-min alkaline treatment, followed by Endo H treatment, immunoblotting was performed with anti-HA and anti-FLAG antibodies or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. The asterisk indicates a nonspecific band. FL, full-length; ΔC, Rim101ΔC.
The C-terminal Region of Rim21 Is Essential for Activation of the Rim101 Pathway
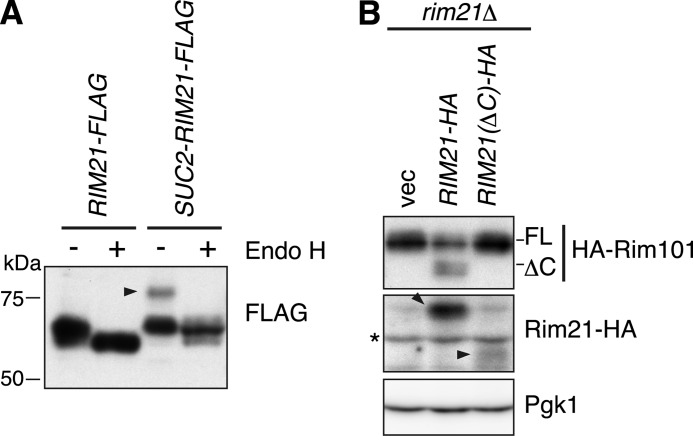
The topology of the N and C termini of Rim21 was biochemically investigated to determine the region essential for pH sensing. Rim21 is predicted to span the membrane six and seven times by the HMMTOP and SOSUI programs, respectively. To analyze the topology of the N terminus, a topological reporter protein, the mature portion of Suc2, was fused to the N terminus of Rim21. The mature portion of Suc2 is rapidly N-glycosylated at multiple sites when translocated in the endoplasmic reticulum lumen, which is topologically equivalent to the extracellular side of the plasma membrane. This system has been successfully applied to determine the topology of membrane proteins (27, 32). The Suc2-Rim21-FLAG fusion protein displayed, upon SDS-PAGE, a slow migrating band at 77 kDa in addition to a band with the predicted molecular mass (Fig. 6A). The additional band shifted down to the predicted band upon deglycosylation with Endo H, indicating that the fusion protein was glycosylated. Because Rim21 is a glycoprotein (Fig. 1A), Rim21-FLAG also showed a downshift upon SDS-PAGE when deglycosylated but to a much lesser degree. This observation strongly suggests that the N terminus of Rim21 is located in the extracellular space. The success of the AID tag experiment described above (Fig. 5) depends on the presence of the AID tag fused to the C terminus of Rim21 in the cytosolic space (31). Thus, Rim21 seems to possess an odd number of transmembrane helices, probably seven, with its N and C termini facing the extracellular and cytosolic spaces, respectively.
FIGURE 6.
Rim21 acidic motif is involved in the regulation of the Rim101 pathway. A, total lysates were prepared from YOK2027 (rim21Δ) cells harboring pOK311 (RIM21-FLAG) or pOK387 (SUC2-RIM21-FLAG) and treated with or without Endo H. Immunoblotting was performed with anti-FLAG antibody. The arrowhead indicates Suc2-Rim21-FLAG glycosylated at the Suc2 moiety. B, total lysates were prepared from SEY6210 (WT) and YOK2027 (rim21Δ) cells harboring pFI1 (HA-RIM101) together with pRS313 (empty vector), pOK313 (RIM21-HA), or pOK419 (RIM21ΔC-HA). After Endo H treatment, immunoblotting was performed with anti-HA antibody or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. Arrowheads indicate Rim21 variants, and the asterisk indicates a nonspecific band. FL, full-length; ΔC, Rim101ΔC.
Although Rim21 appears to have no regions showing similarity to known domains, one prominent feature of Rim21 is that the C-terminal cytosolic tail is rich in acidic amino acid residues. An estimated isoelectric point of the C-terminal tail region is 4.64 as opposed to 6.26 for the entire protein. A cluster of acidic amino acid residues (DEDDDENAEDEDDDE, amino acid residues 496–510) is found near the C terminus of Rim21. To examine a possible involvement of this acidic region in the Rim101 pathway, we engineered a Rim21 variant (Rim21ΔC) that lacks the most C-terminal region including the acidic cluster (amino acid residues 455–534). Cells expressing Rim21ΔC did not activate the Rim101 pathway in response to external alkalization (Fig. 6B), indicating that this region is essential for the Rim101 pathway.
Plasma Membrane Depolarization Activates the Rim101 Pathway
External alkalization causes depolarization of the plasma membrane by collapsing the proton electrochemical gradient across the plasma membrane. For this reason, we examined if plasma membrane depolarization alone, induced by the protonophore CCCP, stimulates the Rim101 pathway without external alkalization. Upon CCCP treatment, the Rim101 pathway was activated in a Rim21-dependent manner (Fig. 7A). Thus, the CCCP-induced activation of the Rim101 pathway occurs through the sensor protein Rim21. Like external alkalization, CCCP treatment induced internalization of Rim21, Dfg16, and Rim9 (supplemental Fig. S4A).
FIGURE 7.
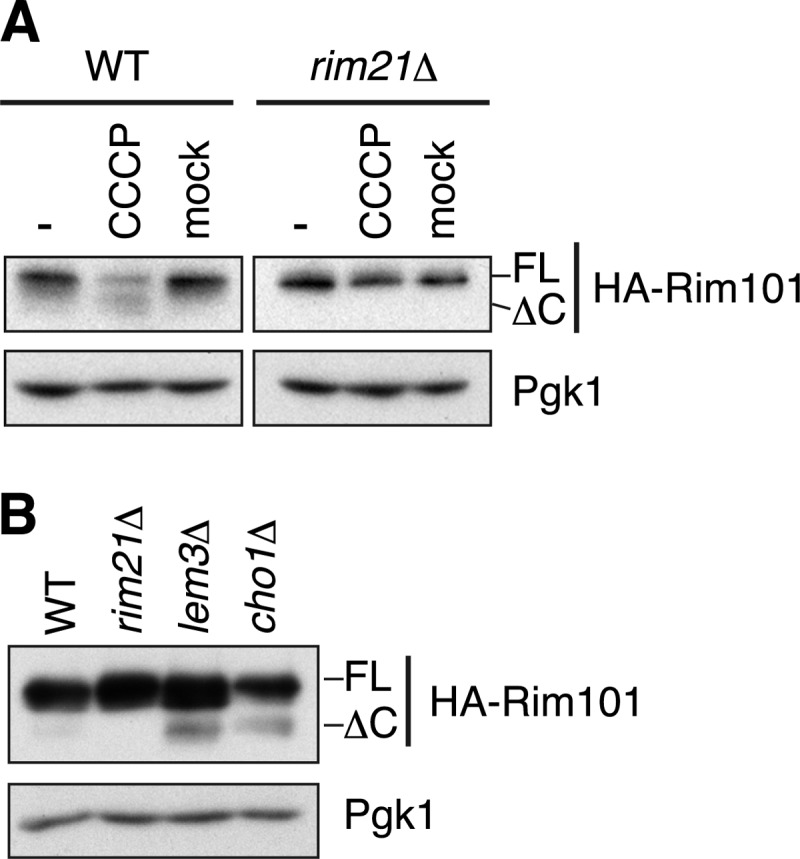
Plasma membrane depolarization activates the Rim101 pathway. A, SEY6210 (WT) and YOK2098 (rim21Δ) cells harboring pFI1 (HA-RIM101) were grown at pH 5.5 and treated with CCCP or ethanol (mock) for 20 min. Immunoblotting was performed with anti-HA antibody or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. B, total lysates were prepared from KCY662 (WT), YOK2098 (rim21Δ), KCY692 (lem3Δ), and KCY1113 (cho1Δ) cells grown in the presence of 1 mm ethanolamine. Immunoblotting was performed with anti-HA antibody or, to demonstrate uniform protein loading, with anti-Pgk1 antibody. FL, full-length; ΔC, Rim101ΔC.
Besides the proton electrochemical gradient, an asymmetric distribution of phospholipids, such as negatively charged PS, across the plasma membrane is known to be important for membrane polarization (33). In the plasma membrane, PS is confined mostly to the inner leaflet, resulting in its asymmetric distribution. We next studied the involvement of PS in regulation of the Rim101 pathway using cho1Δ cells, which do not produce PS due to the missing PS synthase Cho1 (24, 34). In cho1Δ cells, the Rim101 pathway was constitutively activated even without alkaline treatment (Fig. 7B). The constitutive activation of the Rim101 pathway has also been reported in lem3Δ cells (23). Lem3 is required for the asymmetric distribution of phospholipids in the plasma membrane. We confirmed the constitutive activation of the Rim101 pathway in lem3Δ cells. The activation level was comparable with that observed in cho1Δ cells (Fig. 7B). However, the activation efficiency was relatively low in both mutants compared with that in the alkali-treated cells. Such low efficiency may arise from the presence of additional factors that contribute to membrane polarization in these mutants, including other phospholipids (e.g. phosphatidylinositol and phosphoinositides) and the proton electrochemical gradient. Internalization of Rim21–2×GFP and Dfg16/Rim9-GFP in intracellular organelles was also observed in lem3Δ cells, albeit at a low frequency (supplemental Fig. S4B). In cho1Δ cells, however, their internalization was difficult to be evaluated due to their weak signals for an unknown reason (data not shown).
DISCUSSION
In this work, we have obtained some basic biochemical information on the putative sensor molecules Rim21, Dfg16, and Rim9 concerning their localization, complex formation, and interrelationship. Such information could provide a base for future research into the molecular mechanisms for sensing extracellular pH and abnormal membrane lipid asymmetry.
Rim21 was found to act as the sensor molecule in the Rim101 pathway (Fig. 5). Thus, the question is how Rim21 senses external alkalization and altered lipid asymmetry. We have shown the Rim21-dependent activation of the Rim101 pathway, even without external alkalization, by the plasma membrane depolarization induced either by CCCP treatment (Fig. 7A) or by reduction of PS in the inner leaflet of the plasma membrane (Fig. 7B). Thus, one possible hypothesis is that Rim21 senses external alkalization and altered lipid asymmetry by detecting depolarization of the plasma membrane. An alternative hypothesis is that Rim21 detects the reduction of negatively charged lipids, such as PS, in the inner leaflet of the plasma membrane. The membrane depolarization caused by CCCP has been reported to suppress almost completely the inward translocation of phospholipids across the membrane, i.e. phospholipid flip (35, 36). Hence, the membrane depolarization by external alkalization may impair the phospholipid flip, thus leading to the reduction of PS in the inner leaflet of the plasma membrane. It this regard, it is significant that the C-terminal cytosolic region of Rim21, composed of the acidic amino acid cluster, is required for the Rim101 pathway (Fig. 6B). Such a highly acidic motif could play a critical role in the regulation of the Rim101 pathway because it generates a repulsive interaction with the negatively charged lipids in the inner leaflet of the plasma membrane. Other hypotheses are also feasible. For example, the reduction in PS and the membrane depolarization may be simultaneously recognized by different regions of Rim21. In addition, the external alkalization and altered lipid asymmetry may potentially cause the changes in the surface charge of the plasma membrane, which could then be sensed by Rim21. Certainly, more studies are needed.
At 120 min of alkaline treatment, Rim21-HA was synthesized de novo, whereas Dfg16-HA and Rim9-HA were not (Fig. 4B). The promoter region of RIM21 contains two potential Nrg1-binding cis-elements (CCCCT and CCCTC). Nrg1 is a transcription repressor that negatively regulates expression of alkaline response genes (5). In turn, the transcription of NRG1 is directly repressed by Rim101 (5). Thus, activation of the Rim101 pathway leads to expression of genes that are usually repressed by Nrg1. Our result implies that the Rim21 level is regulated, at least in part, by this positive feedback loop. In contrast, the level of Rim8, an arrestin-like protein essential for the Rim101 pathway, is regulated by a negative feedback loop because the transcription of RIM8 is directly repressed by Rim101 (5). It would therefore be interesting to know if the Rim101 pathway is regulated elaborately by such positive and negative feedback loops.
Rim21, Dfg16, and Rim9 displayed a patchy rather than an even distribution in the plasma membrane. The assembly into such clusters may facilitate the efficient transmission of signals of alkaline pH and altered lipid asymmetry to downstream molecules. Immunoblot and microscopic analyses revealed that Rim21, Dfg16, and Rim9 are internalized and degraded upon external alkalization (Fig. 4B). An important issue that should be addressed in the future is whether internalization of the sensor complex, as observed in this work, is an essential process in transducing the signal of external alkalization or whether it is just an attenuation process of the Rim101 pathway. For this purpose, it would be necessary to monitor the Rim101 pathway in several endocytosis mutants with a highly sensitive live imaging system to analyze the actions of these proteins and the downstream molecules at a single molecule level.
Acknowledgments
We thank Dr. T. Maeda for providing a plasmid for expression of HA-Rim101 (pFI1). The yeast strain for the AID system (BY25598) was provided by the National BioResource Project (NBRP) of the Ministry of Education, Culture, Science, Sports and Technology (MEXT), Japan. We are grateful to Dr. T. Toyokuni for editing the manuscript.
This work was supported by Grant-in-aid for Young Scientists (B) 23770135 (to K. O.) and Grant-in-aid for Challenging Exploratory Research 23657120 (to A. K.) from the Japan Society for the Promotion of Science (JSPS) and The Naito Foundation Subsidy for Promotion of Specific Research Projects from The Naito Foundation (to K. O.).

This article contains supplemental Figs. S1–S4.
- IAA
- 3-indoleacetic acid
- PS
- phosphatidylserine
- PE
- phosphatidylethanolamine
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- AID
- auxin-inducible degron
- λ-PPase
- λ-protein phosphatase
- Endo H
- endoglycosidase H.
REFERENCES
- 1. Peñalva M. A., Arst H. N., Jr. (2002) Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66, 426–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peñalva M. A., Arst H. N., Jr. (2004) Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58, 425–451 [DOI] [PubMed] [Google Scholar]
- 3. Davis D. A. (2009) How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr. Opin. Microbiol. 12, 365–370 [DOI] [PubMed] [Google Scholar]
- 4. Causton H. C., Ren B., Koh S. S., Harbison C. T., Kanin E., Jennings E. G., Lee T. I., True H. L., Lander E. S., Young R. A. (2001) Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12, 323–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamb T. M., Mitchell A. P. (2003) The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23, 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamb T. M., Xu W., Diamond A., Mitchell A. P. (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 7. Serrano R., Ruiz A., Bernal D., Chambers J. R., Ariño J. (2002) The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signaling. Mol. Microbiol. 46, 1319–1333 [DOI] [PubMed] [Google Scholar]
- 8. Barwell K. J., Boysen J. H., Xu W., Mitchell A. P. (2005) Relationship of DFG16 to the Rim101p pH response pathway in Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell 4, 890–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W., Mitchell A. P. (1997) Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145, 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tréton B., Blanchin-Roland S., Lambert M., Lépingle A., Gaillardin C. (2000) Ambient pH signaling in ascomycetous yeasts involves homologues of the Aspergillus nidulans genes palF and paIH. Mol. Gen. Genet. 263, 505–513 [DOI] [PubMed] [Google Scholar]
- 11. Hayashi M., Fukuzawa T., Sorimachi H., Maeda T. (2005) Constitutive activation of the pH-responsive Rim101 pathway in yeast mutants defective in late steps of the MVB/ESCRT pathway. Mol. Cell. Biol. 25, 9478–9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. (2008) Arrestin-related ubiquitin ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135, 714–725 [DOI] [PubMed] [Google Scholar]
- 13. Shukla A. K., Xiao K., Lefkowitz R. J. (2011) Emerging paradigms of β-arrestin-dependent seven-transmembrane receptor signaling. Trends Biochem. Sci. 36, 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henne W. M., Buchkovich N. J., Emr S. D. (2011) The ESCRT pathway. Dev. Cell 21, 77–91 [DOI] [PubMed] [Google Scholar]
- 15. Xu W., Smith F. J., Jr., Subaran R., Mitchell A. P. (2004) Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell 15, 5528–5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. (2002) Escrt-III: an endosome-associated hetero-oligomeric protein complex required for MVB sorting. Dev. Cell 3, 271–282 [DOI] [PubMed] [Google Scholar]
- 17. Ito T., Chiba T., Ozawa R., Yoshida M., Hattori M., Sakaki Y. (2001) A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. U.S.A. 98, 4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu W., Mitchell A. P. (2001) Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183, 6917–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiss P., Huppert S., Kölling R. (2009) Analysis of the dual function of the ESCRT-III protein Snf7 in endocytic trafficking and in gene expression. Biochem. J. 424, 89–97 [DOI] [PubMed] [Google Scholar]
- 20. Herranz S., Rodríguez J. M., Bussink H. J., Sánchez-Ferrero J. C., Arst H. N., Jr., Peñalva M. A., Vincent O. (2005) Arrestin-related proteins mediate pH signaling in fungi. Proc. Natl. Acad. Sci. U.S.A. 102, 12141–12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Negrete-Urtasun S., Reiter W., Diez E., Denison S. H., Tilburn J., Espeso E. A., Peñalva M. A., Arst H. N., Jr. (1999) Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol. Microbiol. 33, 994–1003 [DOI] [PubMed] [Google Scholar]
- 22. Calcagno-Pizarelli A. M., Negrete-Urtasun S., Denison S. H., Rudnicka J. D., Bussink H. J., Múnera-Huertas T., Stanton L., Hervás-Aguilar A., Espeso E. A., Tilburn J., Arst H. N., Jr., Peñalva M. A. (2007) Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot. Cell 6, 2365–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikeda M., Kihara A., Denpoh A., Igarashi Y. (2008) The Rim101 pathway is involved in Rsb1 expression induced by altered lipid asymmetry. Mol. Biol. Cell 19, 1922–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hikiji T., Miura K., Kiyono K., Shibuya I., Ohta A. (1988) Disruption of the CHO1 gene encoding phosphatidylserine synthase in Saccharomyces cerevisiae. J. Biochem. 104, 894–900 [DOI] [PubMed] [Google Scholar]
- 25. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 26. Sikorski R. S., Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kihara A., Sakuraba H., Ikeda M., Denpoh A., Igarashi Y. (2008) Membrane topology and essential amino acid residues of Phs1, a 3-hydroxyacyl-CoA dehydratase involved in very long-chain fatty acid elongation. J. Biol. Chem. 283, 11199–11209 [DOI] [PubMed] [Google Scholar]
- 28. Obara K., Sekito T., Ohsumi Y. (2006) Assortment of phosphatidylinositol 3-kinase complexes–Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Mol. Biol. Cell 17, 1527–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamagata M., Obara K., Kihara A. (2011) Sphingolipid synthesis is involved in autophagy in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 410, 786–791 [DOI] [PubMed] [Google Scholar]
- 30. Walther T. C., Brickner J. H., Aguilar P. S., Bernales S., Pantoja C., Walter P. (2006) Eisosomes mark static sites of endocytosis. Nature 439, 998–1003 [DOI] [PubMed] [Google Scholar]
- 31. Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M. (2009) An auxin-based degron system for the rapid depletion of proteins in non-plant cells. Nat. Methods 6, 917–922 [DOI] [PubMed] [Google Scholar]
- 32. Han G., Gable K., Yan L., Natarajan M., Krishnamurthy J., Gupta S. D., Borovitskaya A., Harmon J. M., Dunn T. M. (2004) The topology of the Lcb1p subunit of yeast serine palmitoyltransferase. J. Biol. Chem. 279, 53707–53716 [DOI] [PubMed] [Google Scholar]
- 33. Gurtovenko A. A., Vattulainen I. (2008) Membrane potential and electrostatics of phospholipid bilayers with asymmetric transmembrane distribution of anionic lipids. J. Phys. Chem. B 112, 4629–4634 [DOI] [PubMed] [Google Scholar]
- 34. Letts V. A., Klig L. S., Bae-Lee M., Carman G. M., Henry S. A. (1983) Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc. Natl. Acad. Sci. U.S.A. 80, 7279–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanson P. K., Nichols J. W. (2001) Energy-dependent flip of fluorescence-labeled phospholipids is regulated by nutrient starvation and transcription factors PDR1 and PDR3. J. Biol. Chem. 276, 9861–9867 [DOI] [PubMed] [Google Scholar]
- 36. Stevens H. C., Nichols J. W. (2007) The proton electrochemical gradient across the plasma membrane of yeast is necessary for phospholipid flip. J. Biol. Chem. 282, 17563–17567 [DOI] [PubMed] [Google Scholar]
- 37. Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]