Background: Interactions between environmental conditions and monocyte phenotype are critical for the development of vascular complications in diabetes.
Results: Modulation of ER stress by vitamin D controls monocyte/macrophage phenotype and vascular adhesion.
Conclusion: Vitamin D is a natural ER stress reliever that promotes an anti-inflammatory monocyte/macrophage phenotype.
Significance: Vitamin D is a potential therapy to reduce vascular complications in diabetics.
Keywords: Adhesion, Diabetes, Endoplasmic Reticulum Stress, Monocytes, vitamin D, Monocyte Phenotype
Abstract
Cardiovascular disease is the leading cause of morbidity/mortality in patients with type 2 diabetes mellitus (T2DM), but there is a lack of knowledge about the mechanism(s) of increased atherosclerosis in these patients. In patients with T2DM, the prevalence of 25-hydroxy vitamin D (25(OH)D) deficiency is almost twice that for nondiabetics and doubles the relative risk of developing cardiovascular disease compared with diabetic patients with normal 25(OH)D. We tested the hypothesis that monocytes from vitamin D-deficient subjects will have a proatherogenic phenotype compared with vitamin D-sufficient subjects in 43 patients with T2DM. Serum 25(OH)D level inversely correlated with monocyte adhesion to endothelial cells even after adjustment for demographic and comorbidity characteristics. Vitamin D-sufficient patients (≥30 ng/ml 25(OH)D) had lower monocyte endoplasmic reticulum (ER) stress, a predominance of M1 over M2 macrophage membrane receptors, and decreased mRNA expression of monocyte adhesion molecules PSGL-1, β1-integrin, and β2-integrin compared with patients with 25(OH)D levels of <30 ng/ml. In vitamin D-deficient macrophages, activation of ER stress increased adhesion and adhesion molecule expression and induced an M2-predominant phenotype. Moreover, adding 1,25(OH)2D3 to vitamin D-deficient macrophages shifted their phenotype toward an M1-predominant phenotype with suppressed adhesion. Conversely, deletion of the vitamin D receptor in macrophages from diabetic patients activated ER stress, accelerated adhesion, and increased adhesion molecule expression. The absence of ER stress protein CCAAT enhancer-binding protein homologous protein suppressed monocyte adhesion, adhesion molecule expression, and the M2-predominant phenotype induced by vitamin D deficiency. Thus, vitamin D is a natural ER stress reliever that induced an antiatherogenic monocyte/macrophage phenotype.
Introduction
Nearly 26 million Americans suffer from type 2 diabetes mellitus (T2DM),4 a disease with an increased risk of cardiovascular disease, the leading cause of morbidity and premature mortality in Western populations (1). Very little is known about the mechanisms by which insulin resistance and chronic inflammation promote vascular complications in patients with T2DM. Accumulation of monocyte-derived cells in the vessel wall is a key feature of diabetes-related complications, but it is unclear whether a shift of monocyte subtype influences this vascular inflammation. Peripheral blood monocytes are typically defined by cell surface lipopolysaccharide receptor CD14 and Fcγ receptor III CD16 as CD14hi/CD16− (classical) and CD14lo/CD16+ (nonclassical) (2). The proportion of CD14lo/CD16+ monocytes rises in patients with coronary artery disease compared with healthy controls and significantly correlates with carotid intimal medial thickness in healthy populations (3, 4). In contrast, diabetic patients with coronary artery disease have no difference in the proportion of CD14lo/CD16+ monocytes compared with diabetics or healthy individuals without coronary artery disease, thus raising controversy regarding the importance of these monocyte phenotype markers in vascular disease (5). Monocytes also express the same surface markers used to classify macrophages into the classically activated M1 or alternatively activated M2 subtype, and mounting evidence indicates the importance of these subtypes in the development of atherosclerosis (6, 7). In obese subjects, peripheral monocytes have reduced expression of M2 markers (8). Monocytes from obese type 2 diabetics have lower levels of IL-10 and CD163 expression compared with obese nondiabetics, suggestive of decreased M2 macrophage markers, and this phenotype correlates with increased arterial stiffness (9). However, the contribution of these monocyte phenotypes to atherosclerotic development is unknown. Both the M1 and M2 macrophage subtypes are known to be present in plaques (6). M1 macrophages, induced by IFNγ, release proinflammatory cytokines and generate reactive oxygen and nitrogen intermediates, accelerating additional immune cell recruitment and vascular subendothelial cell remodeling (10). M2 macrophages, induced by IL-4, IL-10, or immunocomplex plus LPS, represent a more heterogeneous group of cells with pro- and anti-inflammatory functions (11), characterized by increased IL-10 secretion and suppressed IL-12 expression (11–13). Current knowledge is evolving but suggests that M1 macrophages perpetuate inflammation associated with atherosclerosis, whereas M2 cells are more likely to become foam cells (8, 14, 15). However, the balance between M1 and M2 monocytes in the promotion of atherosclerosis has not been well characterized.
Monocyte infiltration as part of inflammatory responses first requires the adhesion of monocytes to the endothelium through multiple cell-cell interactions. These include the interaction of circulating monocyte P-selectin glycoprotein ligand 1 (PSGL-1) with P- or E-selectin to tether monocytes to the endothelium, followed by the interaction of monocyte β1- and β2-integrin with intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 to promote tight adhesion (16). In patients with diabetes, high glucose conditions enhance monocyte adhesion to the endothelium by increasing integrin subunit expression on monocytes and increasing secretion of endothelial adhesion molecules (17–20). Interestingly, insulin resistance without hyperglycemia similarly up-regulates adhesion molecules and increases adhesion (21, 22). Insulin resistance is also a potent inducer of prolonged endoplasmic reticulum (ER) stress in monocytes and macrophages (23, 24). The ER is a dynamic membranous organelle that facilitates correct protein modification, folding, and maturation of proteins. Unfolded protein response (UPR) is an adaptive intracellular signaling pathway that responds to ER stress by attenuating global protein translation and degrading unfolded proteins (25, 26). In mouse models of diet-induced insulin resistance and atherosclerosis, up-regulated UPR markers are detected in intimal macrophages at early stages of vascular inflammation, prior to development of fatty streaks or atherosclerotic plaques (27). Suppression of ER stress in the same mouse models decreases vascular inflammation and prevents the development of atherosclerosis (28, 29). Therefore, understanding whether the increased ER stress in monocytes from diabetics generates a subtype that accelerates vascular inflammation will be essential in the generation of therapeutic targets.
Vitamin D deficiency is a largely unacknowledged epidemic associated with incident T2DM and cardiovascular disease (30–32). Deficiency of 25-hydroxy vitamin D (25(OH)D), the principal storage form of vitamin D, is 30% more prevalent in diabetics than in control subjects and nearly doubles the relative risk of developing cardiovascular disease compared with diabetic patients with normal 25(OH)D levels (33, 34). The vitamin D receptor (VDR) and its converting enzyme, 25(OH)D3-1α-hydroxylase, are present in monocytes and macrophages. 1,25-dihydroxy vitamin D (1,25(OH)2D), the active form of vitamin D, facilitates adhesion in monocytic cell lines and stimulates differentiation of myeloid progenitors into macrophages in vitro through the VDR (35, 36). In freshly isolated monocytes from type 2 diabetics or nondiabetic patients, vitamin D prevents production of multiple proinflammatory cytokines (TNFα, IL-1, and IL-6) after stimulation with LPS through inhibition of nuclear factor κ-light chain enhancer of activated B cells (NF-κB) p65 phosphorylation and p38 phosphorylation (37, 38). During monocyte differentiation, the ER reorganizes both structurally and functionally to carry out new cell functions, leading to ER stress (39, 40). We have shown that 1,25(OH)2D3 suppresses ER stress in macrophages from diabetic patients and prevents foam cell formation, indicating that active vitamin D modulates macrophage atherogenic properties through an ER stress-dependent mechanism (41). Furthermore, we have shown that the suppression of ER stress shifts M2-predominant macrophages to M1-predominant cells and decreases foam cell formation, suggesting that regulation of ER stress by vitamin D could be a potential therapy for atherosclerosis.
To determine whether vitamin D has ER stress-dependent effects on innate immune cell properties that influence the proinflammatory state seen in patients with diabetes, we collected peripheral blood monocytes from patients with and without T2DM with varying levels of serum 25(OH)D. We evaluated whether vitamin D level is associated with markers of monocyte subtypes, the relative balance of M1 and M2 cells, and monocyte/macrophage adhesion to fibronectin and human endothelial cells. We also explored the effects of vitamin D on ER homeostasis in regulation of the monocyte/macrophage phenotype and proatherogenic properties in type 2 diabetic patients.
EXPERIMENTAL PROCEDURES
Study Design
We performed a cross-sectional analysis of 43 patients with T2DM and 25 patients without diabetes with varying levels of 25(OH)D. The subjects were voluntarily recruited from the outpatient clinics at Washington University School of Medicine and Barnes-Jewish Hospital (St. Louis, MO). Subjects underwent a single venous blood draw for monocyte isolation, and the 25(OH)D level was measured by radioimmunoassay through DiaSorin (Stillwater, MN).
Isolation and Preparation of Human Monocytes/Macrophages
Peripheral monocytes were isolated by standard Ficoll isolation techniques and selected by CD14 marker positivity (Miltenyi Biotec, Auburn, CA). Monocytes were stabilized for 3 h in 100% serum from the original patient to mimic in vivo conditions prior to any procedures. To induce differentiation of monocytes into vitamin D-deficient or -sufficient macrophages, monocytes were cultured for 5 days in vitamin D-deficient medium (deficient in both 25(OH)D and 1,25(OH)2D; obtained by DMEM plus 10% charcoal/dextran-treated FBS) supplemented with macrophage colony-stimulating factor (Sigma) with or without 1,25(OH)2D3 at 10−8 mol/liter. ER stress was inhibited by adding phenyl butyric acid (PBA; Calbiochem, San Diego, CA) for 16 h to macrophages cultured in vitamin D-deficient medium. ER stress was induced by adding thapsigargin (Sigma) for 24 h to macrophages cultured in vitamin D-supplemented medium.
Isolation of Murine Monocytes
Peripheral blood and bone marrow monocytes were derived from 12-week-old C57BL/6 mice lacking CCAAT enhancer-binding protein homologous protein (CHOP) (Jackson Laboratory, Bar Harbor, ME) and WT C57BL/6 mice fed a vitamin D-deficient diet for 4 weeks. The monocytes were selected by CD11b marker positivity (Miltenyi Biotec). The monocytes were stabilized for 3 h in DMEM plus 10% serum from the original mouse to mimic in vivo conditions prior to any procedures.
Adhesion Assays
96-well plates were coated with human umbilical vein endothelial cells (HUVEC; Lonza, Atlanta, GA; 3 × 105 cells/well) or with fibronectin (Sigma) overnight. The monocytes and macrophages (0.1 × 105 cells/plate) were added to HUVEC or to fibronectin-coated plates and incubated for 4 h at 37 °C. Adhered cells were then washed, fixed in formaldehyde, and stained with crystal violet. Well absorbance was read at 585 nm.
Gene Expression and Western Blot Analysis
Reverse transcription-qPCR analyses were performed by Sybrgreen methodologies (supplemental Table S1). The results were normalized to the housekeeping gene L32. Western blot analyses from macrophage protein extracts were normalized to β-actin expression.
Flow Cytometry of Primary Human Monocytes/Macrophages
Monocytes derived from patients were incubated with antibodies for markers of monocyte differentiation (CD16-FITC and CD14-PE; E-Bioscience, San Diego, CA), M1 macrophage differentiation (CCR7-APC and CD86-PE; E-Bioscience), and M2 macrophage differentiation (CD163-PE and mannose receptor (MR)-PE-CyTM5; BD Pharmingen, San Diego, CA) (supplemental Fig. S1). Antibody specificity for M1 and M2 markers was validated using stimulated macrophages differentiated from vitamin D-sufficient human monocytes to induce the M1 or M2 phenotype (IFNγ/LPS to stimulate M1 and IL-4, IL-10, or immunocomplex plus LPS to stimulate M2; supplemental Fig. S1). Stained cells were analyzed by flow cytometry on a FACStar Plus. The macrophage phenotype ratio (MPR) was calculated as (CCR7 + CD86 expression)/(CD163 + MR expression), all expressed as a percentage of cells positive, to determine the relative abundance of M1 and M2 cell subtypes (see further details in supplemental methods and supplemental Figs. S2 and S3).
Plasmids and Small Interfering RNA
Human monocytes were cultured for 5 days to induce macrophage differentiation in vitamin D-sufficient conditions and then infected with lentivirus containing either VDR-siRNA or control-siRNA. Assays were performed 72 h after recovering from viral infection.
Statistical Analysis
SPSS and GraphPad Prism software were used for statistical calculations. Descriptive variables were expressed as the means ± S.D. for continuous data and as a ratio for categorical data. Experiments were carried out with duplicate or triplicate samples. Analytic data were expressed as the means ± S.E. for continuous variables. Normal distribution of continuous variables was verified graphically. Pearson correlations were used to compare continuous variables. Statistical significance of differences was calculated using a t test for parametric data involving two groups or one-way analysis of variance followed by Tukey's post-test for parametric data involving three or more groups. The differences were considered statistically significant if p ≤ 0.05.
To address possible confounders of the associations between 25(OH)D level and monocyte phenotype caused by demographic characteristics or comorbidities, we performed simple and multiple linear regression models for each monocyte outcome, including adhesion to fibronectin, adhesion to HUVEC, and macrophage phenotype ratio. Demographic information included gender, race, and age. The comorbidity data were first divided by comorbidity type (T2DM, hypertension, dyslipidemia, tobacco use, and obesity) to select the factor from each category with the strongest association with the monocyte outcomes or, if none were significant, that which we judged most likely to be reflective of current disease. For each demographic and comorbidity characteristic, we performed a simple linear regression model with each monocyte outcome and then expanded each model by force-entering 25(OH)D level to determine improvement in the model. For the final model for each monocyte outcome, we performed a multiple linear regression model with two force-entered blocks, the first including the measures of comorbidities (A1c, systolic blood pressure, statin use, any tobacco use, and body mass index (BMI)) and demographics (gender, race, age) and the second including 25(OH)D level to determine the improvement in the model with the addition of vitamin D. We recognize that the final nine factors included in the regression models are greater than typically used for this sample size, but because the 25(OH)D level consistently showed a significant improvement in each individual model, we included both comorbidity and demographic data in the final models.
RESULTS
Population
The initial population consisted of 25 healthy, nondiabetic subjects who were predominantly female and Caucasian. This population had a mean age of 36 ± 12 years, BMI of 26 ± 7 kg/m2, blood pressure of 124/80 ± 17/10 mm Hg, and 25(OH)D level of 26 ± 12 ng/ml. Only 16% had hypertension, 4% had dyslipidemia, and 16% had a current or past history of tobacco use. We also studied 43 obese adult subjects with T2DM, 51% female with race distribution 51% African American and 49% Caucasian. This population had a mean age of 56 ± 8.8 years, BMI of 38 ± 7.7 kg/m2, blood pressure of 135/80 ± 16/10 mm Hg, and 25(OH)D level of 25 ± 12 ng/ml. 41% had a well controlled T2DM with A1c level ≤7.0%, 16% were on insulin therapy, and almost half (48%) had T2DM for more than 5 years. Most were on treatment for hypertension (74%) and/or dyslipidemia (57%), and 18% were active smokers.
Vitamin D Status Correlates with Atherogenic Monocyte Properties
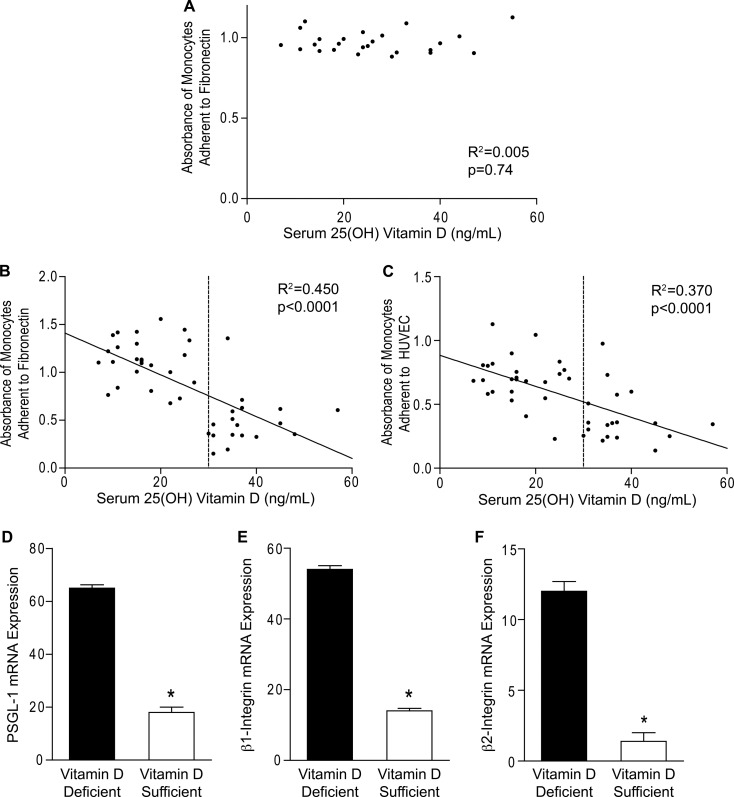
To determine whether vitamin D status is associated with inflammatory properties of monocytes, we performed functional adhesion assays in monocytes from diabetic and nondiabetic patients with varying 25(OH)D levels. In healthy, nondiabetic controls, 25(OH)D level did not correlate with monocyte adhesion to fibronectin (Fig. 1A; R2 = 0.005, p = 0.74). In contrast, in patients with diabetes, monocyte adhesion to both fibronectin and HUVEC was inversely associated with 25(OH)D level (Fig. 1, B and C; R2 = 0.450 and 0.370, respectively, p < 0.001 for both). We also noted that a 25(OH)D level of 30 ng/ml was a natural cutoff delineating high versus low monocyte adhesion in the diabetic patients, despite a lack of difference in total monocyte count or in monocytes as a percentage of white blood cells between the subgroups with 25(OH)D <30 ng/ml versus ≥30 ng/ml. Furthermore, in patients with 25(OH)D level <30 ng/ml, adhesion to fibronectin was significantly higher in diabetics than in nondiabetic controls (1.13 ± 0.047 versus 0.969 ± 0.014, p < 0.05). Conversely, in patients with 25(OH)D levels of ≥30 ng/ml, adhesion to fibronectin was significantly lower in diabetics than in nondiabetic controls (0.439 ± 0.038 versus 0.979 ± 0.031, respectively, p < 0.001), overall suggesting that vitamin D status may link type 2 diabetes to its vascular inflammation.
FIGURE 1.
Serum 25(OH)D correlates with monocyte adhesion in diabetics. A–C, functional adhesion assays performed in monocytes from healthy nondiabetics (A; n = 25) and type 2 diabetics (B and C; n = 43). Scatterplots of correlation between 25(OH)D and absorbance of monocytes adhered to fibronectin (A and B; p = 0.74 for A and p < 0.001 for B) or HUVEC (C; p < 0.001). The solid lines indicate regression lines. The dashed lines indicate 25(OH)D of 30 ng/ml. D–F, qPCR for mRNA of adhesion molecules PSGL-1 (D; n = 6/group), β1-integrin (E; n = 8/group), and β2-integrin (F; n = 8/group) relative to L32 in monocytes from vitamin D-sufficient and vitamin D-deficient diabetics (*, p < 0.001 for all, versus vitamin D-deficient).
To further investigate the correlations between vitamin D and monocyte adhesion, we assessed expression of cell surface adhesion molecules in monocytes from a subset vitamin D-deficient (<30 ng/ml 25(OH)D) or vitamin D-sufficient (≥30 ng/ml 25(OH)D) diabetics. As expected, we found that the mRNA expression of PSGL-1 and β1- and β2-integrin were all higher in monocytes from vitamin D-deficient compared with vitamin D-sufficient diabetics (Fig. 1, D–F; p < 0.001 for all), suggesting increased atherogenic behavior with a lower 25(OH)D level.
Vitamin D Status and Monocyte Adhesion Correlate with Macrophage Phenotype Ratio in Diabetics
Previous studies found no difference in the proportion of CD14lo/CD16+ monocytes between diabetic patients with and without coronary artery disease (5). In this study, we performed flow cytometry for membrane receptors CD14 and CD16 in monocytes from the first 40% of the diabetic patients and found no significant correlation between the proportion of CD14lo/CD16+ cells or CD14hi/CD16− cells and monocyte adhesion (fibronectin, R2 = 0.05 and 0.003, p = 0.35 and 0.82, respectively; HUVEC, R2 = 0.12 and <0.001, p = 0.14 and 0.991, respectively) or 25(OH)D level (R2 = 0.03 and 0.04, p = 0.46 and 0.39, respectively). These data support the lack of correlation between the monocyte CD14/CD16 classification and atherogenesis in diabetics and suggest that the properties defining these monocyte subsets are distinct from those that are affected by vitamin D.
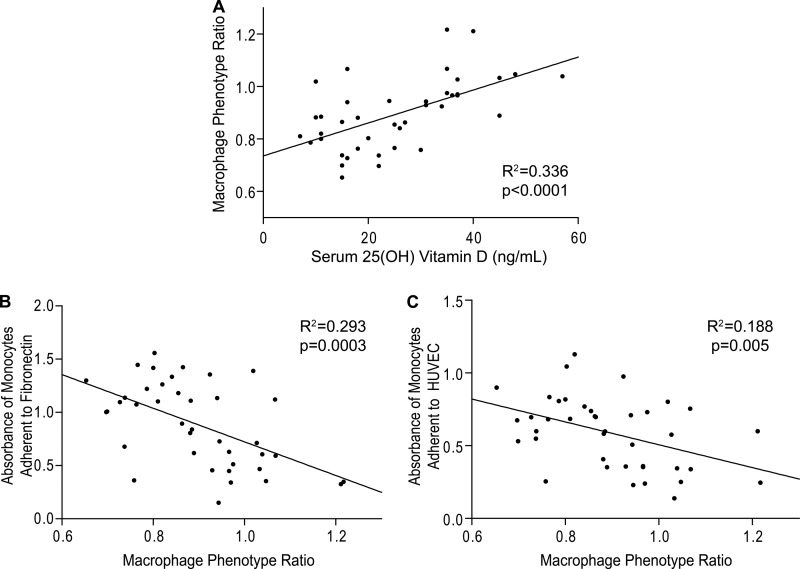
Recently, macrophage membrane markers that classify M1 and M2 subtypes have been found in circulating monocytes (9). Therefore, we explored whether the expression of these cell markers could potentially predict adhesion to the endothelium and explain the effects of vitamin D on monocyte function in diabetics. 25(OH)D level demonstrated a significant inverse correlation with macrophage M1 markers (CCR7 and CD86) and M2 markers (CD163 and MR) individually (R2 = 0.177–0.504, p < 0.01 for all) in diabetics (supplemental Fig. S4), supporting the immunosuppressive effects of vitamin D. To normalize for individual variability in overall marker expression level and provide a more integrative assessment of the overall monocyte/macrophage phenotype, we devised the macrophage phenotype ratio (MPR: (CCR7 + CD86 expression)/(CD163 + MR expression)) as a measure of the phenotype balance (M1 versus M2). The MPR is expected to be greater than 1 with a predominance of M1 markers and less than 1 with a predominance of M2 markers.
We subsequently calculated MPRs for the monocytes of each patient. 25(OH)D level positively correlated with the MPR in diabetics (Fig. 2A; R2 = 0.336, p < 0.001), but not in nondiabetic controls (R2 = 0.104, p = 0.31), revealing that decreasing levels of 25(OH)D are associated with an increased proportion of M2 compared with M1 markers in diabetics. The mean MPR of diabetics with vitamin D deficiency was significantly lower than in those with vitamin D sufficiency (0.83 ± 0.02 versus 1.00 ± 0.03, p < 0.001). In addition, monocytes from vitamin D-deficient diabetics had higher IL-10 and lower IL-12 mRNA expression compared with vitamin D-sufficient monocytes (supplemental Fig. S5, A and B; p < 0.005 for both), confirming the phenotype of an M2-predominant population in vitamin D deficiency. Lower MPR was also significantly associated with increased adhesion to fibronectin and HUVEC in diabetics (Fig. 2, B and C; R2 = 0.293, p < 0.001 and R2 0.188, p < 0.01, respectively). There was no significant relationship between MPR and adhesion in nondiabetic controls (R2 = 0.055, p = 0.46). Thus, vitamin D deficiency in diabetics is associated with a proatherogenic monocyte phenotype characterized by a predominance of M2 macrophage marker expression.
FIGURE 2.
Monocyte receptor phenotype correlates with serum 25(OH)D and monocyte adhesion in type 2 diabetics. Flow cytometry of monocytes from type 2 diabetics was performed to quantify membrane receptor expression. A, scatterplot of Pearson correlation (n = 40) between serum 25(OH)D and MPR (ratio of sum of M1 markers CD86 and CCR7 to sum of M2 markers CD163 and MR) (p < 0.001). B and C, scatterplots of Pearson correlation (n = 40) between MPR and absorbance of monocytes adhered to fibronectin (B; p < 0.001) or HUVEC (C; p < 0.01). The solid lines indicate regression lines.
Vitamin D Status Is Independently Associated with Monocyte Function after Adjustment for Confounders
To ensure that the associations found between 25(OH)D level and monocyte phenotype were not a result of confounding factors, we first performed simple linear regression to model the relationship between each demographic or comorbidity characteristic and each monocyte outcome, including adhesion to fibronectin, adhesion to HUVEC, and macrophage phenotype ratio. Surprisingly, of 16 potential confounders, only statin use had a significant association (p < 0.039) with any of the monocyte outcomes and was only associated with the MPR. For each of five comorbidity types (T2DM, hypertension, dyslipidemia, tobacco, and obesity), we selected the characteristic most predictive of the outcomes, or, in the case where none were significant, that most likely to reflect current disease state, ultimately including hemoglobin A1c, systolic blood pressure, statin use, any history of tobacco use, and BMI. For each of these comorbidity characteristics, as well as the demographic characteristics (gender, race, and age), we force-entered 25(OH)D level into the original simple regression model to determine whether vitamin D status improved the model. In all cases, the 25(OH)D level significantly improved the model, and the initial R-squared values of 0–11% increased by 23–50% over all outcomes (supplemental Table S2), suggesting that vitamin D status is more strongly associated with monocyte behavior than any other single demographic or comorbidity characteristic. Finally, we performed a multiple linear regression model for each monocyte outcome including all of the demographic and selected comorbidity characteristics force-entered into one block and then entered 25(OH)D level in a second block. For monocyte adhesion to fibronectin and to HUVEC, as well as the MPR, 25(OH)D level remained independently associated after adjustment for A1c, systolic blood pressure, statin use, any tobacco use, BMI, gender, race, and age, with an increase in the R-squared value of >30% in each case (supplemental Table S3), suggesting that 25(OH)D status is strongly and independently associated with monocyte properties in patients with diabetes.
1,25(OH)2D3 Suppresses Adhesion and M2 Macrophage Differentiation Induced by Vitamin D Deficiency in Diabetes
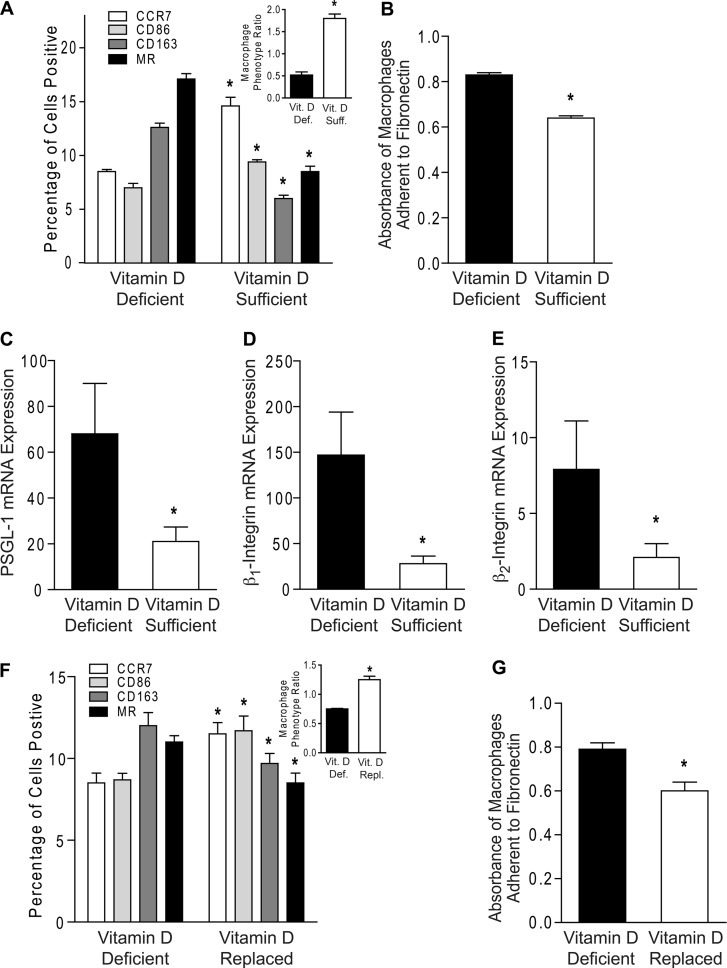
To determine whether vitamin D regulates macrophage adhesion and modifies their phenotype, we assessed M1 and M2 marker expression in monocyte-derived macrophages from diabetic patients cultured in vitamin D-deficient or 1,25(OH)2D3-supplemented conditions. Vitamin D-deficient macrophages expressed 50–70% higher CD163 and MR and 25–40% lower CCR7 and CD86 compared with macrophages cultured in 1,25(OH)2D3-supplemented media (Fig. 3A, p < 0.001 for all receptors). To further characterize the balance of M1 and M2 subtypes, we assessed the MPRs. Vitamin D deficiency resulted in an M2-predominant MPR <1 for all samples, whereas macrophages supplemented with 1,25(OH)2D3 had an M1-predominant MPR >1 (Fig. 3A, inset). In addition, vitamin D-deficient macrophages expressed higher IL-10 and lower IL-12 mRNA compared with 1,25(OH)2D3-supplemented macrophages (supplemental Fig. S5, C and D) consistent with the cytokine profiles predicted by cell surface markers. These data suggest that vitamin D deficiency is characterized by M2-predominant macrophage differentiation. Next, we performed functional adhesion assays in macrophages in both vitamin D conditions. Vitamin D-deficient macrophages had 30% greater adhesion to fibronectin (Fig. 3B; p < 0.001) and accordingly showed higher mRNA expression levels of PSGL-1, β1-integrin, and β2-integrin (Fig. 3, C and D; p < 0.05 for all) compared with 1,25(OH)2D3-supplemented cells, suggesting overall that vitamin D reduces proatherogenic properties in monocytes and macrophages.
FIGURE 3.
Active vitamin D supplementation of macrophages suppresses vitamin D deficiency-induced macrophage adhesion and M2 differentiation. A–E, monocytes from type 2 diabetics were differentiated into macrophages in vitamin D-deficient or 1,25(OH)2D3-supplemented conditions. A, MPR (inset; *, p < 0.001 versus vitamin D-deficient) and flow cytometry quantification of membrane receptors CCR7 (white), CD86 (light gray), CD163 (dark gray), and MR (black) (n = 9/group; *, p < 0.001 versus same receptor in vitamin D-deficient). B, absorbance of macrophages adhered to fibronectin (n = 6/group; *, p < 0.001 versus vitamin D-deficient). C–E, qPCR for mRNA of adhesion molecules PSGL-1 (C; n = 6/group), β1-integrin (D; n = 4/group), and β2-integrin (E; n = 6/group; *, p < 0.05 for all, versus vitamin D-deficient) relative to L32. F and G, macrophages were cultured in vitamin D-deficient media for 5 days and then supplemented with or without 1,25(OH)2D3. F, MPR (inset; *, p < 0.001 versus vitamin D-deficient) and flow cytometry quantification of membrane receptors (n = 12/group; *, p < 0.05 versus same receptor in vitamin D-deficient). G, absorbance of macrophages adhered to fibronectin (n = 3/group; *, p < 0.05 versus vitamin D-deficient). Vit. D Def., vitamin D-deficient; Vit. D Suff., vitamin D-sufficient; Vit. D Repl., vitamin D-replaced.
To clarify whether M2-predominant macrophage differentiation and increased adhesion induced by vitamin D deficiency are reversible, we first differentiated macrophages from diabetics in vitamin D-deficient media for 5 days and then supplemented with 1,25(OH)2D3 or maintained vitamin D-deficient media for an additional 5 days. 1,25(OH)2D3-replaced cells demonstrated a 20–25% increase in CCR7 and CD86, a 20% decrease in CD163 and MR, a significant increase in the MPR to >1, and decreased adhesion to fibronectin when compared with macrophages maintained in vitamin D-deficient conditions (Fig. 3, F and G; p < 0.05 for all), indicating that 1,25(OH)2D3 supplementation reversed macrophage phenotype.
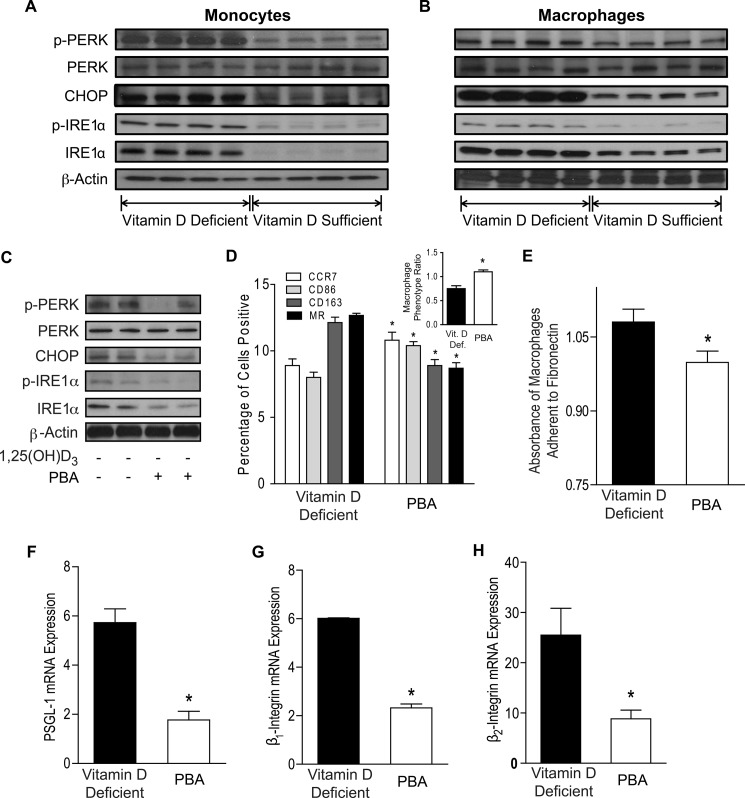
Down-regulation of ER Stress by 1,25(OH)2D3 Prevents Monocyte/Macrophage Adhesion and M2 Differentiation in Cells from Diabetics
We have previously demonstrated that suppression of macrophage ER stress by 1,25(OH)2D3 prevents intracellular cholesterol deposition (41). Thus, we first evaluated whether ex vivo monocytes from vitamin D-deficient nondiabetic controls and diabetics have increased ER stress. Expression of phospho-protein kinase RNA-like endoplasmic reticulum kinase (p-PERK), phospho-inositol-requiring enzyme 1α (p-IRE1α), IRE1α, and CHOP were increased in vitamin D-deficient subjects when compared with monocytes from vitamin D-sufficient subjects in both nondiabetic controls and in diabetics. Furthermore, monocytes from diabetic patients had increased expression of p-PERK, p-IRE1α, and CHOP when compared with those from nondiabetic controls, regardless of vitamin D status (Fig. 4A and supplemental Fig. S6, A and B). Similarly, ER stress protein expression was increased in macrophages from diabetics cultured in vitamin D-deficient versus vitamin D-supplemented conditions (Fig. 4B and supplemental Fig. S6C), suggesting that multiple UPR signaling pathways are up-regulated by vitamin D deficiency and by diabetes.
FIGURE 4.
Down-regulation of ER stress by 1,25(OH)2D3 prevents M2 differentiation and macrophage adhesion. A, ER stress protein expression in monocytes from vitamin D-deficient and vitamin D-sufficient diabetics. B, ER stress protein expression in monocytes from type 2 diabetics differentiated into macrophages in vitamin D-deficient or 1,25(OH)2D3-supplemented conditions. C–H, monocytes from type 2 diabetics were differentiated into macrophages in vitamin D-deficient conditions with or without PBA (protein chaperone). C, ER stress protein expression. D, macrophage phenotype ratio (inset; *, p < 0.001 versus non-PBA-treated) and flow cytometry quantification of membrane receptors CCR7 (white), CD86 (light gray), CD163 (dark gray), and MR (black) (n = 9/group; *, p < 0.05 versus same receptor in non-PBA-treated). E, absorbance of macrophages adhered to fibronectin (n = 12/group; *, p < 0.05 versus non-PBA-treated). F–H, qPCR for mRNA of adhesion molecules PSGL-1 (F), β1-integrin (G), and β2-integrin (H; n = 6/group; *, p < 0.05 for all, versus non-PBA-treated) relative to L32.
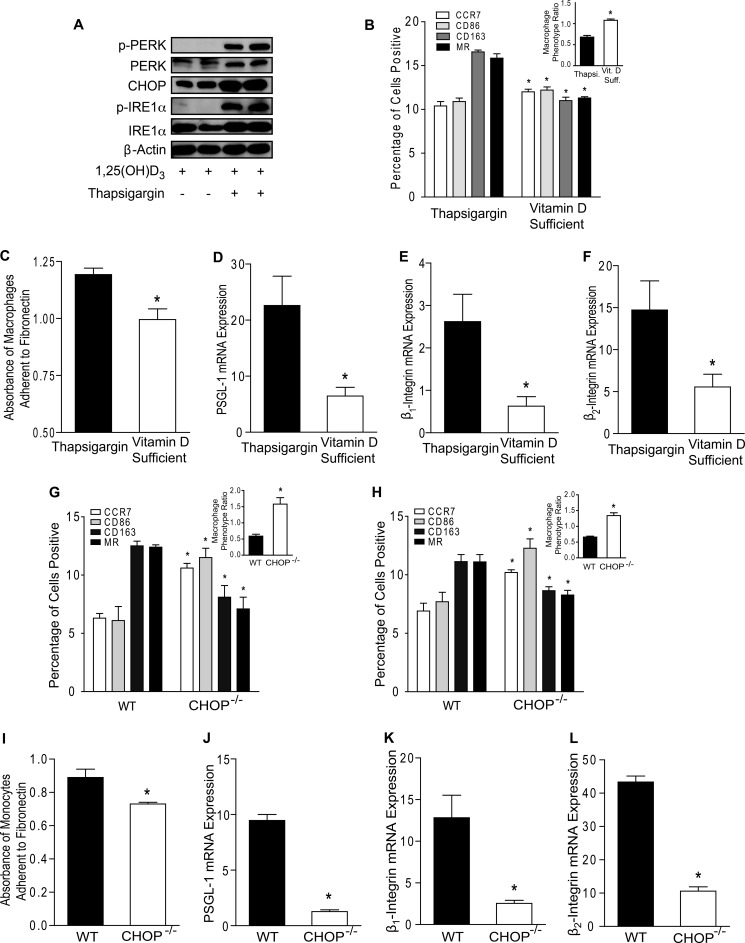
To determine whether ER stress is essential for the M2-predominant differentiation and increased macrophage adhesion induced by vitamin D deficiency, we cultured macrophage colony-stimulating factor-differentiated macrophages from diabetic patients in vitamin D-deficient conditions with or without PBA, an ER stress reliever. PBA treatment down-regulated macrophage ER stress activation as shown by lower p-PERK, p-IRE1α, IRE1α, and CHOP expression (Fig. 4C and supplemental Fig. S7A). PBA increased CCR7 and CD86 and suppressed CD163 and MR membrane expression (Fig. 4D; p < 0.05 for all receptors), shifting the MPR to >1, as well as decreasing IL-10 and increasing IL-12 mRNA expression (Fig. 4D, inset, and supplemental Fig. S7, B and C), compared with non-PBA-treated macrophages, consistent with an M1-predominant expression pattern. In addition, PBA suppressed adhesion to fibronectin (Fig. 4E; p < 0.05) and expression of adhesion molecules PSGL-1, β1-integrin, and β2-integrin, (Fig. 4, F–H; p < 0.05 for all) compared with non-PBA-treated cells. Finally, we stimulated ER stress in macrophages cultured under 1,25(OH)2D3-supplemented conditions by treating with thapsigargin, an ER stress inducer. Thapsigargin-treated macrophages had increased ER stress activation (Fig. 5A and supplemental Fig. S7D), with an M2-predominant phenotype (high MR and CD163 and low CCR7 and CD86 membrane expression) characterized by MPR <1 (Fig. 5B; p < 0.05 for all receptors and MPR) and high IL-10 and low IL-12 mRNA expression (Fig. S7, E and F). Thapsigargin also enhanced adhesion to fibronectin (Fig. 5C; p < 0.005) and increased expression of PSGL-1, β1-integrin, and β2-integrin (Fig. 5, D–F; p < 0.05 for all) compared with 1,25(OH)2D3-supplemented macrophages. Thus, vitamin D acts through ER stress pathways to affect monocyte phenotype.
FIGURE 5.
Induction and suppression of ER stress control regulation of monocyte/macrophage proinflammatory phenotype by vitamin D. A–F, monocytes from type 2 diabetic patients were differentiated into macrophages in 1,25(OH)2D3-supplemented conditions with or without thapsigargin (Thapsi., ER stress inducer). A, ER stress protein expression. B, MPR (inset; *, p < 0.001 versus thapsigargin-treated) and flow cytometry quantification of membrane receptors CCR7 (white), CD86 (light gray), CD163 (dark gray), and MR (black) (n = 9/group; *, p < 0.05 versus same receptor in thapsigargin-treated). C, absorbance of macrophages adhered to fibronectin (n = 12/group; *, p < 0.005 versus thapsigargin-treated). D–F, qPCR for mRNA of PSGL-1 (D), β1-integrin (E), and β2-integrin (F; n = 6/group; *, p < 0.05 for all, versus thapsigargin-treated) relative to L32. G, in peripheral blood monocytes from vitamin D-deficient WT or CHOP−/− mice, MPR (inset; *, p < 0.001 versus WT) and flow cytometry quantification of membrane receptors (n = 6/group; *, p < 0.005 versus same receptor in WT). H–L, CD11b+/CD115+ bone marrow monocytes were harvested from vitamin D-deficient WT or CHOP−/− mice. H, MPR (inset; *, p < 0.001 versus WT) and flow cytometry quantification of membrane receptors (n = 6/group; *, p < 0.005 versus same receptor in WT). I, absorbance of monocytes adhered to fibronectin (n = 3/group; *, p < 0.05 versus WT). J–L, qPCR for mRNA of adhesion molecules PSGL-1 (J; n = 3/group), β1-integrin (K; n = 3/group), and β2-integrin (L; n = 4/group; *, p < 0.05 for all, versus WT) relative to L32. Vit. D Suff., vitamin D-sufficient.
Because PBA is a nonspecific ER stress inhibitor, we studied whether CHOP, a downstream protein of a branch of the UPR induced in advanced murine and human coronary artery plaques, is essential for regulation of monocyte/macrophage adhesion by vitamin D (42, 43). In blood peripheral monocytes and bone marrow (BM) monocytes from vitamin D-deficient mice, the absence of CHOP prevents the M2 phenotype. Circulating monocytes and BM monocytes from vitamin D-deficient WT mice have an MPR <1, consistent with an M2-predominant phenotype, similar to our findings in monocytes from vitamin D-deficient diabetic patients. Conversely, in the absence of CHOP, monocytes and BM monocytes had an M1-predominant with MPR >1 (Fig. 5, G and H, insets) despite vitamin D deficiency when compared with cells from WT mice (Fig. 5, G and H; p < 0.005 for all receptors and MPRs). Moreover, in BM monocytes, the absence of CHOP suppressed adhesion to fibronectin (Fig. 5I; p < 0.05) and decreased mRNA expression of PSGL-1, β1-integrin, and β2-integrin (Fig. 5, J–L; p < 0.05 for all) when compared with WT. Taken together, these data suggest that vitamin D functions as an ER stress reliever in monocytes and macrophages, promoting an antiatherogenic phenotype.
Absence of VDR Signaling Increases Macrophage Adhesion and M2 Differentiation
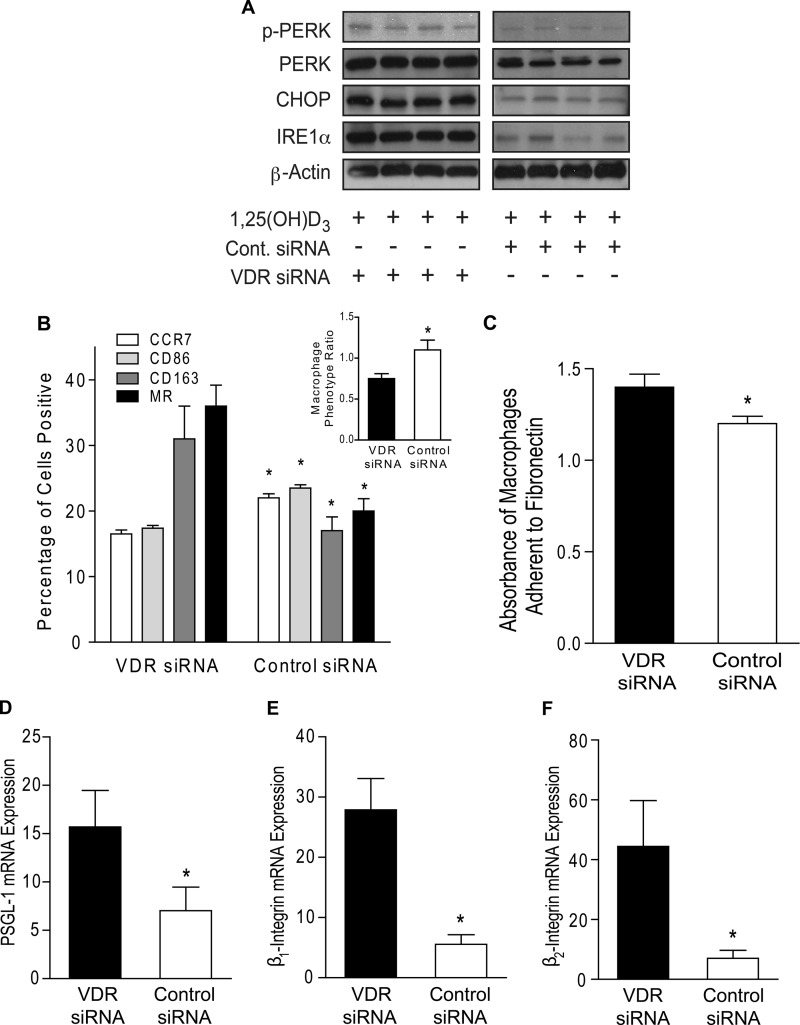
1,25(OH)2D3 acts primarily through the VDR and is known to induce its expression but is also known to have rapid, nongenomic actions upon binding to other proteins at target cells (44). In this study, we demonstrated that vitamin D receptor expression was higher in monocytes from vitamin D-sufficient patients compared with those from vitamin D-deficient patients (supplemental Fig. S8A), regardless of diabetes status, suggesting that higher serum levels of the precursor 25(OH)D generate more 1,25(OH)2D to induce VDR expression. To determine whether the effects of vitamin D on macrophage adhesion in cells from diabetics are VDR-dependent, we infected macrophages cultured in 1,25(OH)2D3-supplemented media with lentivirus containing either VDR-siRNA or control-siRNA hairpins. With attenuated VDR signaling (supplemental Fig. S8B), VDR-siRNA-infected macrophages demonstrated increased expression of ER stress signaling proteins (Fig. 6A and supplemental Fig. S8C), as well as increased CD163 and MR and decreased CCR7 and CD86 membrane expression with a resulting MPR <1 compared with macrophages with intact VDR signaling (Fig. 6B; p < 0.05 for all receptors). Macrophages lacking VDR signaling had increased IL-10 and suppressed IL-12 mRNA expression compared with macrophages with intact VDR signaling (supplemental Fig. S8, D and E), all indicating an M2 expression pattern. Moreover, VDR-siRNA-treated macrophages had increased adhesion to fibronectin (Fig. 6C; p < 0.001) and expression of PSGL-1, β1-integrin, and β2-integrin compared with control siRNA-infected cells (Fig. 6, D–F; p < 0.05 for all), suggesting that VDR signaling reduces macrophage ER stress signaling and shifts the macrophage phenotype toward antiatherogenic properties.
FIGURE 6.
Vitamin D receptor signaling is required for effects of vitamin D on monocyte adhesion. Monocytes from type 2 diabetics were differentiated into macrophages and then infected with either VDR-siRNA or control-siRNA lentivirus in 1,25(OH)2D3-supplemented media. A, ER stress protein expression. B, macrophage phenotype ratio (inset; *, p < 0.05 versus VDR siRNA) and flow cytometry quantification of membrane receptors CCR7 (white), CD86 (light gray), CD163 (dark gray), and MR (black) (n = 12/group; *, p < 0.05 versus same receptor in VDR siRNA). C, absorbance of macrophages adhered to fibronectin (n = 9/group; *, p < 0.001 versus VDR siRNA). D–F, qPCR for mRNA of adhesion molecules PSGL-1 (D), β1-integrin (E), and β2-integrin (F; n = 6/group; *, p < 0.05 for all, versus VDR siRNA) relative to L32.
DISCUSSION
Vitamin D deficiency is associated with T2DM, increased vascular complications, and increased mortality, but causal mechanisms for these associations are unknown. The recruitment of circulating monocytes into the vessel wall is critical to the initiation of diabetes and its complications, a disease state associated with twice the prevalence of vitamin D deficiency compared with nondiabetics. In this study, we demonstrate that serum 25(OH)D level is inversely correlated with monocyte adhesion to endothelial cells in patients with type 2 diabetes. 25(OH)D deficiency (≤30 ng/ml) promotes an M2-predominant phenotype with increased expression of monocyte adhesion molecules PSGL-1, β1-integrin, and β2-integrin. 1,25(OH)2D3 suppression of ER stress decreased monocyte adhesion to endothelial cells, reduced expression of monocyte adhesion molecules, and promoted an M1-predominant phenotype. In macrophages differentiated under vitamin D-deficient conditions, 1,25(OH)2D3 supplementation reversed the M2-predominant macrophage phenotype into an M1-predominant phenotype with lower endothelial adhesion. In addition, absence of the vitamin D receptor prevented 1,25(OH)2D3 suppression of vascular adhesion and the M1 phenotype. These findings demonstrate that vitamin D regulation of ER stress is critical for the innate immune response linked to vascular complications in type 2 diabetic patients.
Although vitamin D is typically recognized for skeletal effects, immune cells express the VDR and are also able to transform 25(OH)D into its active form, which functions as an autocrine/paracrine factor (45). The effects of 25(OH)D status on circulating monocyte adherence to endothelial cells in patients with diabetes are unknown. Previous in vitro studies indicate that active vitamin D maintains monocyte adhesion to fibronectin in cultured nondiabetic human monocytes and stimulates adhesion by up-regulating αl-, αM-, β1-, β2-, and β3-integrins in monocytic cell lines (35, 36, 46). In contrast, we found that increasing in vivo 25(OH)D status in diabetics significantly correlated with decreased monocyte adhesion to both endothelial cells and fibronectin. Limited causative data regarding vitamin D deficiency and cardiometabolic outcomes has led to concern that vitamin D deficiency may be simply a marker of poor health (47), but we found that 25(OH)D status was still strongly inversely associated with monocyte adhesion, even after adjustment for multiple demographic and comorbidity characteristics. We also identified a 25(OH)D level of 30 ng/ml as a natural cutoff delineating high versus low adhesion for the diabetic patients. Circulating monocytes from vitamin D-deficient diabetics (25(OH)D level of <30 ng/ml) had increased expression of cell surface adhesion molecules compared with diabetic patients with 25(OH)D level ≥30 ng/ml. Thus, a 25(OH)D level of 30 ng/ml may be optimal to prevent vascular complications in this population.
Multiple studies in nondiabetics suggest a positive correlation between the proportion of circulating CD14lo/CD16+ monocytes and proinflammatory markers or carotid intima-media thickness (3, 4, 48), but no significant differences are found in the mean percentages of CD14lo/CD16+ monocytes between patients with uncomplicated diabetes, diabetics with cardiovascular disease, and control subjects (5). In this study of diabetic patients, classification of monocyte subtypes based on CD14 and CD16 expression did not correlate with 25(OH)D level or with functional adhesion, suggesting that different monocyte markers could better characterize the functions contributing to the proinflammatory state in diabetics. A recent observation in obese patients with T2DM assessed peripheral monocytes for the macrophage markers that classify M1 and M2 subtypes and found reduced expression of M2 markers compared with obese patients without diabetes; this was associated with increased arterial stiffness (9). A second similar study found reduced expression of M2 markers in all obese patients, regardless of diabetes, compared with lean patients (8). However, no publications have assessed both M1 and M2 markers and used the combination of the two to produce an integrative assessment of circulating monocyte phenotype. Furthermore, the environmental conditions affecting monocyte phenotype and function are unknown. In this study we found that 25(OH)D plays a key role in monocyte phenotype, demonstrated by reduction of both M2 and M1 markers with increased 25(OH)D level, suggesting that vitamin D suppresses both subsets of monocytes. Interestingly, the greater reduction in M2 markers with higher 25(OH)D led to an M1 predominance and higher MPR, but with decreased adhesion and adhesion markers. In apolipoprotein E knock-out mice, early plaque macrophages exhibit a predominantly M2 phenotype, whereas M1 macrophages are dominant in more advanced lesions of aged mice (49), suggesting that the increased adhesion of M2 monocytes to the endothelium may be the origin of early plaque macrophages. We suggest that adequate vitamin D promotes a beneficial monocyte phenotype that may prevent the early stages of vascular inflammation and diabetic complications. These data begin to shed light on the potential mechanisms behind the mounting evidence that correlates vitamin D with the development of diabetes and cardiovascular disease (30–32).
ER stress plays a key role in the development of diabetes and vascular complications (25, 43, 50). In mouse models of diet induced-insulin resistance and atherosclerosis, knock-out of the ER stress protein CHOP or the addition of chemical chaperones to decrease ER stress prevents the development of insulin resistance and atherosclerosis (28, 51). At early stages of vascular inflammation, up-regulated ER stress markers are detected in intimal macrophages even before the formation of atherosclerotic plaques, suggesting that adverse environmental or pathological conditions that increase circulating monocyte ER stress may modulate monocyte infiltration (27). In humans and mice, ER stress is also present in advanced atherosclerotic plaques and is associated with macrophage apoptosis, plaque vulnerability, and acute coronary syndrome (29, 42, 52), suggesting that monocyte/macrophage ER stress is a key regulator in both plaque initiation and progression. We recently demonstrated that suppression of ER stress shifts M2-differentiated macrophages to M1-predominant cells with decreased foam cell formation (15). However, environmental conditions that control monocyte and macrophage phenotype, behavior, and vascular adhesion, an early inflammatory step in atherogenesis, are unknown. We now demonstrate that vitamin D status is a novel key regulator of the ER stress-induced inflammatory phenotype of circulating monocytes and macrophages in patients with diabetes. In circulating monocytes from vitamin D-deficient subjects, we observed activation of ER stress, increased adhesion and adhesion molecule expression, and an M2-predominant phenotype. In this study and our previous studies of macrophages from diabetic patients, VDR signaling activation suppressed ER stress, decreased macrophage foam cell formation by suppressing scavenger receptor CD36 and SR-AI expression, and promoted differentiation into the M1 phenotype (15, 41). Interestingly, other studies indicate that M1 macrophages dominate advanced plaques and are considered to be proinflammatory, whereas M2 macrophages predominate in early plaques, rapidly accumulate small lipid droplets, and are anti-inflammatory, suggesting that vitamin D may induce proinflammatory effects despite suppression of macrophage cholesterol deposition (6, 14, 53–56). However, recent data in mice show that bone marrow transplantation of cells with the absence of the vitamin D receptor increases atherosclerosis, mediated through local activation of the renin angiotensin system in macrophages (57), suggesting that induction of the M1 macrophage phenotype by vitamin D may lead to a net beneficial effect. Additionally, M1 macrophages express membrane receptors that are associated with plaque macrophage egression (58–61). The induction of CCR7 expression in macrophages by vitamin D could be one of the mechanisms for the beneficial effects on atherosclerosis. Therefore, the role of M1/M2 subsets in atherosclerotic progression is likely more complex than the current inflammatory paradigm suggests and seems to be dependent upon the stage and environment of plaque evolution.
Insulin resistance is associated with enhanced monocyte/macrophage adhesion to the endothelium, as well as reduced insulin signaling in macrophages (62). Insulin resistance is known to induce ER stress in monocytes and macrophages (23, 24). In this study, we found that diabetic patients demonstrated increased activation of monocyte/macrophage ER stress when compared with controls, regardless of vitamin D status. Further, we found that vitamin D deficiency, through activation of ER stress, induced monocyte/macrophage adhesion to endothelial cells in diabetics. 1,25(OH)2D3 induces insulin sensitivity and down-regulates ER stress-JNK activity, but only in patients with diabetes (41). Therefore, down-regulation of ER stress might account for the disparity in response to vitamin D between the type 2 diabetic and control groups.
This study demonstrates vitamin D as a natural monocyte/macrophage ER stress reliever that promotes an antiatherogenic monocyte/macrophage phenotype in patients with diabetes. Higher serum 25(OH)D level correlated positively with a monocyte phenotype with higher MPR and antiatherogenic properties. Moreover, reversibility of the proatherogenic macrophage phenotype by vitamin D supplementation highlights vitamin D sufficiency as a potential therapeutic target to modulate monocyte/macrophage ER stress, reduce inflammation, and reduce diabetic complications. Human interventional trials with vitamin D or ER stress suppressers are needed to confirm the effects on atherosclerosis.
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL094818-0 and P30DK079333. This work was also supported by American Diabetes Association Grant 1-12-CT-08.

This article contains supplemental text, Tables S1–S3, and Figs. S1–S8.
- T2DM
- type 2 diabetes mellitus
- IC
- immunocomplex
- LPS
- lipopolysaccharide
- PSGL-1
- P-selectin glycoprotein ligand 1
- ER
- endoplasmic reticulum
- UPR
- unfolded protein response
- 25(OH)D
- 25-hydroxy vitamin D
- VDR
- vitamin D receptor
- 1,25(OH)2D
- 1,25-dihydroxy vitamin D
- PBA
- phenyl butyric acid
- CHOP
- CCAAT enhancer-binding protein homologous protein
- HUVEC
- human umbilical vein endothelial cells
- qPCR
- quantitative PCR
- MR
- mannose receptor
- MPR
- macrophage phenotype ratio
- BMI
- body mass index
- p-PERK
- phospho-protein kinase RNA-like endoplasmic reticulum kinase
- p-IRE1α
- phospho-inositol-requiring enzyme 1α
- BM
- bone marrow.
REFERENCES
- 1. Centers for Disease Control and Prevention (2011) National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, United States Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 2. Passlick B., Flieger D., Ziegler-Heitbrock H. W. (1989) Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 [PubMed] [Google Scholar]
- 3. Schlitt A., Heine G. H., Blankenberg S., Espinola-Klein C., Dopheide J. F., Bickel C., Lackner K. J., Iz M., Meyer J., Darius H., Rupprecht H. J. (2004) CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-α levels. Thromb. Haemost. 92, 419–424 [DOI] [PubMed] [Google Scholar]
- 4. Rogacev K. S., Ulrich C., Blömer L., Hornof F., Oster K., Ziegelin M., Cremers B., Grenner Y., Geisel J., Schlitt A., Köhler H., Fliser D., Girndt M., Heine G. H. (2010) Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 31, 369–376 [DOI] [PubMed] [Google Scholar]
- 5. Patiño R., Ibarra J., Rodriguez A., Yagüe M. R., Pintor E., Fernandez-Cruz A., Figueredo A. (2000) Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am. J. Cardiol. 85, 1288–1291 [DOI] [PubMed] [Google Scholar]
- 6. Bouhlel M. A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., Chinetti-Gbaguidi G. (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 [DOI] [PubMed] [Google Scholar]
- 7. Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages. An immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 8. Bories G., Caiazzo R., Derudas B., Copin C., Raverdy V., Pigeyre M., Pattou F., Staels B., Chinetti-Gbaguidi G. (2012) Diabetes Vasc. Dis. Res. 9, 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satoh N., Shimatsu A., Himeno A., Sasaki Y., Yamakage H., Yamada K., Suganami T., Ogawa Y. (2010) Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients. Effect of pioglitazone. Diabetes Care 33, e7. [DOI] [PubMed] [Google Scholar]
- 10. Tacke F., Alvarez D., Kaplan T. J., Jakubzick C., Spanbroek R., Llodra J., Garin A., Liu J., Mack M., van Rooijen N., Lira S. A., Habenicht A. J., Randolph G. J. (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 12. Gordon S. (2003) Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 13. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization. New molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 14. van Tits L. J., Stienstra R., van Lent P. L., Netea M. G., Joosten L. A., Stalenhoef A. F. (2011) Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages. A crucial role for Krüppel-like factor 2. Atherosclerosis 214, 345–349 [DOI] [PubMed] [Google Scholar]
- 15. Oh J., Riek A. E., Weng S., Petty M., Kim D., Colonna M., Cella M., Bernal-Mizrachi C. (2012) Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J. Biol. Chem. 287, 11629–11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woollard K. J., Geissmann F. (2010) Monocytes in atherosclerosis. Subsets and functions. Nat. Rev. Cardiol. 7, 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fasching P., Waldhausl W., Wagner O. F. (1996) Elevated circulating adhesion molecules in NIDDM–potential mediators in diabetic macroangiopathy. Diabetologia 39, 1242–1244 [DOI] [PubMed] [Google Scholar]
- 18. Jilma B., Fasching P., Ruthner C., Rumplmayr A., Ruzicka S., Kapiotis S., Wagner O. F., Eichler H. G. (1996) Elevated circulating P-selectin in insulin dependent diabetes mellitus. Thromb. Haemost. 76, 328–332 [PubMed] [Google Scholar]
- 19. Kostidou E., Koliakos G., Kaloyianni M. (2009) Increased monocyte alphaL, αM and β2 integrin subunits in diabetes mellitus. Clin. Biochem. 42, 634–640 [DOI] [PubMed] [Google Scholar]
- 20. Shanmugam N., Reddy M. A., Guha M., Natarajan R. (2003) High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52, 1256–1264 [DOI] [PubMed] [Google Scholar]
- 21. Chen N. G., Abbasi F., Lamendola C., McLaughlin T., Cooke J. P., Tsao P. S., Reaven G. M. (1999) Mononuclear cell adherence to cultured endothelium is enhanced by hypertension and insulin resistance in healthy nondiabetic volunteers. Circulation 100, 940–943 [DOI] [PubMed] [Google Scholar]
- 22. Chen N. G., Holmes M., Reaven G. M. (1999) Relationship between insulin resistance, soluble adhesion molecules, and mononuclear cell binding in healthy volunteers. J. Clin. Endocrinol. Metab. 84, 3485–3489 [DOI] [PubMed] [Google Scholar]
- 23. Komura T., Sakai Y., Honda M., Takamura T., Matsushima K., Kaneko S. (2010) CD14+ monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes 59, 634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. (2006) Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell metabolism 3, 257–266 [DOI] [PubMed] [Google Scholar]
- 25. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hotamisligil G. S., Spiegelman B. M. (2000) Diabetes Mellitus (LeRoith D., Taylor S. I., Olefsky J. M. eds) pp. 651–658, Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 27. Zhou J., Lhoták S., Hilditch B. A., Austin R. C. (2005) Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111, 1814–1821 [DOI] [PubMed] [Google Scholar]
- 28. Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tabas I. (2010) The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107, 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pittas A. G., Chung M., Trikalinos T., Mitri J., Brendel M., Patel K., Lichtenstein A. H., Lau J., Balk E. M. (2010) Systematic review. Vitamin D and cardiometabolic outcomes. Ann. Intern. Med. 152, 307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L., Manson J. E., Song Y., Sesso H. D. (2010) Systematic review. Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann. Intern. Med. 152, 315–323 [DOI] [PubMed] [Google Scholar]
- 32. Melamed M. L., Michos E. D., Post W., Astor B. (2008) 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 168, 1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cigolini M., Iagulli M. P., Miconi V., Galiotto M., Lombardi S., Targher G. (2006) Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 29, 722–724 [DOI] [PubMed] [Google Scholar]
- 34. Isaia G., Giorgino R., Adami S. (2001) High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 24, 1496. [DOI] [PubMed] [Google Scholar]
- 35. Polla B. S., Healy A. M., Amento E. P., Krane S. M. (1986) 1,25-Dihydroxyvitamin D3 maintains adherence of human monocytes and protects them from thermal injury. J. Clin. Invest. 77, 1332–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hewison M., Dabrowski M., Faulkner L., Hughson E., Vadher S., Rut A., Brickell P. M., O'Riordan J. L., Katz D. R. (1994) Transfection of vitamin D receptor cDNA into the monoblastoid cell line U937. The role of vitamin D3 in homotypic macrophage adhesion. J. Immunol. 153, 5709–5719 [PubMed] [Google Scholar]
- 37. Du T., Zhou Z. G., You S., Huang G., Lin J., Yang L., Li X., Zhou W. D., Chao C. (2009) Modulation of monocyte hyperresponsiveness to TLR ligands by 1,25-dihydroxy-vitamin D3 from LADA and T2DM. Diabetes Res. Clin. Pract. 83, 208–214 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y., Leung D. Y., Richers B. N., Liu Y., Remigio L. K., Riches D. W., Goleva E. (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 188, 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickhout J. G., Lhotak S., Hilditch B. A., Basseri S., Colgan S. M., Lynn E. G., Carlisle R. E., Zhou J., Sood S. K., Ingram A. J., Austin R. C. (2011) FASEB J. 25, 576–589 [DOI] [PubMed] [Google Scholar]
- 40. Schaefer A., Magócsi M., Stöcker U., Kósa F., Marquardt H. (1994) Early transient suppression of c-myb mRNA levels and induction of differentiation in Friend erythroleukemia cells by the [Ca2+]i-increasing agents cyclopiazonic acid and thapsigargin. J. Biol. Chem. 269, 8786–8791 [PubMed] [Google Scholar]
- 41. Oh J., Weng S., Felton S. K., Bhandare S., Riek A., Butler B., Proctor B. M., Petty M., Chen Z., Schechtman K. B., Bernal-Mizrachi L., Bernal-Mizrachi C. (2009) 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 120, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., Bochaton-Piallat M. L., Mochizuki N., Kitakaze M. (2007) Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116, 1226–1233 [DOI] [PubMed] [Google Scholar]
- 43. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 44. Nemere I., Farach-Carson M. C. (1998) Membrane receptors for steroid hormones. A case for specific cell surface binding sites for vitamin D metabolites and estrogens. Biochem. Biophys. Res. Commun. 248, 443–449 [DOI] [PubMed] [Google Scholar]
- 45. Adams J. S., Ren S., Liu P. T., Chun R. F., Lagishetty V., Gombart A. F., Borregaard N., Modlin R. L., Hewison M. (2009) Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 182, 4289–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwende H., Fitzke E., Ambs P., Dieter P. (1996) Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukocyte Biol. 59, 555–561 [PubMed] [Google Scholar]
- 47. Grey A., Bolland M. (2010) Vitamin D. A place in the sun? Arch. Intern. Med. 170, 1099–1100 [DOI] [PubMed] [Google Scholar]
- 48. Ulrich C., Heine G. H., Gerhart M. K., Köhler H., Girndt M. (2008) Proinflammatory CD14+CD16+ monocytes are associated with subclinical atherosclerosis in renal transplant patients. Am. J. Transplant. 8, 103–110 [DOI] [PubMed] [Google Scholar]
- 49. Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A. T., Clement M., Dussiot M., Levillain O., Graff-Dubois S., Nicoletti A., Caligiuri G. (2010) Macrophage plasticity in experimental atherosclerosis. PLoS One 5, e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gregor M. F., Hotamisligil G. S. (2007) Thematic review series. Adipocyte Biology. Adipocyte stress. The endoplasmic reticulum and metabolic disease. J. Lipid Res. 48, 1905–1914 [DOI] [PubMed] [Google Scholar]
- 51. Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9, 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., Marks A. R., Ron D., Tabas I. (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5, 781–792 [DOI] [PubMed] [Google Scholar]
- 53. Chinetti-Gbaguidi G., Staels B. (2011) Macrophage polarization in metabolic disorders. Functions and regulation. Curr. Opin. Lipidol. 22, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mantovani A., Garlanda C., Locati M. (2009) Macrophage diversity and polarization in atherosclerosis. A question of balance. Arterioscler. Thromb. Vasc. Biol. 29, 1419–1423 [DOI] [PubMed] [Google Scholar]
- 55. Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–617310843666 [Google Scholar]
- 56. Chinetti-Gbaguidi G., Baron M., Bouhlel M. A., Vanhoutte J., Copin C., Sebti Y., Derudas B., Mayi T., Bories G., Tailleux A., Haulon S., Zawadzki C., Jude B., Staels B. (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108, 985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szeto F. L., Reardon C. A., Yoon D., Wang Y., Wong K. E., Chen Y., Kong J., Liu S. Q., Thadhani R., Getz G. S., Li Y. C. (2012) Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol. Endocrinol. 26, 1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feig J. E., Parathath S., Rong J. X., Mick S. L., Vengrenyuk Y., Grauer L., Young S. G., Fisher E. A. (2011) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feig J. E., Pineda-Torra I., Sanson M., Bradley M. N., Vengrenyuk Y., Bogunovic D., Gautier E. L., Rubinstein D., Hong C., Liu J., Wu C., van Rooijen N., Bhardwaj N., Garabedian M., Tontonoz P., Fisher E. A. (2010) LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feig J. E., Rong J. X., Shamir R., Sanson M., Vengrenyuk Y., Liu J., Rayner K., Moore K., Garabedian M., Fisher E. A. (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. U.S.A. 108, 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trogan E., Feig J. E., Dogan S., Rothblat G. H., Angeli V., Tacke F., Randolph G. J., Fisher E. A. (2006) Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 103, 3781–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mita T., Goto H., Azuma K., Jin W. L., Nomiyama T., Fujitani Y., Hirose T., Kawamori R., Watada H. (2010) Impact of insulin resistance on enhanced monocyte adhesion to endothelial cells and atherosclerogenesis independent of LDL cholesterol level. Biochem. Biophys. Res. Commun. 395, 477–483 [DOI] [PubMed] [Google Scholar]