Background: Expression level of chondroitin sulfate (CS) is important in embryonic development. However, its involvement in skeletal myogenesis is unknown.
Results: The CS level is temporally decreased during skeletal muscle development, and its forced reduction enhances myogenic differentiation/regeneration.
Conclusion: Temporal decline in CS levels is required for skeletal muscle differentiation/regeneration.
Significance: Lowering CS abundance is a promising approach for skeletal muscle regenerative therapy.
Keywords: Chondroitin Sulfate, Glycosaminoglycan, Hyaluronate, Muscle Regeneration, Proteoglycan
Abstract
Skeletal muscle formation and regeneration require myoblast fusion to form multinucleated myotubes or myofibers, yet their molecular regulation remains incompletely understood. We show here that the levels of extra- and/or pericellular chondroitin sulfate (CS) chains in differentiating C2C12 myoblast culture are dramatically diminished at the stage of extensive syncytial myotube formation. Forced down-regulation of CS, but not of hyaluronan, levels enhanced myogenic differentiation in vitro. This characteristic CS reduction seems to occur through a cell-autonomous mechanism that involves HYAL1, a known catabolic enzyme for hyaluronan and CS. In vivo injection of a bacterial CS-degrading enzyme boosted myofiber regeneration in a mouse cardiotoxin-induced injury model and ameliorated dystrophic pathology in mdx muscles. Our data suggest that the control of CS abundance is a promising new therapeutic approach for the treatment of skeletal muscle injury and progressive muscular dystrophies.
Introduction
Chondroitin sulfate (CS)2 is widely distributed in extracellular matrices and at cell surfaces in the form of proteoglycans (CSPGs) in which CS side chains are covalently linked to a panel of core proteins. Increasing evidence suggests substantial roles for CS moieties in diverse developmental and/or pathological functions of CSPGs (1, 2). We have previously shown that CS in mammals, or its nonsulfated form in Caenorhabditis elegans, is indispensable for cytokinesis during early embryonic cell division (3, 4). Thus, insufficient CS production at immediate-early stages of division results in undesirable embryonic cell death due to frequent reversion of cytokinesis accompanied by abnormal multinucleation. These findings suggested the seemingly paradoxical notion that a temporal reduction in CS levels was required for normal cell fusion processes to form multinucleated syncytia in somatic cells, including in skeletal myogenesis (5, 6).
Here, we tested this hypothesis and demonstrated the essential requirement of the temporal decline in CS levels for skeletal muscle differentiation and repair processes. Enzymatic removal of CS also ameliorated dystrophic pathology in mdx muscles. Our data suggest that strategies aimed at lowering CS abundance open promising new therapeutic approaches for the treatment of skeletal muscle injury and progressive muscular dystrophies.
EXPERIMENTAL PROCEDURES
Cell Culture
Mouse C2C12 myoblast cells (RIKEN Cell Bank, Tsukuba, Japan) were maintained in DMEM (Sigma) containing 10% FBS. The cDNA encoding mouse HYAL1 (GenBankTM accession number BC021636.1) was subcloned into a pCMV expression vector (Invitrogen). The fidelity of the plasmid construct (pCMV/HYAL1) was confirmed by DNA sequencing. C2C12 cells were transfected with pCMV/HYAL1 using the FuGENETM 6 transfection reagent (Roche Applied Science) according to the manufacturer's instructions. Clonal cell lines (HYAL1 OE cells) were selected and grown in the presence of 1 mg/ml of G418 (Invitrogen). For stable knockdown, shRNA vectors targeting C4ST-1 or HYAL1 (MISSION shRNA, Sigma) were individually transfected into C2C12 cells using FuGENETM 6. Stable transfectants expressing the respective shRNAs (C4ST-1 KD and HYAL1 KD cells) were selected and propagated in the presence of 0.25 μg/ml puromycin. To avoid any side effects of the antibiotics on myogenic differentiation, each stable clone was passaged and maintained in antibiotic-free medium for at least 7 days before the following experiments. To elicit differentiation, parental C2C12 cells and its stable clones were plated on type I collagen-coated coverslips or dishes (Iwaki, Tokyo, Japan) at a cell density of 5,500 cells/cm2 on “day 0,” and the medium was then switched to DM (DMEM supplemented with 2% horse serum) on day 1. The medium was replaced every other day over days 3–8, unless otherwise noted. In some cases, either ChABC (20 mIU, Seikagaku, Tokyo, Japan) or a bacterial hyaluronidase, HAase SD (20 mIU, Seikagaku), was added to the culture medium on day 6. Heat-inactivated enzymes (HI-ChABC and HI-HAase) were also used as controls. Inhibition of PI3K/Akt and ERK5 pathways was performed by a 48-h treatment with chemical drugs, LY294002 (10 μm, Enzo Life Sciences) and U0126 (20 μm, Promega), respectively, on day 5 cultures in DM. LY294002 treatment was conducted in the absence or presence of ChABC (5.0 mIU/ml).
Quantification of Glycosaminoglycans
Glycosaminoglycans (GAGs) from cultured cells were prepared as described previously (7). Briefly, cells were homogenized in acetone and air-dried. The dried materials were exhaustively digested with heat-pretreated actinase E at 60 °C for 48 h. The digest was treated with 5% trichloroacetic acid, and the resultant acid-soluble fraction was extracted with diethyl ether. The aqueous phase was neutralized and adjusted to contain 80% ethanol. The resultant precipitate was dissolved in water and subjected to gel filtration on a PD-10 column (GE Healthcare). The flow-through fractions were collected, evaporated to dryness, and dissolved in water. An aliquot of the sample was digested with either ChABC (5 mIU) or HAase SD (5 mIU) at 37 °C for 2 h. The digests were derivatized with the fluorophore 2-aminobenzamide and were then analyzed by anion-exchange HPLC on a PA-03 column (YMC, Kyoto, Japan) as described previously (8). Identification and quantification of the resulting disaccharides were achieved by comparison with authentic unsaturated CS and HA disaccharides (Seikagaku).
Quantitative RT-PCR
Total RNA was extracted from cells using an RNeasy® mini kit (Qiagen). An aliquot of the total RNA (∼150 ng) was pretreated with an RNase-free DNase and served as a template for cDNA synthesis. Quantitative real-time RT-PCR was performed using a FastStart DNA Master plus SYBR Green I in a LightCycler ST300 (Roche Diagnostics). The primer sequences used are described in supplemental Table 1. The expression level of each mRNA was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and labeled with anti-MHC antibody (A4.1025, Upstate, 1:2,000) followed by development using an M.O.M.TM immunodetection kit (Vector Laboratories) with 3-amino-9-ethylcarbazole (Vector Laboratories) as a chromogen. In some cases, MHC+ cells were visualized by incubation with an Alexa-labeled secondary antibody. After nuclear staining with Hoechst 33342 (Invitrogen), the stained cells were examined using an all-in-one type fluorescence microscope BZ-8000 (Keyence, Osaka, Japan). To quantify multinuclear myotube formation, the number of MHC+ cells was counted in several microscopic areas randomly selected from each of at least three separate cultures under each condition. In addition, the fusion index was calculated by scoring the ratio of the number of nuclei in multinuclear MHC+ cells versus the number of nuclei in the total number of MHC+ cells examined.
To visualize the extra- and/or pericellular distribution of CS moieties or a CSPG core protein, versican, in culture, cells were labeled with anti-CS (CS56, Seikagaku, 1:200) or anti-versican (GAGβ domain, Millipore, 1:100) antibodies before permeabilization. For immunolabeling with the latter antibody, pretreatment of the fixed cells with ChABC (5 mIU, 37 °C for 1 h) was conducted. The processed cells were further treated with 0.2% Triton X-100, and incubated with anti-myogenin (F5D, Santa Cruz Biotechnology, 1:200) or anti-HYAL1 (G-12, Santa Cruz Biotechnology, 1:200) antibodies followed by a mixture of appropriate Alexa-labeled secondary antibodies.
Immunoblotting
The medium of the differentiating C2C12 culture was replaced with serum-free DMEM on day 3 or 5. Forty eight-hour conditioned media (CM) were recovered from the respective cultures on day 5 or 7. The CM corresponding to 60 μg of cellular proteins were digested with ChABC (5 mIU) at 37 °C for 1 h. Each sample was subjected to SDS-PAGE, transferred onto PVDF membrane (GE Healthcare), and incubated with primary antibodies to versican (GAGβ domain, 1:1,000) or biglycan (ab49701, Abcam, 1:1,000). The blot was treated with HRP-conjugated anti-rabbit IgG and developed with ECL detection system (GE Healthcare).
For assessment of the Akt phosphorylation levels in response to the reduction in CS abundance, cell extracts from the day 7 cultures of parental C2C12 cells, which were left undisturbed or exposed to ChABC (2.5–5.0 mIU/ml) from day 6, were examined by immunoblot using polyclonal antibodies to Akt (Cell Signaling, 1:1,000) and phospho-Akt (Ser-437, Cell Signaling, 1:1,000).
In Situ Hybridization
Digoxigenin-labeled RNA probes were transcribed in vitro using T7 or SP6 RNA polymerase, with the linearized pGEM®-T EASY vector carrying mouse Hyal1 cDNA fragment (∼1.2-kbp). Parental C2C12 cells on day 5 in DM culture were fixed with 4% paraformaldehyde, acetylated in 0.25% acetic anhydride in 0.1 m triethanolamine for 10 min, and treated with 0.2 m HCl for 10 min. They were prehybridized with a hybridization buffer (50% formamide, 5× SSC, 500 μg/ml yeast tRNA, 100 μg/ml herring sperm DNA, 100 μg/ml heparin, 1× Denhardt's solution, 0.1% Tween 20, and 5 mm EDTA) for 6 h, followed by hybridization with the digoxigenin-labeled probes prepared as above at 60 °C for 16 h. To remove excess unhybridized probes, extensive washing was performed with the following solutions: 1) 5× saline sodium citrate (SSC) for 1 min; 2) 2× SSC, 50% formamide at 65 °C for 30 min; 3) 2× SSC at 65 °C for 10 min; 4) RNase A (20 μg/ml in 10 mm Tris-HCl, 1 mm EDTA, 0.5 m NaCl) at 37 °C for 1 h; 5) 2× SSC at 65 °C for 10 min; 6) 2× SSC, 50% formamide at 65 °C for 30 min; 7) 0.2× SSC, 0.1% Tween 20 at 65 °C for 30 min. The cells were subsequently immersed in a blocking solution (150 mm maleate, 100 mm NaCl, 0.1% Tween 20, 2% blocking reagent (Roche Applied Science), pH 7.5) containing 5% sheep serum for 1 h, incubated with anti-digoxigenin Fab fragments conjugated with alkaline phosphatase (Roche Applied Science, 1:1,000 in the blocking solution containing 5% sheep serum) at 4 °C overnight, and developed with an alkaline phosphatase substrate, BM Purple (Roche Applied Science). The stained cells were refixed in 4% paraformaldehyde and then processed for immunolabeling with anti-myogenin or anti-CS antibodies.
Animal Models
Five- to 6-week-old wild-type C57BL/10 and mdx (C57BL/10 genetic background) mice were obtained from Japan SLC Inc. (Hamamatsu, Japan) and Clea Japan Inc. (Tokyo, Japan), respectively. Thirty μl of ChABC solution in saline (equivalent to 60 mIU) was injected into the left tibialis anterior (TA) muscles of mice. The right muscles were used as vehicle-treated controls. Injury was induced via injection of 30 μl of cardiotoxin (CTX) from Naja mossambica mossambica (10 μm in saline, Sigma) into the TA muscles of wild-type mice. In the CTX injury model, co-injection of 30 μl of a mixture containing CTX (10 μm) plus ChABC (60 mIU) and administration of ChABC (60 mIU) on day 3 after CTX injection were also performed. Mice were sacrificed at 3, 7, and/or 14 days following the first intramuscular injection, and TA muscles were isolated and frozen in isopentane that was cooled by liquid nitrogen. Mice were kept in an environmentally controlled clean room at the Institute of Laboratory Animals, Kobe Pharmaceutical University. All experiments were conducted according to institutional ethics guidelines for animal experiments.
Histology and Immunohistochemistry
Serial frozen sections (10 μm thick) were cut, labeled with anti-laminin (Sigma, rabbit IgG, 1:400) plus anti-CS (CS56, 1:200) or anti-CS stub (2-B-6, mouse IgG1, Seikagaku, 1:200) antibodies followed by mixtures of appropriate Alexa-labeled secondary antibodies, and counterstained with Hoechst 33342. Fluorescent images were obtained using BZ-8000 (Keyence). In some cases, the Hoechst 33342 signal was pseudo-colored red. The cross-sectional area (CSA) of individual myofibers with centralized nuclei within injured regions of TA muscles from at least four wild-type mice under each condition was measured using a morphological analysis software (Mac SCOPE, Mitani Corp., Fukui, Japan). The mdx pathology was evaluated by scoring the percentage of centrally nucleated myofibers over the total number of fibers in at least five adjacent microscopic fields obtained from each TA muscle section from two to four mdx mice under each condition.
Statistical Analysis
Student's t test was used for determination of statistical significance throughout the study. Differences were considered significant with a p value less than 0.05.
RESULTS
Temporal Reduction in CS Levels Is Required for Myogenic Differentiation
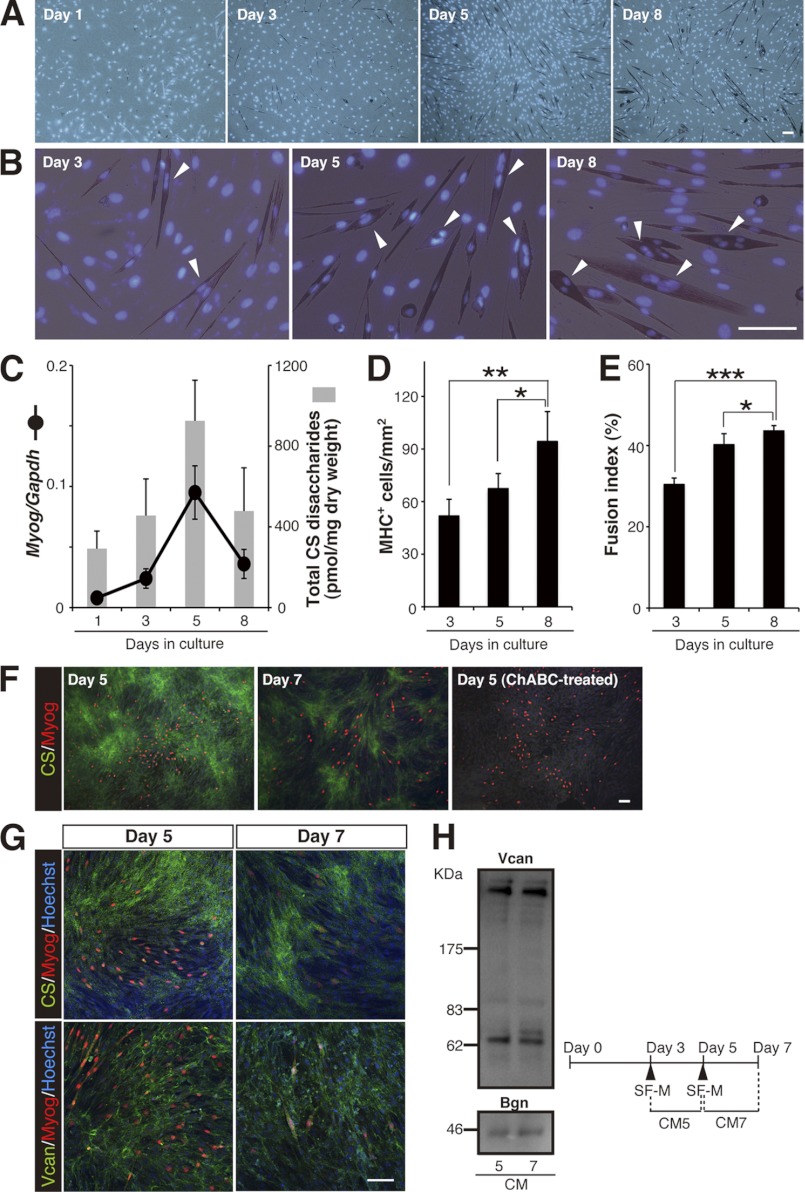
To test our assumption, we took advantage of an in vitro culture system, in which mouse C2C12 myoblasts can be induced to differentiate into multinucleated myotubes by serum withdrawal (9). Following culture in DM containing 2% horse serum, the level of a muscle contractile protein, the myosin heavy chain (MHC), gradually increased over 8 days of C2C12 cell differentiation, as assessed by immunostaining (Fig. 1, A and D). Nuclear counterstaining indicated that the number of mononuclear MHC+ cells on day 3 of the DM culture was greater than that on days 5 and 8, in which a higher number of cells had two or more nuclei (Fig. 1B). The fusion index of MHC+ cells (the proportion of the number of nuclei in multinucleated MHC+ cells versus the total number of nuclei in MHC+ cells) was therefore adopted as a benchmark of multinuclear myotube formation in this study (Fig. 1E). Based on this criterion, vigorous myoblast fusion was considered to occur from day 5 onward under our culture conditions. This conclusion was strongly supported by analysis of the expression profile of Myogenin (Myog) transcripts (Fig. 1C). Myogenin is a late myogenic regulatory factor that initiates terminal differentiation to form multinuclear myotubes, and its expression is known to be transiently up-regulated just before MHC accumulation and to decline thereafter as the rate of myoblast fusion increases (10). Notably, biochemical analysis of CS in the differentiating C2C12 cultures revealed a good correlation between CS abundance and Myog expression (Fig. 1C). In support of the biochemical data, immunocytochemical analysis showed that a robust fluorescent signal for extracellular CS was detected on day 5 in DM culture, and its signal intensity on day 7 declined (Fig. 1F). Of interest was that the CS immunoreactivity was relatively lower around Myog+ cells, despite a uniform distribution of versican, a major CSPG core protein in skeletal muscles (11), on the cell layer (Fig. 1, F and G). Furthermore, no apparent difference exists in the core protein contents between the conditioned media on days 5 and 7 (Fig. 1H), indicating that the decreased CS reactivity around Myog+ cells is not due to the reduced expression and/or deposition of CSPG core proteins carrying CS. Collectively, these findings suggest that a temporal reduction in CS abundance is involved in myogenic differentiation processes, including in multinuclear myotube formation.
FIGURE 1.
Reduction in CS abundance correlates with the progression of multinuclear myotube formation in C2C12 myoblasts. A, temporal pattern of MHC immunostaining (brownish red) in parental C2C12 cells cultured in DM. Cell nuclei were stained with Hoechst 33342 (blue). Scale bar, 100 μm. B, higher magnification of the images in A. Arrowheads indicate MHC+ cells with two or more nuclei. Scale bar, 100 μm. C, changes in the levels of the Myog transcript (normalized to that of Gapdh) and of accumulated CS (estimated as the amount of total CS disaccharides) during myogenic differentiation of parental C2C12 cells (n = 10, Myog expression; n = 6, CS abundance; at each time point). Error bars represent S.D. D and E, the degree of myogenesis, including multinuclear myotube formation, was quantified based on MHC+ cell density (D) and on the fusion index of MHC+ cells (E) in parental C2C12 cell culture in DM at each time point (n = 4, for each time point; results are expressed as means ± S.D.; *, p < 0.05; **, p < 0.005; ***, p < 0.001). F–H, CS immunoreactivity was lower around Myog+ cells. Parental C2C12 cells on days 5 and 7 in DM culture were immunostained with anti-CS (green in F and G, upper panels), anti-versican core protein (Vcan, green in G, lower panels), and anti-Myog (red in F and G) antibodies. Cell nuclei were counterstained in blue with Hoechst 33342. Scale bar, 100 μm. The surrounding milieu of Myog+ cells, where versican core protein was uniformly distributed, was less immunoreactive for CS. ChABC treatment of the fixed cells before incubation with the anti-CS antibody eliminated the CS reactivity, confirming the specificity of the staining. H, temporal changes in the contents of CSPG core proteins in conditioned media (CM). Differentiating, parental C2C12 cells were incubated in serum-free medium (SF-M) for 48 h according to the time schedule shown at the bottom. Western blot analysis of CM5 and CM7 showed that expression levels of the core proteins, versican and biglycan (Bgn), are indistinguishable.
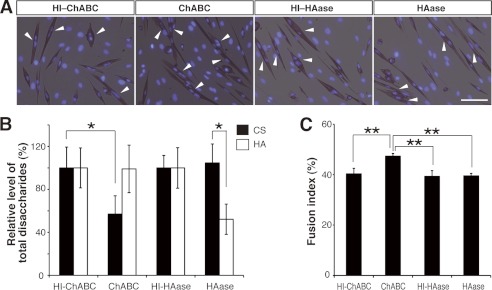
To confirm the importance of CS levels in syncytial myotube formation, we examined the impact of forced down-regulation of CS in differentiating C2C12 cultures. As expected, 24-h treatment of day 6 cultures with a bacterial CS-degrading enzyme, chondroitinase ABC (ChABC), which resulted in an ∼50% reduction in the CS level (Fig. 2B), led to a significant increase in the fusion index on day 7 in DM culture (Fig. 2, A and C). However, ChABC can cleave not only CS but also, to a lesser extent, HA (another GAG that is a major ECM component) in vitro. Although there was no apparent change in the HA level in the ChABC-treated culture, we further analyzed the possibility that the myogenic effect of ChABC treatment might be caused by a modest decrease in HA (Fig. 2B). However, unlike ChABC, addition of a bacterial hyaluronidase (HAase) that specifically degrades HA to the culture medium did not enhance C2C12 fusion (Fig. 2, A and C), even though a 24-h exposure of the cells to the enzyme resulted in selective removal of a high amount of HA (Fig. 2B). This result ruled out the possible involvement of HA in myogenic fusion.
FIGURE 2.
ChABC treatment enhanced myogenic differentiation of C2C12 cells. A–C, parental C2C12 cells on day 6 in DM were treated with either ChABC or a bacterial HA-degrading enzyme. HI enzyme preparations were also tested as negative controls. A, after 24 h of treatment, cells were labeled with anti-MHC (brownish red) antibody and Hoechst 33342 (blue). Arrowheads denote MHC+ cells with more than two nuclei. Scale bar, 100 μm. B, quantification of the amounts of GAGs in the bacterial enzyme-treated C2C12 cell cultures (n = 3 for each group; results are expressed as means ± S.D.; *, p < 0.05). C, fusion index of MHC+ cells was calculated from the experiments in A (n = 4 for each group; results are expressed as means ± S.D.; **, p < 0.001).
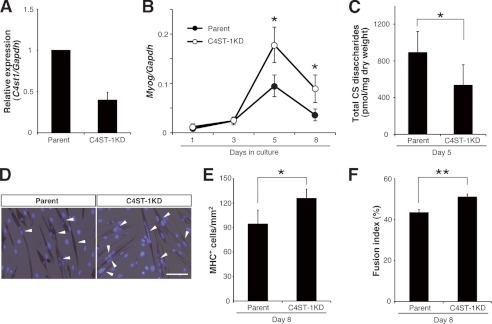
To further confirm that myoblast fusion is accelerated under conditions in which CS levels are decreased, we generated stable clones of C2C12 cells in which chondroitin 4-O-sulfotransferase-1 (C4ST-1) expression was knocked down (Fig. 3A). C4ST-1 is a CS biosynthetic enzyme, whose deficiency or experimental knockdown has been reported to lead to a drastic decrease in cellular and whole-body levels of CS (12–15). Consistent with these previous reports, the level of CS in C4ST-1 knockdown (KD) C2C12 cultures was lower than in the parental cells (Fig. 3C). C4ST-1 depletion enhanced myogenic differentiation resulting in significant increases in MHC+ cell density and in the fusion index on day 8 in DM culture (Fig. 3, D–F). Although the expression levels of Myog were significantly higher in C4ST-1 KD cells than in parental C2C12 cells from day 5 onward, it should be noted that there was no obvious difference in temporal Myog expression patterns. Thus, Myog transcripts reached a maximum level on day 5 and subsequently declined in both cell types (Fig. 3B), possibly indicating a minor contribution of CS reduction to the early phase processes before Myog-triggered differentiation. These data provide strong support for the hypothesis that lowering CS levels promotes myogenic terminal differentiation during which myoblast fusion occurs. However, despite their regulatory potentials in CS abundance, endogenous expression pattern of C4st1 and other CS biosynthetic enzymes did not necessarily match the temporal level of CS in the differentiating C2C12 cultures (supplemental Fig. 1).
FIGURE 3.
Knockdown of C4ST-1 in C2C12 cells reduced CS abundance and promoted myogenesis. A, relative expression level of the C4st1 transcript (normalized to that of Gapdh) in parental C2C12 and C4ST-1 KD cells. The values are averages of two independent experiments and were normalized to that of parental C2C12 cells. B, temporal expression profiles of the Myog transcript (normalized to that of Gapdh) during myogenic differentiation of parental C2C12 and C4ST-1 KD cells (n = 4, for each time point per group; results expressed as means ± S.D.; *, p < 0.001). C, CS levels in C4ST-1 KD cells were significantly reduced compared with their levels in parental C2C12 cells (n = 4 for each group; results are expressed as means ± S.D.; *, p < 0.05). D, representative images of MHC immunostaining (brownish red) of parental C2C12 cells and C4ST-1 KD cells on day 8 in DM culture. Cell nuclei were labeled with Hoechst 33342 (blue). Arrowheads indicate multinuclear MHC+ cells. Scale bar, 100 μm. E and F, the degree of myogenesis was quantified based on the MHC+ cell density (E) and the fusion index of MHC+ cells (F) in parental C2C12 cells and C4ST-1 KD cells cultured in DM for 8 days (n = 3 for each group; results are expressed as means ± S.D.; *, p < 0.05; **, p < 0.005).
CS Levels in Myogenesis Are Regulated by a Cell-autonomous Catabolic Mechanism
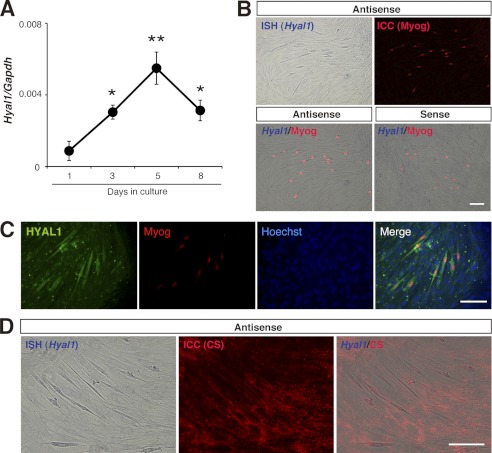
Given that change in CS abundance is temporally controlled during myogenic differentiation, we expected that catabolic mechanism(s) that are involved in CS degradation would also be temporally activated. We therefore analyzed the mRNA expression profile of members of the mammalian hyaluronidase (HYAL) family, HYAL1–4 (Fig. 4A and supplemental Fig. 1). Although these enzymes are widely known as HA-degrading enzymes, some of these enzymes, including HYAL1 and HYAL4, have CS degrading activities (16, 17). Intriguingly, we found that the expression of Hyal1 was apparently synchronous with that of Myog (Figs. 1C and 4A). In situ hybridization and immunocytochemistry further revealed that Hyal1 mRNA and its gene products were preferentially expressed in the Myog+ cells (Fig. 4, B and C) and that the surrounding area of cells with a higher level of Hyal1 was less immunoreactive for CS (Fig. 4D). In contrast, although HYAL4 was recently the first CS-specific hydrolase to be identified in mammals (18), its transcripts were not detected in differentiating C2C12 cells. These observations led us to investigate the functional relevance of HYAL1 for the temporal regulation of CS abundance and skeletal muscle differentiation.
FIGURE 4.
HYAL1 expression coincides with progression of C2C12 myogenesis. A, temporal expression pattern of the Hyal1 transcript (normalized to that of Gapdh) during myogenic differentiation of parental C2C12 cells (n = 4 for each time point; error bars represent S.E.; *, p < 0.05, day 1 versus day 3 or 8; **, p < 0.01, day 1 versus day 5). B, in situ hybridization (ISH) for Hyal1 expression in parental C2C12 cells on day 5 in DM culture. Signal for Hyal1 (purple) was evident in the Myog+ cells (red) that were identified by immunocytochemistry (ICC). No signal was detected by using a sense probe for Hyal1. C, HYAL1 expression (green) in Myog+ cells (red) was also detected by immunocytochemistry. Cell nuclei were labeled with Hoechst 33342 (blue). D, immunofluorescent signal for CS (red) was relatively lower around Hyal1+ cells (purple). Scale bars in B–D, 100 μm.
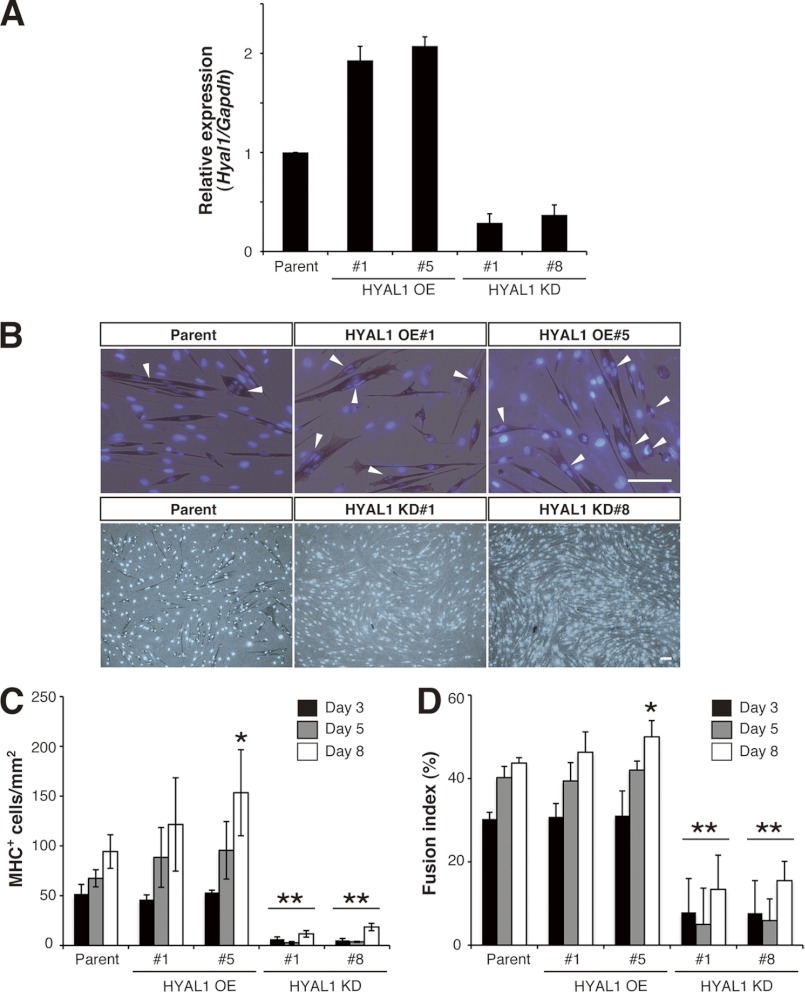
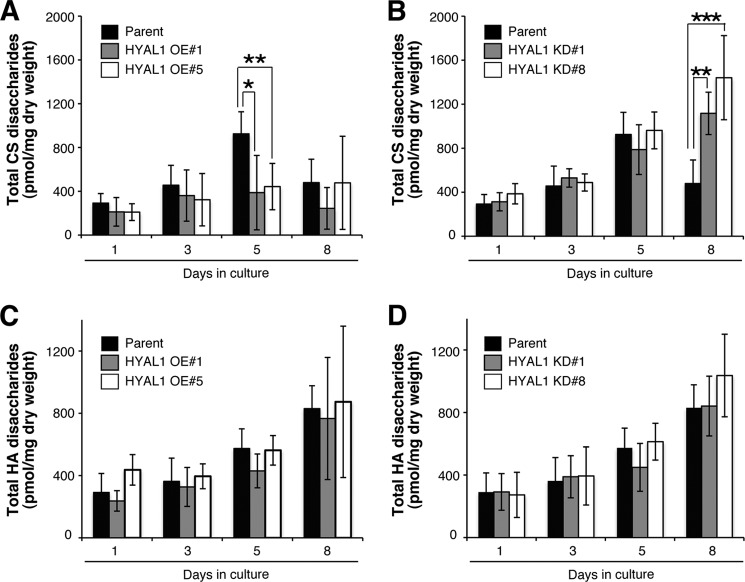
To this end, we first established stable clones of C2C12 cells that overexpressed HYAL1 (HYAL1 OE cells) (Fig. 5A). Unlike the transient elevation in CS abundance in parental C2C12 cultures over time, CS levels in HYAL1 OE cultures remained essentially unaltered over the 8-day time course, mainly due to a significantly lower CS level than parental cells on day 5 (Fig. 6A). Consistent with their low level of CS, multinuclear MHC+ myotubes were more frequently observed on day 8 in HYAL1 OE cultures compared with parental cells (Fig. 5B). Indeed, the two myogenic parameters, the density of MHC+ cells and the fusion index, were both higher in HYAL1 OE cells compared with parental C2C12 cells at each time point during differentiation, and these differences were statistically significant on day 8 (Fig. 5, C and D). These observations imply that HYAL1 can cleave CS even at a cellular level, and can function as a positive regulator of myoblast fusion rather than a regulator of the initiation of differentiation. As a second approach to confirm the role of HYAL1 in differentiation, we also generated C2C12 cells in which HYAL1 was stably knocked down (HYAL1 KD cells) (Fig. 5A). In support of the above hypotheses, knockdown of HYAL1 led to significantly enhanced accumulation of CS on day 8 in DM culture (Fig. 6B) and resulted in developmental arrest or delay, as judged by a marked drop in myogenic indices (Fig. 5, B–D). Surprisingly, despite the well described preference of HAYL1 for HA in vitro, neither HAYL1 overexpression nor its knockdown affected cellular HA levels in differentiating C2C12 cultures (Fig. 6, C and D). Because expression profiles of relevant genes involved in HA production/degradation were also essentially unaltered by perturbation of Hyal1 (supplemental Fig. 2), the invariant HA levels did not appear to be attributable to the breakdown of HA production/degradation system. In addition, unlike CS abundance, the temporal levels of expressed cellular HA, which showed a gradual increase over time of culture, did not match those of Hyal1 expression (Figs. 4A and 6, C and D). These data further support the interpretation that CS rather than HA may be a preferred physiological substrate of HYAL1. Alternatively, compared with HA turnover, CS abundance might be more sensitive to functional level of HYAL1, because of functional compensation of HA degradation by other HYAL family members.
FIGURE 5.
Involvement of HYAL1 function in the myogenesis of C2C12 cells. A–D, constitutive OE and knockdown of HYAL1 in C2C12 cells promoted and suppressed myogenic differentiation, respectively. A, relative expression level of the Hyal1 transcript (normalized to that of Gapdh) in parental C2C12, HYAL1 OE (#1 and #5), and HYAL1 KD (#1 and #8) cells. Values are the average of two independent experiments and were normalized to that of parental C2C12 cells. Error bars indicate S.D. B, representative images of MHC immunostaining (brownish red) in parental C2C12 cells and its stable clones on day 8 in DM. Nuclei were stained with Hoechst 33342 (blue). Arrowheads indicate multinuclear MHC+ cells. Scale bars in top and bottom panels, 100 μm. C and D, degree of myogenesis was quantified based on the MHC+ cell density (C) and on the fusion index of MHC+ cells (D) in parental C2C12 cells and its stable clones in DM culture at each time point (n = 5, parental cells; n = 3, OE #1; n = 4, OE #5; n = 3, KD #1; n = 3, KD #8; at each time point; results are expressed as means ± S.D.; *, p < 0.05; **, p < 0.001).
FIGURE 6.
CS abundance is regulated by HYAL1 expression levels. A and B, constitutive OE of HYAL1 in C2C12 cells abrogated the accumulation of CS on day 5 in DM culture (A), whereas its knockdown (KD) caused elevated CS levels on day 8 (B) (n = 6, parental cells; n = 3, OE#1; n = 4, OE#5; n = 6, KD#1; n = 6, KD#8; at each time point, results are expressed as means ± S.D.; *, p < 0.05; **, p < 0.005; ***, p < 0.001). C and D, HA contents were essentially unaltered in both HYAL1 OE (C) and KD (D) cells (n = 4, parental cells; n = 3, OE#1; n = 4, OE#5; n = 5, KD#1; n = 5, KD#8; at each time point, results are expressed as means ± S.D.).
Reduction in CS Abundance Enhances Intracellular Signaling Required for Muscle Cell Fusion
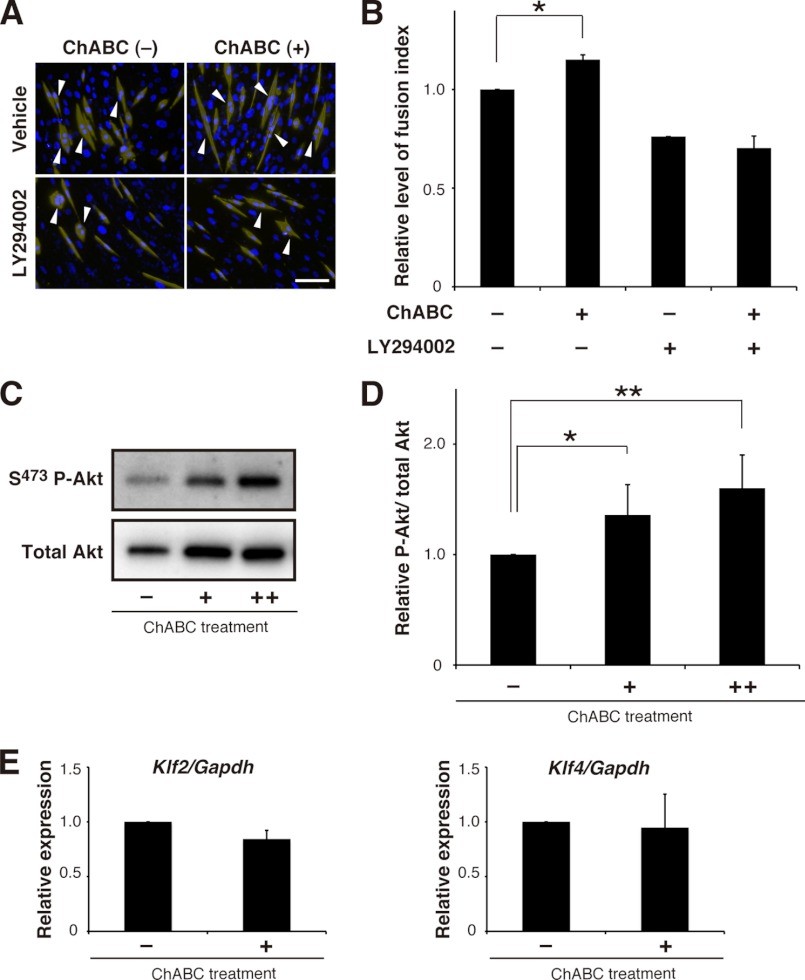
Recent advance in biological functions of CS has demonstrated that CS behaves as extracellular signaling molecules and/or modulators (19–23). Therefore, alteration of CS abundance might also affect intracellular signaling pathways that have been reported to regulate skeletal muscle differentiation. To address this issue, we focused on the phosphoinositide 3-kinase (PI3K)/Akt (protein kinase B) pathway, which is of utmost importance for myoblast differentiation into fused myofibers (24–26). Indeed, in differentiating C2C12 cultures, treatment with a pharmacological PI3K inhibitor, LY294002, from day 5 to 7 brought about a 25% decline in the fusion index when compared with vehicle controls (Fig. 7, A and B), confirming the involvement of PI3K/Akt pathway in myogenic fusion processes. To determine whether the reduction in CS abundance links activation of this pathway, phosphorylation levels of Akt were assessed from the day 7 cultures following 24 h of treatment with ChABC that sufficed for induction of C2C12 fusion (Fig. 2). Of note, such treatment significantly enhanced the Akt phosphorylation levels in a concentration-dependent fashion (Fig. 7, C and D). Conversely, the ChABC-induced cell-cell fusion was abrogated in the presence of LY294002 (Fig. 7, A and B). These results indicate that PI3K/Akt pathway is part of intracellular machinery in response to CS reduction that stimulates myogenic fusion.
FIGURE 7.
Reduction in CS abundance affects intracellular signaling pathway responsible for myogenesis of C2C12 cells. A and B, 48-h treatment of LY294002 suppressed both conventional and ChABC-induced fusion processes of parental C2C12 cells on day 7 cultures in DM. Cells on day 7 were labeled with anti-MHC (pseudocolor, yellow) antibody and Hoechst 33342 (blue). Arrowheads in A indicate multinuclear MHC+ cells. Scale bar in A, 100 μm. The relative fusion index of MHC+ cells (B) was calculated from the experiments in A (n = 3, parental cells; results are expressed as means ± S.E.; *, p < 0.005). C and D, activation of PI3K/Akt pathway in response to ChABC treatment. Parental C2C12 cells on day 6 in DM were incubated for 24 h in the absence (−) or presence of ChABC (+, 2.5 mIU/ml; ++, 5.0 mIU/ml). The phosphorylation levels of Akt were immunodetected using antibodies to Akt and phospho-Akt (Ser-437) (C). D, relative Akt phosphorylation levels (phosphorylated Akt (P-Akt)/total Akt) were calculated by densitometric quantification of the Western blots (n = 3 for each group; results are expressed as means ± S.D.; *, p < 0.05; **, p < 0.01). E, parental C2C12 cells on day 6 incubated in the presence (+) or absence (−) of ChABC (20 mIU). After 24 h of treatment, the expression level of Klf2/4 was analyzed and normalized to that of Gapdh (n = 3, for each group; results expressed as means ± S.E.). Although the 24-h treatment with ChABC led to a significant increase in the fusion index on day 7 in our culture condition (Fig. 2, A–C), no significant change in expression of Klf2/4 was detected.
In view of the latest report on a key role of the ERK5/Klf pathway, one of mitogen-activated protein kinase (MAPK) cascades, in muscle cell fusion (27), we also expected and examined its functional relevance to the reduction in CS abundance. Although ERK5-dependent cell fusion has been shown to correlate with transcriptional up-regulation of Klf2 and Klf4 (27), their up-regulation was not reproduced by ChABC treatment of parental C2C12 cultures in DM (Fig. 7E). Alternative approaches also yielded no evidence for the direct involvement of the ERK5/Klf pathway in our culture system (supplemental Fig. 3).
ChABC Treatment Boosts Myofiber Regeneration and Ameliorates mdx Pathology
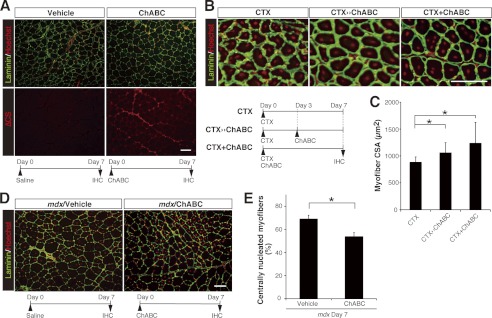
For potential clinical extrapolation of our in vitro findings, we next determined whether intentional induction of CS breakdown by utilizing ChABC could accelerate skeletal muscle regeneration in vivo, because this process also requires the fusion of newly generated myocytes with each other or with existing myofibers (28–30). In the intact tibialis anterior (TA) muscles of the hind limbs of wild-type mice, CS immunoreactivity was detected preferentially around individual myofibers, especially laminin positive-basal lamina and endomysium (supplemental Fig. 4). Intramuscular injection of ChABC into the TA muscles resulted in an extensive decrease in the level of CS on day 7 following injection, which was visualized by an anti-CS stub antibody (Fig. 8A). No differences were detected in the histological appearance of the TA muscles between ChABC- and vehicle-treated control mice (Fig. 8A). Notably, the scarcity of centrally nucleated myofibers, which are indicative of regeneration, suggested that ChABC treatment per se does not result in detrimental effects such as necrotic/apoptotic cell death and/or inflammation of myofibers and that the transient removal of CS from uninjured, normal muscles cannot trigger regenerative processes. We therefore assayed the in vivo effect of ChABC treatment of a widely used experimental mouse model for muscle regeneration in which acute muscle damage is induced by intramuscular administration of CTX. CTX is a cobra venom that causes muscle degeneration. However, this degeneration is reversible, and muscle architecture is later regenerated (28, 31). At 7 days after CTX-induced injury, centrally nucleated (newly regenerated) myofibers were reproducibly observed in the injured TA muscles of all groups of mice, whether they were given saline (CTX alone) or ChABC, administered by one of the two dosing regimens (Fig. 8B). Notably, quantitative analysis of the CSA of individual centrally nucleated myofibers revealed that a single (Day 0) or two (Day 0 and Day 3) ChABC injections together with CTX administration on Day 0 led to a significant augmentation of the size of newly generated myofibers, which is indicative of enhanced regeneration (Fig. 8C). These results indicate that ChABC can act as a potent inducer of muscle regeneration only under the restricted condition where muscle regeneration programs have already been activated.
FIGURE 8.
Intramuscular administration of ChABC is effective for skeletal muscle regeneration and improvement of mdx pathology in mice. A, transverse TA muscle sections from vehicle- and ChABC-treated wild-type mice were immunostained with anti-laminin (green) plus anti-CS stub (ΔCS, red) antibodies. Cell nuclei were stained with Hoechst 33342 and pseudo-colored red. The time schedule of injection and immunohistochemistry (IHC) is shown at the bottom of each panel. ChABC treatment degrades CS chains and produces characteristic CS-stub structures (ΔCS) around individual myofibers. Centrally nucleated myofibers were not observed in either ChABC-treated muscles or in the control. Scale bar, 100 μm. B and C, ChABC treatment promoted regeneration of wild-type TA muscles injured by CTX. B, time schedules of CTX injection with/without ChABC injection and immunohistochemistry (bottom) and representative images of lesion areas of CTX-treated TA muscles from wild-type mice. Most myofibers labeled with anti-laminin antibody (green) had central nuclei (red). Scale bar, 100 μm. C, CSA of all of the centrally nucleated myofibers within injured regions of the CTX-treated TA muscles was measured (CTX, n = 4 (the number of TA muscles analyzed), 2,381 fibers (total number of fibers analyzed); CTX ≫ ChABC, n = 4, 2,545 fibers; CTX + ChABC, n = 5, 2,552 fibers). Note a significant increment in the CSA in both ChABC-treated muscles compared with the control group treated with CTX alone (results are expressed as means ± S.D.; *, p < 0.01, CTX versus CTX ≫ ChABC or CTX + ChABC). D and E, ChABC treatment of TA muscles of mdx mice. D, transverse TA muscle sections from vehicle- and ChABC-treated mdx mice were labeled with anti-laminin antibody (green) and Hoechst 33342 (pseudocolor, red). The time schedule of injection and IHC is shown at the bottom of each panel. Scale bar, 100 μm. E, fraction of centrally nucleated myofibers (% of total fibers) within each TA muscle section obtained from mdx mice on day 7 post-treatment was measured (n = 5, for each group; results are expressed as means ± S.D.; *, p < 0.005).
The potential utility of ChABC for enhancement of muscle regeneration prompted us to further evaluate whether elimination of CS might also ameliorate dystrophic phenotypes in dystrophin-deficient mdx mice, which are a model of Duchenne muscular dystrophy (32–34). Because mdx pathology, which is characterized by widespread degeneration of skeletal myofibers that is accompanied by recuperative regeneration of new fibers with centralized nuclei, generally begins at 3 weeks of age and its severity peaks at 6 weeks of age (34, 35), we administered a single dose of ChABC or vehicle alone to TA muscles of 5–6-week-old mdx mice, and assessed the treated muscles histologically 3, 7, and 14 days later. Consistent with typical mdx pathology, vehicle-treated control mice displayed a high proportion of centrally nucleated myofibers, and the values obtained at all three time points were essentially the same (supplemental Fig. 5), suggesting that a nearly constant rate of degeneration and regeneration persisted in skeletal muscles throughout the period of study. Importantly, ChABC treatment resulted in a remarkable reduction in the proportion of centrally nucleated myofibers at day 7 post-injection (Fig. 8, D and E). The proportion of centrally nucleated myofibers in ChABC-treated muscles reverted to the vehicle control level on day 14 following injection (supplemental Fig. 5). The combined data indicate that a single dose of ChABC can exert a beneficial effect on, and transiently reduce, dystrophic pathology in mdx mice.
DISCUSSION
We have demonstrated here novel functions of CS in the modulation of skeletal muscle differentiation and repair processes, both of which cells undergo fusion. These data may help clarify the essential requirement of CS for embryonic cell division (3, 4). In support of our hypothesis, all methods that lowered CS levels in differentiating C2C12 cells resulted in acceleration of multinuclear myotube formation. Moreover, confirmation of the involvement of HYAL1 in myogenesis suggested that endogenous HYAL1 might catabolize endogenous CS and provided convincing evidence that the observed temporal decline in CS is not accidental but is finely regulated through cell autonomous mechanism(s). It should be noted that HYAL1 is thought to be a lysosomal enzyme (16, 36). A most recent study has reported that both HYAL1 and β-hexosaminidases, other lysosomal glycosidases, are functionally redundant in CS breakdown in vivo (37). Together with the consistent expression of β-hexosaminidases, Hexa and Hexb, during myogenesis of C2C12 cells (supplemental Fig. 1), this might explain the no myogenic phenotypes in Hyal1-null mutations (38) and indicates the functional requirement of more complex systems for effective cleavage of CS in vivo. In terms of this, the reduction in extracellular/pericellular CS might also be regulated via endocytic uptake and/or recycling pathways. Indeed, recycling pathways have been reported to regulate myoblast fusion (39, 40).
Our identification of a signaling pathway responsive to the decline in CS abundance gives valuable insight into the role of CS as a critical extracellular signaling cue that can be recognized by myogenic cell lineages and regulates their differentiation. The PI3K/Akt pathway has been reported to be involved not only in in vitro myogenesis but also in skeletal muscle regeneration (24–26, 41, 42). Notably, activation of Akt signaling has been shown to promote myofiber regeneration after CTX-induced injury and to counteract mdx pathogenesis (42). Therefore, the regenerative effects of ChABC on skeletal muscles in vivo might be also exerted, at least in part, via PI3K/Akt pathway. Further investigations are needed to understand the molecular basis for CS functions in cell-cell fusion and skeletal muscle regeneration processes.
Because of the widely accepted axon-growth inhibitory function of CS in the injured adult central nervous system, approaches for treatment of brain injury using ChABC have recently received attention (43, 44). Although the precise molecular basis of the skeletal muscle differentiation/repair that is boosted by removal of CS is currently unknown, we propose an additional applicability of ChABC for regenerative therapy of skeletal muscles, because ChABC treatment did not influence uninjured normal muscles but did lead to significant histological improvement of injured muscles even following injection of a singe dose. In conclusion, strategies aimed at enhancing the removal of CS could provide promising approaches for high specificity treatment of acute muscle injury and muscular dystrophies with minimum side effects.
This work was supported in part by the Scientific Promotion Fund from the Japan Private School Promotion Foundation, Grant-in-aid for Scientific Research (C) 24590132 (to T. M.), Scientific Research on Innovative Areas 23110003 (to H. K.), and Supported Program for the Strategic Research Foundation at Private Universities (2012–2016) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.

This article contains supplemental Figs. 1–5 and Table 1.
- CS
- chondroitin sulfate
- C4ST-1
- chondroitin 4-O-sulfotransferase-1
- ChABC
- chondroitinase ABC
- CSA
- cross-sectional area
- CTX
- cardiotoxin
- DM
- differentiation medium
- GAG
- glycosaminoglycan
- HA
- hyaluronan
- HAase
- bacterial hyaluronidase
- HI
- heat-inactivated
- HYAL
- mammalian hyaluronidase
- MHC
- myosin heavy chain
- Myog
- myogenin
- OE
- overexpression
- PG
- proteoglycan
- TA
- tibialis anterior.
REFERENCES
- 1. Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 13, 612–620 [DOI] [PubMed] [Google Scholar]
- 2. Uyama T., Kitagawa H., Sugahara K. (2007) in Comprehensive Glycoscience (Kamerling J. P., ed) Vol. 3, pp. 79–104, Elsevier, Amsterdam [Google Scholar]
- 3. Mizuguchi S., Uyama T., Kitagawa H., Nomura K. H., Dejima K., Gengyo-Ando K., Mitani S., Sugahara K., Nomura K. (2003) Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 423, 443–448 [DOI] [PubMed] [Google Scholar]
- 4. Izumikawa T., Kanagawa N., Watamoto Y., Okada M., Saeki M., Sakano M., Sugahara K., Sugihara K., Asano M., Kitagawa H. (2010) Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J. Biol. Chem. 285, 12190–12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rochlin K., Yu S., Roy S., Baylies M. K. (2010) Myoblast fusion. When it takes more to make one. Dev. Biol. 341, 66–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pavlath G. K. (2010) Spatial and functional restriction of regulatory molecules during mammalian myoblast fusion. Exp. Cell Res. 316, 3067–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamada S., Okada Y., Ueno M., Iwata S., Deepa S. S., Nishimura S., Fujita M., Van Die I., Hirabayashi Y., Sugahara K. (2002) Determination of the glycosaminoglycan-protein linkage region oligosaccharide structures of proteoglycans from Drosophila melanogaster and Caenorhabditis elegans. J. Biol. Chem. 277, 31877–31886 [DOI] [PubMed] [Google Scholar]
- 8. Kinoshita A., Sugahara K. (1999) Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high performance liquid chromatography. Application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal. Biochem. 269, 367–378 [DOI] [PubMed] [Google Scholar]
- 9. Yaffe D., Saxel O. (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 [DOI] [PubMed] [Google Scholar]
- 10. Wright W. E., Sassoon D. A., Lin V. K. (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617 [DOI] [PubMed] [Google Scholar]
- 11. Carrino D. A., Sorrell J. M., Caplan A. I. (1999) Dynamic expression of proteoglycans during chicken skeletal muscle development and maturation. Poult. Sci. 78, 769–777 [DOI] [PubMed] [Google Scholar]
- 12. Klüppel M., Wight T. N., Chan C., Hinek A., Wrana J. L. (2005) Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 132, 3989–4003 [DOI] [PubMed] [Google Scholar]
- 13. Uyama T., Ishida M., Izumikawa T., Trybala E., Tufaro F., Bergström T., Sugahara K., Kitagawa H. (2006) Chondroitin 4-O-sulfotransferase-1 regulates E disaccharide expression of chondroitin sulfate required for herpes simplex virus infectivity. J. Biol. Chem. 281, 38668–38674 [DOI] [PubMed] [Google Scholar]
- 14. Mizumoto S., Mikami T., Yasunaga D., Kobayashi N., Yamauchi H., Miyake A., Itoh N., Kitagawa H., Sugahara K. (2009) Chondroitin 4-O-sulfotransferase-1 is required for somitic muscle development and motor axon guidance in zebrafish. Biochem. J. 419, 387–399 [DOI] [PubMed] [Google Scholar]
- 15. Izumikawa T., Okuura Y., Koike T., Sakoda N., Kitagawa H. (2011) Chondroitin 4-O-sulfotransferase-1 regulates the chain length of chondroitin sulfate in co-operation with chondroitin N-acetylgalactosaminyltransferase-2. Biochem. J. 434, 321–331 [DOI] [PubMed] [Google Scholar]
- 16. Csoka A. B., Frost G. I., Stern R. (2001) The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 20, 499–508 [DOI] [PubMed] [Google Scholar]
- 17. Stern R., Jedrzejas M. J. (2006) Hyaluronidases. Their genomics, structures, and mechanisms of action. Chem. Rev. 106, 818–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneiwa T., Mizumoto S., Sugahara K., Yamada S. (2010) Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology 20, 300–309 [DOI] [PubMed] [Google Scholar]
- 19. Mikami T., Yasunaga D., Kitagawa H. (2009) Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 284, 4494–4499 [DOI] [PubMed] [Google Scholar]
- 20. Shen Y., Tenney A. P., Busch S. A., Horn K. P., Cuascut F. X., Liu K., He Z., Silver J., Flanagan J. G. (2009) PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nadanaka S., Kinouchi H., Taniguchi-Morita K., Tamura J., Kitagawa H. (2011) Down-regulation of chondroitin 4-O-sulfotransferase-1 by Wnt signaling triggers diffusion of Wnt-3a. J. Biol. Chem. 286, 4199–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyata S., Komatsu Y., Yoshimura Y., Taya C., Kitagawa H. (2012) Persistent cortical plasticity by up-regulation of chondroitin 6-sulfation. Nat. Neurosci. 15, 414–422 [DOI] [PubMed] [Google Scholar]
- 23. Koike T., Izumikawa T., Tamura J., Kitagawa H. (2012) Chondroitin sulfate-E fine-tunes osteoblast differentiation via ERK1/2, Smad3, and Smad1/5/8 signaling by binding to N-cadherin and cadherin-11. Biochem. Biophys. Res. Commun. 420, 523–529 [DOI] [PubMed] [Google Scholar]
- 24. Rommel C., Bodine S. C., Clarke B. A., Rossman R., Nunez L., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 25. Wilson E. M., Rotwein P. (2007) Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 282, 5106–5110 [DOI] [PubMed] [Google Scholar]
- 26. Roffe S., Hagai Y., Pines M., Halevy O. (2010) Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells. Effect on myotube fusion. Exp. Cell Res. 316, 1061–1069 [DOI] [PubMed] [Google Scholar]
- 27. Sunadome K., Yamamoto T., Ebisuya M., Kondoh K., Sehara-Fujisawa A., Nishida E. (2011) ERK5 regulates muscle cell fusion through Klf transcription factors. Dev. Cell 20, 192–205 [DOI] [PubMed] [Google Scholar]
- 28. Chargé S. B., Rudnicki M. A. (2004) Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 [DOI] [PubMed] [Google Scholar]
- 29. Wagers A. J., Conboy I. M. (2005) Cellular and molecular signatures of muscle regeneration. Current concepts and controversies in adult myogenesis. Cell 122, 659–667 [DOI] [PubMed] [Google Scholar]
- 30. Tedesco F. S., Dellavalle A., Diaz-Manera J., Messina G., Cossu G. (2010) Repairing skeletal muscle. Regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 120, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goetsch S. C., Hawke T. J., Gallardo T. D., Richardson J. A., Garry D. J. (2003) Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol. Genomics 14, 261–271 [DOI] [PubMed] [Google Scholar]
- 32. Bulfield G., Siller W. G., Wight P. A., Moore K. J. (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. U.S.A. 81, 1189–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffman E. P., Brown R. H., Jr., Kunkel L. M. (1987) Dystrophin. The protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 [DOI] [PubMed] [Google Scholar]
- 34. Coulton G. R., Morgan J. E., Partridge T. A., Sloper J. C. (1988) The mdx mouse skeletal muscle myopathy. I. A histological, morphometric and biochemical investigation. Neuropathol. Appl. Neurobiol. 14, 53–70 [DOI] [PubMed] [Google Scholar]
- 35. McArdle A., Edwards R. H., Jackson M. J. (1994) Time course of changes in plasma membrane permeability in the dystrophin-deficient mdx mouse. Muscle Nerve 17, 1378–1384 [DOI] [PubMed] [Google Scholar]
- 36. Harada H., Takahashi M. (2007) CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 282, 5597–5607 [DOI] [PubMed] [Google Scholar]
- 37. Gushulak L., Hemming R., Martin D., Seyrantepe V., Pshezhetsky A., Triggs-Raine B. (2012) Hyaluronidase 1 and β-hexosaminidase have redundant functions in hyaluronan and chondroitin sulfate degradation. J. Biol. Chem. 287, 16689–16697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin D. C., Atmuri V., Hemming R. J., Farley J., Mort J. S., Byers S., Hombach-Klonisch S., Csoka A. B., Stern R., Triggs-Raine B. L. (2008) A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum. Mol. Genet. 17, 1904–1915 [DOI] [PubMed] [Google Scholar]
- 39. Grant B. D., Donaldson J. G. (2009) Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doherty K. R., Demonbreun A. R., Wallace G. Q., Cave A., Posey A. D., Heretis K., Pytel P., McNally E. M. (2008) The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J. Biol. Chem. 283, 20252–20260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim M. H., Kay D. I., Rudra R. T., Chen B. M., Hsu N., Izumiya Y., Martinez L., Spencer M. J., Walsh K., Grinnell A. D., Crosbie R. H. (2011) Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum. Mol. Genet. 20, 1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zanou N., Schakman O., Louis P., Ruegg U. T., Dietrich A., Birnbaumer L., Gailly P. (2012) Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 287, 14524–14534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bradbury E. J., Moon L. D., Popat R. J., King V. R., Bennett G. S., Patel P. N., Fawcett J. W., McMahon S. B. (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 [DOI] [PubMed] [Google Scholar]
- 44. Silver J., Miller J. H. (2004) Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 [DOI] [PubMed] [Google Scholar]