Abstract
Chlorophyll (Chl) biosynthesis in chill (7°C)- and heat (42°C)-stressed cucumber (Cucumis sativus L. cv poinsette) seedlings was affected by 90 and 60%, respectively. Inhibition of Chl biosynthesis was partly due to impairment of 5-aminolevulinic acid biosynthesis both in chill- (78%) and heat-stress (70%) conditions. Protochlorophyllide (Pchlide) synthesis in chill- and heat-stressed seedlings was inhibited by 90 and 70%, respectively. Severe inhibition of Pchlide biosynthesis in chill-stressed seedlings was caused by inactivations of all of the enzymes involved in protoporphyrin IX (Proto IX) synthesis, Mg-chelatase, and Mg-protoporphyrin IX monoester cyclase. In heat-stressed seedlings, although 5-aminolevulinic acid dehydratase and porphobilinogen deaminase were partially inhibited, one of the porphyrinogen-oxidizing enzymes, uroporphyrinogen decarboxylase, was stimulated and coproporphyrinogen oxidase and protoporphyrinogen oxidase were not substantially affected, which demonstrated that protoporphyrin IX synthesis was relatively more resistant to heat stress. Pchlide oxidoreductase, which is responsible for phototransformation of Pchlide to chlorophyllide, increased in heat-stress conditions by 46% over that of the control seedlings, whereas it was not affected in chill-stressed seedlings. In wheat (Triticum aestivum L. cv HD2329) seedlings porphobilinogen deaminase, Pchlide synthesis, and Pchlide oxidoreductase were affected in a manner similar to that of cucumber, suggesting that temperature stress has a broadly similar effect on Chl biosynthetic enzymes in both cucumber and wheat.
Plants play a very important role in our lives and the environment has a significant role in plant growth and development. In different parts of the globe, especially between 20° and 30° latitudes north and south of the equator, the temperature decreases to 4°C to 8°C in the winter and increases to 40°C to 44°C in the summer. Therefore, several plant species, including annual crop plants, are exposed to both chill and heat stress during their lifetime. When plants are exposed to low- or high-temperature stress, Chl biosynthesis is affected (van Hasselt and Strikwerda, 1976; Feierabend, 1977). Biosynthesis of porphyrins and particularly that of Chl during early greening stages of seedlings has been elucidated in detail (Tripathy and Rebeiz, 1986, 1987, 1988; Hukmani and Tripathy, 1992, 1994; for recent reviews, see Leeper et al., 1991; Richards, 1992; von Wettstein et al., 1995; Porra, 1997). It is important to understand exactly how temperature stress impairs Chl biosynthesis. This will have profound importance in the generation of improved crop varieties resistant to temperature stress.
There are a few reports that demonstrate that low or high temperature reduces Chl biosynthesis. Illumination of etiolated seedlings of maize at low temperature resulted in reduced Chl accumulation, and impairment of Chl biosynthesis was paralleled by an aberrant development of the thylakoid membranes (van Hasselt and Strikwerda, 1976; Hodgins and van Huystee, 1986a). A slight photoconversion of Pchlide to Chl was possible at 12°C (van Huystee and Hodgins, 1989). Chlorosis in maize was due to impaired synthesis of ALA, and incubation of leaf tissue at low temperature in ALA resulted in reduced porphyrin synthesis (Hodgins and van Huystee, 1986b). Heat-bleached rye leaves had the capacity to convert ALA to Pchlide, although they had plastidic ribosome deficiency, suggesting that the enzymes involved have to be synthesized on cytoplasmic ribosomes (Feierabend, 1977). In heat-bleached, plastidic ribosome-deficient primary leaves of rye and oat, Chl synthetase activity was partially reduced (40%), although tRNAGlu was found in substantial amounts in bleached rye plastids (Hess et al., 1992). A shift in the ratio of Chl a and Chl b was observed in heat-bleached Euglena gracilis (Thomas and Ortiz, 1995).
Although the above data are available, there has not been any systematic and comparative study on the effect of heat and chill stress on the detailed Chl biosynthetic pathway. Therefore, in the present study the effects of chill (7°C) and heat (42°C) stress on several enzymes of the Chl biosynthetic pathway were studied in the dicotyledonous horticultural plant cucumber and, when required, were compared with the monocotyledonous crop plant wheat.
MATERIALS AND METHODS
Plant Material
Cucumber (Cucumis sativus L. cv Poinsette) and wheat (Triticum aestivum L. cv HD2329) were used as the experimental materials. The seeds were obtained from the Indian Agricultural Research Institute, New Delhi.
Plant Growth Conditions
The seeds were treated with 0.1% HgCl2 solution for 5 min and then washed with tap water three times and spread over a single layer of moist germination paper. Cucumber and wheat seedlings were grown in the dark at 25°C for 4 and 6 d, respectively, and were transferred to low (7°C) and high (42°C) temperatures for various lengths of time. One set of seedlings remained at 25°C under identical conditions for a comparison (control).
ALA Content
Five pairs of cotyledons were preincubated in 60 mm LA in 50 mm phosphate buffer, pH 6.0, for 4 h in the dark at 7°C, 25°C, and 42°C. After the preincubation, cotyledons were exposed to white fluorescent light (30 μmol m−2 s−1) for 3 and 6 h while controls were left in the dark. Each time five pairs of cotyledons were weighed and hand homogenized in a prechilled mortar and pestle in 5 mL of 1 m sodium acetate buffer (pH 4.6). The homogenate was centrifuged at 10,000 rpm (12,000g) for 10 min and supernatant was taken for assay. The assay mixture consisted of 0.1 mL of supernatant, 0.4 mL of distilled water, and 25 μL of acetylacetone. The assay medium was mixed properly and heated in a boiling water bath for 10 min. Then the extract was cooled at room temperature, and an equal volume of modified Ehrlich's reagent was added and vortexed for 2 min. After 10 min of incubation, absorbance of the extract was measured at 555 nm and ALA content was determined from the standard curve of ALA (Harel and Klein, 1972).
ALAD
Two pairs of cotyledons, harvested from etiolated cucumber seedlings grown at different temperature regimes, were weighed and hand homogenized in 5 mL of 0.1 m Tris and 0.01 m mercaptoethanol solution, pH 7.6, in a mortar and pestle at 4°C. Homogenate was centrifuged at 10,000 rpm in a rotor (12,000g, model SM24, Sorvall) in a centrifuge (model RC5C, Sorvall) at 4°C. The supernatant was taken for ALAD assay. The crude enzyme extracts were assayed for ALAD activity according to the method of Shemin (1962) with a slight modification. The enzyme activity was determined by measuring the amount of PBG formed in 1 mL of the reaction mixture. The incubation mixture consisted of 60 mm Tris, 0.2 mm ALA, 1 mm EDTA, 15 mm MgCl2, 0.5% BSA (w/v), and 0.33 m Suc, pH 7.5, and the enzyme preparation. After 10 min of preincubation, the reaction was started by adding the substrate ALA and incubation was carried out for 1 h at 28°C. The amount of PBG formed was calculated using the absorption coefficient (6.2 × 104 m−1 cm−1) (Hukmani and Tripathy, 1994).
PBGD
Two pairs of cucumber cotyledons, harvested from etiolated cucumber seedlings grown at different temperature regimes, were weighed and hand homgenized in 5 mL of 0.1 m Tris buffer (pH 7.6) at 4°C. Homogenate was centrifuged at 10,000 rpm (12,000g) for 10 min. Supernatant was taken for PBGD activity assay. The crude enzyme extracts were assayed for PBGD activity. The enzymatic activity was measured by determining the amount of uroporphyrin formed in 1 mL of reaction mixture consisting of 0.1 m Tris-HCl, 2.5 mm EDTA, 15 mm MgCl2, and 0.1% BSA (w/v), pH 7.5. The reaction was carried out for 1 h at 37°C. An aliquot of 0.85 mL of this reaction mixture was taken, to which 0.25 mL of 5 n HCl was added to stop the reaction. The porphyrinogens that were formed were oxidized in porphyrins by adding 0.1 mL of 0.1% benzoquinone to methanol. After 20 min at 4°C samples were centrifuged. A 0.1-mL aliquot was taken, to which 0.9 mL of 1 n HCl was added. Absorbance was measured at 405 nm (absorption coefficient = 5.48 × 105 m−1 cm −1) (Hukmani and Tripathy, 1994).
Preparation of Urogen III, Coprogen III, and Protogen IX
Sodium amalgam was prepared by adding 18.31 g of mercury to 0.55 g of freshly cut and heated sodium metal under nitrogen gas (Jacobs and Jacobs, 1982). Reduction of porphyrins was carried out according to the method of Poulson and Polglosse (1974) with certain modifications. Porphyrinogens were freshly prepared by reducing porphyrins, namely uroporphyrin III, coproporphyrin III, or Proto IX. Porphyrins were dissolved in 0.1 n KOH consisting of 20% ethanol. Oxygen present in the solution was removed by flushing the solution with nitrogen gas. To this solution 5 g of 3% sodium amalgam was added and flushing the solution with the stream of nitrogen was continued. The reduction was carried out for 2 to 3 min; the solution becomes colorless within this time period and the reduction was continued for 2 to 3 min more to ensure complete reduction of the porphyrin. Immediately, the contents were filtered. The pH of the porphyrinogen solution was adjusted to 7.5 with dilute acetic acid.
Proto IX Synthesis from Urogen III, Coprogen III, and Protogen IX
Dark-grown cucumber seedlings were transferred to different temperature regimes, and intact plastids were isolated from cotyledons over a 50% percoll gradient. The plastids were lysed in lysis buffer consisting of 10 mm Tris-HCl and 2.5 mm Na2EDTA (pH 7.7). Lysed plastids were centrifuged at 5000 rpm for 3 min in a rotor (3000g, model SS-34, Hitachi) in a centrifuge (Hitachi) at 4°C. The supernatant was used for the enzyme assay. The reaction mixture (0.3 mL) consisted of 100 mm Tris (pH 7.5), 15 mm MgCl2, 5 mm DTT, 0.1% BSA (w/v), 0.1 mL of enzyme preparation, and 0.03 mL of urogen, coprogen, or protogen. Incubation was carried out for 1 h at 28°C (Jacobs and Jacobs, 1982). The reaction was terminated by adding 1.5 mL of 90% acetone. Pigments were quantified by spectrofluorometry (Rebeiz et al., 1975; Hukmani and Tripathy, 1992).
Mg-Chelatase
Etioplasts were isolated at 4°C using a modification of the previous method (Tripathy and Rebeiz, 1986). Five grams of cucumber cotyledons harvested from etiolated cucumber seedlings grown at different temperature regimes was hand homogenized in a prechilled mortar and pestle with 13 to 15 gentle strokes under a safe green light in 15 mL of chilled isolation buffer consisting of 0.5 m Suc, 20 mm Hepes, 1 mm MgCl2, 1 mm Na2EDTA, and 0.2% BSA (w/v) at a room-temperature pH of 7.7. The homogenate was passed through four layers of cheesecloth and centrifuged at 1200 rpm (200g) for 2 min. The supernatant was decanted and further centrifuged at 4000 rpm (2000g) for 7 min. All of the centrifugation was done in an SS-34 rotor at 4°C using a Hitachi centrifuge. The pelleted plastids were gently suspended with the help of a paint brush in suspension buffer containing 0.5 m Suc, 0.2 m Tris-HCl, 20 mm MgCl2, 2.5 mm Na2EDTA, 20 mm ATP, 20 mm NAD, and 8 mm Met at a room-temperature pH of 7.7. Incubation of the 0.3-mL reaction mixture consisting of 0.1 mL of chloroplast suspension, 0.1 mL of suspension buffer, 5 μL of Mg-Proto in 80% acetone, 5 μL of Proto IX, and 95 μL of distilled water was carried out at 28°C for 1 h, and 1.7 mL of ice-cold 90% ammonical acetone was added to stop the reaction. Samples were centrifuged at 5000g for 2 min to remove the pellet and an equal amount of hexane was added to the supernatant. The top hexane layer was removed and the bottom layer was again extracted with one-third of the volume of hexane. The top hexane layer was discarded and the bottom HEAR was taken for an estimation of MPE by spectrofluorometry (Hukmani and Tripathy, 1992).
MPE Cyclase
Plastids isolated from control, chill-, and heat-stressed cucumber seedlings were suspended in suspension buffer consisting of 0.5 m Suc, 0.2 m Tris-HCl, 20 mm MgCl2, 2.5 mm EDTA, and 20 mm ATP (pH 7.7). The reaction mixture (0.3 mL) consisted of 0.1 mL of chloroplast suspension, 0.1 mL of suspension buffer, 0.5 mm S-adenosyl Met, 5 μL of Mg-Proto in 80% acetone, and 95 μL of distilled water. The pH of the reaction mixture was adjusted to 7.7 with 1 n NaOH. The incubation was carried out at room temperature (25°C) for 1 h in the dark and 1.7 mL of ice-cold 90% ammonical acetone was added to stop the reaction. The HEAR was prepared from the acetone extract and synthesis of Pchlide was estimated by spectrofluorometry.
Net Synthesis of Pchlide
Three-day-old etiolated cucumber seedlings grown at 25°C were transferred to 7, 42, and 25°C for 48 h in dark. To empty the endogenous Pchlide pool, seedlings were exposed to cool-white fluorescent light (30 μmol m−2 s−1) for 10 min at their respective temperatures, and 10 pairs of cotyledons were excised immediately after the light treatment, weighed, and homogenized in 90% ammonical acetone. After light exposure, seedlings were kept in the dark at their respective temperatures and cotyledons were harvested after 3, 6, and 12 h. On each data point three replicates of 10 pairs of cotyledons were hand homogenized in 20 mL of ice-cold 90% ammonical acetone at 4°C, and homogenate was centrifuged at 10,000 rpm (12,000g) for 10 min. Supernatant was taken for the HEAR preparation and accumulation of Pchlide was measured spectrofluorometrically. Dry weight was measured after keeping 100 mg of cotyledon tissue for 72 h in an oven maintained at 80°C, and data points were corrected for loss of moisture both at low and high temperatures. The net synthesis of Pchlide was calculated by deducting the Pchlide value recorded after 10 min of light exposure from those recorded at 3-, 6-, and 12-h dark intervals.
POR
Etiolated cucumber seedlings (3 d old) grown at 25°C were transferred to 7, 42, and 25°C for 48 h in dark, and 10 pairs of cotyledons were harvested, weighed, and homogenized in 90% ammonical acetone in the dark. Subsequently, seedlings were exposed to cool-white fluorescent light (30 μmol m−2 s−1) for 10 min and cotyledons were harvested, weighed, and homogenized in 90% ammonical acetone. Acetone tissue homogenates were centrifuged at 10,000 rpm in an SS-34 rotor (12,000g) for 10 min at 4°C, and from supernatant HEAR was prepared. Pchlide contents of cucumber cotyledons before and after light exposure were estimated from the HEAR fraction by spectrofluorometry (Rebeiz et al., 1975; Hukmani and Tripathy, 1992). Three replicates were taken for each data point. The percent phototransformation of Pchlide to Chlide in different temperatures was calculated as: ([(Pchlide content before phototransformation) − (Pchlide content after phototransformation)]/Pchlide content before phototransformation) × 100.
Enzymatic Activities in Wheat Seedlings
Etiolated wheat seedlings (6 d old) grown at 25°C were transferred to 7, 42, and 25°C for 24 h in the dark and enzymatic activities were monitored. Longer exposure (48 h) to high temperature (42°C) almost killed the wheat tissue. All of the protocols followed for wheat seedlings were identical to that of cucumber.
Spectrofluorometry
Fluorometric estimation of pigments was done using a photon-counting fluorometer (model 8000C, SLM, Rochester, NY). Channel A (sample) and channel C (reference) were adjusted to 20,000 counts s−1 using a tetraphenylene butadiene block as a standard and excited at 348 nm, and fluorescence emission was monitored at 422 nm. The HEAR samples were excited at 400, 420, and 440 nm and emission spectra were recorded in a ratio mode from 580 to 700 nm. Excitation and emission-slit widths were 4 nm. Emission spectra were corrected for photomultiplier response. Using appropriate equations the concentration of plant tetrapyrroles was quantified and values expressed as nanomoles per milligram of protein of per hour (Rebeiz et al., 1975; Hukmani and Tripathy, 1992).
Chl and Protein Estimation
Chl and protein ware estimated according to the methods of Porra et al. (1989) and Lowry et al. (1951), respectively.
Chemicals
All porphyrin intermediates were purchased from Porphyrin Products (Logan, UT). DTT, ALA, ATP, NAD, Benzoquinone, LA, and Hepes were purchased from Sigma. All other chemicals were purchased from Merck (Darmstadt, Germany), Sd Fine Chemicals (Mumbai, India), BDH (Poole, UK), and Qualigens (Mumbai, India).
RESULTS
Chl Content
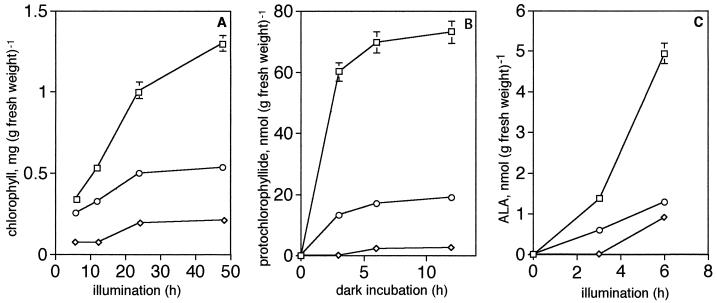
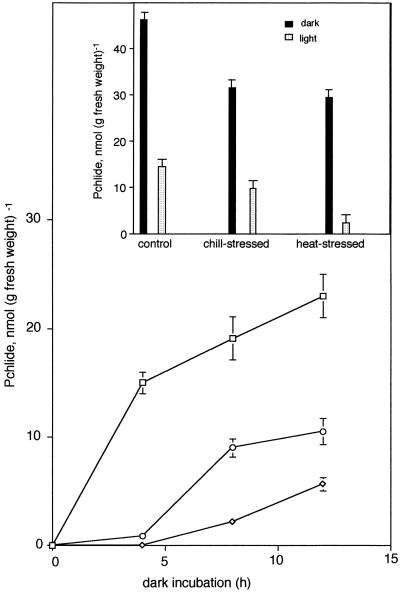
Four-day-old etiolated cucumber seedlings grown at 25°C were transferred to 7°C (chill stressed), 25°C (control), and 42°C (heat stressed) and exposed to cool-white fluorescent light (30 μmol m−2 s−1) for 6, 12, 24, and 48 h. The maximum amount of Chl synthesis was observed in control seedlings at 48 h of light exposure. Chl synthesis was inhibited by 60 and 90% in heat- and chill-stressed seedlings, respectively. An initial lag period of up to 12 h was observed in chill-stressed cucumber (Fig. 1A).
Figure 1.
Biosynthesis of Chl (A), Pchlide (B), and ALA (C) in control (25°C, □), chill-stressed (7°C, ⋄), and heat-stressed (42°C, ○) cucumber seedlings. Experimental details are as in Methods. Each data point is the mean of three replicates, error bars represent sd, and missing error bars indicate that they are smaller than the symbols.
Chl Biosynthetic Intermediates
To probe if inhibition of Chl biosynthesis in chill- and heat-stressed seedlings was due to reduced synthesis of tetrapyrrolic intermediates, etiolated cucumber seedlings grown at 25°C were kept for 48 h in chill- or heat-stressed conditions in the dark, and accumulation of Proto IX, MPE, and Pchlide was measured at low- or high-temperature regimes. Only the net synthesis of Pchlide could be monitored, since the amounts of MPE and Proto IX accumulated in cucumber cotyledons were negligible. In the control, the maximum amount of Pchlide was synthesized during the first 3 h (Fig. 1B). Net synthesis of Pchlide was near the maximum after 12 h of dark incubation. On the contrary, chill-stressed seedlings did not synthesize any Pchlide during the first 3 h and accumulated only small amounts of Pchlide after 12 h of dark incubation. Compared with control seedlings, Pchlide synthesis was inhibited by 90 and 70% in chill- and heat-stressed seedlings, respectively.
ALA Content
To study the mechanism of inhibition of Chl and Pchlide biosynthesis at different temperatures, the biosynthesis of ALA, the precursor of Chl, was monitored in chill- and heat-stressed conditions. ALA synthesis in the presence of LA was almost linear up to 6 h of illumination in control and heat-stressed seedlings. For the first 3 h ALA synthesis was completely inhibited in chill-stressed cucumber seedlings. As compared with the controls, the net synthesis of ALA was severely reduced by 78 and 70% in chill- and heat-stressed seedlings, respectively (Fig. 1C).
To understand in detail the mechanism of impairment of Chl synthesis and Pchlide biosynthesis in cucumber in chill- and heat-stressed conditions, the effects of low (7°C) and high (42°C) temperatures on different enzymes involved in Chl biosynthesis were studied.
ALAD
The ALAD that synthesizes PBG from two molecules of ALA was determined in etiolated cucumber seedlings kept at 7, 25, and 42°C for 48 h in the dark. As compared with the control, the enzyme activity decreased by 24 and 45% in chill- and heat-stressed seedlings, respectively (Fig. 2A).
Figure 2.
ALAD (A) and PBGD (B) activities measured in control (25°C) and chill (7°C)- and heat (42°C)-stressed cucumber seedlings. Experimental details are as in Methods. Each data point is the mean of three replicates and error bars represent sd.
PBGD
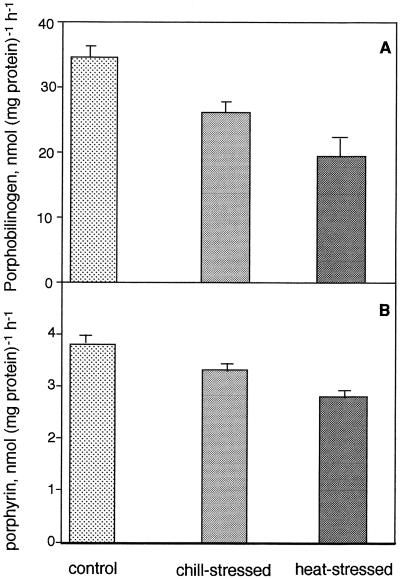
The next step in the Chl biosynthetic pathway is the conversion of PBG to urogen, which is catalyzed by PBGD. The enzyme activity was estimated by measuring the amount of porphyrin synthesis from PBG in cucumber seedlings kept at 7, 25, and 42°C for the last 48 h in the dark. Compared with the control, the enzyme activity was reduced by 13 and 28% in chill- and heat-stressed seedlings, respectively, suggesting that the PBGD activity was affected more in heat than in chill stress (Fig. 2B).
Proto-IX Synthesis from Urogen, Coprogen, and Protogen
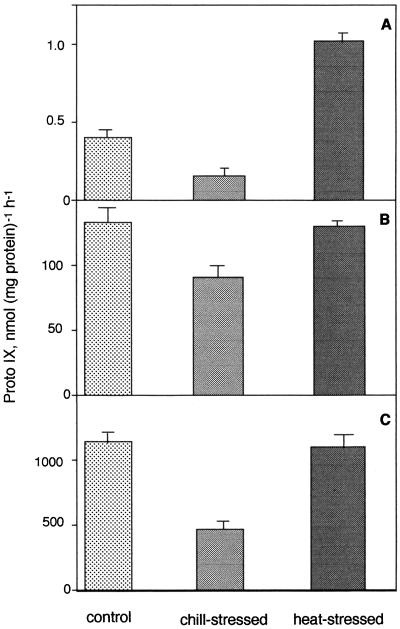
The subsequent steps in the Proto IX biosynthetic pathway are the conversion of urogen III to coprogen III mediated by UDC, the synthesis of protogen IX from coprogen III by coprogen oxidase, and finally the conversion of protogen to Proto IX by Protox. As compared with the controls, in chill-stressed seedlings Proto IX synthesis from urogen was severely reduced by 65%, whereas in heat-stressed seedlings the activity increased by 155% (Fig. 3A). Proto-IX synthesis from coprogen III was also reduced by 34% in chill-stressed seedlings. However, in heat-stressed seedlings, Proto IX synthesis from coprogen III was not affected (Fig. 3B). Protox activity was reduced by 60% in chill-stressed seedlings, whereas there was no significant change in enzyme activity in heat-stressed seedlings (Fig. 3C).
Figure 3.
UDC (A), coprogen oxidase (B), and Protox (C) activities measured in control (25°C) and chill (7°C)- and heat (42°C)-stressed cucumber seedlings. Experimental details are as in Methods. Each data point is the mean of three replicates and error bars represent sd.
Mg-Chelatase
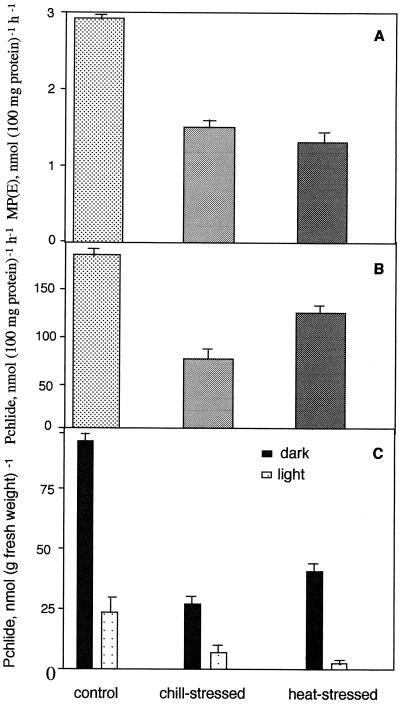
Mg-chelatase is the first enzyme in the Mg branch of the Chl biosynthetic pathway that inserts Mg into Proto IX to form Mg-Proto IX (Gorchein, 1972; Pardo et al., 1980; Jensen et al., 1996). In this experiment Proto IX was used as a substrate and Mg-chelatase activity was measured as the amount of Mg-Proto IX and its monoester synthesized in the plastids isolated from cucumber seedlings. As compared with the controls, the enzyme activity decreased by 60% in chill-stressed and by 65% in heat-stressed seedlings (Fig. 4A).
Figure 4.
Mg-chelatase (A), MPE cyclase (B), and POR (C) activities measured in control (25°C) and chill (7°C)- and heat (42°C)-stressed cucumber seedlings. Experimental details are as in Methods. Each data point is the mean of three replicates and error bars represent sd.
S-Adenosyl Met Methyl Transferase Plus MPE-Cyclase
To analyze the effect of temperature stress on Mg-Proto:S-adenosyl Met methyl transferase and MPE cyclase (Hinchigeri et al., 1981; Bollivar and Beale, 1996), plastids isolated from cucumber seedlings grown at different temperatures were incubated with Mg-Proto IX and S-adenosyl l-Met and synthesis of Pchlide was monitored. Pchlide synthesis was reduced by 60 and 36% in chill- and heat-stressed seedlings, respectively (Fig. 4B).
POR
POR mediates the light-catalyzed reduction of Pchlide to Chlide, which is a key regulatory step in the biosynthesis of Chl in oxygenic photosynthetic organisms (Griffiths, 1978; von Wettstein et al., 1995). Four-day-old-etiolated cucumber seedlings grown at 25°C were transferred to 7°C or 42°C, and were kept in the dark for 48 h. They were illuminated for 10 min in light to phototransform accumulated Pchlide to Chlide. The phototransformation of Pchlide to Chlide was 75% in control and chill-stressed seedlings and 95% in heat-stressed seedlings (Fig. 4C).
Effect of Temperature Stress on Certain Enzymes Involved in the Chl Biosynthetic Pathway in Wheat
To compare the effects of chill and heat stress on Chl biosynthesis in dicot and monocot seedlings, wheat seedlings were used and the activities of PBGD and POR were monitored.
PBG Deaminase Activity in Wheat
Six-day-old-etiolated wheat seedlings grown at 25°C were transferred to 7, 25, and 42°C for 24 h in the dark. PBGD activity in chill- and heat-stressed seedlings declined by 12 and 41%, respectively (data not shown). This demonstrated that both wheat and cucumber seedlings showed a similar pattern of reduction of PBGD activity in response to temperature stress.
Pchlide Synthesis in Wheat Seedlings Grown at Different Temperatures
The net synthesis of Pchlide in control wheat seedlings increased after 4 to 12 h of dark incubation. However, there was a lag in Pchlide synthesis for 4 h in chill- and heat-stressed seedlings after which the rate of Pchlide synthesis increased. As compared with control seedlings, in chill-stressed seedlings Pchlide accumulation was reduced by 80%, whereas in heat-stressed seedlings reduction was 65% (Fig. 5). Chill-stressed wheat seedlings had a higher accumulation of Pchlide after 12 h of dark incubation than that of cucumber. This suggested that Chl biosynthesis in wheat as compared with cucumber was relatively more resistant to chill stress.
Figure 5.
Pchlide synthesis and POR activity (inset) in control (25°C, □) and chill (7°C, ⋄)- and heat (42°C, ○)-stressed wheat seedlings. Experimental details are as in Methods. Each data point is the mean of three replicates, error bars represent sd, and missing error bars indicate that they are smaller than the symbols.
POR in Wheat
The percent phototransformation of Pchlide to Chlide was taken as an index of POR activity in wheat seedlings grown at different temperatures in the dark. Six-day-old etiolated wheat seedlings grown at 25°C were transferred to 7°C, 25°C, or 42°C and were kept in the dark for 24 h. They were illuminated for 10 min in light to phototransform accumulated Pchlide to Chlide. The phototransformation of Pchlide to Chlide was 70% in chill-stressed and control seedlings and 90% in heat-stressed wheat seedlings (Fig. 5, inset). POR activity had a similar response in heat-stressed cucumber seedlings.
DISCUSSION
Illumination of cucumber seedlings in chill- and heat-stress conditions resulted in inhibition of Chl biosynthesis by 90 and 60%, respectively (Fig. 1A), demonstrating that inhibition of Chl biosynthesis is higher in chill-stress than in heat-stress conditions. Reduced synthesis of ALA, the committed precursor of Chl, in chill- and heat-stressed seedlings (Fig. 1C) demonstrates that inhibition of Chl biosynthesis is partly due to impairment of ALA biosynthesis. ALA biosynthesis in cucumber was inhibited to a similar extent both in chill (78%)- and heat (70%)-stress conditions (Fig. 1C). However, as stated above, the inhibition of Chl biosynthesis under identical conditions in chill- and heat-stressed seedlings was 90 and 60%, respectively. To account for the discrepancy between the inhibition of ALA and Chl biosynthesis in chill- and heat-stress conditions, Pchlide synthesis was monitored. Compared with control seedlings, Pchlide synthesis was inhibited by 90 and 70% in chill- and heat-stressed seedlings, respectively (Fig. 1B), demonstrating that inhibition of Chl biosynthesis was mostly due to impairment of ALA and Pchlide biosynthesis.
Enzymes involved in Proto IX and Pchlide biosynthesis were differentially affected by chill and heat stress. The ALAD was reduced by 24 and 45% in chill- and heat-stressed seedlings (Fig. 2A). Higher inhibition of ALAD activity in the heat-stress condition may be due to impairment of the enzyme. Similar to ALAD, PBGD activity was also reduced more in heat (28%)- than in chill (13%)-stress conditions (Fig. 2B).
Heat stress did not impair Protox, although chill stress significantly inhibited its activity (Fig. 3). Heat stress also did not inhibit coprogen oxidase or UDC, rather, the latter was highly stimulated in high-temperature-grown seedlings. The differential inhibition pattern of Proto IX synthesis from urogen, coprogen, and protogen in chill stress suggested that both UDC and coprogen oxidase were inhibited in low-temperature-grown seedlings. These data demonstrated that Proto IX biosynthesis is relatively insensitive to heat stress as compared with chill stress.
Mg-chelatase and Mg-Proto:S-adenosyl Met methyl transferase plus MPE cyclase were inhibited by both chill and heat stress (Fig. 4, A and B). POR activity increased in heat-stress conditions by 46% over that of the control (Fig. 4C). Increase in the activity of POR in heat-stressed conditions may be due to increased synthesis of the enzyme and/or conversion of nonphototransformable Pchlide to the transformable form. POR activity is not affected in chill-stressed seedlings. This is contrary to the previous report (van Huystee and Hodgins, 1989) of inhibition of phototransformation of Pchlide to Chlide in chill-stressed conditions. This discrepancy may be due to a different methodology followed by the authors. They used exogenous substrate ALA to accumulate Pchlide and then phototransform Pchlide to Chlide by exposing plants to light. However, it is known that certain amounts of Pchlide synthesized from exogenous substrate ALA are nonphototransformable (Chakraborty and Tripathy, 1992).
As shown in Figure 1, the net synthesis of Pchlide was inhibited by 90% in chill stress and 70% in heat stress. Severe inhibition of Pchlide biosynthesis in chill-stressed seedlings is caused by a decline in Proto IX, Mg-Proto synthesis, and reduced conversion of Mg-Proto to Pchlide due to impairment of all of the enzymes involved in Proto IX synthesis and inhibition of Mg-chelatase and MPE cyclase activities. However, in heat-stressed seedlings, although ALAD and PBGD are partially inhibited, porphyrinogen-oxidizing enzymes were either stimulated (UDC) or unaffected (coprogen oxidase and Protox). Therefore, synthesis of Proto IX in heat-stressed seedlings is likely to be less affected than that in chill-stressed seedlings. Although heat-stressed seedlings had reduced activity of Mg-chelatase and S-adenosyl Met methyl transferase plus MPE cyclase, they accumulated higher amounts of Pchlide than chill-stressed seedlings, probably due to relatively higher synthesis of the substrate Proto IX. Taking into account the degree of inhibition of Pchlide biosynthesis in chill (90%) and heat (70%) stress, and stimulation of POR activity resulting in increased phototransformation of Pchlide to Chlide in heat stress, the calculated percent inhibition of Chl biosynthesis in chill (92%)- and heat (60%)-stressed seedlings matches well with actual inhibition of the amount of Chl measured in chill (90%)- and heat (60%)-stress conditions.
To compare the effects of growth temperature on Chl biosynthesis in dicot and monocot seedlings, the activities of a few enzymes of the Chl biosynthetic pathway were monitored in wheat seedlings and compared with that of cucumber seedlings under similar temperature-stress conditions.
In etiolated wheat seedlings, the PBGD activity was inhibited by 13% in chill-stress conditions and 42% in heat-stress conditions. This is similar to the pattern of inhibition of the PBGD reaction, observed in chill- and heat-stressed cucumber seedlings. Pchlide synthesis was reduced by 80% in chill-stressed seedlings, whereas in heat-stressed seedlings the reduction was only 60% (Fig. 5). A similar pattern of POR activity was observed in chill- and heat-stressed wheat seedlings as that in cucumber seedlings. These results suggest that temperature stress has a broadly similar effect on Chl biosynthetic enzymes in cucumber (dicot) and wheat (monocot).
Inhibition or stimulation of different enzymes involved in porphyrin biosynthesis in chill- or heat-stressed seedlings may be due to impairment or posttranslational modifications of the enzymes in vivo. Temperature stress might have caused reduced or increased synthesis of the enzymes or corresponding mRNA. Further investigations are needed to ascertain the mechanism of inhibition or stimulation of enzymes in chill- and heat-stressed seedlings.
Abbreviations:
- ALA
5-aminolevulinic acid
- ALAD
5-aminolevulinic acid dehydratase
- Chl
chlorophyll
- Chlide
chlorophyllide
- coprogen
coproporphyrinogen
- HEAR
hexane extracted acetone residue solvent mixture
- LA
levulinic acid
- MPE
Mg-protoporphyrin monoester
- PBG
porphobilinogen
- PBGD
porphobilinogen deaminase
- Pchlide
protochlorophyllide
- protogen
protoporphyrinogen
- POR
Pchlide oxidoreductase
- Proto IX
protoporphyrin IX
- Protox
protogen oxidase
- UDC
urogen decarboxylase
- urogen
uroporphyrinogen
Footnotes
This work was supported by the Council of Scientific and Industrial Research (grant no. 38-922/97/EMR-II) and the Department of Science and Technology, Government of India (grant no. DST/SP/SO/A 49-95 to B.C.T.).
LITERATURE CITED
- Bollivar DW, Beale SI. The chlorophyll biosynthetic enzyme Mg-protoporphyrin IX monomethyl ester (oxidative) cyclase. Plant Physiol. 1996;112:105–114. doi: 10.1104/pp.112.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Tripathy BC. Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiol. 1992;98:7–11. doi: 10.1104/pp.98.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J. Capacity for chlorophyll synthesis in heat-bleached 70S ribosome-deficient rye leaves. Planta. 1977;135:83–88. doi: 10.1007/BF00387980. [DOI] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides: studies with whole cells. Biochem J. 1972;127:97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths WT. Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem J. 1978;174:681–692. doi: 10.1042/bj1740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E, Klein S. Light dependent formation of 5-aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972;49:364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Hess WR, Blank-Huber M, Fieder B, Borner T, Rudiger W. Chlorophyll synthetase and chloroplast tRNAglu are present in heat-bleached, ribosome-deficient plastids. J Plant Physiol. 1992;139:427–430. [Google Scholar]
- Hinchigeri SB, Chan JCS, Richards WR. Purification of S-adenosyl l-methionine magnesium protoporphyrin methyltransferase by affinity chromatography. Photosynthetica. 1981;15:351–359. [Google Scholar]
- Hodgins R, van Huystee RB. Porphyrin metabolism in chill stressed maize (Zea mays L.) J Plant Physiol. 1986a;125:325–336. [Google Scholar]
- Hodgins RR, van Huystee RB. Delta-aminolevulinic acid metabolism in chill-stressed maize (Zea mays L.) J Plant Physiol. 1986b;126:257–268. [Google Scholar]
- Hukmani P, Tripathy BC. Spectrofluorometric estimation of intermediates of chlorophyll biosynthesis: protoporphyrin IX, Mg-protoporphyrin, and protochlorophyllide. Anal Biochem. 1992;206:125–130. doi: 10.1016/s0003-2697(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Hukmani P, Tripathy BC. Chlorophyll biosynthetic reactions during senescence of excised barley (Hordeum vulgare L. cv IB 65) leaves. Plant Physiol. 1994;105:1295–1300. doi: 10.1104/pp.105.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NJ, Jacobs JM. Assay for enzymatic protoporphyrinogen oxidation, a late step in heme synthesis. Enzyme. 1982;28:862–866. doi: 10.1159/000459103. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Willows RD, Petersen BL, Vothknecht UC, Stummann BM, Kannangara GC, von Wettstein D, Henningsen KW. Structural genes for Mg-chelatase subunits in barley: xantha-F, -G and -H. Mol Genet. 1996;250:383–394. doi: 10.1007/BF02174026. [DOI] [PubMed] [Google Scholar]
- Leeper FJ. Intermediate steps in biosynthesis of chlorophylls. In: Scheer H, editor. Chlorophylls. Boca Raton, FL: CRC Press; 1991. pp. 407–431. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Pardo AD, Chereskin BM, Castelfranco PA, Franceschi VR, Wezelman BE. ATP requirement for Mg-chelatase in developing chloroplasts. Plant Physiol. 1980;65:956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ. Recent progress in porphyrin and chlorophyll biosynthesis. Photochem Photobiol. 1997;65:492–516. [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Poulson R, Polglasse WJ. Aerobic and anaerobic coproporphyrinogen oxidase activities in extracts from Saccharomyces cerevisiae. J Biol Chem. 1974;249:6367–6371. [PubMed] [Google Scholar]
- Rebeiz CA, Matheis JR, Smith BB, Rebeiz CC, Dayton DF. Chloroplast biogenesis: biosynthesis and accumulation of Mg-protoporphyrin IX monoester and longer wavelength metalloporphyrins by greening cotyledons. Arch Biochem Biophys. 1975;166:446–465. doi: 10.1016/0003-9861(75)90408-7. [DOI] [PubMed] [Google Scholar]
- Richards WR. Biosynthesis of chlorophyll chromophores of pigmented thylakoid proteins. In: Sundqvist C, Ryberg M, editors. Pigment-Protein Complexes in Plastids: Synthesis and Assembly. San Diego, CA: Academic Press; 1992. pp. 91–178. [Google Scholar]
- Shemin D. 5-Aminolevulinic acid dehydratase from Rhodopseudomonas sphaeroides. Methods Enzymol. 1962;5:883–884. [Google Scholar]
- Thomas EJ, Ortiz W. Loss of chloroplast transcripts for proteins associated with PSII: an early event during heat-bleaching in Euglena gracilis. Plant Mol Biol. 1995;27:317–325. doi: 10.1007/BF00020186. [DOI] [PubMed] [Google Scholar]
- Tripathy BC, Rebeiz CA. Chloroplast biogenesis: demonstration of monovinyl and divinyl monocarboxylic routes of chlorophyll biosynthesis in higher plants. J Biol Chem. 1986;261:13556–13564. [PubMed] [Google Scholar]
- Tripathy BC, Rebeiz CA (1987) Non-equivalence of glutamic and 5-aminolevulinic acids as substrates for protochlorophyllide and chlorophyll biosynthesis in darkness. In J Biggins, ed, Progress in Photosynthesis Research, Vol 4. Nijhoff Publishers, Boston, MA, pp 439–443
- Tripathy BC, Rebeiz CA. Chloroplast biogenesis 601: conversion of divinyl protochlorophyllide to monovinyl protochlorophyllide in green(ing) barley, a dark monovinyl/light divinyl plant species. Plant Physiol. 1988;87:89–94. doi: 10.1104/pp.87.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt PR, Strikwerda JT. Pigment degradation in discs of the thermophilic Cucumis sativus as affected by light, temperature, sugar application and inhibitors. Plant Physiol. 1976;37:253–257. [Google Scholar]
- Van Huystee RB, Hodgins RR. Chlorophyll synthesis from protochlorophyll (ide) in chill-stressed maize (Zea mays L.) J Exp Bot. 1989;40:431–435. [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7: 1039–1057 [DOI] [PMC free article] [PubMed]