Background: Amino acids and cell Ca2+ are potent regulators of autophagy that are thought to act independently of each other.
Results: Withdrawal of essential amino acids increases cytosolic Ca2+ and subsequently activates autophagy via a CaMKK-β-AMPK pathway to ULK1 and mTORC1.
Conclusion: A Ca2+-dependent pathway regulates autophagy under amino acid starvation.
Significance: This new pathway would contribute to better understand autophagy regulation.
Keywords: Amino acid, Autophagy, Calcium, Cellular Regulation, Protein Degradation, AMPK, CAMKK-β, ULK1, mTORC1
Abstract
Autophagy is the main lysosomal catabolic process that becomes activated under stress conditions, such as amino acid starvation and cytosolic Ca2+ upload. However, the molecular details on how both conditions control autophagy are still not fully understood. Here we link essential amino acid starvation and Ca2+ in a signaling pathway to activate autophagy. We show that withdrawal of essential amino acids leads to an increase in cytosolic Ca2+, arising from both extracellular medium and intracellular stores, which induces the activation of adenosine monophosphate-activated protein kinase (AMPK) via Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). Furthermore, we show that autophagy induced by amino acid starvation requires AMPK, as this induction is attenuated in its absence. Subsequently, AMPK activates UNC-51-like kinase (ULK1), a mammalian autophagy-initiating kinase, through phosphorylation at Ser-555 in a process that requires CaMKK-β. Finally, the mammalian target of rapamycin complex C1 (mTORC1), a negative regulator of autophagy downstream of AMPK, is inhibited by amino acid starvation in a Ca2+-sensitive manner, and CaMKK-β appears to be important for mTORC1 inactivation, especially in the absence of extracellular Ca2+. All these results highlight that amino acid starvation regulates autophagy in part through an increase in cellular Ca2+ that activates a CaMKK-β-AMPK pathway and inhibits mTORC1, which results in ULK1 stimulation.
Introduction
Cellular homeostasis is maintained by the proper balance between the biosynthesis and the degradation of macromolecules and organelles. Macroautophagy, hereafter simply called autophagy, is a main catabolic process for the clearance of intracellular components in lysosomes (1). Under full nutrient conditions, autophagy remains at low basal levels in most cells, whereas under starvation and other stress conditions it rises to play a main role in cell survival by delivering nutrients and damaged cell components to lysosomes (2, 3). The most important negative regulators of autophagy are hormonal (e.g. insulin) and nutritional (e.g. amino acids) (1, 4). The serine-threonine kinase mammalian target of rapamycin complex C1 (mTORC1)3 is to date the best known sensor for the availability of energy and nutrients. For example, it is negatively and positively regulated, respectively, by the adenosine monophosphate-activated protein kinase (AMPK) and the insulin signaling pathways. AMPK is activated by various kinases, including the Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β) (5), and its inhibitory effect on mTORC1 occurs via phosphorylation of TSC2 in the tuberous sclerosis complex TSC1/2, which has GTPase-activating protein activity toward its substrate, the Ras-family GTP-binding protein Rheb (6). Even though the involvement of AMPK in the inhibition of mTORC1 is now well established, its direct role inducing autophagy has been only recently described by reporting its involvement in the phosphorylation of ULK1 (UNC-51-like kinase), a mammalian ortholog of the yeast protein kinase Atg1 that is required to initiate autophagy (7). In the presence of nutrients, mTORC1 has also the ability to prevent ULK1 activation by phosphorylating this protein at a residue that is different from those phosphorylated by AMPK, disrupting in this way the interaction between ULK1 and AMPK (8).
Ca2+ ion is a major intracellular second messenger regulating many physiological functions in the cells, such as secretion, contraction, metabolism, gene transcription, death, etc., and is also involved in some pathological processes (9, 10). Although the spatial and temporal distribution of Ca2+ in the cytosol, mitochondria, endoplasmic reticulum (ER), and nucleus determines one of the most commonly recognized and well studied intracellular signals (11, 12), its role in autophagy is hitherto poorly understood. A pioneering study demonstrated the importance of Ca2+ storage within intracellular compartments, rather than cytosolic Ca2+, for autophagy stimulation (13). However, another report provided evidence that, at least under certain conditions, autophagy is inhibited when cytosolic Ca2+ increases (14), whereas others reported that rises in cytosolic Ca2+ stimulate autophagy (5). It is believed that the positive effects of Ca2+ on autophagy occur via activation of AMPK and are mTORC1-dependent (5), whereas the inhibition of autophagy by Ca2+ does not require AMPK and is independent of mTORC1 (14). Thus, apparently conflicting results exist concerning the role of cytosolic Ca2+ and intracellular stores of Ca2+ in autophagy.
We have recently found that three Ca2+ binding proteins, copine 1, annexin A1, and annexin A5, translocate under essential amino acid starvation to lysosomal membranes. This translocation occurs in a Ca2+-dependent manner, at least for annexin A5, which was also shown to stimulate autophagy (15). This led us to consider a possible link between autophagy induction under this condition and intracellular Ca2+. Thus, we investigated in this work the dependence on cellular Ca2+ of autophagy induced by essential amino acid starvation. We demonstrate for the first time that withdrawal of amino acids provokes an increase in cytosolic Ca2+ that originates from both extracellular and, to a larger extent, intracellular stores. We also describe a pathway by which amino acid starvation could activate autophagy through Ca2+ signaling.
EXPERIMENTAL PROCEDURES
Materials
Minimum essential medium, Dulbecco's modified Eagle's medium (DMEM), human insulin, 3-methyladenine, EGTA (ethylene glycol tetraacetic acid), ionomycin, insulin, and NH4Cl were purchased from Sigma. Minimum essential medium, amino acids 50×, fetal bovine serum (FBS), fura-2AM, and fluo-3AM were supplied by Molecular Probes, Invitrogen. Leupeptin was from Peptide Institute, Inc., and rapamycin was from Calbiochem. The following antibodies were used: anti-microtubule-associated protein1 light chain 3 (anti-LC3) from Nanotools, anti-CaMKK-β and anti-CaMKK-α from Santa Cruz Biotechnology, anti-AMPK/P-AMPK (Thr-172), anti-p70S6K/P-p70S6K (Thr-389), anti-ULK1/P-ULK1 (Ser-555)/P-ULK (Ser-757), and anti-mTOR from Cell Signaling, and anti-β-actin and horseradish peroxidase-labeled secondary antibodies from Sigma. Radioisotopes were obtained from Amersham Biosciences. The CaMKK-α/β inhibitor STO-609 and BAPTA-AM (1,2-bis(2-aminophenoxy) ethane-N,N,N,N-tetraacetic acid tetrakis(acetoxymethyl ester)) were from Tocris Bioscience. Other reagents, purchased from Sigma, Invitrogen, or Calbiochem, were of analytical grade.
Cell Culture, Treatments, and Transfections
NIH3T3 mouse embryonic fibroblasts and HeLa cells, obtained from the European Collection of Animal Cell Cultures, were grown at 37 °C in a humidified atmosphere of 5% (v/v) CO2, air in DMEM or minimum essential medium with 10% heat-inactivated FBS and 1% penicillin and streptomycin. Normal human skin fibroblasts 3349B were obtained from the Coriell Institute for Medical Research (Camden, NJ) and were grown as described (4). Mouse embryonic fibroblasts (MEFs) double knock-out (KO) for AMPKα1/2 subunits (α1−/− and α2−/−) and wild type controls, kindly provided by Dr. B. Viollet (Institut Cochin, INSERM U1016, CNRS UMR 8104, Université Paris Descartes, Dept. of Endocrinology, Metabolism and Cancer, 24 rue du Faubourg Saint Jacques, 75014 Paris), were grown as described previously (16). Krebs-Henseleit medium (KH, 18.4 mm NaCl, 4.75 mm KCl, 1.19 mm KH2PO4, 2.54 mm MgSO4, 2.44 mm CaCl2·2H2O, 28.6 mm NaHCO3, 20 mm glucose) with 10 mm Hepes, pH 7.4, was used for high proteolysis (starvation) conditions. For low proteolysis conditions, insulin (0.1 μm), and essential amino acids (at two times the concentration present in the growth media (4)) were added to KH. Incubations without extracellular Ca2+ were carried out in the same KH but without CaCl2 and supplemented with 100 μm EGTA.
For RNAi-mediated inhibition of the expression of CaMKK-β, CaMKK-α, and AMPK genes, cells were transfected 72 h before analysis with small interfering RNAs (siRNAs) using X-tremeGENE siRNA Transfection Reagent (Roche Applied Science) according to the manufacturer's instructions. The siRNAs that target mouse CaMKK-β mRNA and the negative controls were purchased from Ambion Inc., whereas those that target AMPK were purchased from Santa Cruz Biotechnology. All of them were tested and used at a final concentration of 15 nm.
Measurements of Intracellular Ca2+
For cytosolic Ca2+ imaging, cells were loaded with 5 μm fura-2AM for 30 min at 37 °C in KH. Fura-2AM fluorescence was measured at 340 and 380 nm excitation, and emission was detected with a 520 emission filter at intervals of 5 s. Analysis was done using Hamamatsu or MetaMorph/MetaFluor Analyst (Universal Imaging Corp., Downingtown, PA). Assays were carried out on a Zeiss Axiovert 200 inverted microscope equipped either with cooled CCD digital cameras or as part of a Zeiss LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
For Ca2+ measurements by flow cytometry, cells were loaded with 5 μm fluo-3AM for 30 min, detached with trypsin-EDTA, washed once, and resuspended (106 cells/ml) in the appropriate medium. In each experiment, 10,000 cells were analyzed by fluorescence-activated cell sorting (FACS) using a 488 ± 20-nm band-pass filter and a Cytomics FC 500 flow cytometer (Beckman Coulter).
Measurement of Intracellular Protein Degradation
NIH3T3 cells were incubated for 48 h in fresh full medium with 2 μCi/ml [3H]valine followed by a 24-h chase in fresh full medium containing 10 mm l-valine to degrade short-lived proteins (17). Then, degradation of long-lived proteins was measured for 4 h with the indicated treatments. Total and autophagic protein degradation were calculated as previously described (17).
General Procedures
The immunoblotting procedures were carried out as before (17). Phospho-specific antibodies were always used in the first round. After treating the membranes with stripping buffer (0.25 m glycine, pH 2.3), they were probed using the antibodies that recognize the total amount of the protein of interest. Detection of low signals was carried out with the Amersham Biosciences ECL Prime Western blotting Detection Reagent. Comparisons between different conditions, after calculating mean and S.D. values were by Student's t test. p values were considered significant at p < 0.0005 (***), p < 0.005 (**), and p < 0.05 (*).
RESULTS
Withdrawal of Amino Acids Increases Intracellular Ca2+ Levels
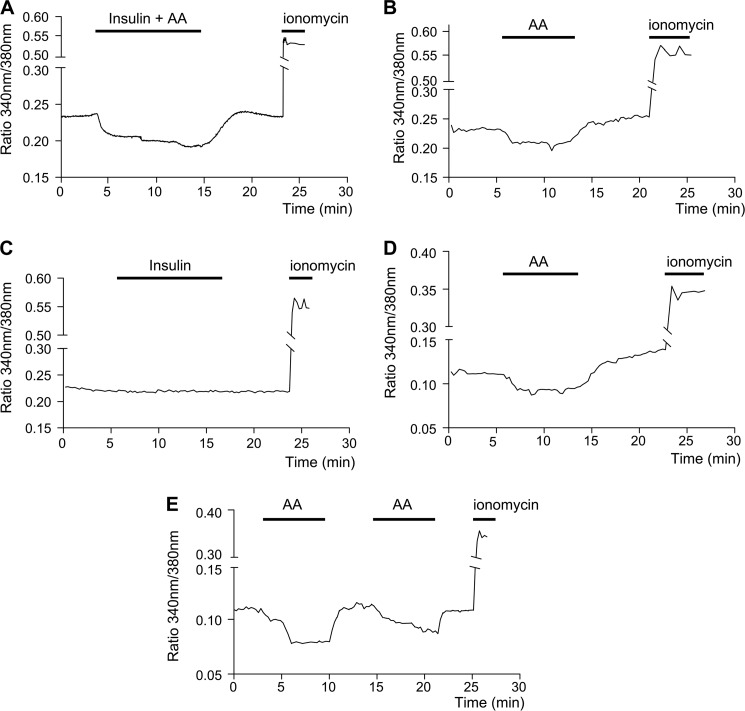
To test whether cytosolic Ca2+ levels ([Ca2+]c) vary under conditions that produce high (KH alone) and low (KH plus insulin and amino acids) proteolysis, we measured their kinetic changes under these conditions in fura-2AM-loaded NIH3T3 cells. We found that in the cells incubated with KH, [Ca2+]c are significantly higher without insulin and amino acids than in the presence of both proteolytic regulators (Fig. 1A and Table 1). The KH-induced [Ca2+]c increase is characterized by a rapid initial rise that quickly becomes stabilized. We also measured, separately, the effects of insulin and amino acids; amino acids induce a decrease in [Ca2+]c, whereas insulin does not provoke any change (Fig. 1, B and C, and Table 1).
FIGURE 1.
Withdrawal of amino acids raises cytosolic Ca2+, derived from both extracellular and intracellular stores. For Ca2+ measurements, fura-2AM-loaded NIH3T3 cells were imaged as described under “Experimental Procedures.” The mean values from ≥40 cells from eight independent experiments are shown. The [Ca2+]c are compared in cells incubated as indicated in KH with and without amino acids (AA) and 0.1 μm insulin either together (A) or separately (B and C, respectively). In D the effect of amino acids on [Ca2+]c was measured in cells incubated in KH without CaCl2 and containing 100 μm EGTA. Panel E shows a representative experiment where a second round of amino acid addition and withdrawal was carried out in cells incubated as in D. To assess the viability of the cells, ionomycin (1 μm) was used as a positive control in each experiment.
TABLE 1.
Effects of insulin and/or amino acids on [Ca2+]c
Fura-2AM-loaded NIH3T3 cells were incubated in KH with and without the indicated stimuli as in Fig.1, A–D. Changes in [Ca2+]c recording (ratio 340 nm/380 nm) were measured before (2.5 min), during (10 min), and at the end of the stimulus (20 min). The effects of stimulus addition and withdrawal were calculated from these data as indicated in the corresponding column, and the differences in the values at these times were found to be statistically significant at p < 0.0005 (***). NS, no significant difference). n corresponds to the number of analyzed cells from different cultures with a positive response to ionomycin.
| Stimulus | Ratio 340 nm/380 nm |
n | ||||
|---|---|---|---|---|---|---|
| 2.5 min | 10 min | 20 min | Addition (10–2.5 min) | Withdrawal (20–10 min) | ||
| AA | 0.240 ± 0.010 | 0.208 ± 0.007 | 0.252 ± 0.009 | −0.032 ± 0.003*** | 0.044 ± 0.002*** | 363 |
| Insulin | 0.228 ± 0.005 | 0.227 ± 0.004 | 0.226 ± 0.005 | −0.001 ± 0.001 NS | 0.001 ± 0.001 NS | 349 |
| AA + Insulin | 0.231 ± 0.009 | 0.198 ± 0.008 | 0.241 ± 0.006 | −0.033 ± 0.001*** | 0.043 ± 0.002*** | 352 |
| AAa | 0.121 ± 0.004 | 0.089 ± 0.005 | 0.125 ± 0.004 | −0.032 ± 0.001*** | 0.036 ± 0.001*** | 365 |
a Cells incubated in KH as above, but without CaCl2 and supplemented with 100 μm EGTA.
To examine whether the increase in [Ca2+]c observed under high proteolysis conditions (KH alone) was due to extracellular Ca2+ influx or to release of Ca2+ from an intracellular store, cells were incubated in KH without extracellular Ca2+ and containing the cell-impermeant Ca2+ chelator EGTA. As shown in Fig. 1D, the addition or withdrawal of amino acids from a Ca2+-free medium produces changes in [Ca2+]c similar to those observed in the presence of extracellular Ca2+ (see Fig. 1A and Table 1) but that occur to a lower extent. The same effects were observed with and without extracellular Ca2+ in HeLa cells (supplemental Fig. S1, A and B) and in 3349B human fibroblasts (supplemental Fig. S1, C and D). Moreover, a second round of amino acid withdrawal still produces an increase in [Ca2+]c (Fig. 1E and supplemental Fig. S1, A and B).
These data indicate that although the observed rise in [Ca2+]c when the cells are incubated without essential amino acids depends in part on Ca2+ entry from the extracellular medium, this increase mainly comes from intracellular Ca2+ stores.
Withdrawal of Amino Acids Activates AMPK in a CaMKK-β-dependent Way
Amino acid removal activates autophagy by complex mechanisms still not fully understood (1, 18). In addition, it is also known that both extracellular- and ER-derived Ca2+ induce autophagy through the activation of AMPK by Ca2+/CaMKK (5). Therefore, we asked if this signaling pathway was also implicated in the observed effects of amino acid starvation on Ca2+ levels or, in other words, if both groups of separate observations could be related.
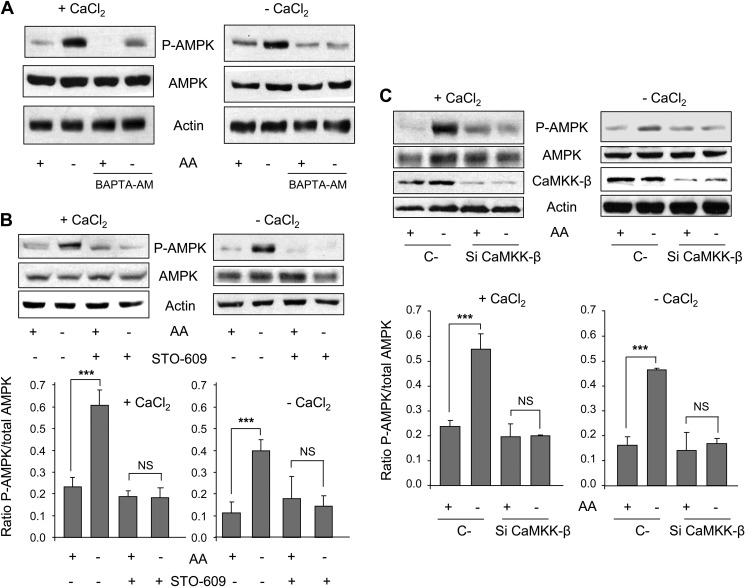
First, we determined in our experimental system whether or not amino acid starvation activates AMPK, analyzing its Thr-172 phosphorylation. Under amino acid starvation, phosphorylation of AMPK is slightly lower in the absence (−CaCl2) of extracellular Ca2+ than in its presence (+CaCl2) (supplemental Fig. S2, B and C). This is in agreement with the observed effects of amino acid starvation on [Ca2+]c in cells incubated in a Ca2+-free medium (Fig. 1D) and also with previous reports showing that Ca2+ plays a crucial role in the activation of this kinase (5, 19). Furthermore, withdrawal of amino acids with and without extracellular Ca2+ activates AMPK (Fig. 2A, see the first and second lanes in +CaCl2 and −CaCl2). To analyze the possibility of a Ca2+-dependent activation of AMPK by withdrawal of amino acids, we used the intracellular Ca2+ chelator BAPTA-AM. Chelation of intracellular Ca2+ by BAPTA-AM diminishes this activation in the presence of extracellular Ca2+ and eliminates it in its absence, indicating a Ca2+-dependent activation of AMPK by amino acid starvation (Fig. 2A). Activation of AMPK can be mainly accomplished via its phosphorylation by the tumor suppressor kinase LKB1 (when energy levels are low) and by CaMKK-β and to a lesser extent by CaMKK-α (in response to an increase in [Ca2+]c) (19, 20). As demonstrated above, withdrawal of amino acids increases [Ca2+]c. Thus, we speculated that this effect could activate AMPK in NIH3T3 cells through CaMKK-α/β. To test this hypothesis, we first treated the cells with a CaMKK-α/β inhibitor (STO-609) (21). As shown in Fig. 2B (see the two last bands in +CaCl2 and −CaCl2), STO-609 abolishes the activation of AMPK produced in the absence of amino acids and with and without extracellular Ca2+. To further confirm these effects, we carried out the same experiments but now using CaMKK-β-specific siRNAs. The observed AMPK phosphorylations in CaMKK-β-silenced cells (Fig. 2C) are fully consistent with the results obtained with STO-609 (Fig. 2B). To distinguish between the involvement of CaMKK-β and CaMKK-α in this pathway, we compared the silencing effect of each one. A possible higher expression of one CaMKK in response to the knockdown of the other is ruled out in supplemental Fig. S2A. In general, CaMKK-α knockdown produces much less effect than that of CaMKK-β (supplemental Fig. S2, B and C). In contrast to CaMKK-β, silencing of CaMKK-α does not fully abolish the activation of AMPK produced by amino acid starvation. Under CaMKK-α silencing, this activation persists in the absence of extracellular Ca2+ and is abrogated in its presence. This suggests that the activation of AMPK under amino acid starvation by CaMKK-α depends on extracellular Ca2+ and not on Ca2+ derived from intracellular stores. Taken together, these results show that in NIH3T3 cells, withdrawal of amino acids activates AMPK by a signaling pathway involving Ca2+ and the CaMKKs, mainly the β isoform and to a lesser extent the α.
FIGURE 2.
Withdrawal of amino acids activates AMPK phosphorylation in a CaMKK-β-dependent way. A, NIH3T3 cells were grown for 2 h in KH and AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2) in the presence or absence of 10 μm BAPTA-AM. In the last 30 min of incubation, AA were removed or not, as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-AMPK, anti-P-AMPK (Thr-172) and, as a loading control, anti-actin antibodies. B, NIH3T3 cells were grown for 60 min in KH and AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2) in the presence or absence of 25 μm STO-609. In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using the same antibodies as in A. NS, not significant. C, NIH3T3 cells were transfected with CaMKK-β (Si CaMKK-β) or with negative control (C-) siRNAs. After 72 h, cells were incubated as above and analyzed by immunoblot using the same antibodies as in A plus anti-CaMKK-β. Densitometric measurements of the ratio of P-AMPK to total AMPK from four independent experiments in each case are shown below in B and C. Differences were found to be statistically significant at p < 0.0005 (***). NS, no significant difference.
AMPK Is Involved in the Induction of Autophagy by Amino Acid Starvation
To elucidate a possible role of AMPK in the inhibition of autophagy by amino acids, we took advantage of the availability of AMPK KO MEFs (see “Experimental Procedures”). Autophagy was first assessed by the levels of LC3-II, the processed and lipidated form of LC3-I that is recruited to autophagosomes (22). As LC3-II is degraded in autolysosomes, a well established procedure to investigate autophagosome formation is based on the inhibition with various agents of lysosomal proteases and/or of the fusion process between lysosomes/endosomes and autophagosomes (23). Under these conditions, the levels of LC3-II are indicative of the formation rate of autophagosomes i.e. the autophagic flux (2, 24, 25). Here, we used leupeptin and ammonium chloride (17, 26, 27), but similar results were obtained with 500 nm bafilomycin A (data not shown). Because LC3-I is reported to be less stable and less immunoreactive than LC3-II (28), we used the LC3-II/actin ratio to assess autophagy (28, 29). Fig. 3A shows that in WT (wild type) MEFs under amino acid starvation, LC3-II levels increase 3 times in the presence of extracellular Ca2+ and slightly less (2.7 times) in its absence. These values are reduced in AMPK KO MEFs to 2.3 (23% reduction) and 1.4 (48% reduction) times, respectively. These data suggest that AMPK is involved in the activation of autophagy by amino acid starvation, especially in the absence of extracellular Ca2+. To further confirm these results, we carried out pulse-chase experiments to analyze the degradation of long-lived proteins by autophagy using its inhibitor 3-methyladenine (17, 25). Similar rates of autophagic degradation under amino acid starvation were observed in AMPK WT and KO cells, possibly because of mechanisms that compensate for the lack of AMPK to assure the survival of the cells under this stress condition. Results show that the inhibitory effect of amino acids on autophagy is greater in WT than in KO cells (Fig. 3B), confirming a requirement of AMPK in the regulation of autophagy by amino acids. Thus, in WT MEFs, in the presence and absence of extracellular Ca2+, withdrawal of amino acids increases autophagy 11.3 and 9.1 times, respectively, whereas in AMPK KO MEFs, these increases are only 2.7 and 2.3 times. Moreover, and consistent with the findings that Ca2+ induces autophagy in an AMPK-dependent way (5), only WT but not AMPK KO MEFs show a moderate increase in the rate of autophagic degradation when extracellular Ca2+ is present (Fig. 3B, compare the fourth and eighth columns in the histogram).
FIGURE 3.
Autophagy induced by amino acid starvation is AMPK-dependent. A, AMPK KO and WT MEFs were incubated for 4 h in KH supplemented with AA plus 100 μm leupeptin and 20 mm NH4Cl and in the presence (+) or not (−) of extracellular CaCl2 (in the latter case, supplemented with 100 μm EGTA). In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-LC3 (-I and -II), anti-AMPK and, as a loading control, anti-actin antibodies. Densitometric measurements from six independent experiments are shown on the right. LC3-II bands were normalized to the corresponding actin bands. B, exponentially growing AMPK KO and WT MEFs were metabolically labeled with [3H]valine for 48 h, chased for 24 h, and then switched to KH with or without CaCl2 (in the last case, supplemented with 100 μm EGTA) and containing or not AA for 4 h. Data are from four independent experiments with duplicated samples and are expressed as the percentage of the labeled proteins that are degraded per hour by autophagy in the different conditions. C, NIH3T3 cells were transfected with AMPK (Si AMPK) or with negative control (C-) siRNAs. After 72 h cells were incubated as above with or without 100 μm leupeptin (Leup) and 20 mm NH4Cl and analyzed by immunoblot using the same antibodies as in A. Densitometric measurements from two different blots are shown below. In A–C, stars indicate differences that were found to be statistically significant at p < 0.0005 (***) and p < 0.05 (*). D, AMPK KO and WT MEFs (upper panels) and NIH3T3 cells previously transfected with AMPK (Si AMPK) or with negative control (C-) siRNAs (lower panels) were grown for 60 min in KH plus AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2). In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-mTOR, anti-p70S6K, anti-P-p70S6K (Thr-389) and, as a loading control, anti-actin antibodies. In the lower panel, low and high exposure (exp.) blots are shown.
Next we tested whether AMPK silencing by specific siRNAs has similar effects on autophagy induction by amino acid starvation. We found that AMPK down-regulation considerably decreases the levels of LC3-II in the absence and particularly in the presence of inhibitors of lysosomal proteases (Fig. 3C), in agreement with its well established role in the activation of autophagy (7, 8). Furthermore, and in accordance with the previous results, the induction of autophagy by withdrawal of amino acids is attenuated under AMPK silencing both in the presence (2.2 times in C-siRNA-transfected cells versus 1.6 times in silenced cells) and in the absence (1.8 times in C-siRNA transfected cells versus 1.1 times in silenced cells) of extracellular Ca2+ (Fig. 3C).
Although results were qualitatively similar in AMPK-silenced cells and AMPK KO MEFs, the autophagic responses were milder in the latter. In this regard AMPK is known to inhibit the activity of mTORC1, a complex with a well known inhibitory function in autophagy. Therefore, we compared the effects of AMPK silencing and KO on mTORC1 activity, measured by the Thr-389 phosphorylation of the ribosomal protein S6 kinase 1 (p70S6K) (30). The results show that with or without extracellular Ca2+ and in contrast to AMPK-silenced cells, mTORC1 activity is almost abolished in AMPK KO MEFs (Fig. 3D). This could explain the lower autophagy response in these cells. Fig. 3D also shows that these differences are not due to variations of mTORC1 levels. Therefore, AMPK KO MEFs have apparently developed a compensatory mechanism for the permanent lack of AMPK based on a decrease in mTORC1 activity. This assures a high level of autophagy for the survival of the cells under stress conditions, such as amino acid starvation. In summary, all these data show that withdrawal of amino acids with or without extracellular Ca2+ induces autophagy in an AMPK-dependent way.
Withdrawal of Amino Acids Activates Autophagy in a CaMKK-β-dependent Way
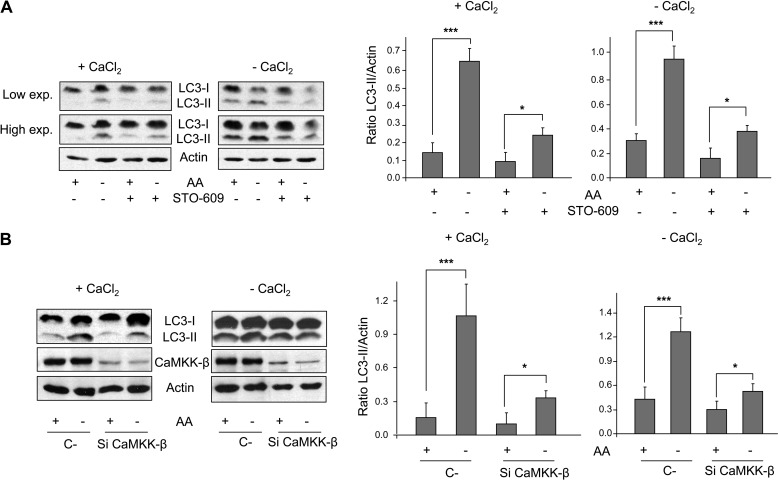
Withdrawal of essential amino acids activates AMPK via CaMKK-β (see Fig. 2) and the induced autophagy requires, at least in part, AMPK (Fig. 3). Therefore, we next investigated whether CaMKK-β alters autophagosome formation under this stimulus. Results show that the rise of LC3-II levels under amino acid starvation considerably diminishes when CaMKK-β is inhibited with STO-609 or silenced with specific siRNAs both in the presence and absence of extracellular Ca2+ (Fig. 4, A and B). This indicates that withdrawal of amino acids activates autophagy via a CaMKK-β-dependent pathway. Interestingly, the effects are greater after CaMKK-β down-regulation than after AMPK KO and silencing (see Fig. 3). Therefore, it is possible that CaMKK-β also mediates the activation of autophagy by amino acid starvation following other pathways independent of AMPK. Anyway, these findings show that CaMKK-β remains as an important sensor of the withdrawal of amino acids that mediates in the activation of AMPK and of autophagy under these conditions.
FIGURE 4.
CaMKK-β is involved in the activation of autophagy by amino acid starvation. A, NIH3T3 cells were grown for 60 min in KH and AA with (+CaCl2) or without CaCl2 (in the last case, supplemented with 100 μm EGTA, −CaCl2) plus 100 μm leupeptin and 20 mm NH4Cl and in the presence or absence of 25 μm STO-609. In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-LC3 (-I and -II) and, as a loading control, anti-actin antibodies. Low and high exposure (exp.) blots are shown. B, NIH3T3 cells were transfected with CaMKK-β (Si CaMKK-β) or with negative control (C-) siRNAs. After 72 h, cells were incubated as above and analyzed by immunoblot using the same antibodies as in A plus anti-CaMKK-β. Densitometric measurements of the LC3-II/actin ratios from three independent experiments in each case are also shown on the right of A and B. Differences were found to be statistically significant at p < 0.0005 (***) and p < 0.05 (*).
Withdrawal of Amino Acids Phosphorylates ULK1 in a CaMKK-β-dependent Way
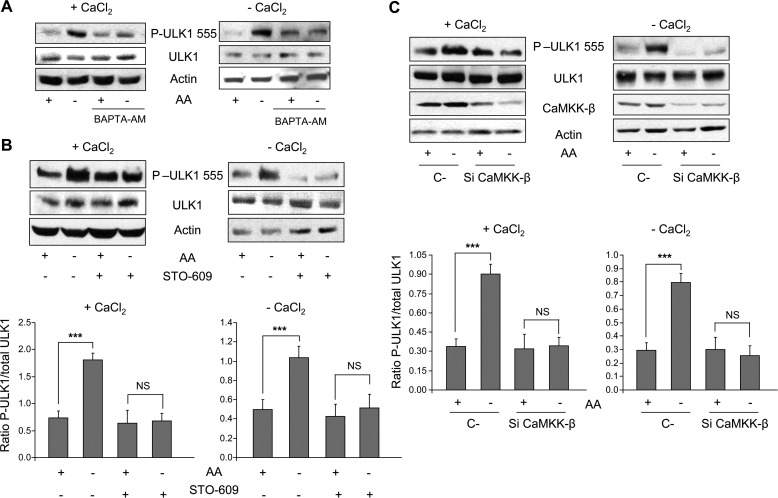
The results described above show that amino acid starvation induces autophagy by an AMPK-dependent mechanism (see Fig. 3). Because AMPK has been shown to activate autophagy by Ser-555 phosphorylation of ULK1 (7), we analyzed the effect of the withdrawal of amino acids on this phosphorylation. Under these conditions, the Ser-555 phosphorylation of ULK1 increases both in the presence and absence of extracellular Ca2+ (Fig. 5A, see the first and second lanes in +CaCl2 and −CaCl2). This phosphorylation is Ca2+-dependent because it is eliminated by BAPTA-AM (Fig. 5B, see two last bands in +CaCl2 and −CaCl2). Next, and given that withdrawal of amino acids activates AMPK in a CaMKK-β-sensitive manner (see Fig. 2) and that CaMKK-β is important for autophagy induction by amino acid starvation (see Fig. 4), we investigated the effect of CaMKK-β on the phosphorylation of ULK1 at Ser-555. Interestingly, when CaMKK-β was inhibited with STO-609 or down-regulated with specific siRNAs (Fig. 5, B and C, see the two last bands in +CaCl2 and −CaCl2), the effect of amino acid starvation on Ser-555 phosphorylation of ULK1 was abolished. These findings together with those shown before, indicate that Ca2+-CaMKK-β-dependent activation of AMPK by withdrawal of amino acids correlates with the phosphorylation at Ser-555 of ULK1, a modification that induces autophagy.
FIGURE 5.
Withdrawal of amino acids increases ULK1 phosphorylation at Ser-555 in a CaMKK-β-dependent manner. A, NIH3T3 cells, grown and treated as described in the legend to Fig. 2A, were analyzed by immunoblot using anti-ULK1, anti-P-ULK1 (Ser-555), and as a loading control, anti-actin antibodies. B and C, NIH3T3 cells were grown and treated as described in the legend to Fig. 2, B and C, respectively, and were analyzed by immunoblot using the same antibodies as in A plus anti-CaMKK-β in C. In B and C, densitometric measurements of the ratio of P-ULK1 (Ser-555) to total ULK1 from four independent experiments in each case are shown below. Differences were found to be statistically significant at p < 0.0005 (***). NS, no significant differences.
Inactivation of mTORC1 by Withdrawal of Amino Acids Involves Intracellular Ca2+ and CaMKK-β
Because ULK1 activation is positively and negatively regulated by AMPK and mTORC1, respectively (8, 31), we decided to determine if mTORC1 activity is affected by the Ca2+-CaMKK-β-AMPK pathway under withdrawal of amino acids by analyzing the phosphorylation of p70S6K at Thr-389. Amino acid removal inactivated mTORC1 in the presence and absence of extracellular Ca2+ (Fig. 6A, see the first and second lanes in +CaCl2 and −CaCl2). The inactivation of mTORC1 by amino acid starvation was abrogated when intracellular Ca2+ was previously chelated with BAPTA-AM (Fig. 6A, see the third lane), an effect that was lost in the presence of rapamycin (Fig. 6A, see the last lane). This supports that inactivation of mTORC1 by withdrawal of amino acids is Ca2+-dependent. The raise of intracellular Ca2+ levels under amino acid starvation occurs upstream of mTORC1, as this effect was insensitive to rapamycin, even during the second round of amino acid addition and removal. This was shown by both cytosolic Ca2+ imaging in fura-2AM loaded cells (Fig. 6B) and FACS analysis in fluo-3AM-loaded cells (Fig. 6C), in which the increase in [Ca2+]c under amino acid withdrawal was also in accordance with previous results (see Fig. 1D). As expected, BAPTA-AM canceled these effects (Fig. 6C).
FIGURE 6.
Ca2+ and CaMKK-β are also implicated in mTORC1 inactivation by amino acid starvation. A, NIH3T3 cells were grown for 2 h in KH plus AA with (+CaCl2) or without CaCl2 and supplemented with 100 μm EGTA (−CaCl2) in the presence or absence of 10 μm BAPTA-AM. When indicated, rapamycin (100 nm) was used during the last hour as a control. In the last 30 min of incubation, AA were removed or not as indicated. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-p70S6K, anti-P-p70S6K (Thr-389), anti-ULK1, anti-P-ULK1 (Ser-757), and as a loading control, anti-actin antibodies. B, fura-2AM-loaded NIH3T3 cells incubated for 1 h with rapamycin (100 nm) were imaged as described under “Experimental Procedures.” The effect of two rounds of AA addition and withdrawal on [Ca2+]c was measured in cells incubated in KH without CaCl2 and containing 100 μm EGTA. The mean values and S.D. at each time point of more than 50 cells from two different cultures are shown. No significant differences were observed in the effects of addition/withdrawal of AA on [Ca2+]c with cells not treated with rapamycin. Ionomycin (1 μm) was used as a positive control. C, NIH3T3 cells were previously incubated for 2 h in KH supplemented with AA with or without BAPTA-AM (10 μm). When indicated, rapamycin (100 nm) was used during the last hour as a control. Subsequently, AA were removed (KH) or not (AA) as indicated, and cells were loaded with 5 μm fluo-3AM for 30 min. The fluorescence intensity of fluo-3AM in the cells was measured by flow cytometry. Values are the means and S.D. from two separate experiments with quadruplicate samples. The fluorescence emitted by 10,000 cells in each case is showed below. D, NIH3T3 cells were grown and treated as described in the legend to Fig. 2B. Cell extracts (75 μg of protein) were analyzed by immunoblot using anti-p70S6K, anti-P-p70S6K (Thr-389), and as a loading control, anti-actin antibodies. E, NIH3T3 cells were transfected with CaMKK-β (Si CaMKK-β) or with negative control (C-) siRNAs. After 72 h, cells were incubated as above and analyzed by immunoblot using the same antibodies as in A plus anti-CaMKK-β. Densitometric measurements of the ratio of P-p70S6K to total p70S6K from four independent experiments in each case are also shown on the right of D and E. In E, low and high exposure (exp.) blots are shown. Differences were found to be statistically significant at p < 0.0005 (***) and p < 0.005 (**). NS, no significant differences.
Moreover, when CaMKK-β was inhibited with STO-609 or down-regulated with specific siRNAs, the differences in p70S6K phosphorylation with or without amino acids were attenuated in the presence of extracellular Ca2+ (Fig. 6, D and E, respectively, see the two last bands in +CaCl2) and almost fully abolished in its absence (Fig. 6, D and E, respectively, see the two last bands in −CaCl2CaCl2). Therefore, the CaMKK-β-AMPK signaling pathway is also involved here in mTORC1 inactivation. However, there is a difference in the effects with or without extracellular Ca2+. In the latter case, the Ca2+-CaMKK-β-AMPK pathway is essential for the amino acid effects on mTORC1, whereas when extracellular Ca2+ is present other pathways appear also to be involved. Because mTORC1 has been recently reported to prevent ULK1 activation by phosphorylating it at Ser-757 (8), the effects of amino acid withdrawal on ULK1 phosphorylation by mTORC1 were also analyzed. As expected, phosphorylation of ULK1 at Ser-757 diminishes under amino acid starvation both with and without extracellular Ca2+, and BAPTA-AM restores this rapamycin-dependent phosphorylation (Fig. 6A). Taken together, these data support a model where amino acid starvation inhibits mTORC1 in a Ca2+-dependent manner, which leads to ULK1 activation and consequently to autophagy induction.
DISCUSSION
Despite the well established role of amino acids in the regulation of autophagy, the detailed molecular mechanisms are still not fully understood (1, 32, 33). Activation of autophagy by amino acid starvation is the subject of a complex regulation via several signaling pathways (18). The hitherto most important step in the induction of autophagy by deprivation of amino acids is the inhibition of mTORC1. This kinase when inactivated dissociates from an important complex that initiates autophagy and contains, among other proteins, the AuTophagy related Gene 13 (Atg13), the focal adhesion kinase interacting protein 200 (FIP200), and the kinase ULK1/2, which has been identified as a substrate of AMPK (7, 8, 31). Under these conditions, autophagy becomes stimulated (34, 35). However, how amino acid starvation inactivates mTORC1 is still poorly understood. This is in part due to the diversity of amino acids and to the variety of their metabolisms in different cells (4, 32, 36, 37). Examples of the signaling pathways involved in mTORC1 inhibition by amino acid starvation are PI3K-Akt-mTORC1 (34, 35) and Ras-Raf-MEK-ERK (38–40). In addition, mTORC1-independent pathways in the regulation of autophagy by amino acid starvation have been also postulated (for review, see Refs. 18 and 33). The findings presented in this work support that a Ca2+-dependent pathway is operative in the activation of autophagy by essential amino acid starvation. This pathway involves CaMKK-β-AMPK-ULK1 and also mTORC1 signaling.
The results reported herein show for the first time that withdrawal of essential amino acids induces a rise in [Ca2+]c. This Ca2+ originates from extracellular and intracellular stores. The intracellular stores responsible of this Ca2+ release are unknown, and although other sources such as lysosomes cannot be excluded, it is possible that the ER is implicated, as it is the most important organelle that supplies Ca2+ to the cell (12). In fact, it has been reported that amino acid starvation causes the release of Bcl-2 from ER to induce autophagy (41) and that the association of Bcl-2 to the ER decreases the exit of Ca2+ from ER and inhibits autophagy. Another study showed that amino acids increase [Ca2+]c, which enhances the binding of Ca2+/calmodulin to hVps34. This results in a rise in phosphatidylinositol 3-phosphate levels and in an enhanced signaling by the mTORC1 complex (42). Because we observed the same increase in [Ca2+]c when the amino acids were added to the medium without adjusting the pH to physiological conditions (data not shown), it is possible that the Ca2+ increase observed in that study is a physiological response to a low pH. Moreover, a more recent report in HEK293T cells suggests that the described interaction of Ca2+/Calmodulin with hVps34 does not directly affect hVps34 activity (43). Therefore, the mechanisms by which amino acids control intracellular Ca2+ homeostasis remain to be studied.
AMPK inhibits the mTORC1 complex (6), and Ca2+ activates AMPK via CaMKK-β (5, 19). Based on these premises, we propose that the well established activation of autophagy by amino acid starvation occurs in part via the Ca2+-CaMKK-β-AMPK pathway. This model (Fig. 7) is supported by the results showing that under amino acid starvation, Ca2+ and CaMKK-β are required for the activation of AMPK and for the inhibition of mTORC1, stimulating in this way ULK1 for an increased formation of autophagosomes.
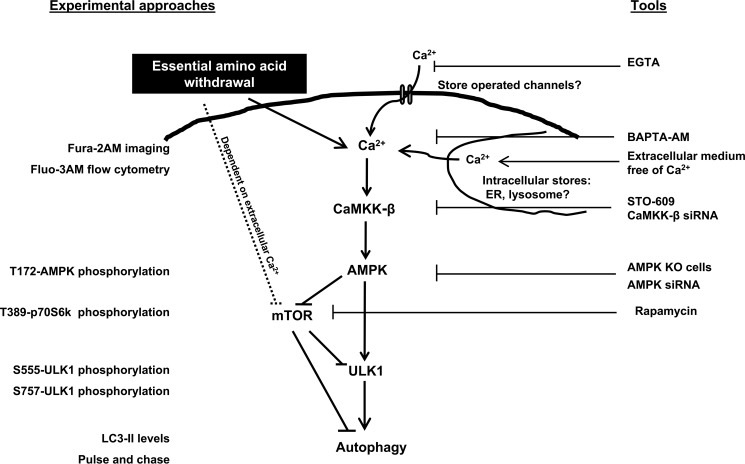
FIGURE 7.
Suggested Ca2+-dependent signaling pathway from amino acid starvation to autophagy. Detection methods/experimental approaches and assays/tools used in this study to monitor the indicated events are shown, respectively, on the left (marked in blue) and on the right (marked in red).
Another important output for CaMKK-β-dependent activation of AMPK by amino acid starvation is the Ser-555 phosphorylation of ULK1, which is required for the initiation of autophagy. Active CaMKK-β is fully required for this phosphorylation, in accordance with the results showing the involvement of CaMKK-β in the activation of autophagosome formation by amino acid starvation. However, CaMKK-β inactivation does not fully abolish the autophagy induced by withdrawal of amino acids, suggesting that this condition also induces autophagy by pathways independent of ULK1.
mTORC1 is also involved in the Ca2+-CaMKK-β-AMPK pathway stimulated by amino acid starvation. In the presence of extracellular Ca2+, CaMKK-β seems to be partially involved in the inhibition of mTORC1 mediated by amino acid starvation, which suggests the involvement of other pathways in this inhibition. In fact and as mentioned above, several mTORC1-dependent pathways have already been reported for the regulation of autophagy by amino acid starvation (38–40). However, in the absence of extracellular Ca2+, CaMKK-β becomes fully indispensable. It is then conceivable that Ca2+ provided by the extracellular medium contributes to the effect of amino acid starvation on mTORC1 activity independently of Ca2+-CaMKK-β-AMPK. In contrast, Ca2+ provided by intracellular stores acquires an exclusive role in the regulation of mTORC1 by amino acid starvation via CaMKK-β.
In summary, this study proposes a pathway for the regulation of autophagy by amino acid starvation involving Ca2+, CaMKK-β, AMPK, ULK1, and mTORC1. Thus, intracellular Ca2+ levels remain an important signal in the cell response to the availability of amino acids. As amino acids have different metabolisms, it would be important to identify those that change intracellular Ca2+ levels as well as the possible existence of an intra- or extracellular Ca2+-dependent sensor for these amino acids.
Acknowledgments
We thank Asunción Montaner for technical assistance and Benoit Viollet for providing the AMPK-deficient and wild-type mouse embryonic fibroblasts.
This work was supported by Ministerio de Educación y Ciencia Grant BFU2011-22630 and Fundació Marató TV3 Grant 100130).

This article contains supplemental Figs. 1 and 2.
- mTORC1
- mammalian target of rapamycin complex 1
- AMPK
- AMP-activated protein kinase
- [Ca2+]c
- cytosolic Ca2+ levels
- CaMKK-β
- Ca2+/calmodulin-dependent kinase kinase-β
- ER
- endoplasmic reticulum
- LC3
- microtubule-associated protein1 light chain 3
- MEF
- mouse embryonic fibroblast
- ULK1
- UNC-51-like kinase
- KH
- Krebs-Henseleit medium
- AA
- amino acids
- p70S6K
- phosphorylation of the ribosomal protein S6 kinase 1.
REFERENCES
- 1. Knecht E., Aguado C., Cárcel J., Esteban I., Esteve J. M., Ghislat G., Moruno J. F., Vidal J. M., Sáez R. (2009) Intracellular protein degradation in mammalian cells. Recent developments. Cell. Mol. Life Sci. 66, 2427–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lum J. J., Bauer D. E., Kong M., Harris M. H., Li C., Lindsten T., Thompson C. B. (2005) Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 [DOI] [PubMed] [Google Scholar]
- 3. Onodera J., Ohsumi Y. (2005) Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 280, 31582–31586 [DOI] [PubMed] [Google Scholar]
- 4. Esteban I., Aguado C., Sánchez M., Knecht E. (2007) Regulation of various proteolytic pathways by insulin and amino acids in human fibroblasts. FEBS Lett. 581, 3415–3421 [DOI] [PubMed] [Google Scholar]
- 5. Høyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., Bianchi K., Fehrenbacher N., Elling F., Rizzuto R., Mathiasen I. S., Jäättelä M. (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase β, and Bcl-2. Mol. Cell 25, 193–205 [DOI] [PubMed] [Google Scholar]
- 6. Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 7. Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R. J. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J., Kundu M., Viollet B., Guan K. L. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perez-Terzic C. M., Chini E. N., Shen S. S., Dousa T. P., Clapham D. E. (1995) Ca2+ release triggered by nicotinate adenine dinucleotide phosphate in intact sea urchin eggs. Biochem. J. 312, 955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling. Dynamics, homeostasis, and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 11. Hajnóczky G., Davies E., Madesh M. (2003) Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 304, 445–454 [DOI] [PubMed] [Google Scholar]
- 12. Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. (2008) Calcium and apoptosis. ER mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon P. B., Holen I., Fosse M., Røtnes J. S., Seglen P. O. (1993) Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J. Biol. Chem. 268, 26107–26112 [PubMed] [Google Scholar]
- 14. Williams A., Sarkar S., Cuddon P., Ttofi E. K., Saiki S., Siddiqi F. H., Jahreiss L., Fleming A., Pask D., Goldsmith P., O'Kane C. J., Floto R. A., Rubinsztein D. C. (2008) Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 4, 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghislat G., Aguado C., Knecht E. (2012) Annexin A5 stimulates autophagy and inhibits endocytosis. J. Cell Sci. 125, 92–107 [DOI] [PubMed] [Google Scholar]
- 16. Laderoute K. R., Amin K., Calaoagan J. M., Knapp M., Le T., Orduna J., Foretz M., Viollet B. (2006) 5'-AMP-activated protein kinase (AMPK) is induced by low oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 26, 5336–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuertes G., Martín De Llano J. J., Villarroya A., Rivett A. J., Knecht E. (2003) Changes in the proteolytic activities of proteasomes and lysosomes in human fibroblasts produced by serum withdrawal, amino acid deprivation, and confluent conditions. Biochem. J. 375, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang R. C., Levine B. (2010) Autophagy in cellular growth control. FEBS Lett. 584, 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 20. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 21. Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. (2002) STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 22. Tanida I., Minematsu-Ikeguchi N., Ueno T., Kominami E. (2005) Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1, 84–91 [DOI] [PubMed] [Google Scholar]
- 23. Codogno P., Meijer A. J. (2005) Autophagy and signaling. Their role in cell survival and cell death. Cell Death Differ 12, 1509–1518 [DOI] [PubMed] [Google Scholar]
- 24. Levine B., Klionsky D. J. (2004) Development by self-digestion. Molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 [DOI] [PubMed] [Google Scholar]
- 25. Mizushima N., Yoshimori T., Levine B. (2010) Methods in mammalian autophagy research. Cell 140, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubinsztein D. C., Cuervo A. M., Ravikumar B., Sarkar S., Korolchuk V., Kaushik S., Klionsky D. J. (2009) In search of an “autophagomometer.” Autophagy 5, 585–589 [DOI] [PubMed] [Google Scholar]
- 27. Niemann A., Takatsuki A., Elsässer H. P. (2000) The lysosomotropic agent monodansylcadaverine also acts as a solvent polarity probe. J. Histochem. Cytochem. 48, 251–258 [DOI] [PubMed] [Google Scholar]
- 28. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., Blum J. S., Bredesen D. E., Brodsky J. L., Brumell J. H., Brunk U. T., Bursch W., Camougrand N., Cebollero E., Cecconi F., Chen Y., Chin L. S., Choi A., Chu C. T., Chung J., Clarke P. G., Clark R. S., Clarke S. G., Clavé C., Cleveland J. L., Codogno P., Colombo M. I., Coto-Montes A., Cregg J. M., Cuervo A. M., Debnath J., Demarchi F., Dennis P. B., Dennis P. A., Deretic V., Devenish R. J., Di Sano F., Dice J. F., Difiglia M., Dinesh-Kumar S., Distelhorst C. W., Djavaheri-Mergny M., Dorsey F. C., Droge W., Dron M., Dunn W. A., Jr., Duszenko M., Eissa N. T., Elazar Z., Esclatine A., Eskelinen E. L., Fesus L., Finley K. D., Fuentes J. M., Fueyo J., Fujisaki K., Galliot B., Gao F. B., Gewirtz D. A., Gibson S. B., Gohla A., Goldberg A. L., Gonzalez R., Gonzalez-Estevez C., Gorski S., Gottlieb R. A., Haussinger D., He Y. W., Heidenreich K., Hill J. A., Hoyer-Hansen M., Hu X., Huang W. P., Iwasaki A., Jaattela M., Jackson W. T., Jiang X., Jin S., Johansen T., Jung J. U., Kadowaki M., Kang C., Kelekar A., Kessel D. H., Kiel J. A., Kim H. P., Kimchi A., Kinsella T. J., Kiselyov K., Kitamoto K., Knecht E., Komatsu M., Kominami E., Kondo S., Kovacs A. L., Kroemer G., Kuan C. Y., Kumar R., Kundu M., Landry J., Laporte M., Le W., Lei H. Y., Lenardo M. J., Levine B., Lieberman A., Lim K. L., Lin F. C., Liou W., Liu L. F., Lopez-Berestein G., Lopez-Otin C., Lu B., Macleod K. F., Malorni W., Martinet W., Matsuoka K., Mautner J., Meijer A. J., Melendez A., Michels P., Miotto G., Mistiaen W. P., Mizushima N., Mograbi B., Monastyrska I., Moore M. N., Moreira P. I., Moriyasu Y., Motyl T., Munz C., Murphy L. O., Naqvi N. I., Neufeld T. P., Nishino I., Nixon R. A., Noda T., Nurnberg B., Ogawa M., Oleinick N. L., Olsen L. J., Ozpolat B., Paglin S., Palmer G. E., Papassideri I., Parkes M., Perlmutter D. H., Perry G., Piacentini M., Pinkas-Kramarski R., Prescott M., Proikas-Cezanne T., Raben N., Rami A., Reggiori F., Rohrer B., Rubinsztein D. C., Ryan K. M., Sadoshima J., Sakagami H., Sakai Y., Sandri M., Sasakawa C., Sass M., Schneider C., Seglen P. O., Seleverstov O., Settleman J., Shacka J. J., Shapiro I. M., Sibirny A., Silva-Zacarin E. C., Simon H. U., Simone C., Simonsen A., Smith M. A., Spanel-Borowski K., Srinivas V., Steeves M., Stenmark H., Stromhaug P. E., Subauste C. S., Sugimoto S., Sulzer D., Suzuki T., Swanson M. S., Tabas I., Takeshita F., Talbot N. J., Talloczy Z., Tanaka K., Tanaka K., Tanida I., Taylor G. S., Taylor J. P., Terman A., Tettamanti G., Thompson C. B., Thumm M., Tolkovsky A. M., Tooze S. A., Truant R., Tumanovska L. V., Uchiyama Y., Ueno T., Uzcategui N. L., van der Klei I., Vaquero E. C., Vellai T., Vogel M. W., Wang H. G., Webster P., Wiley J. W., Xi Z., Xiao G., Yahalom J., Yang J. M., Yap G., Yin X. M., Yoshimori T., Yu L., Yue Z., Yuzaki M., Zabirnyk O., Zheng X., Zhu X., Deter R. L. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizushima N., Yoshimori T. (2007) How to interpret LC3 immunoblotting. Autophagy 3, 542–545 [DOI] [PubMed] [Google Scholar]
- 30. Pullen N., Dennis P. B., Andjelkovic M., Dufner A., Kozma S. C., Hemmings B. A., Thomas G. (1998) Phosphorylation and activation of p70s6k by PDK1. Science 279, 707–710 [DOI] [PubMed] [Google Scholar]
- 31. Egan D., Kim J., Shaw R. J., Guan K. L. (2011) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim E. (2009) Mechanisms of amino acid sensing in mTOR signaling pathway. Nutr. Res. Pract. 3, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meijer A. J. (2008) Amino acid regulation of autophagosome formation. Methods Mol. Biol. 445, 89–109 [DOI] [PubMed] [Google Scholar]
- 34. Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J. L., Oshiro N., Mizushima N. (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung C. H., Jun C. B., Ro S. H., Kim Y. M., Otto N. M., Cao J., Kundu M., Kim D. H. (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20, 1992–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suryawan A., Hawes J. W., Harris R. A., Shimomura Y., Jenkins A. E., Hutson S. M. (1998) A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 68, 72–81 [DOI] [PubMed] [Google Scholar]
- 37. Brosnan J. T., Brosnan M. E. (2006) Branched-chain amino acids. Enzyme and substrate regulation. J. Nutr. 136, 207S–211S [DOI] [PubMed] [Google Scholar]
- 38. Ogier-Denis E., Pattingre S., El Benna J., Codogno P. (2000) Erk1/2-dependent phosphorylation of Gα-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J. Biol. Chem. 275, 39090–39095 [DOI] [PubMed] [Google Scholar]
- 39. Pattingre S., Bauvy C., Codogno P. (2003) Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J. Biol. Chem. 278, 16667–16674 [DOI] [PubMed] [Google Scholar]
- 40. Shaw R. J., Cantley L. C. (2006) Ras, PI(3)K, and mTOR signalling controls tumorcell growth. Nature 441, 424–430 [DOI] [PubMed] [Google Scholar]
- 41. Pattingre S., Tassa A., Qu X., Garuti R., Liang X. H., Mizushima N., Packer M., Schneider M. D., Levine B. (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, 927–939 [DOI] [PubMed] [Google Scholar]
- 42. Gulati P., Gaspers L. D., Dann S. G., Joaquin M., Nobukuni T., Natt F., Kozma S. C., Thomas A. P., Thomas G. (2008) Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 7, 456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yan Y., Flinn R. J., Wu H., Schnur R. S., Backer J. M. (2009) hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem. J. 417, 747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]