Background: The BMP signaling pathway modulates the expression of protein coding genes and non-coding RNAs.
Results: BMP4-Smad pathway represses the transcription of miR-302∼367 cluster and depresses the expression of the type II BMP receptor.
Conclusion: BMP4 treatment facilitates the BMP signaling pathway by down-regulation of miR-302.
Significance: Autoregulatory mechanism of control of BMP signaling pathway via miRNA and its target.
Keywords: Bone Morphogenetic Protein (BMP), MicroRNA, Receptor Serine Threonine Kinase, Smooth Muscle, Transcription Regulation, Smad, miR-302
Abstract
The signaling pathway mediated by BMPs plays an essential role during development as well as the maintenance of homeostasis in adult. Aberrant activation or inactivation of BMP signaling can lead to developmental defects and various human disorders. To fine-tune its activity, BMP signaling is regulated both positively and negatively by extrinsic and intrinsic regulatory factors that modulate binding of ligand to the receptors, and the activity of receptors and their dedicated signal transducers, the Smad proteins. Upon BMP binding to the receptor complex, Smad proteins translocate to the nucleus and modulate gene expression transcriptionally by directly associating with the promoter region of target genes, or post-transcriptionally through modulation of microRNA (miRNA) synthesis. In this study, we demonstrate that BMP signaling down-regulates transcription of the miRNA-302∼367 gene cluster. We show that the type II BMP receptor (BMPRII) is a novel target of miR-302. Upon overexpression, miR-302 targets a partially complementary sequence localized in the 3′-untranslated region (UTR) of BMPRII transcripts and leads to destabilization of the transcripts and down-regulation of BMP signaling. We propose that the negative regulatory loop of BMP4-miR-302-BMPRII is a potential mechanism for the maintenance and fine-tuning of the BMP signaling pathway in various systems.
Introduction
The TGFβ-family of growth factors, including TGFβ, nodals, activins, and bone morphogenetic protein 4 (BMP4),3 play an essential role during development and maintenance of homeostasis in multicellular organisms (1). For example, TGFβ and BMP4 transmit a critical signal that regulates the phenotype of vascular smooth muscle (vSMCs) and endothelial cells (ECs) (2, 3). Abnormality in these signaling pathways can lead to vascular disorders, such as pulmonary artery hypertension (PAH) and hereditary hemorrhagic telangiectasia (HHT) (4). TGFβ and BMP4 bind to specific sets of heteromeric receptor complexes, the type I and type II receptors, which are both serine/threonine kinases (4). Upon ligand stimulation, the type I receptor kinase phosphorylates the downstream signal transducer Smad proteins, which then translocate to the nucleus and modulate gene expression as they bind DNA in a sequence specific manner and modulate transcription (5). Recently, we found that Smads also regulate the biogenesis of small noncoding miRNAs (6). miRNA biogenesis begins by transcription of miRNA genes by RNA polymerase II (RNA pol II) into long primary miRNA transcripts (pri-miRNAs), which undergo two sequential processing events to generate mature miRNAs (7). In the first processing, pri-miRNAs are cropped by RNase III Drosha to generate precursor miRNAs (pre-miRNAs) in the nucleus. After translocation to the cytoplasm, pre-miRNAs undergo a secondary cropping by RNase III Dicer, which generates mature miRNAs (7). Smad proteins can control the biogenesis of a subset of miRNA either transcriptionally or post-transcriptionally, at the first stage of processing by Drosha in the nucleus (8).
The miR-302∼367 cluster encodes several miR-302 family members, including miR-302a/b/c/d and miR-367, which are highly expressed in embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), but also expressed in other cell types at lower level (9–13). The level of expression of miR-302 is tightly regulated during the differentiation of ESCs and iPSCs: the highest expression of miR-302 is observed in undifferentiated cells and declines rapidly as they begin to differentiate (12). This observation suggests that the miR-302 family of miRNAs might play a role in the maintenance of pluripotency or self-renewal. In agreement with its expression timeline, miR-302 is transcriptionally controlled by ESC-specific factors, such as Nanog, Oct4, and Sox2 (14–16). It is unclear, however, how miR-302 level is regulated in cell types other than ESs and iPSCs since Nanog, Oct4, and Sox2 are expressed only in undifferentiated cells. In this study, we demonstrate that transcription of the miR-302∼367 gene cluster is repressed, upon BMP treatment, by a Smad protein complex in a histone deacetylase (HDAC)-dependent manner. We report that BMPRII transcripts are targeted by the miR-302 family of miRNAs. These results indicate that down-regulation of miR-302 by BMP signaling leads to derepression of BMPRII expression, which in turn augment responsiveness to BMP signaling in vSMCs.
EXPERIMENTAL PROCEDURES
Cell Culture
Human primary pulmonary artery smooth muscle cells were purchased from Lonza (CC-2581) and maintained in Sm-GM2 media (Lonza) containing 5% fetal bovine serum (FBS). COS7, C3H10T1/2, and P19 cells were maintained in Dulbecco's modified Eagle media (DMEM) supplemented with 10% FBS. Cells were cultured at 37 °C in the presence of 5% CO2.
Quantitative Reverse Transcriptase-PCR (qRT-PCR)
Total RNAs were isolated using TRizol (Invitrogen) and reverse transcribed using first-strand cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. qRT-PCR analysis was performed in triplicates using the SYBR Green master mix (Applied Biosystems). mRNA level was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For a quantitation of miRNAs, TaqMan MicroRNA assay kit (Applied Biosystems) was used according to manufacturer's instructions and results were normalized to U6 snRNA. Pri-miR-302∼367: 5′-TGAATCCAATTTACTTCTCCA-3′ and 5′-TCCTTTAACCTGTAACAAGC-3′; Pri-miR-221: 5′-GCAACTGCTGCACAAATACC-3′ and 5′-TTGATAAAGGGCTGCTGGAC-3′; Pri-miR-380: 5′-CTGGAGGTGATGCTAGTGAG-3′ and 5′-GCCAGATCAGTGTGTCTCC-3′; ASMA: 5′-GCGTGGCTATTCCTTCGTTA-3′ and 5′-ATGAAGGATGGCTGGAACAG-3′; SM22α: 5′-AACAGCCTGTACCCTGATGG-3′ and 5′-CGGTAGTGCCCATCATTCTT-3′; CNN: 5′-AGCTAAGAGAAGGGCGGAAC-3′ and 5′-CATCTGCAGGCTGACATTGA-3′; Smad4: 5′-AAGGTGAAGGTGATGTTTG-3′ and 5′-GAGCTATTCCACCTACTGAT-3′; Id3: 5′-GGAGCTTTTGCCACTGACTC-3′ and 5′-TTCAGGCCACAAGTTCACAG-3′; HDAC1: 5′-CCAAATGCAGGCGATTCCT-3′ and 5′-AGAATCGGAGAACTCTTCCTCACA-3′. Primer sets for Smad1, Smad5, BMPR1A, BMPR1B, and TβRII have been published previously (17,18).
Luciferase Reporter Constructs
Luciferase reporter constructs containing promoter regions of miR-302c, -974-Luc, and -525-Luc were previously published (19). SBE-Luc and SBE(Mut)-Luc constructs were generated by PCR amplification of −185 to +47 of human miR-302∼367 promoter region. SBE(Mut) contains mutations in the SBE sequence as follows, 5′-GAAACC-3′. Predicted miR-302c MRE sequence of BMPRII [MRE(WT)]and its mutated sequence [MRE(Mut)] were cloned into the pIS0 vector (Addgene) containing the luciferase gene. 5′-GTTTTTTTTAAATAAAGCACTTT-3′ and 5′-AAAGTGCTTTATTTAAAAAAAAC-3′ were used for MRE(WT) and 5′-GTTTTTTTTAAATAAAGGTGATT-3′ and 5′-AATCACCTTTATTTAAAAAAAAC-3′ were used for MRE(Mut).
Luciferase Assay
Cos7 cells were transfected with luciferase reporter constructs using Fugene6 (Roche) and a β-galactosidase (β-gal) expression plasmid as an internal transfection control. Twenty-four hours later, cells were treated with BMP4 or transfected using RNAi Max (Invitrogen) with 5 nm miR-302c mimic or control miRNA mimic. Luciferase assays were carried out, and luciferase activities were presented after normalization to β-galactosidase activities.
RNA Interference
Smad4#1siRNA: 5′-CCUGAGUAUUGGUGUUCCAUUGCUU-3′ (Stealth siRNA, Invitrogen), Smad4#2 siRNA: MISSION® esiRNA targeting human SMAD4 (esiRNA1; Sigma-Aldrich) and Smad1#1, #2, and Smad5#1, #2 siRNAs were described previously (20). Non-targeting scrambled siRNA (Qiagen) was used as a negative control#1 (catalogue# 1027280) and #2 (catalogue# 1027281). HDAC1#1 siRNA: 5′-UCCGUAAUGUUGCUCGAUGUU-3′ and HDAC1#2 siRNA: 5′-UGAACGAUCCUAUCCGCCA-3′. The siRNAs were transfected at 40 nm using RNAi Max (Invitrogen) according to manufacturer's instruction.
miRNA Mimic and Antisense Oligonucleotides against miRNA
Chemically modified double-stranded RNAs designed to mimic the endogenous mature miR-302c and negative control miRNA were purchased from Ambion. 2′-O-methyl modified RNA oligonucleotides complementary to miRNA (anti-miR) or GFP (control) sequence were purchased from IDT. 5 nm miRNA mimics or 50 nm anti-miRs were transfected using RNAi Max (Invitrogen) according to manufacturer's directions. Anti-GFP: 5′-AAGGCAAGCUGACCCUGAAGU-3′. Anti-miR-302c: 5′-CCACUGAAACAUGGAAGCACUUA-3′.
Immunoblotting
Cells were lysed in TNE buffer and total cell lysates were separated by SDS-PAGE, transferred to PVDF membranes (Millipore), immunoblotted with antibodies, and visualized using an enhanced chemiluminescence detection system (Amersham Biosciences). Antibodies used for immunoblotting are: anti-GAPDH antibody (2E3–2E10, Abnova), and anti-human BMPRII antibodies (#6979, Cell Signaling). Anti-α-smooth muscle actin antibodies were previously described (20). Protein bands were quantitated by image analysis software, ImageJ (rsbweb.nih.gov/ij/).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed as described (18). After BMP4 stimulation for 4 h, soluble chromatin was prepared from PASMC following crosslinking with 1% formaldehyde for 10 min, sonication, and centrifugation. Soluble chromatin was incubated with anti-Smad4 antibody (Clone H-552, Santa Cruz), anti-phosphoSmad1/5 antibody (41D10, Cell Signaling), or rabbit IgG as negative control. miR-302 primer: 5′-CAGGATCATACATTCCCTGA-3′ and 5′-TAGTTCCCAAAGATTCGTGT-3′ and a control primer (>2 kb upstream): 5′-TACAAAATGAGCTGGGCGTGG-3′ and 5′-TATACTCCAGCCTGAGTGA-3′ were used. As a control for Smad4/1/5 binding site, PCR primers for human miR-143∼145 promoter were used (21).
Cell Proliferation and Migration Assay
Cell proliferation assay and scratch wound migration assay was performed in PASMCs as described previously (22, 23).
Statistical Analysis
The results presented are an average of at least three experiments each performed in triplicate with standard errors. Statistical analyses were performed by analysis of variance, followed by Tukey's multiple comparison test or by Student's t test as appropriate, using Prism 4 (GraphPAD Software Inc.). p values of <0.05 were considered significant and are indicated with asterisks.
RESULTS
Down-regulation of the miR-302 Cluster by BMP4
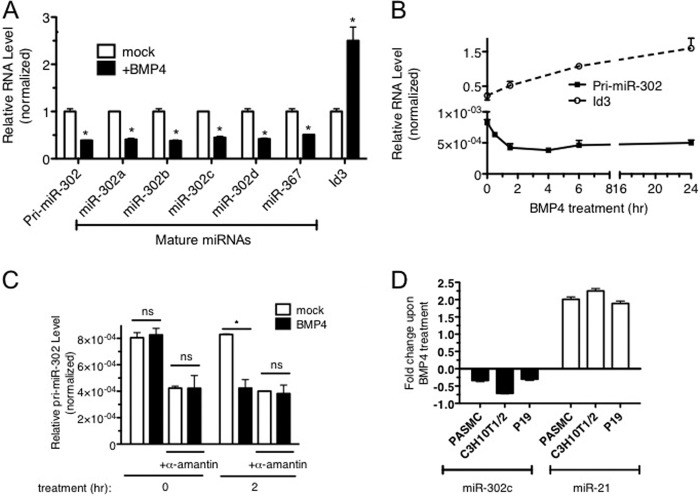
miRNA expression profiling analysis in pulmonary artery smooth muscle cells (PASMCs) indicated that multiple members of the miR-302 family of miRNAs (miR-302a/b/c) are repressed upon BMP4 stimulation (3 nm) for 24 h (data not shown) (24). As four members of the miR-302 family of miRNAs (miR-302a/b/c/d) and miR-367 are encoded in the miR-302∼367 gene cluster and transcribed as a single transcript, we hypothesized that BMP4 signaling may regulate the entire miR-302∼367 gene cluster. qRT-PCR analysis in PASMC confirmed the miRNA expression profiling result and showed a decrease in miR-302a/b/c/d and miR-367 to ∼50% of the basal level upon BMP4 stimulation (Fig. 1A). The mRNA of Id3, a transcriptional target of BMP4-Smad signaling (25), was increased ∼2.5-fold, indicating a successful treatment of cells with BMP4 (Fig. 1A). The level of primary miR-302∼367 cluster transcripts was decreased to less than 40% upon BMP4 treatment, similarly to the levels of mature miR-302a/b/c/d and miR-367 (Fig. 1A, pri-miR-302). A time-course analysis of pri-miR-302 in parallel with Id3 mRNA (control) exhibits a rapid reduction of pri-miR-302 within 2 h after BMP4 treatment (Fig. 1B), indicating that down-regulation of miR-302a/b/c/d and miR-367 is likely to occur at the transcriptional level. To test this possibility, PASMCs were treated with the RNA polymerase II (RNA pol II) inhibitor α-amanitin prior to 2 h BMP4 stimulation, followed by qRT-PCR analysis of pri-miR-302. α-Amanitin treatment abolished BMP4 treatment-induced repression of pri-miR-302 (Fig. 1C). A similar result was obtained with another RNA pol II inhibitor, actinomycin D (data not shown). Repression of the miR-302 family by BMP4 is not cell type-specific as it was observed not only in PASMCs but also mouse mesenchymal C3H10T1/2 cells or mouse embryonic carcinoma p19 cells, in which miR-302 is expressed at higher levels than PASMCs (Fig. 1D). miR-21 is shown as a control for BMP4 treatment as it is induced by BMP4 (Fig. 1D) (20). These results demonstrate that transcriptional repression of the miR-302 gene cluster by BMP4 signaling occurs in both differentiated and less differentiated cells.
FIGURE 1.
Transcriptional regulation of miR-302∼367 cluster by BMP4. A, total RNAs were harvested from PASMCs treated with 3 nm BMP4 for 24 h and expression levels of pri-miR-302 or Id3 mRNA relative to GAPDH and mature miR-302a-d and miR-367 relative to U6 snRNA were measured by qRT-PCR. Data represent the expression levels in BMP4-stimulated cells relative to untreated cells in triplicates. *, p < 0.01. B, PASMCs were treated with 3 nm BMP4, and total RNAs were harvested at different time points. mRNA levels of pri-miR-302 and Id3 relative to GAPDH were measured by qRT-PCR in triplicates. Data represent mean ± S.E. C, PASMCs were treated with α-amanitin for 4 h prior to the stimulation with 3 nm BMP4 for 2 h. Level of pri-miR-302 relative to GAPDH was measured by qRT-PCR. Data represent mean ± S.E. *, p < 0.01. ns: statistically not significant. D, different types of cells, including PASMC, C3H10T1/2, and P19, were treated with 3 nm BMP for 24 h, and total RNAs were subjected to qRT-PCR analysis of miR-302c and miR-21 relative to U6 snRNA in triplicates. Data represent fold changes of the expression levels in BMP4-treated cells in comparison with untreated cells in triplicates. *, p < 0.01.
Smad-dependent Transcriptional Repression of miR-302
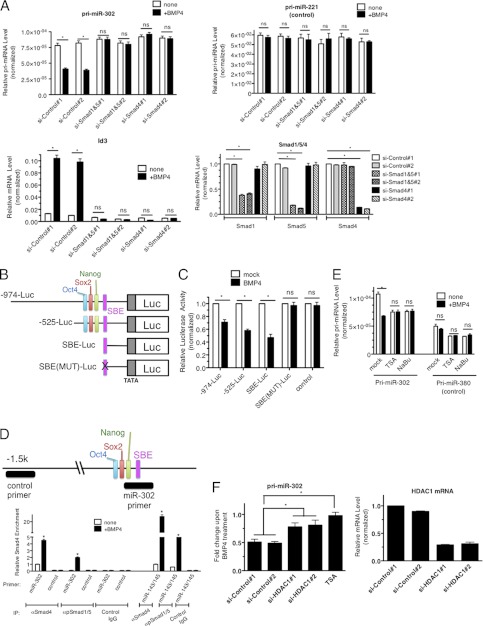
To examine whether transcriptional repression of miR-302 by BMP4 is mediated by the signal transducers of the BMP signaling pathway, Smad1/5 or Smad4, the levels of these proteins were reduced by two different small interfering RNAs (siRNAs) (si-Smad1/5#1, #2, or si-Smad4#1,#2). qRT-PCR analysis indicated a knock down of each Smad by siRNA (Fig. 2A, Smad1/5/4). Both si-Smad1/5 and si-Smad4 abolished induction of Id3 by BMP4 (Fig. 2A, Id3), indicating an inhibition of Smad activity (Fig. 2A, Id3). Similarly, BMP4-induced down-regulation of pri-miR-302 was blunted by both si-Smad1/5 and si-Smad4, suggesting that Smad proteins are essential for transcriptional repression (Fig. 2A, pri-miR-302).
FIGURE 2.
Transcriptional repression of miR-302 by BMP4 is Smad-dependent. A, PASMCs were transfected with non-targeting control siRNAs (si-Control#1 and #2), mixture of siRNAs against Smad1 and Smad5 (si-Smad1&5#1 and #2) or Smad4 (si-Smad4#1 and #2), followed by 3 nm BMP stimulation for 24 h. Levels of pri-miR-302, pri-miR-221 (control), Id3, Smad1, Smad4, or Smad5 mRNAs relative to GAPDH were measured by qRT-PCR. Data represent mean ± S.E. *, p < 0.01. ns: statistically not significant. B, schematic diagram of luciferase reporter constructs containing human miR-302∼376 gene promoter region. C, Luciferase activity was examined in COS7 cells using the luciferase vector containing either long −974-Luc (−974-Luc) or short −525-Luc (−525-Luc). An empty luciferase vector (control) was used as a control. Relative luciferase activity of each construct with BMP4 stimulation relative to unstimulated are shown. Data represent mean ± S.E. *, p < 0.01. ns: statistically not significant. D, schematic diagram of the miR-302∼367 promoter region which is recognized by the control or miR-302 ChIP primer is indicated as a black bar. PASMCs were stimulated with BMP4 for 4 h and subjected to ChIP assay using anti-Smad4 antibody. qRT-PCR was then performed to measure enrichment of DNA fragment by primers, such as control, miR-302, or miR-143/145 promoter region. The data were plotted as relative enrichment to input. Error bars indicate S.E. *, p < 0.01. E, PASMCs were treated with HDAC inhibitors; TSA or NaBu prior to BMP4 stimulation and total RNAs were isolated. Levels of pri-miR-302 or pri-miR-380 relative to GAPDH were quantitated. Data represent mean ± S.E.; *, p < 0.01. ns: statistically not significant. F, PASMCs were transfected with control siRNA #1,2 or siRNA against HDAC1#1,2, followed by BMP4 treatment. Levels of pri-miR-302 and HDAC1 mRNA relative to GAPDH were quantitated. Data represent mean ± S.E. *, p < 0.01.
To map the miR-302 cluster promoter region required for the BMP4-Smad-dependent repression, luciferase constructs containing different promoter regions (-974-Luc construct (−974 to +50) and −525-Luc construct (−525− to +50)) (19) were transfected into COS7 cells (Fig. 2B). As shown in Fig. 2C, the −525-Luc construct is sufficient to recapitulate BMP4-mediated repression. Multiple positive regulatory elements, including Oct4, Sox2, and Nanog binding sites, have been previously mapped to this region, (Fig. 2B) (14). Computational analysis identified an evolutionarily conserved Smad binding element (SBE, 5′-CAGACC-3′) at position -191 (supplemental Fig. S1 and Fig. 2B). To test the involvement of this putative SBE in miR-302 cluster repression, we generated constructs containing the wild type SBE sequence from the miR302 promoter (SBE-Luc) or a mutated SBE sequence (5′-GAAACC-3′) [SBE(Mut)-Luc] (Fig. 2B) and subjected them to the reporter assay. While the SBE-Luc construct was repressed by BMP4 treatment, similarly to the −525-Luc or −974-Luc reporters, mutation in the SBE abolished regulation by BMP4 (Fig. 2C), pinpointing the −191 SBE as a critical target of BMP4-mediated repression of the miR-302∼367 cluster. To investigate whether a Smad1/5-Smad4 complex was directly recruited to the −191 SBE upon BMP4 treatment, we performed a ChIP assay. Genomic DNA fragments associated with phospho-Smad1/5 or Smad4 were immunoprecipitated with appropriate antibodies, followed by PCR amplification with either a primer set recognizing the region containing the −191 SBE (miR-302 primer, Fig. 2D, top) or a control primer set that recognizes a sequence >2 kb upstream of the SBE (Fig. 2D, top). A primer set recognizing the miR-143/145 promoter was included as control because of its previously demonstrated association with a Smad complex (21). As negative controls, DNA fragments precipitated with nonspecific rabbit IgGs were also subjected to amplification with miR-302, control or miR-143/145 primers (Fig. 2D). The ChIP assay demonstrated BMP4-induced recruitment of phospho-Smad1/5 and Smad4, to the miR-302 promoter, as well as to the miR-143/145 promoter (Fig. 2D). It is known that Smad proteins can repress transcription through recruitment of HDACs to promoter regions. To examine the potential involvement of HDACs in the repression of miR-302∼367 transcription, cells were treated with the HDAC inhibitors trichostatin A (TSA) or sodium butyrate (NaBu), followed by BMP4 stimulation. Down-regulation of pri-miR-302 by BMP4 was abolished upon treatment with these HDAC inhibitors (Fig. 2E), suggesting that the recruitment of HDACs is required for transcriptional repression of miR-302 by Smads. It has been previously shown that Smad1/Smad4 complex-mediated recruitment of HDAC1 is responsible for the BMP-dependent transcriptional repression of the Nkx3.2 gene (26). HDAC1 belongs to class I HDACs, which are inhibited by TSA and NaBu. To examine the potential role of HDAC1 in the repression of miR-302 upon BMP4 treatment, endogenous HDAC1 in PASMCs was reduced by two independent siRNAs to <30% (Fig. 2F). Under these conditions, repression of miR-302 by BMP4 was greatly reduced, but not abolished (Fig. 2F). This result suggests that HDAC1 is involved in the transcriptional repression of miR-302∼367 by BMP4; however, it is plausible that other members of class I or class II HDACs might also be necessary.
BMPRII Is a Novel Target of miR-302c
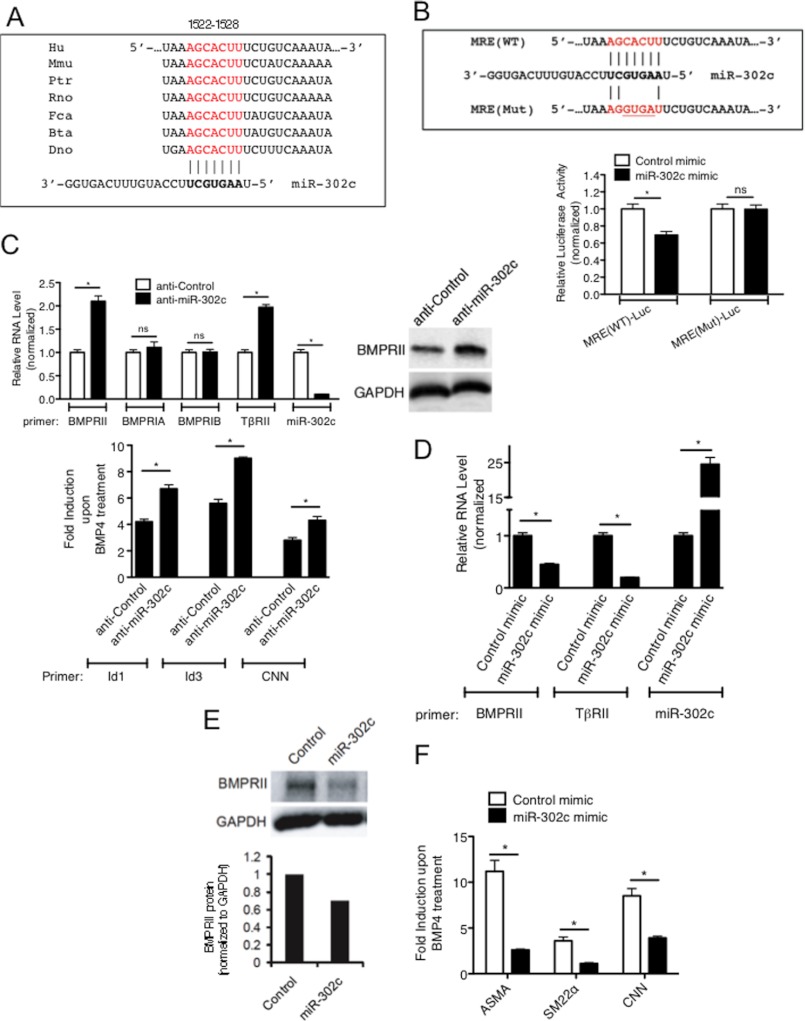
In a search for potential targets of the miR-302∼367 family of miRNAs using the TargetScan target prediction algorithm (supplemental Fig. S2), we discovered an evolutionarily conserved miRNA recognition element (MRE) partially complementary to miR-302a-e (Fig. 3A) in the 3′-UTR of the type II BMP receptor (BMPRII) gene. To examine whether this predicted MRE is targeted by miR-302c, we generated a luciferase reporter construct containing the putative 21-nt miR-302 MRE sequence at the 3′-end of the luciferase cDNA [MRE(WT)-Luc] (Fig. 3B). We also designed a control construct [MRE(Mut)-Luc] with 4-nt mutations in the miR-302 MRE sequence (Fig. 3B, upper panel). These reporter constructs were co-transfected into COS7 cells with 2-O-methylated RNA oligonucleotides corresponding to either the miR-302c sequence (miR-302c mimic) or a GFP sequence as control (Control mimic). The luciferase activity of MRE(WT)-Luc was reduced ∼30% upon cotransfection with miR-302c, while the activity of the mutated construct was not affected (Fig. 3B, lower panel), demonstrating that the miR-302 MRE in the BMPRII mRNA 3′-UTR can be targeted by miR-302c and possibly other members of the miR-302 family of miRNAs.
FIGURE 3.
miR-302 family targets BMPRII gene. A, miR-302 MRE sequence found in the 3′-UTR of BMPRII mRNA are conserved in different mammals. Hu: human, Mmu: mouse, Ptr: chimpanzee, Rno: rat, Fca: cat, Bta: cow, and Dno: armadillo. B, sequence of wild type miR-302 MRE [MRE(WT)] and mutated MRE [MRE(Mut)] cloned into the 3′-UTR of the luciferase gene is shown (upper panel). Luciferase activity was examined in COS7 cells transfected with MRE(WT)-Luc or MRE(Mut)-Luc with miR-302 mimic or control mimic. Luciferase activity with miR-302 mimic relative to the activity with control mimic is shown. C, PASMCs were transfected with 50 nm antisense against GFP (anti-Control) or anti-miR-302c, followed by BMP4 treatment. Levels of BMPRII, BMPRIA, BMPRIB, TβRII, Id1, Id3, or CNN mRNAs relative to GAPDH and miR-302c relative to U6 snRNA were quantitated by qRT-PCR analysis. Fold induction of Id1, Id3, or CNN mRNAs upon BMP4 treatment is plotted (bottom panel). Data represent mean ± S.E. of three independent experiments. *, p < 0.01. ns: statistically not significant. Total cell lysates were subjected to immunoblot analysis of endogenous BMPRII and GAPDH (loading control) (top right panel). D, PASMCs were transfected with 5 nm Control mimic or miR-302c mimic, followed by qRT-PCR expression analysis of BMPRII or TβRII mRNAs, relative to GAPDH and miR-302c relative to U6 snRNA. Data represent mean ± S.E.; *, p < 0.01. E, total cell lysates of PASMC transfected with 5 nm Control mimic or miR-302c mimic were subjected to immunoblot analysis of endogenous BMPRII and GAPDH (loading control). Relative intensity of the band of BMPRII and GAPDH protein are quantitated by ImageJ and shown at the bottom panel. F, PASMCs were transfected with Control mimic or miR-302c mimic, followed by qRT-PCR expression analysis to quantitate ASMA, SM22α, and CNN mRNAs relative to GAPDH.
To assess whether endogenous miR-302 regulates the expression of BMPR2, miR-302c was inhibited by transfection of antisense RNA oligonucleotides complementary to the miR-302c sequence (anti-miR-302c) in PASMCs. Anti-miR-302c reduced the level of miR-302c to less than 10% of the basal level (Fig. 3C, miR-302c). The level of type II TGFβ receptor (TβRII) mRNA, a previously validated target of miR-302c (9), was elevated ∼2-fold by anti-miR-302c, presumably due to derepression by inactivation of miR-302c (Fig. 3C, TβRII, top panel). Increased expression of TβRII led to an increase in the induction of the target gene Smad7 by TGFβ (supplemental Fig. S3). Similarly to TβRII, BMPRII mRNA (Fig. 3C, BMPRII, top left panel), as well as its protein (Fig. 3C, top right panel) were elevated ∼2-fold upon anti-miR-302c transfection, while the mRNAs of type IA or IB BMP receptors (BMPRIA or BMPRIB) were unchanged (Fig. 3C, top left panel). The induction of BMP target genes Id1, Id3, and calponin1 (CNN) upon BMP4 treatment was enhanced in anti-miR-302c-transfected cells in comparison with control cells, suggesting that increased expression of BMPRII translates into an augmented downstream pathway signaling (Fig. 3C, bottom panel). Conversely, when miR-302c mimic was transfected, BMPRII and TβRII mRNA levels were reduced to 55 and 20% of the basal level, respectively (Fig. 3D). Immunoblot analysis confirmed that miR-302c led to a reduction of BMPRII protein (Fig. 3E). These results demonstrate that BMPRII is a novel target of miR-302c. To examine the functional consequence of miR-302c-dependent down-regulation of BMPRII on the BMP signaling pathway, BMP4 induction of contractile genes, such as ASMA, CNN, and SM22 was examined upon overexpression of miR-302c or control. As shown in Fig. 3F, the induction of all 3 contractile genes was reduced upon miR-302c mimic transfection (Fig. 3F). This result demonstrates that forced expression of miR-302c negatively regulates the BMP signaling pathway, likely due to the decrease in BMPRII level (Fig. 3F).
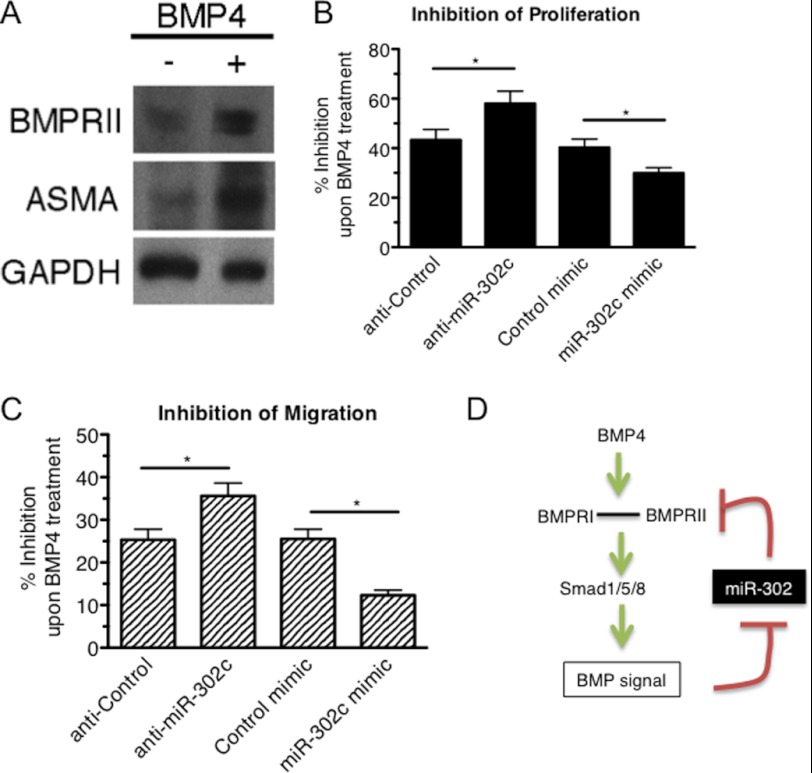
To examine whether the BMP4-mediated down-regulation of miR-302 modulates BMPRII expression, we stimulated PASMCs with BMP4 for 24 h, followed by immunoblot analysis of BMPRII (Fig. 4A). ASMA is shown as positive control of BMP treatment (Fig. 4A). As shown in Fig. 4A, BMPRII protein was elevated upon BMP4 treatment (Fig. 4A). Next we examined whether the BMP4-miR-302-BMPRII axis affects the modulation of vSMC phenotype by BMP4. We have previously shown that BMP4 inhibits proliferation and migration of PASMCs and promotes a highly differentiated, contractile vSMC phenotype (17, 21–23). PASMCs were transfected with miR-302c mimic or anti-miR-302c, followed by BMP4 stimulation and cell counting (Fig. 4B) or scratch-wound migration assay (Fig. 4C). BMP4-mediated inhibition of proliferation and migration was augmented upon transfection of anti-miR-302c, while miR-302 mimic transfection reduced the inhibitory effects of BMP4 (Fig. 4, B and C). In summary, these results support a model in which the BMP4-miR-302-BMPRII axis modulates the Smad signaling pathway and affects vSMC phenotype (Fig. 4D).
FIGURE 4.
Functional significance of the BMP-miR-302-BMPRII axis in the BMP signaling. A, immunoblot analysis of BMPRII, ASMA, and GAPDH (loading control) in PASMC with or without BMP4 treatment. B, PASMCs were transfected with anti-Control, anti-miR-302c, Control mimic, or miR-302c mimic, starved for 24 h, followed by treatment with BMP4 for 48 h. Cells were tryspinized and counted using a hemacytometer. The relative number of cells with BMP4 treatment compared with untreated cells was plotted as % inhibition of proliferation. Means ± S.E. of triplicate measurements of three independent experiments; *, p < 0.05. C, PASMCs transfected with anti-Control, anti-miR-302c, Control mimic or miR-302c mimic were subjected to the scratch wound assay in the presence or absence of BMP4. Results are shown as the relative migration distance with BMP4 treatment compared with untreated cells and plotted as % inhibition of migration upon BMP4 treatment; mean ± S.E. of triplicate measurements of three independent experiments; *, p < 0.05. D, schematic diagram of a positive regulatory loop of the BMP signaling pathway via the miR-302-BMPRII axis.
DISCUSSION
The BMP signaling pathway exhibits various biological activities via transcriptional regulation of protein-coding genes by the Smad signal transducers. In general, Smads associate with different co-factors and modulate gene transcription both positively and negatively (5). In addition to protein coding genes, several miRNAs have been identified as transcriptional targets of TGFβ-specific Smads, such as miR-216, miR-29, miR-143/145, and let-7a/d (8). In this study, we demonstrate that transcription of the miR-302∼367 gene cluster is repressed by the BMP signaling pathway in a Smad-dependent manner. miR-302 is most abundantly expressed in pluripotent hESCs and its expression rapidly declines upon differentiation. Exogenous expression of miR-302∼367 cluster miRNAs can directly reprogram somatic cells without the use of any transcription factors to generate pluripotent cells, suggesting a critical role of miR-302 and related miRNAs in the induction and maintenance of pluripotency (9, 27, 28). A genome-wide identification of miR-302 targets in human ESCs (hESCs) using a photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation (PAR-CLIP) method identified miR-302 target genes that act as antagonists of BMP signaling (13), such as TOB2, DAZAP2, and SLAIN1, and suggested that one of the mechanisms by which miR-302 maintains pluripotency in hESCs is through repression of BMP inhibitors and up-regulation of BMP signaling (13). The same study also found BMPRII as one of the transcripts associated with miR-302 expression in hESCs, but did not confirm a functional relationship between miR-302 and BMPRII (13). In addition to the maintenance of pluripotency, miR-302 plays a role in lineage choice upon induction of differentiation in hESCs (12). A previous study suggests that miR-302 up-regulates BMP signaling to inhibit neural differentiation (29). These result hints to a fine-balancing act of miR-302 for lineage choice during the differentiation of mesoderm: maintaining high enough levels of BMP signaling to prevent unintended neural induction but low enough levels to avoid trophectoderm and mesendodermal induction, presumably by raising the threshold for differentiation. Our finding that miR-302 is capable of down-regulating both the mediator and the inhibitors of BMP signaling suggests that (i) modulation of miR-302 can result in either reduction or activation of BMP signaling depending on the cellular context and levels of expression of miR-302 targets and (ii) miR-302 can cell-autonomously modulate the BMP signal.
It has been shown that miR-302 targets the type II receptor of TGFβs (TβRII) and enhances the efficiency of reprogramming of somatic cells by inhibiting the TGFβ-mediated epithelial-to-mesenchymal transition (EMT) (9). A more recent study demonstrates that miR-302 targets Lefty-1 and Lefty-2, which are both inhibitors of the TGFβ/nodal signaling pathway, and promotes the TGFβ/nodal signaling to balance between pluripotency and germ layer specification, similarly to the control of BMP signaling pathway by miR-302 (15). This bifunctional role of miR-302 in the regulation of the TGFβ/nodal pathway through targeting both the mediator and the inhibitors of the TGFβ/nodal pathway is very similar to the regulation of BMP signaling by miR-302. It is likely that the significant roles of miR-302 during reprogramming of somatic cells and early embryogenesis are presumably due to the versatility of the miR-302 family of miRNAs in modulating both BMP and TGFβ signaling pathways positively and negatively. Members of the TGFβ family of growth factors, such as BMPs and nodals, act as morphogens, with different concentrations responsible for mediating different tissue types in distinct spatial order during embryogenesis (30). Morphogen-regulated pathways have to be tightly controlled in order to transmit a distinct signal by orchestrating context- and spatial-specific changes in gene expression. We speculate that the regulatory loop of BMP, miR-302 and targets of miR-302 enables miR-302 to control the intensity and the duration of the TGFβ or BMP signal, which is critical for exhibiting precise gene expression profiles and cellular outcomes.
Although the miR-302 family of miRNAs is expressed at a low level in differentiated cells in general, it can be elevated upon specific stimuli or pathological conditions. Our study suggests that abnormal elevation of miR-302 can lead to down-regulation of contractile genes in vSMCs, which is often observed in the medial layer of the remodeled vasculature in PAH or restenosis patients (3). miRNAs of the miR-302∼367 cluster are also highly expressed in germ cell tumors (GCTs) and serum samples from patients with GCTs (31, 32). Higher expression of miR-302 is also observed in cancer stem cells (33). Our study sheds light on a mechanism by which miR-302 and its family of related miRNAs may be induced in GCTs or other tumors in a BMP-dependent manner and on how miR-302 may play a role in tumorigenesis through modulating the activities of the BMP and TGFβ signaling pathway.
Acknowledgments
We thank Dr. Menéndez for miR-302 promoter-luciferase constructs. We thank members of the Hata/Lagna laboratory for critical discussion and advice.
This work was supported, in whole or in part, by NHLBI, National Institutes of Health Grant HL093154, the LeDucq Foundation, and the American Heart Association (to A. H.).

This article contains supplemental Figs. S1–S3.
- BMP
- bone morphogenetic protein
- miRNA
- microRNA
- HDAC
- histone deacetylase
- qRT-PCR
- quantitative reverse transcriptase-PCR
- PASMC
- pulmonary artery smooth muscle cell
- MRE
- miRNA recognition element
- PAR-CLIP
- photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation
- EMT
- epithelial-to-mesenchymal transition
- GCT
- germ cell tumor
- ESC
- embryonic stem cell
- EC
- endothelial cell
- vSMC
- vascular smooth muscle cell
- UTR
- untranslated region.
REFERENCES
- 1. Hogan B. L. (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580–1594 [DOI] [PubMed] [Google Scholar]
- 2. Davis-Dusenbery B. N., Wu C., Hata A. (2011) Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 31, 2370–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 4. ten Dijke P., Arthur H. M. (2007) Extracellular control of TGFβ signaling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 8, 857–869 [DOI] [PubMed] [Google Scholar]
- 5. Massagué J., Gomis R. R. (2006) The logic of TGFβ signaling. FEBS Lett. 580, 2811–2820 [DOI] [PubMed] [Google Scholar]
- 6. Davis-Dusenbery B. N., Hata A. (2010) Mechanisms of control of microRNA biogenesis. J. Biochem. 148, 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim V. N., Han J., Siomi M. C. (2009) Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 8. Blahna M. T., Hata A. (2012) Smad-mediated regulation of microRNA biosynthesis. FEBS Lett. 586, 1906–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subramanyam D., Lamouille S., Judson R. L., Liu J. Y., Bucay N., Derynck R., Blelloch R. (2011) Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 29, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson K. D., Venkatasubrahmanyam S., Jia F., Sun N., Butte A. J., Wu J. C. (2009) MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev 18, 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin S. L., Chang D. C., Chang-Lin S., Lin C. H., Wu D. T., Chen D. T., Ying S. Y. (2008) Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 14, 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipchina I., Studer L., Betel D. (2012) The expanding role of miR-302–367 in pluripotency and reprogramming. Cell Cycle 11, 1517–1523 [DOI] [PubMed] [Google Scholar]
- 13. Lipchina I., Elkabetz Y., Hafner M., Sheridan R., Mihailovic A., Tuschl T., Sander C., Studer L., Betel D. (2011) Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes Dev. 25, 2173–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosa A., Brivanlou A. H. (2011) A regulatory circuitry comprised of miR-302 and the transcription factors OCT4 and NR2F2 regulates human embryonic stem cell differentiation. EMBO J. 30, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barroso-delJesus A., Lucena-Aguilar G., Sanchez L., Ligero G., Gutierrez-Aranda I., Menendez P. (2011) The Nodal inhibitor Lefty is negatively modulated by the microRNA miR-302 in human embryonic stem cells. FASEB J. 25, 1497–1508 [DOI] [PubMed] [Google Scholar]
- 16. Barroso-del Jesus A., Lucena-Aguilar G., Menendez P. (2009) The miR-302–367 cluster as a potential stemness regulator in ESCs. Cell Cycle 8, 394–398 [DOI] [PubMed] [Google Scholar]
- 17. Lagna G., Ku M. M., Nguyen P. H., Neuman N. A., Davis B. N., Hata A. (2007) Control of phenotypic plasticity of smooth muscle cells by BMP signaling through the myocardin-related transcription factors. J. Biol. Chem. 282, 37244–37255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neuman N. A., Ma S., Schnitzler G. R., Zhu Y., Lagna G., Hata A. (2009) The four-and-a-half LIM domain protein 2 regulates vascular smooth muscle phenotype and vascular tone. J. Biol. Chem. 284, 13202–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barroso-delJesus A., Romero-López C., Lucena-Aguilar G., Melen G. J., Sanchez L., Ligero G., Berzal-Herranz A., Menendez P. (2008) Embryonic stem cell-specific miR302–367 cluster: human gene structure and functional characterization of its core promoter. Mol. Cell Biol. 28, 6609–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis B. N., Hilyard A. C., Lagna G., Hata A. (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis-Dusenbery B. N., Chan M. C., Reno K. E., Weisman A. S., Layne M. D., Lagna G., Hata A. (2011) Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J. Biol. Chem. 286, 28097–28110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan M. C., Hilyard A. C., Wu C., Davis B. N., Hill N. S., Lal A., Lieberman J., Lagna G., Hata A. (2010) Molecular basis for antagonism between PDGF and the TGFβ family of signaling pathways by control of miR-24 expression. EMBO J. 29, 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang H., Davis-Dusenbery B. N., Nguyen P. H., Lal A., Lieberman J., Van Aelst L., Lagna G., Hata A. (2012) Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J. Biol. Chem. 287, 3976–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis B. N., Hilyard A. C., Nguyen P. H., Lagna G., Hata A. (2010) Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol. Cell 39, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollnagel A., Oehlmann V., Heymer J., Rüther U., Nordheim A. (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 274, 19838–19845 [DOI] [PubMed] [Google Scholar]
- 26. Kim D. W., Lassar A. B. (2003) Smad-dependent recruitment of a histone deacetylase/Sin3A complex modulates the bone morphogenetic protein-dependent transcriptional repressor activity of Nkx3.2. Mol. Cell Biol. 23, 8704–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuo C. H., Deng J. H., Deng Q., Ying S. Y. (2012) A novel role of miR-302/367 in reprogramming. Biochem. Biophys. Res. Commun. 417, 11–16 [DOI] [PubMed] [Google Scholar]
- 28. Lin S. L., Chang D. C., Lin C. H., Ying S. Y., Leu D., Wu D. T. (2011) Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 39, 1054–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosa A., Spagnoli F. M., Brivanlou A. H. (2009) The miR-430/427/302 family controls mesendodermal fate specification via species-specific target selection. Dev. Cell 16, 517–527 [DOI] [PubMed] [Google Scholar]
- 30. Hogan B. L., Blessing M., Winnier G. E., Suzuki N., Jones C. M. (1994) Growth factors in development: the role of TGF- related polypeptide signaling molecules in embryogenesis. Dev. Suppl, 53–60 [PubMed] [Google Scholar]
- 31. Murray M. J., Halsall D. J., Hook C. E., Williams D. M., Nicholson J. C., Coleman N. (2011) Identification of microRNAs From the miR-371∼373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am. J. Clin. Pathol. 135, 119–125 [DOI] [PubMed] [Google Scholar]
- 32. Palmer R. D., Murray M. J., Saini H. K., van Dongen S., Abreu-Goodger C., Muralidhar B., Pett M. R., Thornton C. M., Nicholson J. C., Enright A. J., Coleman N. (2010) Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 70, 2911–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Golestaneh A. F., Atashi A., Langroudi L., Shafiee A., Ghaemi N., Soleimani M. (2012) miRNAs expressed differently in cancer stem cells and cancer cells of human gastric cancer cell line MKN-45. Cell Biochem. Funct. 30, 411–418 [DOI] [PubMed] [Google Scholar]