Background: Copper trafficking in Gram-negative bacteria supplies cuproenzymes via transporters and periplasmic chaperones.
Results: Symbiotic nitrogen fixation, denitrification, and copper-starved growth depend on a periplasmic, copper-binding protein named PcuC.
Conclusion: The pcuC mutant phenotypes are caused by defects in copper-containing respiratory enzymes.
Significance: Research on cytochrome oxidase biogenesis in α-proteobacteria, the extant relatives of mitochondria, helps to understand how mitochondria assemble the respiratory chain.
Keywords: Bacterial Genetics, Bioenergetics, Copper, Cytochrome Oxidase, Metalloproteins, Respiratory Chain, Symbiosis, Trafficking, Transcriptomics, Symbiotic Nitrogen Fixation, Bacterial Respiration, Copper Acquisition
Abstract
Microarray analysis of Bradyrhizobium japonicum grown under copper limitation uncovered five genes named pcuABCDE, which are co-transcribed and co-regulated as an operon. The predicted gene products are periplasmic proteins (PcuA, PcuC, and PcuD), a TonB-dependent outer membrane receptor (PcuB), and a cytoplasmic membrane-integral protein (PcuE). Homologs of PcuC and PcuE had been discovered in other bacteria, namely PCuAC and YcnJ, where they play a role in cytochrome oxidase biogenesis and copper transport, respectively. Deletion of the pcuABCDE operon led to a pleiotropic phenotype, including defects in the aa3-type cytochrome oxidase, symbiotic nitrogen fixation, and anoxic nitrate respiration. Complementation analyses revealed that, under our assay conditions, the tested functions depended only on the pcuC gene and not on pcuA, pcuB, pcuD, or pcuE. The B. japonicum genome harbors a second pcuC-like gene (blr7088), which, however, did not functionally replace the mutated pcuC. The PcuC protein was overexpressed in Escherichia coli, purified to homogeneity, and shown to bind Cu(I) with high affinity in a 1:1 stoichiometry. The replacement of His79, Met90, His113, and Met115 by alanine perturbed copper binding. This corroborates the previously purported role of this protein as a periplasmic copper chaperone for the formation of the CuA center on the aa3-type cytochrome oxidase. In addition, we provide evidence that PcuC and the copper chaperone ScoI are important for the symbiotically essential, CuA-free cbb3-type cytochrome oxidase specifically in endosymbiotic bacteroids of soybean root nodules, which could explain the symbiosis-defective phenotype of the pcuC and scoI mutants.
Introduction
This report deals with copper routing to membrane-bound heme-copper cytochrome oxidases in a Gram-negative bacterium. Based on the presence of different copper sites, two groups of heme-copper oxygen reductases must be distinguished (1, 2): (i) those that carry only the high-spin heme-CuB center on subunit I, which is buried in the membrane part and forms the active site for O2 reduction to H2O (3), and (ii) those that carry in addition a binuclear Cu-Cu center (CuA) on subunit II, which is exposed to the periplasm and serves as a recipient of the electrons delivered by periplasmic c-type cytochromes (4, 5). Hence, members of the second group are cytochrome oxidases, represented prominently by the mitochondrial and bacterial aa3-type oxygen reductases, whereas most of the bacterial quinol oxidases belong to the first group. The bacterial cbb3-type cytochrome oxidase is in a class of its own (6). Although it lacks the CuA center, electrons are delivered to the heme-CuB site on subunit I (FixN or CcoN) via the c-type cytochromes FixO/CcoO and FixP/CcoP, which constitute subunits II and III of the enzyme complex (7). In contrast to mitochondria, which have only one energy-conserving respiratory chain with one terminal oxidase, bacteria often employ a branched respiratory chain terminating with disparate oxidases, allowing them to efficiently adapt to a wide range of environmental oxygen concentrations (8, 9). The symbiotic nitrogen-fixing bacterium Bradyrhizobium japonicum, the organism investigated here, reflects this complexity most notably because it possesses up to eight different terminal oxidases. Two of these dominate (i.e. cytochrome aa3 in oxically grown cells and cytochrome cbb3 in symbiosis) (10).
The question of how copper is delivered to and assembled in the CuA and CuB centers has been addressed not only in mitochondria but also in Gram-negative bacteria, primarily in members of the α-proteobacteria (e.g. Rhodobacter species, B. japonicum, Paracoccus denitrificans) because they are phylogenetically the closest extant relatives of mitochondria. Certain conserved functions seem to emerge for each of the following four biogenesis factors. (i) CoxG, also termed CtaG, is a homolog of the mitochondrial Cox11 protein (10–14). The protein is a membrane-anchored Cu(I) chaperone with the copper-binding domain facing the periplasm or the mitochondrial intermembrane space. It plays a role in the assembly of the CuB center of cytochrome aa3 (12). For unknown reasons, however, CoxG does not appear to be required for the symbiotically essential cbb3-type oxidase in B. japonicum, although this enzyme also carries a CuB center (10). (ii) FixI, also called CcoI or CtpA, might be the unknown factor for CuB insertion into cytochrome cbb3. If it is a copper-translocating P1B-type ATPase, as previously proposed (15, 16), this protein might work as an uptake system destined to insert copper into subunit I. Although copper import has yet to be proven unequivocally, the dependence of cytochrome cbb3 assembly on FixI (or its paralogs) is well documented (16–18). (iii) ScoI, also called SenC or PrrC, is a homolog of the mitochondrial copper chaperone Sco1 (for a review, see Ref. 19). Like CoxG, the ScoI protein is anchored to the bacterial cytoplasmic membrane or the mitochondrial inner membrane, where the copper-binding globular domain protrudes into the periplasm or the intermembrane space. Work with the Bacillus subtilis (20) and the mitochondrial Sco1 proteins has suggested a role in the metallation of the CuA site on subunit II of the aa3-type cytochrome oxidase (19). Subsequent work with B. japonicum ScoI has adopted this view, supported by the fact that a scoI knock-out mutant was clearly defective in cytochrome aa3 but not in cytochrome cbb3 when the latter was tested in cells that had been grown micro-oxically or anoxically (10). The symbiosis defect caused by the B. japonicum scoI mutant remained enigmatic (10, 21). Other reports have shown, however, that SenC and PrrC play a role in the formation of active cytochrome cbb3 (22–24). (iv) A more recently discovered copper chaperone is PCuAC, which rivals Sco1 in its function to metallate the CuA site. Abriata et al. (25) designed in vitro experiments that documented the direct transfer of Cu(I) to the CuA site of the Thermus thermophilus ba3 oxidase. Sco1 functioned in this assay as a disulfide reductase to maintain the correct oxidation state of the subunit II cysteine ligands. In vivo data with Rhodobacter sphaeroides confirmed that PCuAC and Sco1 co-participate in the assembly of a functional CuA center in cytochrome aa3 (24). Intriguingly, the authors of this study noticed a role of PCuAC also in the formation of the CuB center of cytochrome cbb3. Homologous genes for PCuAC-like proteins had been recognized in the B. japonicum genome (10, 21), but detailed studies on their biochemical function had not been done.
The present work was initiated with the idea of finding new genes for copper acquisition in B. japonicum. Incidentally, transcriptome analyses of copper-starved cells uncovered an operon that also contained the gene for a PCuAC-like protein. What ensued was an extensive genetic and biochemical investigation that proved its identity as a copper protein and provided evidence for its role in the biogenesis of both the aa3- and cbb3-type cytochrome oxidases.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Media, and Growth Conditions
Escherichia coli was grown in Luria-Bertani (LB) medium (26) containing the following concentrations of antibiotics, if necessary: ampicillin, 200 μg/ml; kanamycin, 30 μg/ml; spectinomycin, 20 μg/ml; tetracycline, 10 μg/ml. B. japonicum was routinely cultivated in a peptone-salts-yeast extract medium supplemented with 0.1% l-arabinose (27, 28). Buffered Vincent's minimal medium (BVM),2 here defined as vitamin-free modified Vincent's minimal medium (29, 30) supplemented with trace elements (31), 10 mm MOPS (final pH adjusted to 6.8 with 2 m NH3), and 0.3% l-arabinose, was alternatively used. This medium contains 20 nm CuSO4. Glassware was treated overnight with 0.1 m HCl and rinsed thoroughly with double-distilled H2O when used for experiments on copper limitation, and 10 μm BCS and 1 mm ascorbate were added. Yeast extract-mannitol medium (YEM) (32) supplemented with 10 mm KNO3 was used for anoxic growth (nitrate respiration). Where appropriate, antibiotics were added to these final concentrations: kanamycin, 100 μg/ml; spectinomycin, 100 μg/ml; streptomycin, 50 μg/ml; tetracyclin, 50 μg/ml (solid media) or 25 μg/ml (liquid media). B. japonicum strains used in this work are listed in Table 1; E. coli strains are listed in supplemental Table S1.
TABLE 1.
B. japonicum strains used in this work
| Strain | Relevant genotype or phenotype | Source or Ref. |
|---|---|---|
| 110spc4 | Spr wild type | Ref. 27 |
| COX132 | Spr Kmr coxA::Tn5 | Ref. 33 |
| 3613 | Spr Kmr fixN::Tn5 | Ref. 34 |
| 2574 | Spr Kmr ΔscoI::aphII (same orientation) | Ref. 10 |
| 6611 | Spr Kmr ΔpcuABCDE::aphII (same orientation) | This work |
| 6612 | Spr Kmr ΔpcuABCDE::aphII (opposite orientation) | This work |
| 6620 | Spr Smr Δblr7088::Ω (same orientation) | This work |
| 6621 | Spr Smr Δblr7088::Ω (opposite orientation) | This work |
| 6611-20 | Spr Kmr Smr ΔpcuABCDE::aphII (same orientation), Δblr7088::Ω (same orientation) | This work |
| 6611-21 | Spr Kmr Smr ΔpcuABCDE::aphII (same orientation), Δblr7088::Ω (opposite orientation) | This work |
| 6632 | Spr Tcr pSUP202pol4 chromosomally integrated in 110spc4 | This work |
| 6611-32 | Spr Kmr Tcr pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-33 | Spr Kmr Tcr pcuABCDE on pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-34 | Spr Kmr Tcr pcuAB[ΔC]DE on pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-6630 | Spr Kmr Tcr pcuABCD[ΔE] on pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-1654 | Spr Kmr Tcr pcuABC[ΔD]E on pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-1650 | Spr Kmr Tcr pcuA[ΔB]CDE on pSUP202pol4 chromosomally integrated in 6611 | This work |
| 6611-1653 | Spr Kmr Tcr pcu[ΔA]BCDE on pSUP202pol4 chromosomally integrated in 6611 | This work |
Mutant Constructions
Detailed information on plasmids and primers is given in supplemental Tables S1 and S2, respectively. For both the ΔpcuABCDE and Δblr7088 marker replacement mutants, the upstream and downstream flanking regions of the target genomic sequences were amplified and cloned into pBluescript SK(+) (Stratagene, La Jolla, CA). The aphII gene encoding kanamycin resistance or the Ω cassette encoding streptomycin resistance (ΔpcuABCDE and Δblr7088 mutants, respectively) was then inserted in both orientations between the upstream and downstream flanking regions. The DNA constructs were excised and inserted into the suicide plasmid pSUP202pol4 (35), yielding plasmids pRJ6611, pRJ6612, pRJ6620, and pRJ6621. Mobilization of these plasmids into B. japonicum 110spc4 was carried out via E. coli S17-1 and followed by screening for double recombination events. The resulting strains 6611 (ΔpcuABCDE, same orientation of the aphII gene) and 6612 (ΔpcuABCDE, opposite orientation) carry a deletion between positions 5,408,844 and 5,414,217 of the genome (Fig. 1). Strains 6620 (Δblr7088, same orientation of the Ω cassette) and 6621 (Δblr7088, opposite orientation) are deleted between positions 7,804,884 and 7,805,644. For the generation of double mutants 6611-20 (ΔpcuABCDE, same orientation; Δblr7088, same orientation) and 6611-21 (ΔpcuABCDE, same orientation; Δblr7088, opposite orientation), the plasmid pRJ6611 was mobilized into strains 6620 and 6621, respectively.
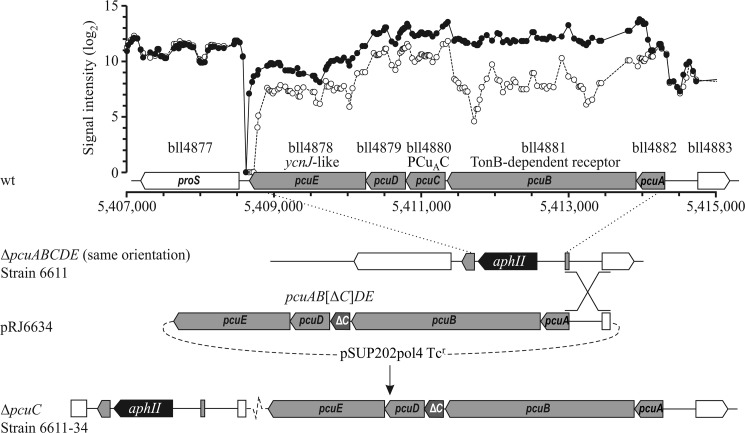
FIGURE 1.
Map of the pcuABCDE gene cluster. Gene names and relevant homologies (if available) are given below the gene numbers. Genome coordinates are reported below the gene map. The top part of the scheme shows the transcription levels of B. japonicum wild-type cells grown in copper excess BVM (2 μm CuSO4; open circles) and in copper starvation BVM (5 nm copper; closed circles) obtained from microarray analyses. The bottom part shows the genotype of the ΔpcuABCDE::aphII strain (same orientation) and the strategy used to partially complement it with an in-frame pcuC-deleted version of the operon (pcuAB[ΔC]DE), resulting in a ΔpcuC genotype. Analogous strategies were used to construct ΔpcuA, ΔpcuB, ΔpcuD, and ΔpcuE strains.
Complementation of ΔpcuABCDE
The genome sequence comprising the pcuABCDE operon and its upstream region necessary for a single crossover event (corresponding to genome coordinates 5,408,585–5,414,850), with the addition of appropriate restriction sites, was cloned into vector pGEM-T Easy (Promega, Madison, WI), yielding plasmid pRJ6626. The restriction map of pRJ6626 allowed excision of operon fragments via simple digestions followed by self-ligation: FseI treatment deleted the entire operon, leaving its 5′ region to give an empty vector insertion for control (pRJ6631); SphI was used to excise pcuE (pRJ6627); MlsI/OliI were used to in-frame delete pcuB (pRJ6628). When in-frame deletion of genes was not possible with the aforementioned strategy, alternative approaches were applied. Substitution of the naturally occurring HindIII/FspAI fragment with a shorter PCR-generated fragment, including the natural HindIII site on its 3′ end and an added FspAI site on its 5′ end, was carried out to obtain an in-frame pcuD deletion (pRJ1651). Analogously, natural AbsI/EcoRV restriction sites were exploited for the pcuC in-frame deletion (pRJ6629). An overlapped extension PCR (36) was used to obtain a fragment carrying a pcuA in-frame deletion, which substituted the full-length gene via BamHI/SpeI restriction sites (pRJ1652). SpeI/PsiI fragments from pRJ6626, pRJ6631, pRJ6627, pRJ1651, pRJ6629, pRJ6628, and pRJ1652 were inserted into a SpeI/SmaI-linearized pSUP202pol4 suicide plasmid, yielding pRJ6633, pRJ6632, pRJ6630, pRJ1654, pRJ6634 (Fig. 1), pRJ1650, and pRJ1653, respectively. These plasmids were then mobilized into B. japonicum 6611 via E. coli S17-1, followed by screening for single recombination events (Fig. 1). Plasmid pRJ6632 (empty vector control) was mobilized into B. japonicum 110spc4 as well. All of the resulting strains are listed in Table 1.
Determination of Copper Atoms in Copper-free BVM
The concentration of copper in CuSO4-free BVM prepared with treated glassware was determined by graphite furnace atomic absorption spectroscopy. The instrument used was an AAnalyst800 (PerkinElmer Life Sciences) equipped with a transversely heated graphite furnace and longitudinal Zeeman background correction system. A hollow cathode lamp was chosen as the radiation source. A temperature program was applied to the furnace for solvent drying (110 °C for 30 s followed by 130 °C for 30 s under 250 ml/min argon flow), matrix pyrolysis (1,200 °C for 20 s under 250 ml/min argon flow), and atomization (2,100 °C for 5 s). Copper atoms were detected during the last step by measuring absorption at 324.8 nm. A three-point standard addition procedure was applied for the quantification of copper in the analyzed samples. 10-μl samples were mixed with a matrix modifier solution (∼500 ng of MgNO3 and PdNO3/g of sample) and different amounts of a copper standard solution (0, 0.9, 1.8, and 2.9 ng of copper/g with samples containing up to 3.5 ng; 0, 9, 18, and 29 ng/g with samples containing more than 3.5 ng of copper) in a final volume of 30 μl. Each measurement was repeated three times. All solutions were prepared in 0.5% HNO3. The instrumental limit of detection was 0.05 ng of copper/g of sample.
RNA Isolation and cDNA Synthesis
Cell harvesting, RNA extraction, cDNA synthesis for microarray, and quantitative RT-PCR (qRT-PCR) were performed as described (37).
qRT-PCR
RNA from aerobically grown B. japonicum 110spc4, 6611-33, and 6611-34 was isolated in order to monitor the expression level of pcuD by qRT-PCR, similarly to what had been described previously (38). In order to analyze the influence of copper on the expression of genes pcuA and pcuB, RNA was extracted from B. japonicum 110spc4 grown in standard BVM or in copper-free BVM supplemented with 10 μm BCS and 1 mm ascorbic acid. Details of the primers used are listed in supplemental Table S2.
Primer Extension
Mapping of the transcription start site of the pcuABCDE operon was carried out as described previously (39) using the primer named “primer_extension_1” (supplemental Table S2). RNA was isolated from B. japonicum 110spc4 grown aerobically in standard BVM or in copper-free BVM supplemented with 10 μm BCS and 1 mm ascorbic acid. The sequencing ladder was obtained by sequencing plasmid pRJ6336 (supplemental Table S1) with the same primer that was used for the extension reaction.
Microarrays
Transcriptomics experiments were carried out as described previously (40) using a custom-designed Affymetrix GeneChip® (37). The chip was hybridized with cDNA obtained from B. japonicum 110spc4 grown aerobically either in BVM containing 2 μm CuSO4, in copper-free BVM (5 nm copper), or in copper-free BVM supplemented with 10 μm BCS and 1 mm ascorbic acid. Three biological replicates were prepared for each condition. Data were analyzed using GeneSpring GX 7.3.1 software (Agilent, Santa Clara, CA). The data were filtered for probe sets that were called “present” or “marginal” in at least two of three replicas, and Student's t test with a p value threshold of 0.01 was applied. Differential expression in a comparison of two conditions was defined when the -fold change value was larger than 2 or smaller than −2. Data sets generated in this work are deposited in the GEO database with the record number GSE40437.
Plant Growth
Sterilization of soybean seeds (Glycine max (L.) Merr. cv. Williams), cultivation of plants, and nitrogenase activity measurements were performed as described previously (10, 41–43). Plants were evaluated for nodulation and nitrogen fixation 21 days after the infection with the appropriate B. japonicum strains.
Bacteroid Isolation
Bacteroids were isolated from root nodules and separated from plant material as described previously (44, 45).
Nitrate and Nitrite Detection in the Growth Medium
Adapted versions of described methods were used (46). Cells were removed from the growth medium by centrifugation. For nitrate detection, a 0.1-ml sample of 10-fold diluted supernatant was mixed with 0.1 ml of 1% sulfamic acid and 0.8 ml of a 1:1 mixture of 98% H2SO4 and 85% H3PO4 (v/v). After a 10-min incubation at room temperature, 0.1 ml of 0.12% (w/v) 2,6-dimethyl phenol in 100% (v/v) acetic acid was added. Absorption at 334 nm was recorded after a 90-min incubation at room temperature. A standard curve was recorded from 0 to 10 mm KNO3 in YEM. For nitrite determination, a 0.4-ml sample of 80-fold diluted supernatant was mixed with 0.4 ml of 1% (w/v) sulfanilamide in 7.4% (v/v) HCl and 0.4 ml of 0.02% (w/v) N-(1-naphthyl)ethylenediamine. Absorption at 540 nm was measured after a 30-min incubation at room temperature. A standard curve was taken from 0 to 10 mm NaNO2 in YEM.
Determination of Cytochrome c Oxidase Activity in Membrane Fractions
Preparation of the membrane fraction and determination of cytochrome c oxidase activity were carried out as described previously (10, 47), starting either from culture-grown cells or from purified bacteroids. Concentration of solubilized membrane proteins was determined with the Bradford method (48), using a Bio-Rad assay with bovine serum albumin as the standard.
Immunological Techniques
Rabbit antibodies specific for CoxA and CoxB proteins were available from previous work (10, 49). Western blot analyses were carried out on membrane proteins (35 μg/lane) separated by SDS-PAGE (50) and blotted as described previously (49). Anti-rabbit IgG(H+L)-horseradish peroxidase conjugate (Bio-Rad) and a peroxidase chemiluminescence detection kit (Thermo Fisher Scientific, Waltham, MA) were used to detect bands containing primary antibodies bound to CoxA and CoxB.
Purification of PcuC and Its Derivatives
Details of the plasmids and primers are listed in supplemental Tables S1 and S2, respectively. For the periplasmic expression construct, the B. japonicum genomic sequence corresponding to the pcuC gene product (amino acids 25–173), excluding the predicted signal peptide, was amplified, adding a Strep-tag II sequence and a TEV protease recognition site (51) at the 5′ end, and cloned into pEC415 (52). This resulted in a plasmid for expression of PcuC that was additionally fused to the E. coli OmpA signal peptide. For cytoplasmic expression, DNA for the soluble part of PcuC was cloned into pKL1 to fuse it with a His10 tag followed by a TEV recognition site. Derivatives of PcuC in which His79, Met90, His113, Met115, and His113 plus Met115 residues are substituted by alanine were obtained by exchanging the wild-type sequence of the cytoplasmic expression construct with synthetic fragments (custom-synthesized by Eurofins MGW Operon, Ebersberg, Germany) bearing the point mutations. A shortened version of pcuC missing the coding region for the 15-amino acid, methionine-rich C terminus was created by PCR and by inserting a stop codon at the 3′ end. This was cloned into pKL1. Dense E. coli BL21 (DE3) precultures containing the described expression vectors or the TEV protease expression vector pRK793 (53) were used to inoculate 1-liter main cultures. Cells were grown at 37 °C until they reached an optical density (A600) of 0.6; the expression was then induced by adding a final concentration of 0.1% (w/v) arabinose or 0.1 mm isopropyl-β-d-1-thiogalactopyranoside for the periplasmic PcuC expression and for the other expression constructs, respectively. Cultures were transferred to 30 °C, and after 2 h, cells were collected by centrifugation and disrupted by means of three passages at 9,000 p.s.i. through a French press. The periplasmically expressed protein was purified with a Strep-Tactin-Sepharose column (IBA GmbH, Göttingen, Germany) according to the supplier's protocol. To obtain apo-PcuC, the purified protein was incubated with an ∼1,000-fold molar excess of BCS, and the buffer was eventually exchanged with a PD-10 desalting column (GE Healthcare). The poly-His-tagged proteins were purified via nickel-nitrilotriacetic acid-agarose columns (Qiagen, Hilden, Germany). Cleavage of the tag was achieved by treatment with TEV protease, which was later removed with a nickel-nitrilotriacetic acid-agarose column. Purity of the protein and the copper binding status were verified with electrospray ionization MS measurements performed at the Functional Genomics Center Zurich.
Tests for Cu(I) Binding to PcuC
PcuC from cytoplasmic expression was used for the experiment, which proved to be copper-free according to electrospray ionization MS analysis. Alternatively, apo-PcuC was obtained from periplasmic expression as described above. The titration buffer was obtained by filtering 100 mm HEPES, pH 7, 10 mm NaCl through a Chelex 100 chelating ion exchange resin (Bio-Rad) into 0.1 m HCl-treated glassware in order to ensure a maximally possible absence of free copper. The protein elution buffer was exchanged with titration buffer using a PD-10 desalting column. The preparation of Cu(BCS)23− solution and the titration of apo-PcuC on the Cu(BCS)23− complex was carried out as described (54), except that titration buffer was used. The experiment was performed in both anoxic and oxic conditions. In the first case, the solutions were prepared in an anaerobic glove box (Coy Laboratory Products, Grass Lake, MI) with a maximal oxygen concentration of 80 ppm, and the UV-visible spectra were recorded on an Agilent diode array photometer. In the latter case, CuCl was dissolved directly into a BCS solution, and UV-visible spectra were recorded on a Hitachi U-3300 spectrophotometer (Hitachi, Tokyo, Japan).
Bioinformatics Analyses
BLAST was used for homology searches. Multiple alignments were done with ClustalW and visualized with ESPript 2.2. The presence and cleavability of signal peptide were predicted with SignalP. The extinction coefficient of purified proteins was calculated by ProtParam. Homology modeling of the PcuC amino acid sequence on the TtPCuAC three-dimensional structure (Protein Data Bank code 2K6W) was done using SWISS-MODEL.
RESULTS
Comparative Transcription Profile of Cells Grown at Different Copper Concentrations
Copper-limiting growth conditions were thought to cause an induction of genes possibly involved in copper uptake and sorting. This rationale in mind, we performed microarray analyses on B. japonicum cells grown in three variations of the BVM minimal medium. Variant 1 contained 2 μm CuSO4 (copper excess). Variant 2 was prepared in HCl-treated glassware without any copper added (copper starvation). The residual copper concentration in this copper starvation medium was analyzed by graphite furnace atomic absorption spectroscopy and determined to be 5 nm. Variant 3 (extreme copper limitation) was prepared like variant 2 but with the addition of 10 μm BCS and 1 mm ascorbic acid where BCS chelates Cu(I) selectively, and ascorbic acid reduces any Cu(II) to Cu(I). Changes in the transcription profiles were recorded by the pairwise comparison of cells grown in variant 2 versus variant 1 and in variant 3 versus variant 2 (supplemental Tables S3 and S4, respectively). Only a small set of genes were differentially up- or down-regulated when copper-starved cells were compared with cells grown in copper excess (supplemental Table S3). Most notably, five genes located adjacent to each other on the B. japonicum genome displayed an increased expression: bll4882 to bll4878; supplemental Table S3). For reasons that will become evident from subsequent research (see below), the five genes were named pcuA, pcuB, pcuC, pcuD, and pcuE (mnemonic of proteins for Cu trafficking). The genes with decreased expression either are of unknown function or, not surprisingly, play a role in copper resistance. Extreme copper limitation (variant 3 versus variant 2) did not further enhance the expression of the five pcu genes. Instead, another cluster of adjacent genes was strongly up-regulated: bll0889 to bll0883, which code for unidentified transport functions (supplemental Table S4). Incidentally, the list also includes the copper chaperone ScoI. As expected, the pairwise comparison of variant 3 versus variant 1 (not shown) included again all of the differentially expressed genes listed in supplemental Table S3. Taken together, copper-limiting growth conditions have led to the derepression of genes potentially involved in copper acquisition. In this work, we have focused on the investigation of the pcuABCDE gene cluster.
The pcuABCDE Genes Are Transcribed as an Operon
The short distances between the ORFs of the pcuABCDE cluster already suggested an operon organization in which the five genes would be co-regulated and co-transcribed. The tiling-like design of the oligonucleotides on the gene chip used for microarrays (37) allowed us to confirm this inference because, consistently, transcript levels in copper-starved cells were higher throughout the entire pcuABCDE cluster as compared with copper-rich conditions (Fig. 1). Complementary information was obtained by applying qRT-PCR and primer extension techniques. For these experiments, RNA was extracted from wild-type cells grown in extreme copper limitation and from cells grown in standard BVM. When cDNA corresponding to pcuA and pcuB was produced thereof for qRT-PCR, the data reflected a respective up-regulation of 3.4 ± 0.8- and 33.3 ± 6.2-fold in copper limitation. The numbers compared well with the transcriptomics data. Likewise, a primer extension product of the mRNA 5′ end was seen only in copper-limited cells (Fig. 2). The transcription start site was thus mapped at nucleotide position 5,414,300 in the B. japonicum genome, which lies at a distance of 21 bp upstream of the pcuA start codon. Sequence inspection of the associated promoter region did not allow predictions to be made on the possible mode of transcriptional regulation.
FIGURE 2.
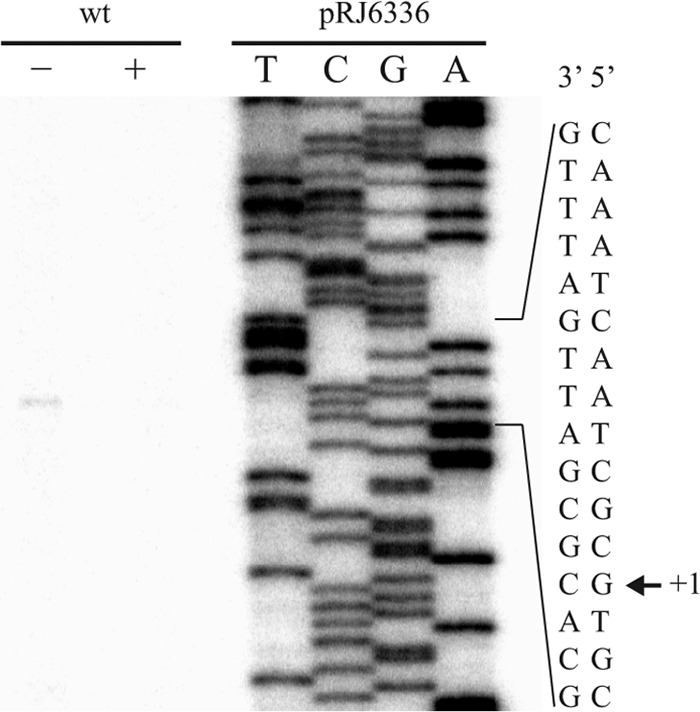
Transcription start site mapping of pcuA. Total RNA from B. japonicum wild-type strain grown in standard BVM (0.02 μm CuSO4; plus sign) or in extreme copper limitation BVM (containing 10 μm BCS and 1 mm ascorbic acid; minus sign). Extension products were obtained with the 32P-labeled primer named “primer_extension_1” and separated on a 6% denaturing polyacrylamide gel. The sequencing ladder was generated with plasmid pRJ6336 and the same primer. Part of the promoter region is shown on the right, and the transcription start site (+1) is indicated (arrow).
Predicted Proteins Encoded by the pcuABCDE Operon Suggest a Role in Copper Trafficking
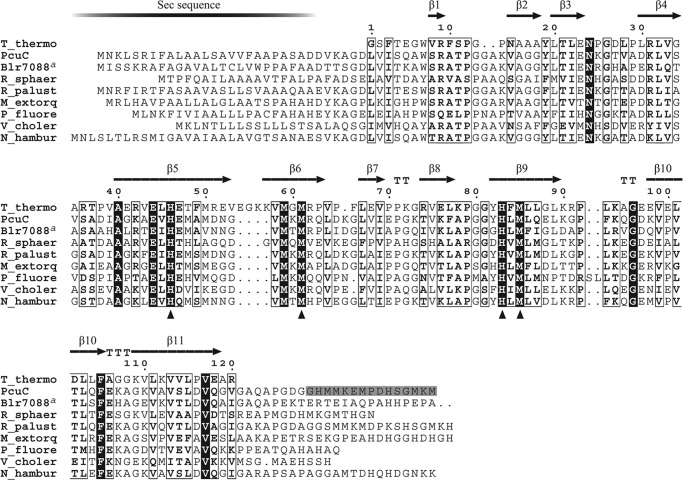
The putative gene products can be grouped into two categories, soluble and membrane-bound proteins. PcuA, PcuC, and PcuD are predicted soluble, periplasmic proteins because all three have an N-terminal sequence for Sec-dependent secretion. PcuA is conserved only in the order Rhizobiales and does not contain a noteworthy amino acid motif other than a CXXC sequence. PcuC is clearly homologous to the known copper chaperone PCuAC (e.g. sharing 36% identity with the T. thermophilus PCuAC protein; Fig. 3). PcuD is well conserved in bacteria, occasionally fused N-terminally with PcuC, but neither is its function known, nor does it contain a conspicuous amino acid domain or motif. The PcuB and PcuE proteins are membrane-integral proteins. PcuB is a member of the TonB-dependent receptor protein family, which implies a location in the outer membrane by virtue of the β-barrel structure. The high majority of the characterized members of this family are siderophore-iron or tetrapyrrole-metal importers (55). It was tempting to speculate that PcuB might be a receptor for the uptake of a copper-chelate complex through the B. japonicum outer membrane. PcuE is most likely a cytoplasmic membrane protein made up of an N-terminal CopC-like domain and a C-terminal CopD-like domain. The conserved CopC and CopD proteins have first been described in Pseudomonas syringae as part of the copper resistance (export) system (56). By contrast, the B. subtilis YcnJ protein, which shares 27% identity with PcuE, is a CopCD-like fusion protein that was reported to have a copper acquisition function (57). In conclusion, the proteins encoded by the B. japonicum pcuABCDE operon appeared as strong candidates for playing a role in copper import and sorting.
FIGURE 3.
Multiple alignment of PCuAC amino acidic sequences. The secondary structure of T. thermophilus PCuAC extracted from its crystal structure (Protein Data Bank code 2K6W) is shown above the alignment. The numbering refers to the T. thermophilus PCuAC sequence. B. japonicum PcuC and Blr7088, R. sphaeroides 2.4.1 RSP_2017, Rhodopseudomonas palustris HaA2 RPB_2549, Methylobacterium extorquens PA1 Mext_1379, Pseudomonas fluorescens Pf0-1Pfl01_0598, Vibrio cholerae HC-02A1 VCHC02A1_2964, and Nitrobacter hamburgensis X14 Nham_2200 sequences are included in the alignment. Similarity and identity columns are shown as empty boxes and white on black type, respectively. Copper-binding residues of TtPCuAC are marked with triangles; the corresponding residues of PcuC are His79, Met90, His113, and Met115. The gray-shaded amino acids at the C-terminal end of the PcuC sequence are those that were removed to test if they bind copper. a, the C-terminal 118-amino acid domain of Blr7088 was omitted in the alignment.
The ΔpcuABCDE Strains Have a Pleiotropic Phenotype
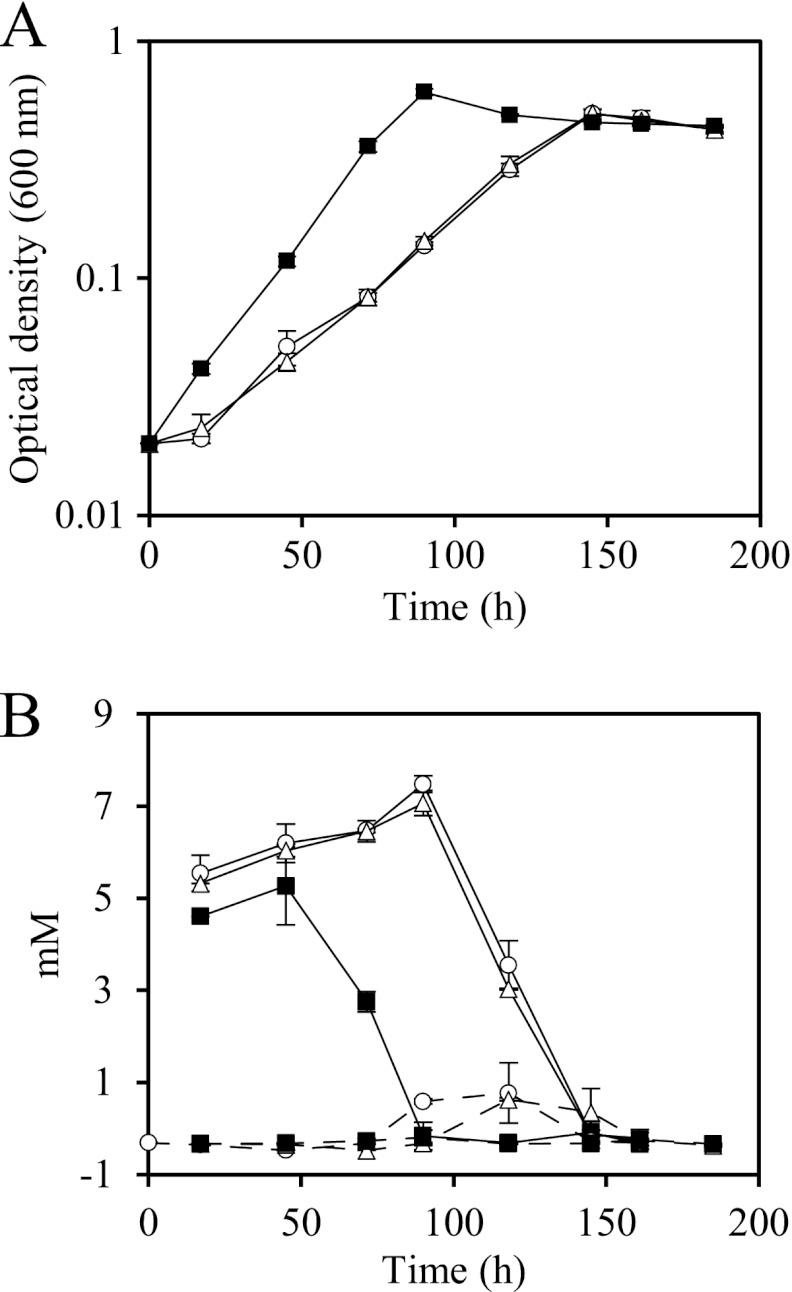
Two deletion mutants were created by replacing the entire operon sequence with a kanamycin resistance cassette in both orientations relative to the deleted genes, yielding strains 6611 (same orientation; Fig. 1) and 6612 (opposite orientation; Table 1). Aerobic growth was measured in different copper concentrations. The B. japonicum wild type and the two ΔpcuABCDE strains had an identical growth behavior in standard complete and minimal media or in copper starvation medium under oxic conditions (data not shown). However, growth of the mutants was impaired in extreme copper limitation (Fig. 4A; here shown for strain 6611). Also, under conditions of anoxic nitrate respiration (YEM plus NO3−, 0.2 μm CuSO4), the growth rate of the operon mutants was diminished when compared with the wild type (Fig. 5A). Concomitantly, a delayed consumption of nitrate and a transient accumulation of nitrite were observed in the mutants (Fig. 5B). This could be interpreted to mean that, in the mutants, the copper-dependent nitrite reductase (NirK) (58) is insufficiently supplied with the copper cofactor. In fact, attempts at limiting the copper concentration in such anoxic cultures led to a cessation of growth already with the wild type, making it impossible to further elaborate discriminative mutant phenotypes with respect to the denitrification pathway.
FIGURE 4.
Decreased growth of the operon mutant ΔpcuABCDE under copper starvation is due to loss of pcuC. Strains were grown in BVM medium under extreme copper limitation (no copper added; 10 μm BCS and 1 mm ascorbic acid added). Aerobic growth was measured from a starting optical density of 0.02 until stationary phase, and values represent the average optical density of cultures measured in triplicate. A, wild-type B. japonicum (filled squares) and ΔpcuABCDE strain 6611 (open circles). B, single-gene deletion strains ΔpcuE (open squares), ΔpcuD (open triangles), ΔpcuC (filled circles), ΔpcuB (open diamonds), and ΔpcuA (crosses). Error bars, S.D.
FIGURE 5.
Anaerobic growth, NO3− consumption, and transient NO2− accumulation in the ΔpcuABCDE mutant. The following strains were tested: wild type (closed squares), 6611 (ΔpcuABCDE insert in the same orientation; open circles), and 6612 (ΔpcuABCDE insert in the opposite orientation; open triangles). A, growth curves in anaerobic YEM containing 10 mm KNO3. B, concentrations (in mm) of NO3− (continuous lines) and NO2− (dashed lines) in the medium during growth. All of the measurements were taken in triplicates. Error bars, S.D.
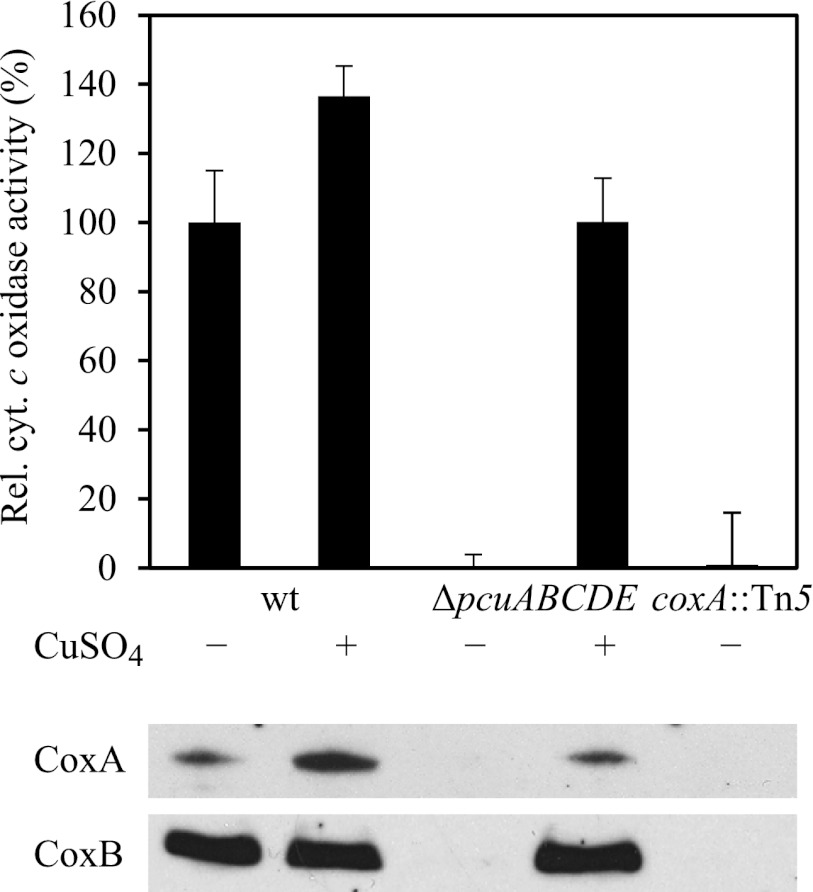
Another important phenotype observed with the ΔpcuABCDE mutants was the complete lack of activity of the aa3-type cytochrome oxidase, which is the predominant respiratory oxygen reductase in oxically grown B. japonicum wild type. The membrane protein fraction was isolated from oxically grown strains, and the ability to oxidize reduced horse heart cytochrome c was measured. As shown in Fig. 6, oxidase activity in wild type is due to cytochrome aa3 because membrane proteins extracted from a coxA mutant (Tn5 insertion in aa3-subunit-I gene) have almost no activity. Cytochrome c oxidase activity of both ΔpcuABCDE strains (here shown for 6611) is as low as that of the coxA mutant, indicating an involvement of the operon in cytochrome aa3 biogenesis. The wild-type phenotype could be restored by growing the cells in medium containing 50 μm CuSO4. Western blot analyses of all of these membrane extracts (Fig. 6, bottom) revealed an absence of both subunits I and II in the ΔpcuABCDE mutant, whereas they were detectable again in cells grown in copper excess. We tentatively concluded, therefore, that the pcuABCDE operon is involved in providing copper for the aa3-type cytochrome c oxidase.
FIGURE 6.
Effect of the pcuABCDE deletion on aa3-type cytochrome c oxidase activity. The relative cytochrome c oxidase activity of aerobically grown wild type (wt) and strains 6611 (ΔpcuABCDE) and COX132 (coxA::Tn5) is shown in the top panel. Wild-type activity corresponds to 0.206 μmol of horse heart cytochrome c oxidized/mg of membrane protein/min. 50 μm CuSO4 was added to the peptone-salts-yeast extract medium where indicated (+). Western blot analysis for the detection of subunits I and II of the cytochrome aa3 (CoxA and CoxB, respectively) was carried out on the same membrane proteins (bottom panels). Error bars, S.D.
Finally, as we routinely tested any B. japonicum mutant generated in our laboratory for its symbiotic efficiency, a 75% decrease of symbiotic nitrogen fixation activity (measured with the acetylene reduction test) was recorded in soybean root nodules induced with the ΔpcuABCDE strains (Table 2). The possible reason for this strong defect will be addressed further below (see “PcuC and ScoI Are Required for the Symbiotically Essential cbb3-Type Cytochrome Oxidase”). The addition of excess copper to the plant growth medium did not help to abrogate the defect.
TABLE 2.
Symbiotic properties of ΔpcuABCDE mutants inoculated on soybean
Mutants were tested in parallel with the wild type in three independent experiments. Shown here are data from one representative experiment.
| Strain | Relevant genotypea | Number of nodules | Nodule dry weight | Nitrogenase activityb | Relative Fix activityc |
|---|---|---|---|---|---|
| mg | %/min/g | % | |||
| 110spc4 | Wild type | 20.4 ± 4.0 | 2.4 ± 0.7 | 3.0 ± 0.7 | 100 ± 22.3 |
| 6611 | ΔpcuABCDE-a | 25.8 ± 7.8 | 1.3 ± 0.3 | 0.8 ± 0.5 | 25.9 ± 16.9 |
| 6612 | ΔpcuABCDE-b | 23.3 ± 5.1 | 1.4 ± 0.4 | 0.7 ± 0.4 | 24.4 ± 12.5 |
a Strain 6611 has insert in the same orientation (-a) and strain 6612 in the opposite orientation (-b).
b Nitrogenase activity is expressed as percentage of C2H4 formed/min/g.
c Relative nitrogen fixation activity is expressed as a percentage of wild type.
pcuC Is the Only Essential Gene of the pcuABCDE Operon for All Tested Functions
Having established a pleiotropic phenotype associated with the operon deletion, it was necessary to find out how many and which of the five genes are responsible. For this purpose, a comprehensive complementation strategy was applied in which either all five genes or five minus one were recombined back into the deletion mutant 6611. The latter constructs consisted of operons in which a markerless in-frame deletion was generated in each of the five genes, leaving the other four intact (see the ΔpcuC example in Fig. 1). This resulted in a set of five strains with single gene deletions plus the controls (vector insertion, complete operon; Table 1). All strains were then tested for most of the phenotypes reported in the preceding section. Surprisingly, the ΔpcuC deletion proved to be the only responsible mutation for the decreased cytochrome c oxidase activity (Table 3), and this defect was restored to wild-type activity in cells grown with copper excess (Table 3). Deletions of pcuA, pcuB, pcuD, or pcuE did not cause a defect. Likewise, the ΔpcuC strain was the only one that exhibited an impaired aerobic growth in conditions of copper limitation (Fig. 4B), and it was the only one that had a decreased symbiotic nitrogen fixation activity (Table 3). The ΔpcuC phenotype was unlikely to be the result of polar effects of the deletion on the downstream operon genes, because the deletions of pcuE and pcuD themselves had no effect. Nevertheless, we ascertained by qRT-PCR that DNA downstream of the pcuC in-frame deletion was fully transcribed (data not shown).
TABLE 3.
Complementation of ΔpcuABCDE (strain 6611) and effects of single-gene deletions on the phenotypes
Activities of the mutant strains are expressed as a percentage of wild-type activity.
| Strain | Genes added for complementation | Relative cytochrome c oxidase activitya |
Relative Fix activitya | |
|---|---|---|---|---|
| Without 50 μm Cu(SO)4 | With 50 μm Cu(SO)4 | |||
| % | % | % | ||
| 6611 | 16.8 ± 4.7 | 100.1 ± 12.8 | 34.7 ± 13.5 | |
| 6611-32 | Only vector insert | 14.4 ± 5.1 | 90.3 ± 9.8 | NDb |
| 6611-33 | pcuABCDE+ | 106.6 ± 10.5 | ND | ND |
| 6611-6630 | pcuABCD[ΔE]+ | 113.4 ± 9.9 | ND | 112.8 ± 16.8 |
| 6611-1654 | pcuABC[ΔD]E+ | 120.5 ± 18.2 | ND | 127.8 ± 40.9 |
| 6611-34 | pcuAB[ΔC]DE+ | 24.8 ± 4.4 | 100.5 ± 10.1 | 36.1 ± 5.0 |
| 6611-1650 | pcuA[ΔB]CDE+ | 101.0 ± 17.0 | ND | 105.1 ± 8.5 |
| 6611-1653 | pcu[ΔA]BCDE+ | 111.8 ± 19.5 | ND | 99.7 ± 10.3 |
a The average wild-type cytochrome c oxidase activity (100%) is ∼0.31 μmol of horse heart cytochrome c oxidized/mg of membrane protein/min. The average wild-type Fix activity (100%) is 2.82 ± 0.4% C2H4/min/g.
b ND, not determined.
B. japonicum Possesses a Second pcuC-like Gene (blr7088) without an Obvious Function
The predicted Blr7088 protein shares in its N-terminal half 40% identity with PcuC (cf. Fig. 3), whereas the C-terminal extension carries a weakly conserved domain. The blr7088 gene is located next to the respiratory nitrite reductase gene (nirK, blr7089), which might implicate a function of the Blr7088 protein in the biogenesis of the copper-containing nitrite reductase. However, Δblr7088 strains (6620 and 6621; Table 1) were constructed and shown to be unaffected in anoxic growth with nitrate as the terminal electron acceptor. Also, these mutants had none of the phenotypes observed for the ΔpcuABCDE strains, except for a slight delay in aerobic, copper-limited growth. Furthermore, double deletion strains (ΔpcuABCDE plus Δblr7088; Table 1) had phenotypes that were not more severe than those observed for the ΔpcuABCDE strains, except for perhaps an additive impairment in aerobic, copper-limited growth (data not shown). Hence, the function of the Blr7088 protein remains elusive, and there was no indication that it might be able to functionally replace the PcuC protein in a pcuC mutant background. Therefore, research on blr7088 was discontinued.
PcuC Is a Cu(I)-binding PCuAC-like Protein
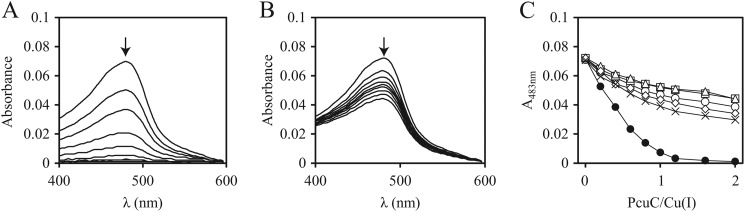
Because pcuC turned out to be the only important gene of the pcuABCDE operon (under the conditions tested), we wished to obtain additional evidence for its role in copper metabolism. The PcuC protein was expressed in E. coli using both periplasmic and cytoplasmic overexpression systems (see “Experimental Procedures”), and the proteins were purified to apparent homogeneity from these compartments. Electrospray ionization MS analyses revealed that cytoplasmically produced PcuC was copper-free, whereas periplasmically expressed PcuC contained one copper ion bound to it. Copper could be removed from the latter by treatment with a >1,000-fold molar excess of BCS but not with EDTA (data not shown). Considering the high specificity of the BCS chelate for Cu(I) (59, 60), this pointed toward a preference of PcuC for Cu(I). Increasing amounts of copper-free PcuC were then mixed as competitors with a constant amount of the Cu(BCS)23− complex, and the decrease of the characteristic 483-nm absorbance peak of the latter (54) was recorded by UV-visible absorption spectroscopy until saturation occurred (Fig. 7A). The result showed that the affinity of PcuC for Cu(I) was higher than that of BCS. Applying the method elaborated by Zhou et al. (54), we arrived at an estimated KD of ≤10−16 m. When the A483 values were plotted against the PcuC/Cu(I) ratio, we observed the disappearance of Cu(BCS)23− after the addition of one equivalent of PcuC, indicating a PcuC/Cu(I) stoichiometry of 1:1 (Fig. 7C, curve with black circles). Similar data were obtained with a PcuC derivative in which the last 15, non-conserved amino acids had been deleted (data not shown). This C-terminal extension is rich in possible copper-binding amino acids (5 Met, 2 His, 1 Glu, 1 Asp; Fig. 3). The unaltered activity of the truncated form precluded a role of this peptide region in copper binding.
FIGURE 7.
PcuC binds one Cu(I) ion via His79, Met90, His113, and Met115. A, UV-visible spectra of incremental addition of apo-PcuC (wild type) to a 7.5 μm Cu(BCS)23− solution. Spectra were taken when 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.6, and 2.0 protein equivalents/copper atom were added. The arrows indicate the direction of the absorption change after the protein addition. B, incremental addition of apo-PcuC(M90A) to a 7.5 μm Cu(BCS)23− solution is shown as an example. An analogous behavior was observed for the other substituted versions of PcuC (data not shown). C, plot of A483 against the ratio of PcuC wild type (closed circles), PcuC(H79A) (open circles), PcuC(M90A) (squares), PcuC(H113A) (triangles), PcuC(M115A) (diamonds), and PcuC(H113A/M115A) (crosses) to total Cu(I). Saturation is observed only when PcuC (wild type) was used in the experiment.
The copper-binding amino acid ligands described for the homologous T. thermophilus PCuAC protein (25) are conserved in PcuC (Fig. 3), and their similar spatial orientation was confirmed by modeling the PcuC primary structure onto the three-dimensional structure of T. thermophilus PCuAC (data not shown). Therefore, copper binding was also tested with purified mutant derivatives of PcuC in which each of the four presumed Cu(I)-binding residues had been substituted with alanine: H79A, M90A, H113A, and M115A. Further, a double replacement derivative was tested (PcuC(H113A/M115A)). The purity and identity of these proteins were documented by mass spectrometry (supplemental Fig. S1). All of the mutant proteins had an impaired capability of removing Cu(I) from the Cu(BCS)23− complex, as shown with PcuC(M90A) as an example (Fig. 7B). The plot in Fig. 7C illustrates that saturation occurred only with wild-type PcuC, whereas a moderate decrease in A483 was observed for the substituted proteins; hence, they bound Cu(I) more weakly than wild-type PcuC. The experiments supported the notion that PcuC binds one Cu(I) ion with high affinity via His79, Met90, His113, and Met115.
PcuC and ScoI Are Required for the Symbiotically Essential cbb3-type Cytochrome Oxidase
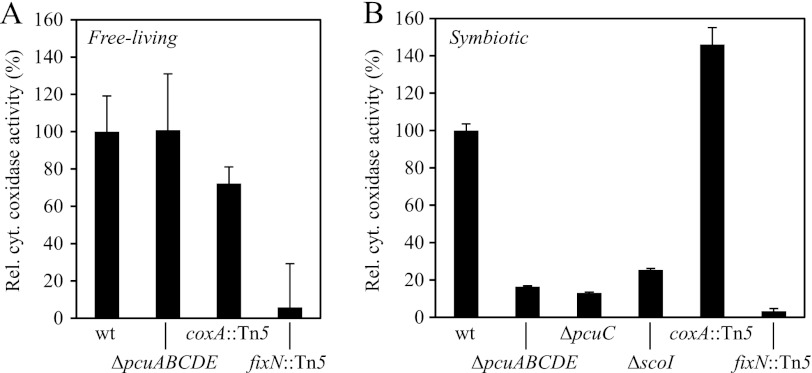
As described above, deletion of the pcuC gene caused a defect in symbiotic nitrogen fixation (Tables 2 and 3). Having shown that the PcuC protein is a periplasmic Cu(I) protein, we asked whether or not it is involved in the biogenesis of the symbiotically essential cbb3-type oxidase. To assess its functionality, cells were first grown anoxically with nitrate as the terminal electron acceptor. Under these conditions, virtually all of the cytochrome c oxidase activity present in wild-type membranes stems from cytochrome cbb3 and not from cytochrome aa3 (Fig. 8A, compare coxA mutant with fixN mutant). The activity measured with membranes extracted from anoxically grown ΔpcuABCDE cells was as high as that from the wild type. It thus appeared as if PcuC was not important for the formation of cytochrome cbb3. Prompted by recent findings of Thompson et al. (24), who reported that PCuAC was a biogenesis factor for cytochrome cbb3 in R. sphaeroides, we tested membrane proteins that had been extracted from endosymbiotic cells. For this purpose, root nodule bacteroids of the wild type and of the deletion mutants 6611 (ΔpcuABCDE) and 6611-34 (ΔpcuC) were separated from plant material via sucrose gradient centrifugation prior to membrane isolation. Bacteroids of coxA and fixN mutants served as positive and negative controls, respectively. The cbb3-type oxidase activity was strongly impaired in membranes of pcuC mutant bacteroids (Fig. 8B). In contrast to the result of Fig. 8A, this now suggested a role of PcuC in cytochrome cbb3 biogenesis and provided a possible explanation for the symbiotic nitrogen fixation defect of pcuC mutants.
FIGURE 8.
PcuC and ScoI are involved in cbb3 oxidase biogenesis in bacteroids. A, relative cytochrome c oxidase activity of anaerobically grown wild type (wt) and strains 6611 (ΔpcuABCDE), COX132 (coxA::Tn5), and 3613 (fixN::Tn5). Wild-type activity corresponds to 0.891 μmol of horse heart cytochrome c oxidized/mg of membrane protein/min. B, relative cytochrome c oxidase activity of bacteroid membrane proteins from wild type (wt) and strains 6611 (ΔpcuABCDE), 6611-34 (ΔpcuC), 2575 (ΔscoI), COX132 (coxA::Tn5), and 3613 (fixN::Tn5). Wild-type activity corresponds to 1.15 μmol of horse heart cytochrome c oxidized/mg of membrane protein/min. Error bars, S.D.
In previous work (10), we found that a B. japonicum scoI mutant had very similar phenotypes as described now for the pcuC mutant, such as the absence of an effect on cbb3-type oxidase activity in cells grown anoxically. The unexpected condition-dependent difference (free-living, anoxic cells versus symbiotic bacteroids) led us to investigate the cbb3 oxidase activity of the scoI mutant also in symbiosis. Indeed, oxidase activity was strongly impaired in membranes of scoI mutant bacteroids (Fig. 8B). Hence, the ScoI protein is important for the formation of cytochrome cbb3 specifically in symbiosis, just like PcuC.
DISCUSSION
Our expectation to discover derepressed genes in B. japonicum cells grown in copper-limited conditions was fulfilled. Which and how many of these are in fact important for copper acquisition is a matter of ongoing and future research. Extreme copper limitation led to the expression of seven clustered genes (bll0889–bll0883), preceded by a predicted transcription activator (bll0890), which codes for two putative cytoplasmic membrane complexes of the ABC transporter family and a major facilitator superfamily transport protein (bll0889). Interestingly, Ekici et al. (61) have reported a major facilitator superfamily-type transporter (CcoA) that is specifically required for cytochrome cbb3 biogenesis in Rhodobacter capsulatus. Although B. japonicum has several ccoA homologs, the bll0889 gene product might play a similar role because of its low-copper inducibility. Undoubtedly, this cluster of B. japonicum genes is worthy of further investigation.
For the present work, we decided to explore the pcuABCDE operon because it attracted attention for the following reasons: (i) it is already induced by more moderate copper starvation conditions (5 nm residual copper in so-called “Cu-free” medium); (ii) it looked as if the five genes are organized and co-regulated as an operon, for which we then obtained experimental evidence; and (iii) the encoded proteins promised to constitute a novel relay system for copper acquisition in a Gram-negative bacterium consisting of a TonB-ExbBD-dependent outer membrane receptor (PcuB), one or two periplasmic copper chaperones (PcuC and perhaps PcuA), and a copper-specific cytoplasmic membrane transporter (PcuE). This idea of a cooperating import system was further strengthened by the pleiotropic phenotype of a pcuABCDE deletion mutant, which suffered defects in copper-limited growth, symbiotic nitrogen fixation, and anoxic denitrification. In a first approximation, these phenotypes were supposed to be caused by a critical shortage of copper for the synthesis of copper-containing enzymes, such as the aa3- and cbb3-type cytochrome oxidases, and the nitrite and nitrous oxide reductases. After a functional gene-by-gene analysis, we demonstrated that the missing pcuC gene alone was responsible for all of the defects. Why the pcuA, pcuB, pcuD, and pcuE genes are functionally inconspicuous remains a mystery at present. Given the substantial induction of expression and clear co-regulation together with pcuC under copper starvation, the precise conditions in which cells will need the other four genes have yet to be identified. The minimal copper concentrations one can reach in laboratory media might still be too high to mimic the copper-limited niches that bacteria may encounter in natural environments. What speaks in favor of this argument is the ease with which the pcuC defect could be abrogated by the simple supplementation of media with more copper, albeit at a different threshold level to achieve phenotypic compensation.
Further investigations focused on pcuC and its product. The results have ascertained the cuproprotein nature of PcuC. The reduced form of copper (Cu+) was found to be bound to purified PcuC in a 1:1 ratio, and the estimated affinity (KD ≤ 10−16 m) compares well with data obtained for other copper chaperones (62). Deduced from the structure of a PcuC-homologous protein (PCuAC) (25), the two histidines and two methionines for copper binding lie in an unusual protein motif (HX10MX22HXM) in which not only the four ligands but also the fairly large spacing is evolutionarily well conserved. We confirmed here by mutational analysis that all four liganding amino acids are needed for efficient copper binding to PcuC. Furthermore, we made sure by truncation that the 15 C-terminal amino acids, which are rich in Met and His, are dispensable. This truncated version might become useful in crystallization studies because efforts to crystallize the full-length PcuC protein have been unsuccessful. In vivo, the PcuC protein was shown here to be involved in the biogenesis of the aa3-type cytochrome oxidase. This was anticipated because Abriata et al. (25) had demonstrated the transfer of Cu(I) from T. thermophilus PCuAC to the CuA site of the ba3-type oxygen reductase in vitro. Less expected was the role that PcuC appears to play in the formation of the cbb3-type oxygen reductase because this oxidase lacks the CuA center. Two independent studies (Ref. 24 and this work) have now arrived at this new function. With an expedient combination of genetic and biochemical approaches, Thompson et al. (24) have recently shown for R. sphaeroides that PCuAC works both for the metallation of the CuA site of its aa3-type oxidase and for the metallation of the CuB site of its cbb3-type oxidase. Furthermore, these authors argued that PCuAC might even be involved to some extent in the formation of the CuB center in the aa3-type oxidase despite the presence of Cox11 (see Introduction), which is still the major player in this process (14). Our own data with B. japonicum were less straightforward because the dependences of cytochrome cbb3 formation on PcuC differed fundamentally in anoxic, culture-grown cells and in symbiotic bacteroids, where a pcuC mutant had no effect in the former but a strong defect in the latter. Formally, we cannot rule out the possibility that a PcuC-compensating protein is synthesized in free-living conditions but not in symbiosis. In any case, we showed that the PcuC-homologous Blr7088 protein was not involved. We rather suspect that the copper concentrations play a decisive role. In order to achieve optimal growth of B. japonicum under denitrifying conditions (even with the wild type), the copper concentration should not fall below 0.2 μm, which might already be high enough to bypass the need for PcuC in the assembly of active cytochrome cbb3. In symbiosis, however, it is possible that such a functional compensation does not work if the copper concentration that is available for bacteroids in root nodules is very low. Unfortunately, it is practically difficult, if not impossible, to assess and distinguish copper contents in separate compartments, such as the cytosol of colonized plant cells in nodules and the symbiosome (i.e. bacteroids plus peribacteroid membrane-surrounded space). Our attempt to abrogate the symbiotic defect by increasing the copper content in the plant growth medium has not been successful. The bottom line of our investigations was that PcuC is important for cytochrome cbb3 formation specifically in endosymbiotic bacteroids. This fact alone is sufficient to explain the low nitrogen fixation activity in pcuC mutant-infected soybean nodules because this bacteroid process depends on mico-oxic respiration via the high affinity cbb3-type oxidase (63). Along the same lines, we now showed that the B. japonicum scoI mutant had a similar condition-dependent phenotype as the pcuC mutants (i.e. the scoI mutation negatively affected cytochrome cbb3 activity in bacteroids (Fig. 8B) but had no effect on this oxidase in cells grown under anoxic, denitrifying conditions (10)). Therefore, we must revise a previous interpretation according to which ScoI was not believed to be an important biogenesis factor for cytochrome cbb3 in B. japonicum (10). Other recent studies have provided good evidence for a role of ScoI-like proteins (SenC) in cytochrome cbb3 formation in P. aeruginosa (22), R. sphaeroides (24), and R. capsulatus (23).
In conclusion, a unifying concept seems to emerge, at least for members of the α-proteobacteria, according to which two proteins (ScoI-like and PcuC-like) are required collectively for the biogenesis of two types of cytochrome oxidases (aa3 and cbb3). To what extent each of the two biogenesis factors is involved in the metallation of the CuA and CuB sites and how they cooperate with each other need to be explored. Lack of ScoI (10) or PcuC (cf. Fig. 6) leads to an apparent destabilization and/or degradation not only of subunit II but also of subunit I of cytochrome aa3. Therefore, one cannot readily attribute an exclusive function to the chaperones in either CuA or CuB formation. Intuitively, one would like to propose that the soluble, periplasmic PcuC protein disseminates copper to the membrane-bound ScoI protein and perhaps to other membrane-bound recipients (e.g. CoxG/Cox11, FixI/CcoI, and CcoA), as has been suggested in part also by others (15, 21, 23, 24). However, more biochemical work, such as establishing a copper transfer assay with purified components in vitro, will be needed as proof.
Acknowledgments
We are most grateful to Rudi Glockshuber, Elisabeth Mohorko, Kaspar Locher, Detlef Günther, Gabriella Pessi, Philipp Christen, and the Functional Genomics Center Zurich for valuable advice and help in various aspects of experimentation and data analysis.
This work was supported by grants from the Swiss National Foundation for Scientific Research and the ETH Zurich.

This article contains supplemental Tables S1–S4 and Fig. S1.
- BVM
- buffered Vincent's minimal medium
- BCS
- bathocuproine disulfonate
- YEM
- yeast extract mannitol medium
- qRT-PCR
- quantitative RT-PCR
- TEV
- tobacco etch virus.
REFERENCES
- 1. García-Horsman J. A., Barquera B., Rumbley J., Ma J., Gennis R. B. (1994) The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 176, 5587–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sousa F. L., Alves R. J., Ribeiro M. A., Pereira-Leal J. B., Teixeira M., Pereira M. M. (2012) The superfamily of heme-copper oxygen reductases. Types and evolutionary considerations. Biochim. Biophys. Acta 1817, 629–637 [DOI] [PubMed] [Google Scholar]
- 3. Abramson J., Riistama S., Larsson G., Jasaitis A., Svensson-Ek M., Laakkonen L., Puustinen A., Iwata S., Wikström M. (2000) The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat. Struct. Biol. 7, 910–917 [DOI] [PubMed] [Google Scholar]
- 4. Iwata S., Ostermeier C., Ludwig B., Michel H. (1995) Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376, 660–669 [DOI] [PubMed] [Google Scholar]
- 5. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 6. Ducluzeau A. L., Ouchane S., Nitschke W. (2008) The cbb3 oxidases are an ancient innovation of the domain bacteria. Mol. Biol. Evol. 25, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 7. Buschmann S., Warkentin E., Xie H., Langer J. D., Ermler U., Michel H. (2010) The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science 329, 327–330 [DOI] [PubMed] [Google Scholar]
- 8. Poole R. K., Cook G. M. (2000) Redundancy of aerobic respiratory chains in bacteria? Routes, reasons, and regulation. Adv. Microb. Physiol. 43, 165–224 [DOI] [PubMed] [Google Scholar]
- 9. Han H., Hemp J., Pace L. A., Ouyang H., Ganesan K., Roh J. H., Daldal F., Blanke S. R., Gennis R. B. (2011) Adaptation of aerobic respiration to low O2 environments. Proc. Natl. Acad. Sci. U.S.A. 108, 14109–14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bühler D., Rossmann R., Landolt S., Balsiger S., Fischer H. M., Hennecke H. (2010) Disparate pathways for the biogenesis of cytochrome oxidases in Bradyrhizobium japonicum. J. Biol. Chem. 285, 15704–15713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Oost J., Haltia T., Raitio M., Saraste M. (1991) Genes coding for cytochrome c oxidase in Paracoccus denitrificans. J. Bioenerg. Biomembr. 23, 257–267 [DOI] [PubMed] [Google Scholar]
- 12. Hiser L., Di Valentin M., Hamer A. G., Hosler J. P. (2000) Cox11p is required for stable formation of the CuB and magnesium centers of cytochrome c oxidase. J. Biol. Chem. 275, 619–623 [DOI] [PubMed] [Google Scholar]
- 13. Cobine P. A., Pierrel F., Winge D. R. (2006) Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta 1763, 759–772 [DOI] [PubMed] [Google Scholar]
- 14. Thompson A. K., Smith D., Gray J., Carr H. S., Liu A., Winge D. R., Hosler J. P. (2010) Mutagenic analysis of Cox11 of Rhodobacter sphaeroides. Insights into the assembly of CuB of cytochrome c oxidase. Biochemistry 49, 5651–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekici S., Pawlik G., Lohmeyer E., Koch H. G., Daldal F. (2012) Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817, 898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hassani B. K., Astier C., Nitschke W., Ouchane S. (2010) CtpA, a copper-translocating P-type ATPase involved in the biogenesis of multiple copper-requiring enzymes. J. Biol. Chem. 285, 19330–19337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Preisig O., Zufferey R., Hennecke H. (1996) The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high affinity cbb3-type cytochrome oxidase. Arch. Microbiol. 165, 297–305 [DOI] [PubMed] [Google Scholar]
- 18. Koch H. G., Winterstein C., Saribas A. S., Alben J. O., Daldal F. (2000) Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J. Mol. Biol. 297, 49–65 [DOI] [PubMed] [Google Scholar]
- 19. Robinson N. J., Winge D. R. (2010) Copper metallochaperones. Annu. Rev. Biochem. 79, 537–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cawthorn T. R., Poulsen B. E., Davidson D. E., Andrews D., Hill B. C. (2009) Probing the kinetics and thermodynamics of copper(II) binding to Bacillus subtilis Sco, a protein involved in the assembly of the CuA center of cytochrome c oxidase. Biochemistry 48, 4448–4454 [DOI] [PubMed] [Google Scholar]
- 21. Arunothayanan H., Nomura M., Hamaguchi R., Itakura M., Minamisawa K., Tajima S. (2010) Copper metallochaperones are required for the assembly of bacteroid cytochrome c oxidase which is functioning for nitrogen fixation in soybean nodules. Plant Cell Physiol. 51, 1242–1246 [DOI] [PubMed] [Google Scholar]
- 22. Frangipani E., Haas D. (2009) Copper acquisition by the SenC protein regulates aerobic respiration in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 298, 234–240 [DOI] [PubMed] [Google Scholar]
- 23. Lohmeyer E., Schröder S., Pawlik G., Trasnea P. I., Peters A., Daldal F., Koch H. G. (2012) The ScoI homologue SenC is a copper-binding protein that interacts directly with the cbb3-type cytochrome oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817, 2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson A. K., Gray J., Liu A., Hosler J. P. (2012) The roles of Rhodobacter sphaeroides copper chaperones PCuAC and Sco (PrrC) in the assembly of the copper centers of the aa3-type and the cbb3-type cytochrome c oxidases. Biochim. Biophys. Acta 1817, 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abriata L. A., Banci L., Bertini I., Ciofi-Baffoni S., Gkazonis P., Spyroulias G. A., Vila A. J., Wang S. (2008) Mechanism of CuA assembly. Nat. Chem. Biol. 4, 599–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller J. H. (1972) Experiments in Molecular Genetics, p. 433, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 27. Regensburger B., Hennecke H. (1983) RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 135, 103–109 [DOI] [PubMed] [Google Scholar]
- 28. Mesa S., Hauser F., Friberg M., Malaguti E., Fischer H. M., Hennecke H. (2008) Comprehensive assessment of the regulons controlled by the FixLJ-FixK2-FixK1 cascade in Bradyrhizobium japonicum. J. Bacteriol. 190, 6568–6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vincent J. M. (1970) A Manual for the Practical Study of Root Nodule Bacteria, Blackwell Scientific, Oxford, UK [Google Scholar]
- 30. Becker A., Bergès H., Krol E., Bruand C., Rüberg S., Capela D., Lauber E., Meilhoc E., Ampe F., de Bruijn F. J., Fourment J., Francez-Charlot A., Kahn D., Küster H., Liebe C., Pühler A., Weidner S., Batut J. (2004) Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol. Plant Microbe Interact. 17, 292–303 [DOI] [PubMed] [Google Scholar]
- 31. Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. (1976) Relation between glutamine synthetase and nitrogenase activities in the symbiotic association between Rhizobium japonicum and Glycine max. Plant Physiol. 57, 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daniel R. M., Appleby C. A. (1972) Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum. Effects on cytochrome P450, other hemoproteins, nitrate, and nitrite reductases. Biochim. Biophys. Acta 275, 347–354 [DOI] [PubMed] [Google Scholar]
- 33. Bott M., Bolliger M., Hennecke H. (1990) Genetic analysis of the cytochrome c-aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol. Microbiol. 4, 2147–2157 [DOI] [PubMed] [Google Scholar]
- 34. Preisig O., Anthamatten D., Hennecke H. (1993) Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. U.S.A. 90, 3309–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fischer H. M., Babst M., Kaspar T., Acuña G., Arigoni F., Hennecke H. (1993) One member of a groESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J. 12, 2901–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higuchi R., Krummel B., Saiki R. K. (1988) A general method of in vitro preparation and specific mutagenesis of DNA fragments. Study of protein and DNA interactions. Nucleic Acids Res. 16, 7351–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hauser F., Pessi G., Friberg M., Weber C., Rusca N., Lindemann A., Fischer H. M., Hennecke H. (2007) Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genomics 278, 255–271 [DOI] [PubMed] [Google Scholar]
- 38. Lindemann A., Moser A., Pessi G., Hauser F., Friberg M., Hennecke H., Fischer H. M. (2007) New target genes controlled by the Bradyrhizobium japonicum two-component regulatory system RegSR. J. Bacteriol. 189, 8928–8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beck C., Marty R., Kläusli S., Hennecke H., Göttfert M. (1997) Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179, 364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mesa S., Reutimann L., Fischer H. M., Hennecke H. (2009) Posttranslational control of transcription factor FixK2, a key regulator for the Bradyrhizobium japonicum-soybean symbiosis. Proc. Natl. Acad. Sci. U.S.A. 106, 21860–21865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hahn M., Hennecke H. (1984) Localized mutagenesis in Rhizobium japonicum. Mol. Gen. Genet. 193, 46–52 [Google Scholar]
- 42. Göttfert M., Grob P., Hennecke H. (1990) Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. U.S.A. 87, 2680–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Göttfert M., Hitz S., Hennecke H. (1990) Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol. Plant Microbe Interact. 3, 308–316 [DOI] [PubMed] [Google Scholar]
- 44. Sarma A. D., Emerich D. W. (2005) Global protein expression pattern of Bradyrhizobium japonicum bacteroids. A prelude to functional proteomics. Proteomics 5, 4170–4184 [DOI] [PubMed] [Google Scholar]
- 45. Delmotte N., Ahrens C. H., Knief C., Qeli E., Koch M., Fischer H. M., Vorholt J. A., Hennecke H., Pessi G. (2010) An integrated proteomics and transcriptomics reference data set provides new insights into the Bradyrhizobium japonicum bacteroid metabolism in soybean root nodules. Proteomics 10, 1391–1400 [DOI] [PubMed] [Google Scholar]
- 46. Nicholas D. J. D., Nason A. (1957) Determination of nitrate and nitrite. Methods Enzymol. 3, 981–984 [Google Scholar]
- 47. Gerhus E., Steinrücke P., Ludwig B. (1990) Paracoccus denitrificans cytochrome c1 gene replacement mutants. J. Bacteriol. 172, 2392–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 49. Loferer H., Bott M., Hennecke H. (1993) Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 12, 3373–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 51. Parks T. D., Leuther K. K., Howard E. D., Johnston S. A., Dougherty W. G. (1994) Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal. Biochem. 216, 413–417 [DOI] [PubMed] [Google Scholar]
- 52. Schulz H., Hennecke H., Thöny-Meyer L. (1998) Prototype of a heme chaperone essential for cytochrome c maturation. Science 281, 1197–1200 [DOI] [PubMed] [Google Scholar]
- 53. Kapust R. B., Tözsér J., Fox J. D., Anderson D. E., Cherry S., Copeland T. D., Waugh D. S. (2001) Tobacco etch virus protease. Mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 14, 993–1000 [DOI] [PubMed] [Google Scholar]
- 54. Zhou L., Singleton C., Le Brun N. E. (2008) High Cu(I) and low proton affinities of the CXXC motif of Bacillus subtilis CopZ. Biochem. J. 413, 459–465 [DOI] [PubMed] [Google Scholar]
- 55. Krewulak K. D., Vogel H. J. (2011) TonB or not TonB. Is that the question? Biochem. Cell Biol. 89, 87–97 [DOI] [PubMed] [Google Scholar]
- 56. Bender C. L., Cooksey D. A. (1986) Indigenous plasmids in Pseudomonas syringae pv. tomato. Conjugative transfer and role in copper resistance. J. Bacteriol. 165, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chillappagari S., Miethke M., Trip H., Kuipers O. P., Marahiel M. A. (2009) Copper acquisition is mediated by YcnJ and regulated by YcnK and CsoR in Bacillus subtilis. J. Bacteriol. 191, 2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Velasco L., Mesa S., Delgado M. J., Bedmar E. J. (2001) Characterization of the nirK gene encoding the respiratory, copper-containing nitrite reductase of Bradyrhizobium japonicum. Biochim. Biophys. Acta 1521, 130–134 [DOI] [PubMed] [Google Scholar]
- 59. Smith G. F., Wilkins D. H. (1953) New colorimetric reagent specific for copper. Anal. Chem. 25, 510–511 [Google Scholar]
- 60. Zak B. (1958) Simple procedure for the single sample determination of serum copper and iron. Clin. Chim. Acta 3, 328–334 [DOI] [PubMed] [Google Scholar]
- 61. Ekici S., Yang H., Koch H. G., Daldal F. (January 31, 2012) Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. MBio [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Banci L., Bertini I., Ciofi-Baffoni S., Kozyreva T., Zovo K., Palumaa P. (2010) Affinity gradients drive copper to cellular destinations. Nature 465, 645–648 [DOI] [PubMed] [Google Scholar]
- 63. Preisig O., Zufferey R., Thöny-Meyer L., Appleby C. A., Hennecke H. (1996) A high affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 178, 1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]