Background: FGF signaling is a critical pathway regulating cell migration during gastrulation.
Results: Inhibition of FGF signaling up-regulates six microRNAs, partially through loss of LIN28B. FGF-regulated microRNAs target proteins important for gastrulation, including serine/threonine and tyrosine kinase receptors.
Conclusion: FGF signaling regulates microRNA abundance to control gastrulation.
Significance: This identifies a new mechanism by which FGF signaling regulates gene expression.
Keywords: Development, Fibroblast Growth Factor (FGF), MicroRNA, RNA Processing, RNA Turnover, Chicken Embryo, Gastrulation, LIN28B
Abstract
FGF signaling plays a pivotal role in regulating cell movements and lineage induction during gastrulation. Here we identify 44 microRNAs that are expressed in the primitive streak region of gastrula stage chicken embryos. We show that the primary effect of FGF signaling on microRNA abundance is to negatively regulate the levels of miR-let-7b, -9, -19b, -107, -130b, and -218. LIN28B inhibits microRNA processing and is positively regulated by FGF signaling. Gain- and loss-of-function experiments show that LIN28B negatively regulates the expression of miR-19b, -130b, and let-7b, whereas negative modulation of miR-9, -107, and -218 appears to be independent of LIN28B function. Predicted mRNA targets of the FGF-regulated microRNAs are over-represented in serine/threonine and tyrosine kinase receptors, including ACVR1, ACVR2B, PDGFRA, TGFBR1, and TGFBR3. Luciferase assays show that these and other candidates are targeted by FGF-regulated microRNAs. PDGFRA, a receptor whose activity is required for cell migration through the primitive streak, is a target of miR-130b and -218 in vivo. These results identify a novel mechanism by which FGF signaling regulates gene expression by negatively modulating microRNA abundance through both LIN28B-dependent and LIN28B-independent pathways.
Introduction
The body plan of vertebrates arises during gastrulation, when through complex morphogenetic movements, the three primary germ layers and the major body axes are formed. A key feature of gastrulation in amniotes is the ingression of cells from the epithelial epiblast through the primitive streak to form the mesoderm and the endoderm cell layers (1). Cells leaving the epiblast undergo an epithelial-to-mesenchymal transition (EMT),2 in which the epithelial phenotype is down-regulated and a migratory phenotype is activated (2). Mesoderm cells initially maintain a migratory phenotype, whereas cells comprising the endoderm re-establish cell-cell junctions and form a contiguous layer at the base of the primitive streak.
Transcriptional profiling has shown that gastrulation and germ layer formation involve the up-regulation and down-regulation of several thousand genes (3, 4). Genes up-regulated in the primitive streak region of avian embryos include members of the FGF, NOTCH, PDGF, EPH, and canonical and noncanonical WNT pathways, negative pathway modulators, and a large number of transcription factors. The FGF, PDGF, and WNT pathways have been shown to regulate primitive streak formation and/or cell migration through the streak (5–8). FGF signaling is required for expression of members from all of these pathways, suggesting that it lies at the top of a hierarchy of signaling cascades.
MicroRNAs (miRs) regulate crucial although still relatively undefined processes during early embryogenesis. Their importance is underscored by the early and severe phenotypes observed when miR processing is inactivated. In sea urchin, loss of Drosha or Dicer blocks gastrulation and greatly reduces expression of endoderm- and mesoderm-specific genes (9). These defects were rescued by the introduction of four miRs. Dicer and DGRC8 null mouse embryos are reduced in size, fail to gastrulate or express mesoderm markers, and exhibit a defect in development of ES cells that form the inner mass (10, 11). Ablation of Dicer in zebrafish leads to cell movement defects during gastrulation and abnormal heart and neural development (12). miRs play an important role in regulating pluripotency in embryonic stem cells (13); however, this function alone is unlikely to account for the embryonic phenotypes observed when dicer or DGRC8 are ablated.
Individual miRs have been shown to regulate specific aspects of EMT in cultured cells (14–18), although the potential function of these and other miRs in regulating EMT and the reverse process MET in vivo remains enigmatic. While investigating gene regulation during avian gastrulation (4), we observed that LIN28B is highly down-regulated following inhibition of FGF signaling. LIN28B and its paralog LIN28A negatively regulate miR processing, in particular the let-7 family of miRs (19, 20). LIN28A inhibits processing of pre-let-7 by Dicer in the cytoplasm, whereas LIN28B blocks the earlier step of pri-let-7 processing in the nucleus by the Drosha-containing Microprocessor complex (21). In general, studies in cancer cell lines and cultured stem cells have shown that repression of let-7 by LIN28A and/or LIN28B is associated with pluripotency and tumorigenesis (22, 23), whereas up-regulation of miR-let-7 is associated with cell differentiation and reduced cell proliferation (13). Almost all of these findings have arisen from tissue culture studies of stem cells or immortalized cell lines. Little information is available about the functions of LIN28 and its relationship to miR processing during early embryogenesis. Here we investigate the relationship between FGF signaling, LIN28B expression, and miR function during avian gastrulation.
EXPERIMENTAL PROCEDURES
Chicken Embryo Culture and Manipulation
Fertile chicken (Gallus gallus) eggs from Hy-Line International (Spencer, IA) were incubated at 37 °C with high humidity until the embryos reached Hamburger-Hamilton (HH) stage 4 (24, 25). For studies of FGF signaling inhibition, embryos placed in New Culture (4, 26) were exposed to 100 μm SU5402 (Pfizer) or dimethyl sulfoxide (DMSO) carrier diluted in cell culture medium supplemented with penicillin and streptomycin. For studies of LIN28B knockdown or forced expression, embryo electroporations were performed as described previously (4, 7) using a plasmid containing the entire coding region cDNA of LIN28B plus a 3′-terminal FLAG epitope tag under control of the chicken β-actin promoter (pBE-LIN28B) or morpholinos (AAGACAGAAAAGGGTCTTACTTGGT) antisense to the 3′ exon-intron boundary of LIN28B exon 2 or the standard control morpholino (GeneTools, Philomath, OR). Embryos were incubated for 5 h at 37 °C. Embryos analyzed by in situ hybridization (ISH) were fixed in 4% paraformaldehyde in PBS, whereas those used for real-time PCR analysis were dissected into lysis buffer (Life Technologies).
Real-time PCR Analysis
RNA was isolated from embryos using the mirVana miR isolation kit (Ambion). First-strand cDNA was prepared using the NCode miR first-strand cDNA synthesis kit (Invitrogen). To generate cDNA suitable for measuring miR precursors, a custom primer corresponding to the 3′ end of the pre-miR sequence was used in the reverse transcriptase reaction in lieu of random hexamers. Real-time PCR analyses were carried out with Maxima SYBR Green quantitative PCR master mix (Fermentas/Thermo Scientific) in a Rotorgene RG6000, using standard protocols and the Rotorgene statistical analysis software. Mature miRs were amplified using primers corresponding to the exact sequence of the miRs, along with the “Universal” primer supplied with the NCode kit (Invitrogen). miR precursors were amplified using primers corresponding to their stem-loop structures, whereas primary miR transcripts were amplified using the 3′ primer of the stem-loop structure and a 5′ primer positioned ∼100 bases upstream of the stem-loop structure (27).
All real-time PCR assays were performed in triplicate and included a no-template control. To normalize the data, each real-time experiment also included measurement of a control gene: ribosomal protein RPL4 for measuring primary miR transcripts and mRNAs, snRNA U6 for mature miRs and pre-miRs, or β-galactosidase (β-gal) for cDNA prepared from transfected HeLa cells.
Microarray Analysis
Samples of RNA isolated from the primitive streak region of HH stage 4 chicken embryos were mixed with spike-in controls and fluorescently labeled with Hy3 and Hy5 dyes using the miRCURY LNA microRNA power labeling kit (Exiqon, Woburn, MA). Samples were hybridized according to the protocols of the miRCURY microRNA array kit, using multispecies array based on miRBase version 11. Hybridized slides were processed and scanned according to standard protocols. Each slide contained four array replicates, which were compared against negative probe controls using an unpaired Student's t test and adjusted for multiple hypothesis testing using custom-built software written in the R statistical computing language.
Whole-mount ISH
Control or experimentally manipulated embryos were fixed in 4% paraformaldehyde overnight at 4 °C. ISH were performed as described (28) with some modifications (29). Detection of miR transcripts was accomplished using LNA probes (Exiqon) labeled with digoxigenin at the 5′ and 3′ ends. ISH using LNA probes was performed as described (30).
HeLa Cell Culture and Luciferase Assays
HeLa cells were grown and passaged in DMEM (Invitrogen) plus 10% fetal bovine serum, supplemented with penicillin and streptomycin. Luciferase reporter constructs were generated from the vector pmiR-Report (Ambion). Constructs contained either the full-length 3′-UTR from the candidate target mRNA or a 50–60-bp miR recognition element (MRE). 12-well culture plates of HeLa cells were grown to 70–80% confluence in 500 μl of antibiotic-free medium and then transfected using standard protocols with 1 μg of luciferase construct and 0.5 μg of a β-gal expression vector per well, using either Lipofectamine 2000 (Invitrogen) or TransPass transfection reagent (New England Biolabs, Ipswich, MA). Cells were harvested 48 h after transfection into Tropix lysis buffer (Applied Biosystems). Luciferase and β-gal activities were measured using the Dual-Light reporter gene assay system (Applied Biosystems). All luciferase assays were run in triplicate, normalized relative to β-gal, and compared with transfections using the parental vector (unmodified pmiR-Report). For experiments using miR mimics (Dharmacon), 100 nm of either a G. gallus miR mimic or a Caenorhabditis elegans miR-167 control mimic were used. The target protector miR-130b morpholino (GeneTools) was transfected at 5 μm. Cells were harvested 30 h after transfection, according to Yekta et al. (31).
Computational Analysis of miR Targets
Lists of evolutionarily conserved miR targets were obtained using a local installation of TargetScan (32) and filtered against mRNA expression data (4) to identify predicted miR mRNA targets expressed in the primitive streak.
Gene Ontology (GO) terms for G. gallus proteins were obtained from the National Center for Biotechnology Information (NCBI). Over-represented GO terms among the targets of each FGF-regulated miR (let-7b, miR-9, miR-19b, miR-107, miR-130b, and miR-218) were identified using a χ-squared test, comparing GO term frequency in the target set versus the frequency of these terms across all proteins, adjusted for multiple hypothesis testing.
RESULTS
Regulation of miR Abundance by FGF Signaling during Gastrulation
To investigate the relationship between FGF signaling and miR function, miR expression in the primitive streak was assessed using a multispecies miR microarray (33). Of the 44 chicken miRs detected significantly above background on the microarray (p ≤ 0.05 after adjustments for multiple hypothesis testing), the expression of 40 miRs was confirmed by real-time PCR analyses (supplemental Table 1). Hybridization signal was detected for four miRs not previously identified in chicken. Expression of the minor (asterisk) sequences for miR-18a and -193b was verified by PCR. The miR-193b strand we detected is designated the major strand in other species. However, on miRBase, this is shown as the minor strand. Expression of sequences corresponding to chicken miR-let7e and -1030i was confirmed by PCR. Because these genes were not annotated on the chicken genome, BLAST analysis was used to identify potential gene locations. A putative miR-let7e gene was identified on chromosome 26, and a putative miR-1030i gene was identified on chromosome 1. These locations are syntenic with their locations in other species. Secondary structure analysis (34) showed that these regions can form stem-loop structures characteristic of miR genes that would permit the processing of their primary transcripts into mature microRNAs (data not shown).
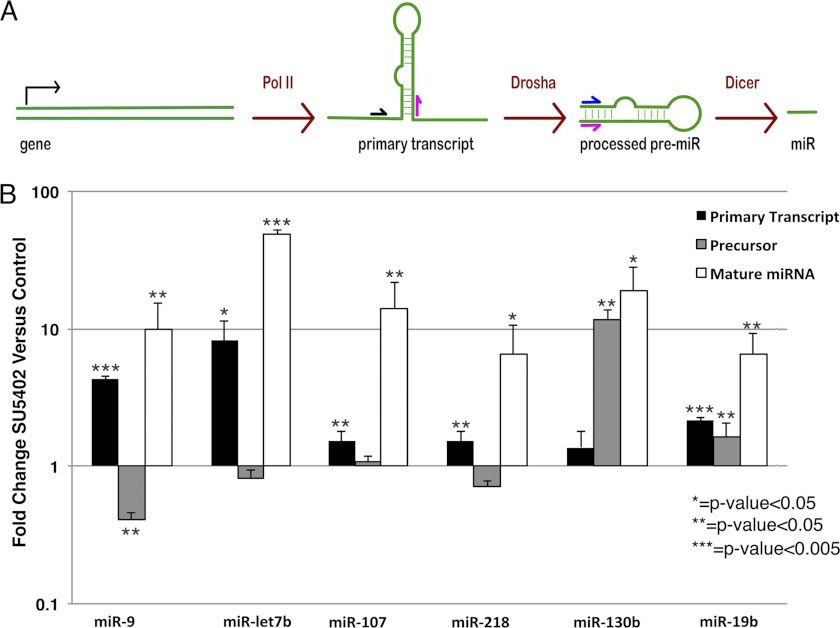
To determine whether FGF signaling influences miR abundance in the primitive streak, miR expression levels were compared in RNA samples isolated from the primitive streak regions of control embryos versus embryos exposed for 5 h to the FGF receptor (FGFR) inhibitor SU5402 (4, 35). The abundance of six miRs was significantly increased following inhibition of FGF signaling, whereas only two miRs showed modest down-regulation (Fig. 1). miR let-7b levels increased almost 50-fold in response to FGF signaling inhibition, whereas the levels of miR-9, -19b, -107, -130b, and -218 increased 6.5–20-fold. miR-let-7e, identified in our microarray screen, was expressed at very low levels that were unchanged by SU5402 treatment. Other let-7 family miRs were expressed at undetectable levels in the gastrula stage chicken embryo (data not shown). ISH detection of miR expression showed that miR-let-7b, -9, -19b, -130b, and -218 were expressed in the epiblast and primitive streak, but at reduced levels in the mesoderm and endoderm (Fig. 1B). miR-107 was not consistently detected by ISH.
FIGURE 1.
Changes in miR expression following inhibition of FGF signaling. A, comparison of miR expression levels in RNA samples from control versus SU5402-treated primitive streak regions of embryos, determined by real-time PCR. All miRs detected in the primitive streak region of gastrula stage embryos (supplemental Table 1) were assessed for changes in expression levels. Only those miRs with a statistically significant change in abundance are shown. Statistical significance was determined using a non-paired t test. Inset shows ISH detection of miR-let-7b in control versus SU5402-treated embryos. miR-let-7b expression was increased throughout the embryo, particularly in the primitive streak and more anteriorly. Error bars indicate S.E. B, ISH analysis of miR expression HH stage 4 embryos. miR-let-7b staining in the primitive streak was variable, with some embryos showing robust streak staining (B), whereas others showed somewhat reduced expression in the streak itself (e.g. control embryo in A).
miR Abundance Is Regulated by FGF Signaling at Multiple Processing Steps
miR abundance can be regulated transcriptionally and at multiple post-transcriptional processing steps (36). To determine how FGF signaling influences miR expression, the relative abundances of primary (pri-miRs), hairpin loop intermediates (pre-miRs), and mature miR transcripts were assessed by real-time RT-PCR using primer pairs designed to amplify each type of miR transcript (Fig. 2A). Comparing RNA samples from primitive streak regions of control versus SU5402-treated embryos, pri-miR-9, let-7b, -107, and -218 were increased following inhibition of FGF signaling, with reduced or no change in pre-miR abundance (Fig. 2B). In contrast, pri- and pre-miR levels for miR-19b were increased, whereas only the pre-miR levels of miR-130b were increased.
FIGURE 2.
FGF signaling regulates miR abundance through transcriptional and post-transcriptional mechanisms. A, simplified pathway of miR processing, showing the location of primers for detecting pri- or pre- miR transcripts. Pol II, RNA polymerase II. B, -fold change in abundance of pri-, pre-miRs, and mature miRs in control versus SU5402-treated embryos. Pri-miR levels are increased for all FGF-regulated miRs except miR-130b. Only miR-130b and -19b show increased pre-miRs levels, whereas miR-9 shows a decrease in pre-miRs. Error bars indicate S.E.
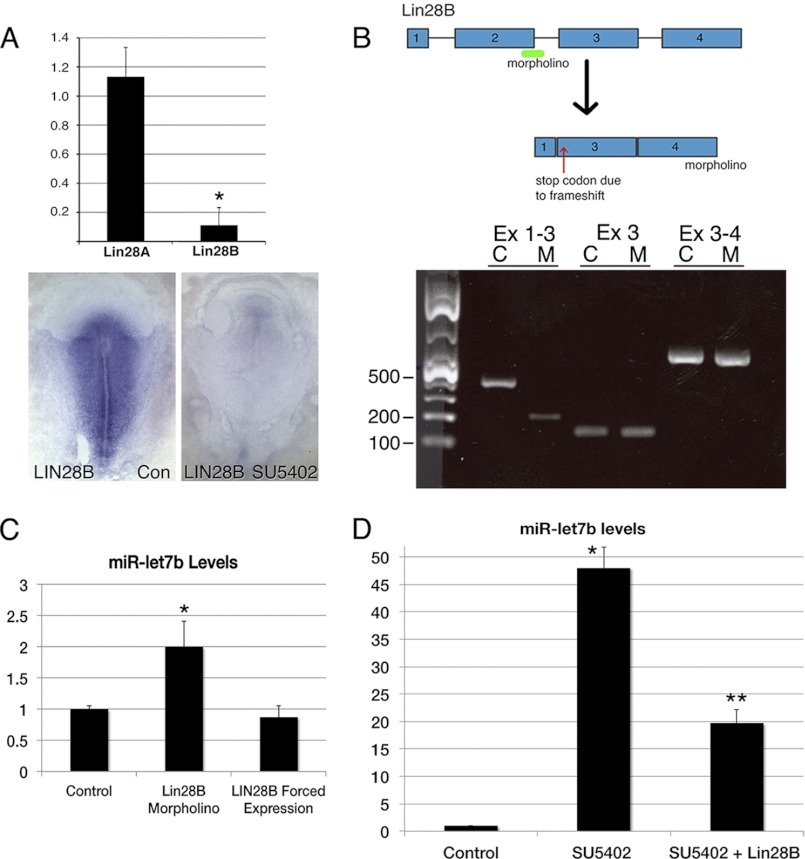
Previous studies have shown that LIN28B can negatively regulate processing of let-7 primary transcripts (21, 37, 38). Because LIN28B is down-regulated following inhibition of FGF signaling in the gastrula stage chicken embryo (Fig. 3A) (4), gain- and loss-of-function experiments were performed to determine whether FGF signaling acts through LIN28B to reduce miR-let-7b abundance. LIN28B knockdown was accomplished using a splice-blocking morpholino that deletes exon 2, introducing a frameshift and a premature stop codon 11 amino acids into exon 3 (Fig. 3B). Electroporation of the LIN28B morpholino into the primitive streak region of control embryos increased mature miR-let-7b levels 2-fold relative to a control morpholino (Fig. 3C). Although LIN28B forced expression had no effect on miR-let-7b levels in untreated embryos (Fig. 3C), forced expression in SU5402-treated embryos significantly reduced miR-let-7b abundance toward control levels (Fig. 3D). These results indicate that LIN28B can modulate miR-let-7b levels in control embryos and that the dramatic (50-fold) increase in mature miR-let-7b following inhibition of FGF signaling is at least partially due to reduced LIN28B expression.
FIGURE 3.
LIN28B regulation of miR abundance. A, real-time RT-PCR comparison of LIN28A and LIN28B mRNA levels in samples from primitive streak regions of control (Con) versus SU5402-treated embryos. LIN28B mRNA levels are reduced 10-fold by inhibition of FGF signaling, whereas LIN28A mRNA levels are unchanged. LIN28B ISH analysis of control versus SU5402-treated embryos. B, top, exon-intron structure of the LIN28B gene, showing the location of the splice blocking morpholino at the exon2-intron-2 boundary that is predicted to cause skipping of exon 2 and introduction of a premature in frame translation stop codon. Bottom, RT-PCR analysis of RNA isolated from HH stage 4 embryos electroporated with a control (C) or LIN28B (M) morpholino. The PCR product generated using primers spanning exons 1–3 shows the expected 382-nucleotide product for the control morpholinos sample and the 194-nucleotide product for the sample electroporated with the LIN28B morpholino, reflecting the absence of exon 2. C, normalized levels of miR-let-7b in samples from embryos electroporated with control or LIN28B-targeting morpholinos. The LIN28B morpholino increases let-7b levels 2-fold. Forced expression of LIN28B has no effect on let-7b levels. D, forced expression of LIN28B in SU5402-treated embryos (SU5402 + LIN28B) reduces let-7b levels relative to SU5402-treated embryos electroporated with a control plasmid. Asterisks indicate changes in mRNA levels that are statistically significant (p < 0.05). Error bars in A, C, and D indicate S.E.
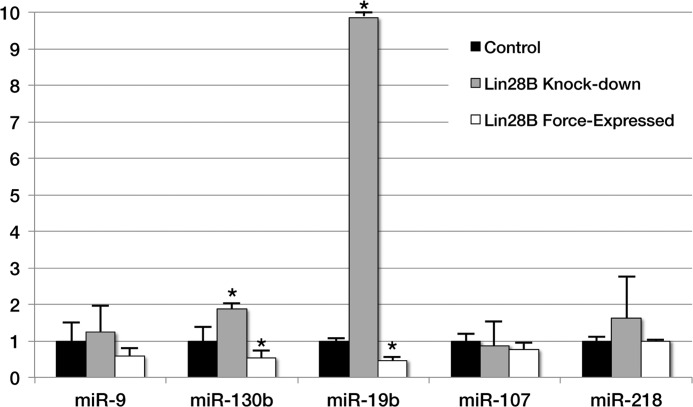
The effects of LIN28B overexpression and knockdown on the levels of other FGF-regulated miR transcripts were also assessed. LIN28B morpholino knockdown decreased, and LIN28B overexpression increased, the levels of miR-130b and -19b, whereas the levels of miR-9, -107, and -218 were unaffected (Fig. 4). Together, these results show that FGF signaling negatively regulates miR abundance through LIN28B-dependent and LIN28B-independent mechanisms.
FIGURE 4.
-Fold change in levels of miR-9, -130b, 19b, -107, and -218 following LIN28B morpholino knockdown or LIN28B forced expression. Levels of miR-130b and -19b increased with LIN28B knockdown and decreased following LIN28B forced expression. Levels of other miRs were unchanged. Asterisks indicate changes in mRNA levels that are statistically significant (p < 0.05). Error bars indicate S.E.
Predicted mRNA Targets of FGF-regulated miRs
To investigate the function of miRs negatively regulated by FGF signaling, bioinformatic analyses were performed to identify their predicted mRNA targets. Transcriptional profiling had previously identified 11,516 genes that are expressed in the primitive streak region of HH stage 4 chicken embryos (4). The 3′-UTRs of these mRNAs were analyzed using the TargetScan algorithm (32, 39, 40) to identify mRNAs containing evolutionarily conserved seed sites for miR-let-7b, -9, -19b,-107, -130b, and -218.
Approximately 580 3′-UTRs of mRNAs detected in the primitive streak contain one or more seed sequences for FGF-regulated miRs (supplemental Table 2). One 3′-UTR contains seed sites for five miRs, 16 contain seed sites for four miRs, 57 contain three seed sites, and 188 contain seed sites for two miRs. USP32, a highly conserved ubiquitin specific peptidase, is the most highly targeted mRNA (seven predicted sites). A cluster of miR-targeted mRNAs codes for proteins that regulate mRNA polyadenylation and deadenylation. CPEB proteins regulate polyadenylation and translation by binding to defined elements in the 3′-UTRs of some mRNAs (41). CNOT proteins are part of the protein complex involved in mRNA deadenylation and decay (42), including miR-mediated RNA degradation (43). CPEB1, CPEB4, and CNOT6L contain seed sites for miR-let-7b, -9, -19b, and -130b, and the 3′-UTR of CNOT7 contains seed sites for miR-19b and -130b.
To gain insight into the biological functions of mRNAs targeted by these miRs, their associated GO was analyzed for over-represented terms. Relative to all genes expressed in the primitive streak, mRNAs containing seed sites for FGF-regulated miRs are over-represented in serine/threonine and tyrosine kinase receptors (supplemental Table 3). These include the BMP type I receptors ACVR1 and ACVR1C, the BMP type II receptors ACVR2A and ACVR2B, plus TGFBR1 and TGFBR3, and PDGFRA. PDGFRA activity is required for cell migration during gastrulation (8).
FGF-regulated miRs Target mRNAs Containing Predicted Seed Sites
To determine whether mRNAs containing predicted miR seed sites are targeted by FGF-regulated miRs, the 3′-UTRs or the miR recognition sites (MRE: miR seed site plus 60 nucleotides of surrounding sequence) from candidate mRNAs were cloned downstream of a luciferase reporter and assayed for repression in HeLa cells. HeLa cells express human miR-let-7b, -9, 19b, and -107 that are identical in sequence to their chicken orthologs (44–46). Human miR-130b differs from the chicken ortholog by several nucleotides and miR-218 is not expressed in HeLa cells, so miR mimics corresponding to chicken miR-130b and -218 were transfected into cells along with the appropriate luciferase reporter and β-gal control vectors to adjust for transfection efficiency.
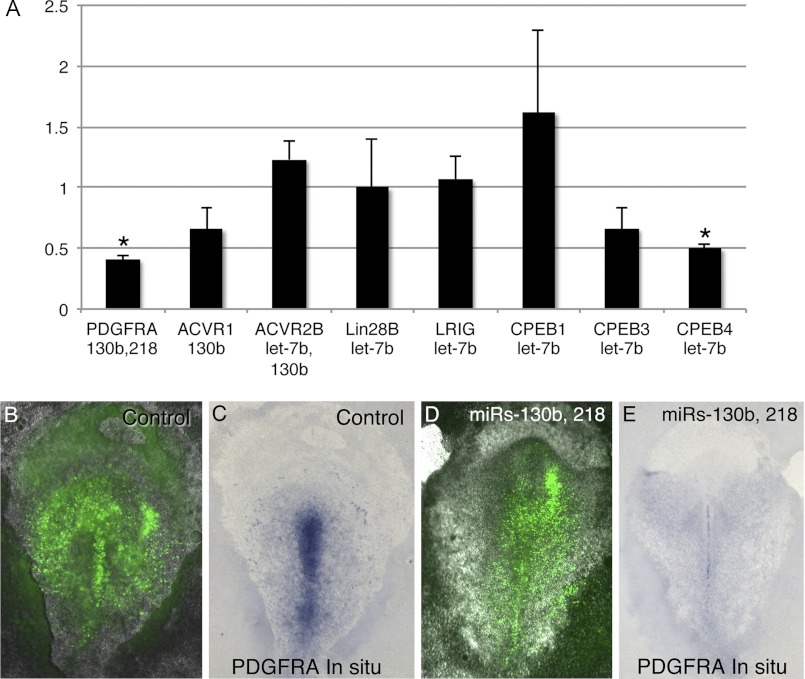
The chicken PDGFRA 3′-UTR contains predicted seed sites for miR-130b and -218. The reporter vector containing the 3′-UTR of PDGFRA was significantly down-regulated by co-transfection of miR-130b and/or miR-218 (Fig. 5A). Although the luciferase vector containing the PDGFRA miR-218 MRE showed a 35% reduction when co-transfected with miR-218, luciferase expression for the vector containing the miR-130b MRE was not reduced following co-transfection with miR-130b. However, an miR-130b seed site target protector morpholino abolished the reduction in luciferase activity observed with the full-length PDGFRA 3′-UTR, demonstrating functionality of the miR-130b seed site (Fig. 5A).
FIGURE 5.
3′-UTR luciferase reporter assays. A, 3′-UTR reporter analysis of the vectors containing the PDGFRA or ACVR1 3′-UTR or an MRE containing miR seed sites plus 50–60 nucleotides of flanking sequence, with or without exogenous miR-130b and/or miR-218. The addition of miRs significantly reduced luciferase activity. Luciferase reporter vectors containing the PDGFRA 3′-UTR or microRNA recognition elements show reduced luciferase activity, except for the vector containing the miR-130b MRE. The addition of a morpholino targeting the miR-130b seed site (target protector) restores luciferase activity of the PDGFRA 3′-UTR vector to control levels. B, 3′-UTR reporter analysis of the vectors containing the ACVR2B 3′-UTR or MREs for each of the miR-let-7b or miR-107 binding sites. C, of seven strongly predicted mRNA targets of miR-let-7b, only COL3A failed to show a reduction in luciferase activity. D, effects of 3′-UTRS and MREs on luciferase mRNA levels. Although all of the 3′-UTRs tested reduce luciferase activity and are targeted by miRs (A–C), only 3′-UTR sequences from PDGFRA, and the CPEB1 and CPEB3 MREs, reduced luciferase mRNA levels. The addition of 3′-UTRs for ACVR1, LRIG1, and CPEB4 increased luciferase mRNA levels. Error bars indicate S.D. in A–C and S.E. in D.
A luciferase vector containing an miR-130b MRE sequence from the ACVR1 3′-UTR showed a 50% reduction following co-transfection with miR-130 (Fig. 5A). The ACVR2B 3′-UTR contains two predicted seed sites each for miR-107 and -let-7b. The human and chicken orthologs of these miRs have identical sequences, and both miRs are expressed in HeLa cells. The addition of the ACVR2B 3′-UTR to the parental luciferase expression vector reduced luciferase activity by ∼40%, and each of the individual MREs reduced luciferase activity relative to the parental vector (Fig. 5B). UTRs of several additional mRNAs showing putative seed sites for miR-let-7b were also tested (Fig. 5C). The addition of 3′-UTRs or miR-let-7b MREs from LIN28B, ABL2, LRIG1, CPEB1, CPEB3, and CPEB4, but not from COL3A, significantly reduced luciferase activity relative to the control vector (Fig. 5C). Together, these results demonstrate direct targeting of numerous mRNAs by FGF-regulated miRs.
To determine whether changes in luciferase activity were due to inhibition of translation or degradation of target mRNAs, transfection assays were repeated, and luciferase mRNA levels were determined by real-time RT-PCR. As shown in Fig. 5D, the addition of miR-130b and -218 led to a reduction of luciferase transcripts that contained the PDGFRA 3′-UTR. Among The targets of miR-let-7b, only the 3′-UTRs of CPEB1 and CPEB3 appeared to induce luciferase transcript degradation. The addition of ACVR1, PDGFRA LRIG1, and CPEB4 3′-UTRs increased luciferase mRNA abundance, although the final protein levels (measured by luciferase activity) were decreased (Fig. 5D).
Analysis of FGF-regulated miRs in Vivo
To investigate whether FGF-regulated miRs target specific mRNAs in vivo, miR overexpression experiments were performed in HH stage 4 embryos. Despite extensive effort, antibodies recognizing the chicken proteins encoded by targeted mRNAs could not be identified, so the levels of targeted mRNAs were assessed. When HH stage 4 embryos were electroporated with mimics for miR-let-7b, -130b, and -218, mature miR levels increased 1350-, 18-, and 60-fold, respectively, as determined by quantitative PCR. Of the mRNA targets and miR mimic combinations tested, PDGFRA mRNA levels were significantly down-regulated by mimics for miR-130b and -218, and CPEB4 mRNA levels were reduced following electroporation of let-7b mimics (Fig. 6A). Although CPEB4 mRNAs are not detectable by whole-mount ISH, PDGFRA mRNAs are readily detectable in the primitive streak (4, 8). Reduction in PDGFRA mRNA levels by miR-130b and -218 was also evident by ISH, whereas a control miR had no effect on PDGFRA ISH staining intensity (Fig. 6, B–E). Although electroporation of mimics for miR-130b and -218 did not detectably alter migration of cells through the primitive streak (data not shown), electroporation of the LIN28B morpholino led to altered cell migration and abnormal embryo development (Fig. 7). Cells containing the LIN28B morpholino were frequently observed aggregated above the primitive streak, with LIN28B-positive few cells in the mesoderm. The mesoderm layer also appeared to contain fewer cells, although cells containing the LIN28B morpholino were observed in the endoderm. Embryos electroporated with the control morpholino were dispersed throughout the mesodermal and endodermal layers.
FIGURE 6.
PDGFRA and CPEB4 mRNAs are targeted for degradation by miRs in vivo. A, real-time PCR analysis comparing mRNA levels of predicted miR targets in samples from primitive streak regions of embryos electroporated with a control miR (cel-167) versus embryos electroporated with miR-let-7b, -130b, and/or -218. Asterisks indicate changes in mRNA levels that are statistically significant (p < 0.05). Error bars indicate S.E. B–E, visualization of PDGFRA mRNA reduction in embryos by overexpression of miRs. B and C, images showing the same embryo electroporated with a control miR mimic (cel-167) or with mimics for miR-130b and -218, visualizing the location of miR mimics (green fluorescence in B and D) and PDGFRA mRNAs by ISH (C and E). Introduction of miR-130b and -218 leads to a reduction of PDGFRA mRNAs in the primitive streak.
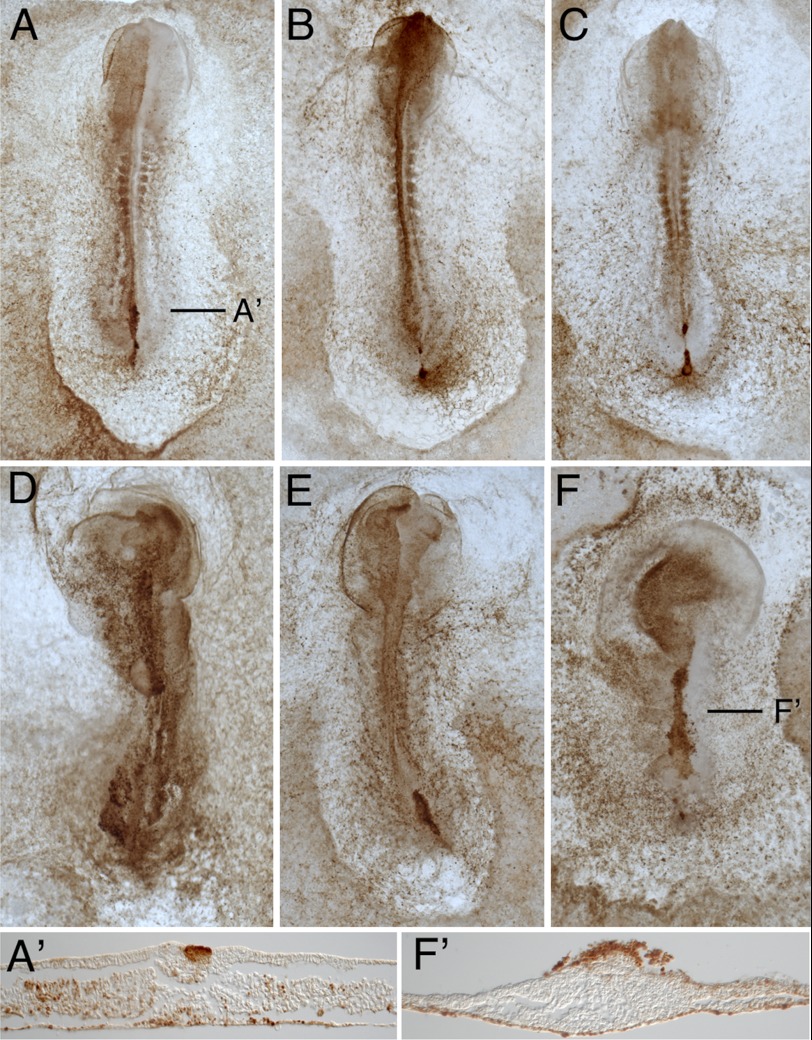
FIGURE 7.
LIN28B knockdown impairs cell migration and embryo development. Fluorescein-conjugated control (cel-167) or LIN28B morpholinos were electroporated into the primitive streak region epiblast of HH stage 4 embryos. Following a 14-h incubation, morpholino-containing cells were visualized using anti-fluorescein-HRP. Embryos electroporated with the LIN28B morpholino (D–F, F′) showed impaired cell migration and development relative to embryos receiving the Control morpholino (A–C, C′).
DISCUSSION
We have shown that FGF signaling regulates gene expression during gastrulation by negatively modulating the abundance of miR-let-7b, -9, -19b, -107, and -218. FGF signaling is required for expression of LIN28B, which gain-and loss-of-function studies have shown inhibits the processing of miR-let-7b, -19b, and -130b. miR seed site prediction in 3′-UTRs of mRNAs expressed in the primitive streak identifies ∼580 potential targets of FGF-regulated miRs. Receptor kinases, including BMP type I and type II receptors, TGFBR1, and PDGFRA, are over-represented among mRNAs targeted by FGF-regulated miRs. Cell culture transfection assays and in vivo experiments confirm the targeting of these mRNAs. These findings show that FGF signaling can regulate signaling pathways and other processes during gastrulation by negatively modulating miR abundance through mechanisms that include up-regulating LIN28B expression.
Previous studies have indirectly linked FGF signaling and LIN28 expression to the generation and self-renewal of cultured stem cells (47–50). Here we find that inhibition of FGF signaling in gastrulating chicken embryos causes a 10-fold decrease of LIN28B mRNA levels, whereas LIN28A mRNA levels are unaffected. Reduced LIN28B levels are at least partially responsible for increased miR abundance because knockdown of LIN28B causes an increase in miR-let7, -19b, and -130b, and forced expression of LIN28B in SU5402-treated embryos reduced miR-let-7b abundance toward control levels. LIN28A can block the processing of miR-let7 family members at the level of intermediate formation or final maturation (51), whereas LIN28B can inhibit processing of miR-let7 by sequestering the primary transcripts (21). Our results show that LIN28B can also regulate processing of miR-19b and -130b. Given the complexity of the miR processing pathway and the unknown potential interplay between LIN28A and LIN28B function, the variety of patterns observed in the relative abundance of primary transcripts versus miR intermediates when comparing control versus SU5402-treated embryos is not surprising. miR-9, -107, and -218 show increased levels following inhibition of FGF signaling without apparent involvement of LIN28B, suggesting that FGF signaling affects their expression through direct or indirect transcriptional repression. A few studies have similarly linked FGF signaling to the down-regulation of miR expression. Inhibition of FGFR1 during zebrafish fin regeneration led to the down-regulation of 22 miRs and up-regulation of 34 miRs (52). In chicken embryos, miR-206 is repressed by FGF4 in somatic skeletal muscle cells (53). It is not known whether the FGF-dependent reduction in miR levels in these two studies is mediated by increased expression of LIN28A or LIN28B. A striking finding in the present study is that the primary effect of FGF signaling on miR expression in gastrulating chicken embryos is to negatively modulate their abundance.
Computational methods identified almost 600 potential targets of FGF-regulated miRs. Predicted targets include several mRNAs (CPEB1, CPEB4, CNOT6, and CNOT7L) that themselves code for proteins that regulate mRNA polyadenylation and deadenylation and mRNA stability. Luciferase assays confirmed the targeting of CPEB1 and CPEB4 by miR-let-7b. A second group of over-represented mRNAs code for serine/threonine and tyrosine kinase receptors that include the BMP type I receptors ACVR1 and ACVR1C, type II receptors ACVR2A and ACVR2B, plus TGFBR1, TGFBR3, and PDGFRA. Targeting of ACVR1, ACVR2B, and PDGFRA was confirmed in cell culture assays, and the ability of miR-130b and -218 to reduce mRNA levels of PDGFRA was confirmed in vivo. Although the ligand specificities in chicken of ACVR1, ACVR1C, ACVR2A, and ACVR2B receptors have not been determined, in other organisms, they can bind nodal and BMP growth factors. In avian embryos, Nodal is expressed along the anterior portion of the primitive streak and BMPs are expressed posteriorly, creating an antagonistic signaling gradient that patterns the emerging mesoderm. A similar expression gradient is observed in most vertebrate embryos (2). The potential targeting of these receptors by FGF-regulated miRs suggests that FGF signaling can influence dorsal ventral patterning. Unfortunately, the lack of antibodies recognizing these receptors in chicken has precluded a more in-depth analysis of this possibility.
PDGFRA controls N-cadherin protein expression through PI 3-kinase and is required for migration of epiblast cells through the primitive streak (8). We have shown both in vitro and in vivo that miR-218 causes a substantial down-regulation of PDGFRA mRNA. Together, these data suggest a mechanism by which FGF signaling-mediated down-regulation of miR-218 expression leads to increased PDGFRA protein levels, permitting cell migration through the primitive streak. The finding that FGF signaling regulates miR abundance during gastrulation adds another layer of complexity to the pivotal role that this pathway plays during embryogenesis.
This work was supported, in whole or in part, by National Institutes of Health Grant 1 P41 HD064559 (to P. B. A.). This work was also supported by an American Heart Association grant (to P. B. A.).

This article contains supplemental Tables 1–3.
- EMT
- epithelial-to-mesenchymal transition
- HH
- Hamburger-Hamilton
- miR
- microRNA
- pre-miR
- precursor miR
- pri-miR
- primary miR
- MRE
- miR recognition element
- ISH
- in situ hybridization(s)
- CPEB
- cytoplasmic polyadenylation element-binding protein
- CNOT6L
- CCR4-NOT transcription complex, subunit 6-like
- BMP
- bone morphogenetic protein
- GO
- Gene Ontology.
REFERENCES
- 1. Stern C. D. (ed) (2004) Gastrulation: From Cells to Embryo, pp. 219–232, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2. Nakaya Y., Sheng G. (2009) An amicable separation: Chick's way of doing EMT. Cell Adh. Migr. 3, 160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alev C., Wu Y., Kasukawa T., Jakt L. M., Ueda H. R., Sheng G. (2010) Transcriptomic landscape of the primitive streak. Development 137, 2863–2874 [DOI] [PubMed] [Google Scholar]
- 4. Hardy K. M., Yatskievych T. A., Konieczka J., Bobbs A. S., Antin P. B. (2011) FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chuai M., Weijer C. J. (2009) Regulation of cell migration during chick gastrulation. Curr. Opin. Genet. Dev. 19, 343–349 [DOI] [PubMed] [Google Scholar]
- 6. Chuai M., Zeng W., Yang X., Boychenko V., Glazier J. A., Weijer C. J. (2006) Cell movement during chick primitive streak formation. Dev. Biol. 296, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hardy K. M., Garriock R. J., Yatskievych T. A., D'Agostino S. L., Antin P. B., Krieg P. A. (2008) Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev. Biol. 320, 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X., Chrisman H., Weijer C. J. (2008) PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development 135, 3521–3530 [DOI] [PubMed] [Google Scholar]
- 9. Song J. L., Stoeckius M., Maaskola J., Friedländer M., Stepicheva N., Juliano C., Lebedeva S., Thompson W., Rajewsky N., Wessel G. M. (2012) Select microRNAs are essential for early development in the sea urchin. Dev. Biol. 362, 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein E., Kim S. Y., Carmell M. A., Murchison E. P., Alcorn H., Li M. Z., Mills A. A., Elledge S. J., Anderson K. V., Hannon G. J. (2003) Dicer is essential for mouse development. Nat. Genet. 35, 215–217 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y., Medvid R., Melton C., Jaenisch R., Blelloch R. (2007) DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 39, 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giraldez A. J., Cinalli R. M., Glasner M. E., Enright A. J., Thomson J. M., Baskerville S., Hammond S. M., Bartel D. P., Schier A. F. (2005) MicroRNAs regulate brain morphogenesis in zebrafish. Science 308, 833–838 [DOI] [PubMed] [Google Scholar]
- 13. Melton C., Judson R. L., Blelloch R. (2010) Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gregory P. A., Bracken C. P., Bert A. G., Goodall G. J. (2008) MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 7, 3112–3118 [DOI] [PubMed] [Google Scholar]
- 15. Sreekumar R., Sayan B. S., Mirnezami A. H., Sayan A. E. (2011) MicroRNA control of invasion and metastasis pathways. Frontiers Genet. 2, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee M. R., Kim J. S., Kim K. S. (2010) miR-124a is important for migratory cell fate transition during gastrulation of human embryonic stem cells. Stem Cells 28, 1550–1559 [DOI] [PubMed] [Google Scholar]
- 17. Wang F. E., Zhang C., Maminishkis A., Dong L., Zhi C., Li R., Zhao J., Majerciak V., Gaur A. B., Chen S., Miller S. S. (2010) MicroRNA-204/211 alters epithelial physiology. FASEB J. 24, 1552–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brabletz S., Brabletz T. (2010) The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO reports 11, 670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viswanathan S. R., Daley G. Q. (2010) Lin28: A microRNA regulator with a macro role. Cell 140, 445–449 [DOI] [PubMed] [Google Scholar]
- 20. Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Selective blockade of microRNA processing by Lin28. Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piskounova E., Polytarchou C., Thornton J. E., LaPierre R. J., Pothoulakis C., Hagan J. P., Iliopoulos D., Gregory R. I. (2011) Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147, 1066–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peter M. E. (2009) Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 8, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyerinas B., Park S. M., Hau A., Murmann A. E., Peter M. E. (2010) The role of let-7 in cell differentiation and cancer. Endocr. Relat. Cancer 17, F19–F36 [DOI] [PubMed] [Google Scholar]
- 24. Hamburger V., Hamilton H. L. (1951) A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 [PubMed] [Google Scholar]
- 25. Hamburger V., Hamilton H. L. (1992)A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231–272 [DOI] [PubMed] [Google Scholar]
- 26. New D. A. T. (1955) A new technique for the cultivation of the chick embryo in vitro. J. Embryol. Exp. Morphol. 3, 326–331 [Google Scholar]
- 27. Schmittgen T. D., Jiang J., Liu Q., Yang L. (2004) A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res. 32, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nieto M. A., Patel K., Wilkinson D. G. (1996) In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 51, 219–235 [DOI] [PubMed] [Google Scholar]
- 29. Antin P. B. (2012) Geisha: Gallus Expression in Situ Hybridization Analysis geisha.arizona.edu/geisha/protocols.jsp [DOI] [PubMed]
- 30. Darnell D. K., Kaur S., Stanislaw S., Konieczka J. H., Yatskievych T. A., Antin P. B. (2006) MicroRNA expression during chick embryo development. Dev. Dyn. 235, 3156–3165 [DOI] [PubMed] [Google Scholar]
- 31. Yekta S., Shih I. H., Bartel D. P. (2004) MicroRNA-directed cleavage of HOXB8 mRNA. Science 304, 594–596 [DOI] [PubMed] [Google Scholar]
- 32. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 33. Kozomara A., Griffiths-Jones S. (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sato K., Hamada M., Asai K., Mituyama T. (2009) CENTROIDFOLD: a web server for RNA secondary structure prediction. Nucleic Acids Res. 37, W277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 [DOI] [PubMed] [Google Scholar]
- 36. Roush S., Slack F. J. (2008) The let-7 family of microRNAs. Trends Cell Biol. 18, 505–516 [DOI] [PubMed] [Google Scholar]
- 37. Newman M. A., Thomson J. M., Hammond S. M. (2008) Lin-28 interaction with the let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piskounova E., Viswanathan S. R., Janas M., LaPierre R. J., Daley G. Q., Sliz P., Gregory R. I. (2008) Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 283, 21310–21314 [DOI] [PubMed] [Google Scholar]
- 39. Grimson A., Farh K. K., Johnston W. K., Garrett-Engele P., Lim L. P., Bartel D. P. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter J. D. (2007) CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285 [DOI] [PubMed] [Google Scholar]
- 42. Mittal S., Aslam A., Doidge R., Medica R., Winkler G. S. (2011) The Ccr4a (CNOT6) and Ccr4b (CNOT6L) deadenylase subunits of the human Ccr4-Not complex contribute to the prevention of cell death and senescence. Mol. Biol. Cell 22, 748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fabian M. R., Sonenberg N., Filipowicz W. (2010) Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 [DOI] [PubMed] [Google Scholar]
- 44. Pillai R. S., Bhattacharyya S. N., Artus C. G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. (2005) Inhibition of translational initiation by Let-7 microRNA in human cells. Science 309, 1573–1576 [DOI] [PubMed] [Google Scholar]
- 45. Guo Y., Chen Y., Ito H., Watanabe A., Ge X., Kodama T., Aburatani H. (2006) Identification and characterization of lin28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384, 51–61 [DOI] [PubMed] [Google Scholar]
- 46. Yeung M. L., Bennasser Y., Myers T. G., Jiang G., Benkirane M., Jeang K. T. (2005) Changes in microRNA expression profiles in HIV-1-transfected human cells. Retrovirology 2, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diecke S., Quiroga-Negreira A., Redmer T., Besser D. (2008) FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs 188, 52–61 [DOI] [PubMed] [Google Scholar]
- 48. Levenstein M. E., Ludwig T. E., Xu R. H., Llanas R. A., VanDenHeuvel-Kramer K., Manning D., Thomson J. A. (2006) Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells 24, 568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Page R. L., Ambady S., Holmes W. F., Vilner L., Kole D., Kashpur O., Huntress V., Vojtic I., Whitton H., Dominko T. (2009) Induction of stem cell gene expression in adult human fibroblasts without transgenes. Cloning and stem cells 11, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin I. I, Thomson J. A. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- 51. Lehrbach N. J., Armisen J., Lightfoot H. L., Murfitt K. J., Bugaut A., Balasubramanian S., Miska E. A. (2009) LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 16, 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yin V. P., Thomson J. M., Thummel R., Hyde D. R., Hammond S. M., Poss K. D. (2008) Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish. Genes Dev. 22, 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sweetman D., Rathjen T., Jefferson M., Wheeler G., Smith T. G., Wheeler G. N., Münsterberg A., Dalmay T. (2006) FGF-4 signaling is involved in mir-206 expression in developing somites of chicken embryos. Dev. Dyn. 235, 2185–2191 [DOI] [PubMed] [Google Scholar]