Background: TG-interacting factor 1 plays a role in radiation-induced injury.
Results: In vitro, TGIF1 overexpression increases stress-induced cytokine expression whereas TGIF1 knockdown limits it.
Conclusion: TGIF1 regulates radiation and TNF-α-induced inflammation in endothelial cells.
Significance: TGIF1 could be a molecular target to limit radiation or TNF-α-induced proinflammatory phenotype in endothelium.
Keywords: Cytokine Induction, Endothelial Dysfunction, Inflammation, Radiation Biology, SMAD Transcription Factor, TGIF
Abstract
The endothelium contributes to the control of the tissue inflammatory response following stress and in particular after exposure to ionizing radiation. We previously showed that the TG-interacting factor 1 (TGIF1) plays a role in radiation-induced normal tissue injury. In this study we hypothesized that this protein could play a role in inflammation. The role of TGIF1 in the stress-induced proinflammatory phenotype was investigated in human endothelial cells. In HUVECs ionizing radiation induces TGIF1 expression as well as a proinflammatory phenotype associated with up-regulation of IL-6, IL-8, CXCL1, MIP-2, and MCP-1. TGIF1 overexpression enhances the radiation-induced proinflammatory phenotype whereas TGIF1 silencing limits both the TNF-α- and radiation-induced overexpression of proinflammatory cytokines. Interestingly, in vivo, in radiation-induced intestinal inflammation in mice, TGIF1 genetic deficiency is associated with a reduced radiation-induced overexpression of proinflammatory molecules. In HUVECs, TNF-α- and radiation-induced NF-κB pathway activation is not influenced by TGIF1 expression, whereas TGIF1 knockdown inhibits both TNF-α- and radiation-induced p38 MAPK pathway activation. This study demonstrates that TGIF1 plays a role in TNF-α- and radiation-induced inflammation and suggests that it could be a target in limiting this event in the vascular compartment.
Introduction
Radiation-induced normal tissue injury is a dose-limiting factor in the treatment of cancer with radiotherapy (1). Early and late side effects limit radiation dose escalation and affect the patient's quality of life. Radiation damage to normal tissues results from insults to all compartments, and tissue scarring involves an orchestrated response of numerous cell types involved in pathophysiological processes such as cell death, coagulation system activation, inflammation, and matrix remodeling (2).
The involvement of transforming growth factor (TGF)-β1 in radiation-induced normal tissue damage has been demonstrated extensively (3, 4). Radiation-induced up-regulation of TGF-β1 expression levels has been shown in various organs, and, in addition to TGF-β1 itself, several studies have shown up-regulation of intracellular effectors of TGF-β1 signaling, in particular the canonical Smad-dependent pathway (5–8). Smad-mediated signals induced by TGF-β1 are tightly regulated by negative feedback mechanisms via inhibitory Smads (I-Smads) (Smad6 and -7) and through the recruitment of transcriptional Smad co-repressors such as c-Ski, SnoN, and TG-interacting factor 1 (TGIF1)3 (9, 10). TGIF1 is a member of the three-amino acid loop extension class of homeodomain proteins that functions as a co-repressor of the TGF-β1 pathway (11). TGF-β1-dependent transcriptional repression by TGIF1 is mediated by direct competition with the co-activator p300/CBP for Smad2 interaction, thereby repressing TGF-β1-activated genes (12).
In addition and independently of Smad signaling, TGIF1 has been reported to be an essential component of the tumor necrosis factor α (TNF-α) cytotoxic program, suggesting a putative role of this protein in the proinflammatory response following exposure to a proinflammatory cytokine (13). The vascular endothelium is a crucial target for radiation (14, 15). Endothelial cell activation and dysfunction participate in the earliest steps of tissue response to radiation exposure and have been implicated in the mechanisms of acute and late tissue damage in different organs (16–18). Vascular endothelium plays an integrative role in the tissue inflammatory response following stress, and TGF-β1 controls the initiation and resolution of inflammatory responses through the regulation of chemotaxis and activation of leukocytes in the periphery, including lymphocytes, natural killer cells, dendritic cells, macrophages, mast cells, and granulocytes (19). Interestingly, we recently showed that TGIF1 plays a role in radiation-induced small intestinal damage (20). Unexpectedly, our previous in vivo and in vitro results show that this Smad co-repressor plays a role in radiation-induced injury independently of the Smad signaling pathway (20).
The role of TGIF1 in vascular inflammation has never been investigated, and we hypothesized that TGIF1 could play a role in the inflammatory response of endothelial cells following proinflammatory stimuli. In this study we demonstrate for the first time that TGIF1 promotes the endothelial cell inflammatory response after exposure to radiation or TNF-α.
EXPERIMENTAL PROCEDURES
Animals and Irradiation Procedure
TGIF1 knock-out mice were maintained on a C57BL/6J background as described previously. TGIF1+/+ and TGIF1−/− were from the same colony (21), and genotype was determined by PCR screening. Localized small intestinal radiation injury was induced by exposure of an intestinal segment to 19 Gy of radiation (7). Briefly, TGIF1+/+ and TGIF1−/− mice were anesthetized with 5% isoflurane and maintained under anesthesia with 1.7% isoflurane during surgery and irradiation procedures. Mice first underwent laparotomy, and a 3-cm-long intestinal segment (10 cm from the ileocecal valve) was exteriorized and exposed to a single dose of 19 Gy (60Co source dose rate 1.2 Gy/min). Sham irradiation was performed by maintaining the exteriorized intestinal segment without radiation exposure. During irradiation, the abdominal organs were covered with gauze moistened with sterile saline solution. After radiation exposure or sham irradiation, the exteriorized segment was returned to the abdominal cavity and peritoneum/abdominal muscles and skin were separately closed with interrupted sutures. Mice were euthanized at 3 days after irradiation, and intestinal segments from sham and irradiated mice were harvested and snap-frozen in liquid nitrogen and stored at −80 °C for RNA isolation. All experiments were conducted in compliance with legal regulations in France for animal experimentation, and protocols were approved by the ethics committee for animal experimentation of the Institute for Radiological Protection and Nuclear Safety.
Immunohistochemistry
Specimens of normal tissue from 12 patients treated for rectal adenocarcinoma with preoperative radiotherapy (45 Gy; 2 or 1.8 Gy by fraction) were obtained following institutional ethics (Gustave Roussy Institute) and French Medical Research Council guidelines. Tumors were surgically resected 5–7 weeks after treatment; for each patient, specimens of normal tissue were taken from the irradiated field adjacent to the tumor and distant from the tumor, making each patient his own control. Briefly, 5-μm sections of formalin-fixed, paraffin-embedded tissue samples were incubated with anti-TGIF1 (sc-17800; Santa Cruz Biotechnology). Biotinylated rabbit anti-mouse IgG and streptavidin/biotinylated-peroxidase kit (DAKO) were used before staining using the DAB substrate kit (DAKO) and counterstained with hematoxylin.
Endothelial Cell Culture, Irradiation, and TNF-α Exogenous Stimulation
Human umbilical vascular endothelial cells (HUVECs) from Lonza (Verviers, Belgium) were cultured in EGM-2-MV medium at 37 °C with 5% CO2. Cells were used between passages 4 and 5 and were irradiated with a 137cesium source (IBL 637; CisBio, Saclay, France; dose rate 1 Gy min−1).
RNA Isolation and RT Real-time PCR
Total RNA was prepared with the total RNA isolation kit (RNeasy Mini Kit; Qiagen). Total RNA integrity was analyzed using Agilent 2100. After quantification on a NanoDrop ND-1000 apparatus (NanoDrop Technologies, Rockland DE), reverse transcription was performed using the High Capacity Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instructions. Predeveloped TaqMan® Gene Expression Assays (Applied Biosystems) were used, and PCR was performed with the ABI PRISM 7900 Sequence detection system (Applied Biosystems). PCR fluorescent signals were normalized to a PCR fluorescent signal obtained from the housekeeping gene GAPDH (for in vitro experiments) or 18S (for in vivo experiments). Relative mRNA quantification was performed by using the comparative ΔΔCT method. For TaqMan Low Density Array experiments, 400 ng of cDNA of samples was loaded onto the port of human immune gene signature array cards (90 genes). Global analyses were performed using RQ Manager and Data Assist software with a selection of three endogenous genes: 18S, ACTB, and GAPDH.
Western Blot Analysis
Proteins lysed in radioimmune precipitation assay buffer supplemented with protease and phosphatase inhibitors were separated by SDS-polyacrylamide gel electrophoresis before transfer onto nitrocellulose membranes. The following protein-specific primary antibodies were used: anti-TGIF1, anti-Myc (9E10) (Santa Cruz Biotechnology); anti-phospho-p65, anti-phospho-p38, anti-p38, anti-phospho-MAPK-activated protein kinase 2 (MAPKAPK-2), anti-MAPKAPK-2, anti-phospho-HSP27, anti-HSP27 (Cell Signaling Technology). Membranes were incubated with HRP-conjugated secondary antibody (Amersham Biosciences) and were developed using enhanced chemiluminescence. Membranes were stripped and reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Biodesign, Saco, ME) to detect GAPDH expression as a loading control.
Transfection of HUVECs
Human siRNA and nontargeting negative control siRNA (ON-TARGETplus SMARTpool TGIF1 L-011404, p38α L-003512, and ON-TARGETplus Nontargeting Pool) were from Dharmacon (Chicago, IL). HUVECs were seeded into 6-well plates for 24 h to reach 50–70% confluence and were transfected with siRNA at a final concentration of 100 nm using Dharmafect according to the manufacturer's protocols. The medium was replaced 24 h after transfection, and cells were irradiated and/or treated with TNF-α (0.1 ng/ml) and processed for further analysis. For plasmid transfection, HUVECs were transiently transfected with empty vector (pCMV6) or pCMV-Myc-TGIF1 using FuGENE HD (Roche Diagnostics) according to the manufacturer's instructions.
Cytokine Protein Measurements
Cytokine concentrations in cell supernatants were measured using ELISA (R&D Systems) according to the supplier's guidelines.
Statistical Analyses
Data are given as means ± S.E. Statistical analyses were performed using ANOVA or Student's t test with a level of significance of p < 0.05.
RESULTS
TGIF1 Immunoreactivity Is Increased in Inflamed Human Rectum following Radiotherapy
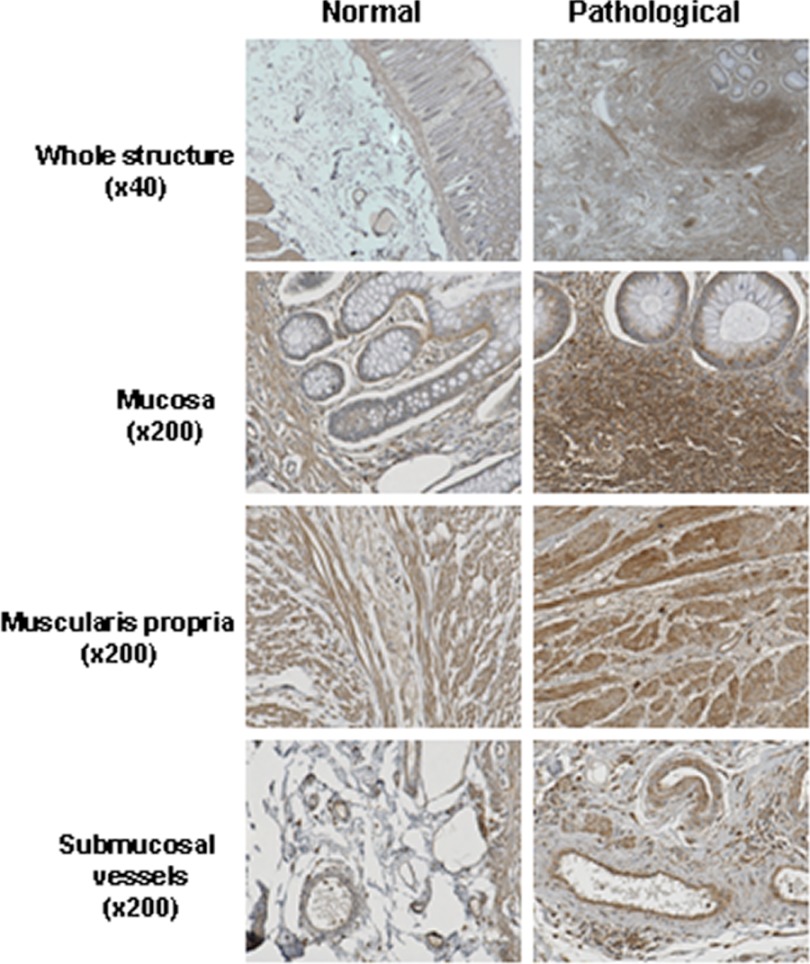
First, to prove the clinical relevance of our hypothesis, we performed a retrospective immunohistochemical study in 12 patients. Normal human rectal tissues were obtained from patients treated with radiotherapy for rectal adenocarcinoma. Tissues were taken in the irradiation field adjacent to the tumor and from macroscopically normal mucosa distant from the tumor. In normal tissues, TGIF1 immunoreactivity is present in muscularis propria and vascular smooth muscle cells, endothelial cells, in resident immunocompetent cells in the submucosa and mucosa, and in the bordering epithelial cells (Fig. 1). In pathological tissues, radiation-induced inflammation is associated with increased TGIF1 immunoreactivity mainly due to lymphoid cells invading the mucosa and submucosa. No significant change in staining intensity was noted concerning muscularis propria and submucosal vessels. These observations suggest that TGIF1 could play a role in radiation-induced tissue inflammation.
FIGURE 1.
TGIF1 immunoreactivity is increased in human rectum after radiotherapy. Pictures are representative of TGIF1 immunostaining of specimens of normal rectal tissue from patients treated with preoperative radiotherapy (n = 12). Tissues were taken distant from the tumor (Normal) and in the irradiation field adjacent to the tumor (Pathological). All images were recorded in normal and pathological areas from the same patient.
TGIF1 Overexpression Enhances the Basal and Radiation-induced Proinflammatory Phenotype of Endothelial Cells
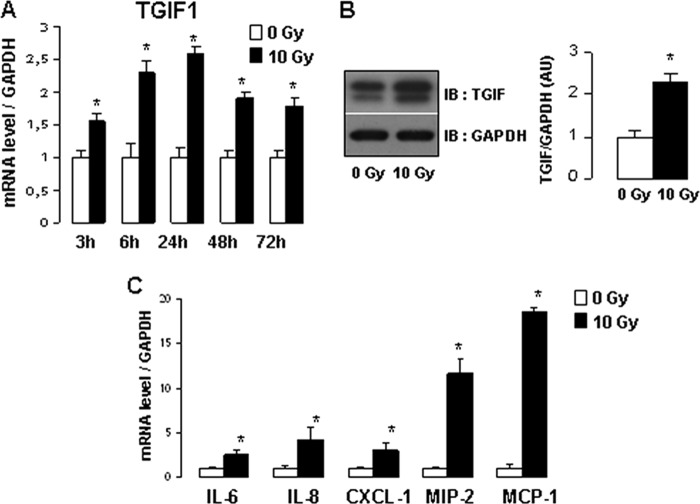
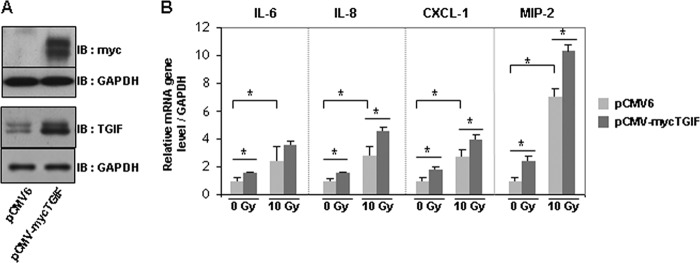
Because the endothelium plays a key role in vascular injury and subsequent inflammatory intestinal damage after radiation, the radiation-induced proinflammatory phenotype was investigated in human endothelial cells. Ionizing radiation induces TGIF1 expression at mRNA and protein levels (Fig. 2, A and B) and a proinflammatory phenotype in HUVECs associated with a significant up-regulation of gene expression of IL-6, IL-8, CXCL1, MIP-2, and MCP-1 in irradiated HUVECs compared with control cells (Fig. 2C). To examine whether overexpression of TGIF1 affects the inflammatory state of endothelial cells, HUVECs were transfected with Myc-TGIF1 expression vector. After transfection, TGIF1 overexpression was confirmed by Western blot analysis (Fig. 3A). TGIF1 overexpression in HUVECs induced a slight increase in the basal mRNA levels of IL-6, IL-8, CXCL1, and MIP-2. TGIF1 overexpression enhanced the radiation-induced up-regulation of IL-8, CXCL1, and MIP-2 genes with no statistically significant variation for IL-6.
FIGURE 2.
Radiation induces expression of TGIF1 and proinflammatory genes in vitro in endothelial cells. A, mRNA levels of TGIF1 3, 6, 24, 48 and 72 h after 10 Gy. B, representative Western blot (IB) of TGIF1 protein expression 24 h after 10 Gy. Relative quantification was performed from four experiments performed in triplicate. C, mRNA levels of IL-6, IL-8, CXCL1, MIP-2, and MCP-1 in HUVECs 3 h after 10 Gy. mRNA levels were calculated relative to GAPDH as housekeeping gene and normalized to a value of 1 for nonirradiated cells. Results are the mean ± S.E. (error bars) of three independent experiments done in triplicate. *, p < 0.05 versus nonirradiated cells.
FIGURE 3.
TGIF1 overexpression stimulates the basal and the radiation-induced proinflammatory phenotype of endothelial cells. A, representative Western blots show total protein cell lysates from HUVECs transfected for 24 h with control vector (pCMV6) or with pCMV-Myc-TGIF1 vector. B, HUVECs were transfected with control vector (pCMV6) or pCMV-Myc TGIF for 24 h and irradiated or not at 10 Gy. mRNA expression levels of IL-6, IL-8, CXCL1, and MIP-2 were measured 3 h after irradiation. Results are ± S.E. (error bars) of two independent experiments done in triplicate. *, p < 0.05.
TGIF1 Knockdown Limits Radiation or TNF-α-induced HUVEC Proinflammatory State
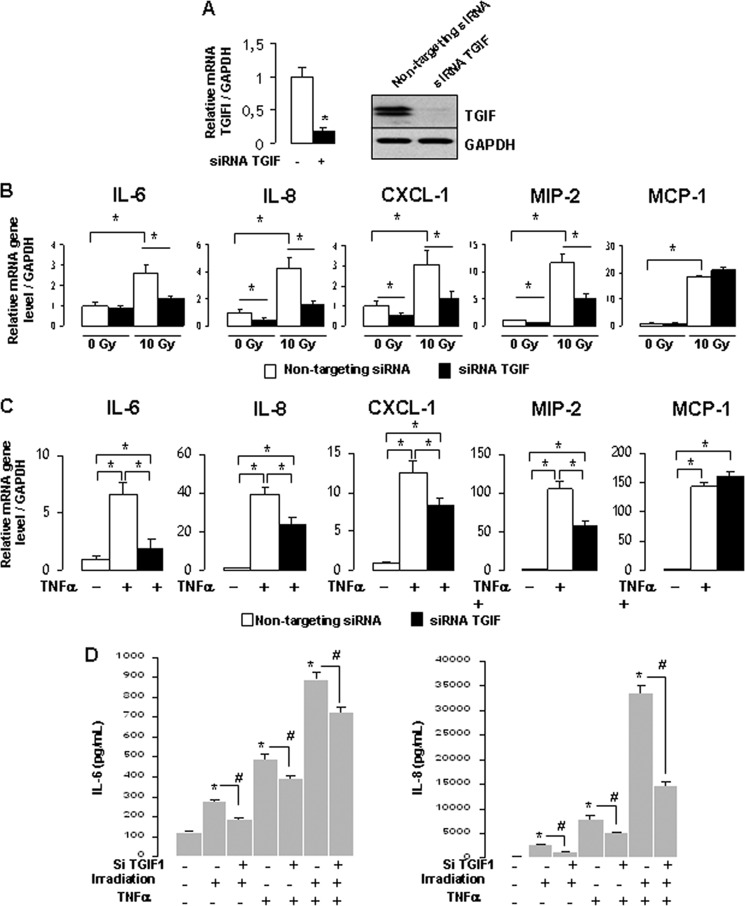
To investigate whether TGIF1 silencing impacts the endothelial inflammatory state after irradiation, TGIF1 expression knockdown was performed in HUVECs using an RNA interference (RNAi) approach. Silencing efficiency was confirmed at mRNA and protein levels and revealed a decrease of up to 90% in TGIF1 expression in HUVECs transfected with siRNA TGIF1 compared with cells transfected with nontargeting siRNA (Fig. 4A). Using a global approach with TaqMan Low Density Arrays, we observed that TGIF1 silencing down-regulated expression levels of numerous radiation-induced proinflammatory genes (supplemental Fig. 1A). By real-time PCR we confirmed that TGIF1 silencing significantly decreased basal expression levels of IL-8, CXCL1, and MIP-2 in HUVECs, with no statistically significant difference for IL-6 and MCP-1 genes. In irradiated cells, TGIF1 silencing limited radiation-induced inflammatory gene expression by 50, 54, 61, and 58% for IL-6, IL-8, CXCL1, and MIP-2, respectively, without any effect on MCP-1 gene expression (Fig. 4B). Next we considered whether the role of TGIF1 is restricted to radiation stress or whether TGIF1 acts as a universal regulator of inflammation. To determine the involvement of TGIF1 in the TNF-α-induced proinflammatory state of HUVECs, cytokine expression in TNF-α-treated cells transfected with nontargeting siRNA or siRNA targeting TGIF1 was compared. TNF-α strongly increased expression of a cascade of proinflammatory genes (Fig. 4C). The global approach with TaqMan Low Density Arrays revealed that TGIF1 silencing down-regulated expression of numerous TNF-α-induced proinflammatory genes (supplemental Fig. 1B). Individual real-time PCR measurements confirmed that TGIF1 silencing limited TNF-α-induced inflammatory gene expression by 70, 45, 31, and 50% for IL-6, IL-8, CXCL1, and MIP-2, respectively (Fig. 4C). To estimate the consequences at the protein level, measurements of IL-6 and IL-8 concentrations in cell supernatants showed that TGIF1 knockdown limited radiation- and/or TNF-α-induced IL-6 and IL-8 overexpression at the protein level (Fig. 4D). These observations were confirmed for CXCL1 secretion in irradiated and TNF-α-treated cells (supplemental Fig. 2).
FIGURE 4.
TGIF1 silencing limits the radiation- and TNF-α-induced proinflammatory state of endothelial cells. A, HUVECs were transfected for 24 h with nontargeting siRNA or siRNA targeting TGIF1. Silencing efficiency was measured at mRNA and protein levels 24 h after transfection. B, HUVECs were transfected with nontargeting siRNA or siRNA targeting TGIF1 and exposed or not to 10-Gy irradiation. mRNA expression levels of IL-6, IL-8, CXCL1, MIP-2, and MCP-1 were measured 3 h after irradiation. C, HUVECs were transfected with nontargeting siRNA or siRNA targeting TGIF1 and treated or not with 0.1 ng/ml TNF-α. mRNA expression levels were measured 3 h after incubation. D, IL-6 and IL-8 protein secretion in supernatant 8 h after irradiation and/or TNF-α exposure is shown. Results are ± S.E. (error bars) of three independent experiments done in triplicate. *, p < 0.05.
TGIF1 Deficiency Limits Radiation-induced Overexpression of Proinflammatory Molecules in Vivo
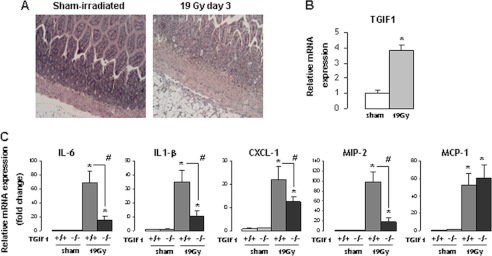
To confirm our in vitro results, we explored the in vivo effects of TGIF1 genetic deficiency on radiation-induced small intestinal inflammation in mice. Three days after a high localized single dose of radiation, the crypt compartment was severely depleted, with crypt abscesses and dense inflammatory infiltrate. The villus compartment was moderately affected (Fig. 5A). In this model, irradiation increased TGIF1 expression (Fig. 5B) and strongly increased the mRNA levels of IL-6, IL-1β, CXCL1, MIP-2, and MCP-1 in total intestinal tissues from irradiated mice compared with nonirradiated sham mice. Interestingly, TGIF1 genetic deficiency disrupted cytokine and chemokine production in irradiated intestinal tissues. Real-time PCR analyses showed a strong inhibition of IL-6 (78%), IL-1β (73%), CXCL1 (41%), and MIP-2 (80%) expression in irradiated intestine from TGIF1−/− mice compared with irradiated intestine from TGIF+/+ mice, with no difference observed for MCP-1 (Fig. 5C).
FIGURE 5.
In vivo, TGIF1 genetic deficiency limits radiation-induced proinflammatory cytokine expression. A, representative hemalum-eosin-safran staining of small intestine 3 days after sham (left) or 19 Gy-localized small intestinal irradiation (right) (original magnification, ×20). B, mRNA TGIF1 level in intestine 3 days after 19 Gy-localized small intestinal radiation exposure in TGIF1+/+ mice. mRNA levels were calculated relative to 18S as housekeeping gene and normalized to a value of 1 for the sham control group (n = 8/group). C, relative mRNA levels of IL-6, IL1-β, CXCL1, MIP-2, and MCP-1 in sham TGIF1+/+ and TGIF1−/− mice or TGIF1+/+ and TGIF1−/− 3 days after 19 Gy-localized small intestinal irradiation. mRNA levels were calculated relative to 18S as housekeeping gene and normalized to a value of 1 for the TGIF1+/+ sham group. Results are the mean ± S.E. (error bars) with n = 8 for sham TGIF1+/+ mice, n = 18 for irradiated TGIF1+/+, and n = 14 for irradiated TGIF1−/− mice. *, p < 0.05 versus TGIF1+/+ sham mice; #, p < 0.05 versus TGIF1+/+ irradiated mice.
The p38 MAPK Pathway Is Involved in TGIF1-dependent Radiation and TNF-α-induced Proinflammatory State in HUVECs
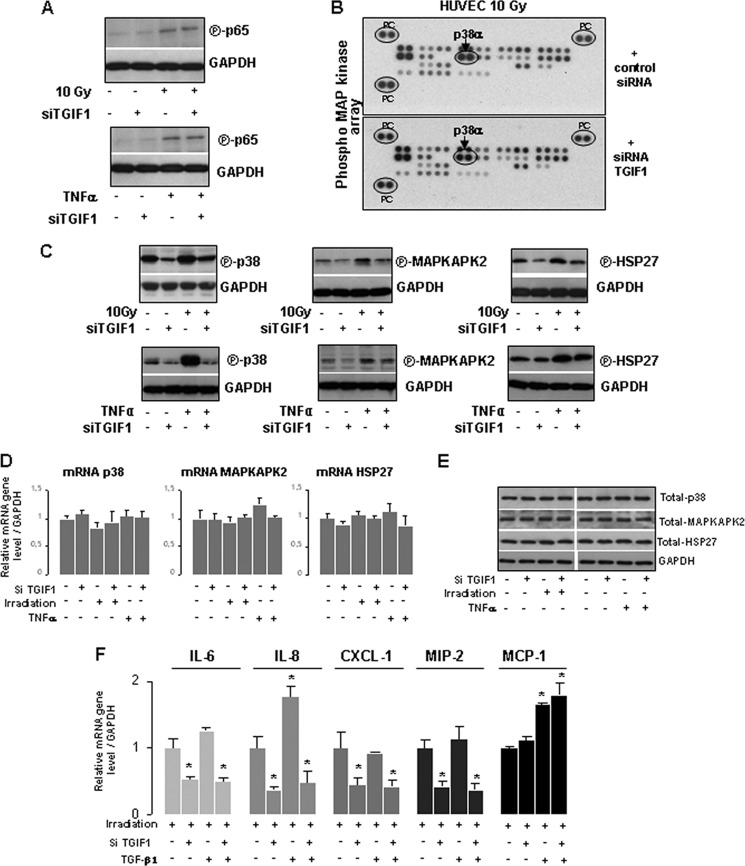
To understand the molecular mechanisms by which TGIF1 controls cytokine expression, we first examined the major proinflammatory signaling pathway, i.e. nuclear factor-κB (NF-κB). Immunoblot analysis was performed to quantify the phosphorylated form of NF-κB subunit p65, which reflects the activation of the NF-κB signaling. Radiation or TNF-α exposure increased the level of phosphorylated p65 in HUVECs, but TGIF1 knockdown had no effect on NF-κB pathway activation (Fig. 6A). Because NF-κB signaling pathway does not seem to be involved in TGIF1-dependent inflammatory gene expression, we hypothesized that the MAPK pathway may be involved. First, we used a global approach with a protein phospho-MAPK array which simultaneously explores the phosphorylation patterns of 23 different MAPKs and other serine/threonine kinases. When we applied a minimum cut-off threshold of 20% change, among the panel of MAPKs, we identified a marked decrease by 32% of the phosphorylation level of p38α in irradiated HUVECs knocked down for TGIF1 compared with irradiated HUVECs transfected with control siRNA (Fig. 6B). The same experiment was performed in TNF-α-treated cells ± siRNA TGIF1 and a decrease by 35% of the phosphorylation level of p38α in TNF-α-treated HUVECs knocked down for TGIF1 compared with TNF-α-treated HUVECs transfected with control siRNA (data not shown). For the other targets, variations were in the ±20% range. To confirm the phospho-MAPK array results, p38 MAPK and its downstream targets MAPKAPK-2 and heat shock protein 27 (HSP27) were monitored by Western blotting using antibodies against the phosphorylated forms of these proteins. Western blot analyses revealed that TGIF1 knockdown was associated with decreased levels of basal phospho-p38 protein and its downstream substrates MAPKAPK-2 and HSP27. Moreover, TGIF1 knockdown inhibited both TNF-α- and radiation-induced p38 MAPK pathway activation by decreasing the phosphorylation level of p38, MAPKAPK-2, and HSP27 (Fig. 6C). Interestingly, mRNA levels and total forms of p38, MAPKAPK-2, and HSP27 were unchanged in all experimental conditions showing that activation of the p38 MAPK pathway is not related to transcriptional modifications (Fig. 6, D and E).
FIGURE 6.
TGIF1 influences the activation of the p38 MAPK pathway. A, HUVECs were transfected with nontargeting siRNA or siRNA TGIF1 for 24 h and irradiated at 10 Gy or treated with 0. 1 ng/ml TNF-α. Total protein prepared from whole cell lysate 1 h after irradiation or 15 min after TNF-α treatment was subjected to immunoblotting. Representative Western blot shows the phosphorylated form of the NFκB subunit p65. B, HUVECs were transfected with nontargeting siRNA or siRNA TGIF1 for 24 h and irradiated at 10 Gy. Cells were lysed 2 h after irradiation, and total protein lysates were applied to the human phospho-MAPK array (PC, positive controls). Results revealed a decrease in the phospho-p38α protein level in HUVECs transfected with siRNA-TGIF1. C, representative Western blots from three independent experiments show the phosphorylated form of p38, MAPKAPK-2, and HSP27 in TNF-α-treated or irradiated HUVECs. D, mRNA levels of p38, MAPKAPK-2, and HSP27 in TNF-α-treated or irradiated HUVECs knockdown or not for TGIF1 are shown. E, representative Western blots from three independent experiments show the total form of p38, MAPKAPK-2, HSP27 in TNF-α-treated or irradiated HUVECs. F, mRNA expression levels of IL-6, IL-8, CXCL-1, MIP-2, and MCP-1 were measured 3 h after irradiation in HUVECs knocked down for TGIF1 or/and treated by 10 ng/ml TGF-β1. Results are ± S.E. (error bars) of three independent experiments done in triplicate. *, p < 0.05 versus irradiated cells.
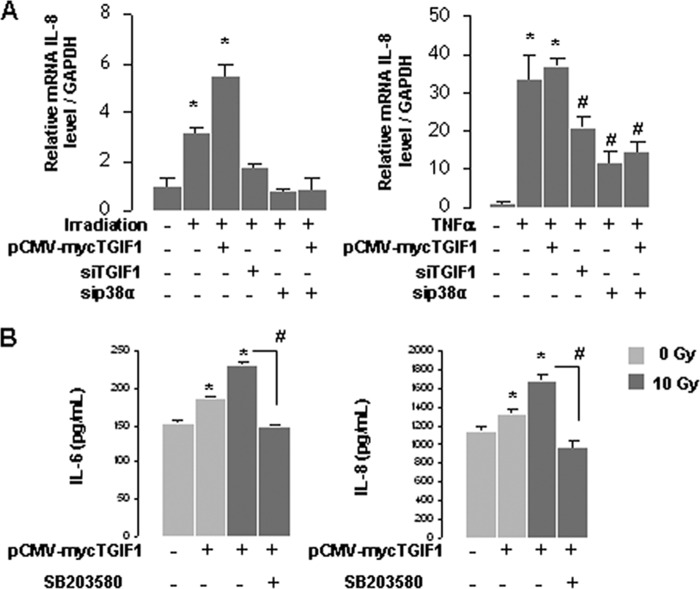
Moreover, because TGIF1 is a Smad co-repressor, we explored whether TGF-β1 could influence the TGIF1-dependent radiation and TNF-α-induced proinflammatory state in HUVECs. Cytokine expression was monitored in irradiated cells knockdown or not for TGIF1 and treated with TGF-β1. Results showed that exogenous TGF-β1 has no effect on the mechanism by which TGIF1 influences cytokine expression. Finally, to prove that p38 is involved in overexpression of proinflammatory cytokines, HUVECs were co-transfected with pCMV-Myc-TGIF and siRNA p38α. p38α silencing blunted TGIF1-dependent transcriptional IL-8 overexpression in both irradiated and TNF-α-treated cells (Fig. 7A). Finally, in irradiated HUVECs overexpressing TGIF1, the p38α/β inhibitor SB203580 limited IL-6 and IL-8 protein secretion (Fig. 7B).
FIGURE 7.
The p38 MAPK pathway is involved in the TGIF1-dependent radiation and TNF-α-induced proinflammatory state in HUVECs. A, HUVECs were transfected with pCMV-myc TGIF1 or siRNA TGIF1 or siRNAp38α for 24 h and irradiated at 10 Gy or treated with 0.1 ng/ml TNF-α. mRNA IL-8 levels were determined by real-time PCR. *, p < 0.05 versus control cells; #, p < 0.05 versus TNF-α-treated cells. B, HUVECs were transfected for 24 h with control or with pCMV-myc-TGIF1 vector and treated or not with the p38α/β inhibitor SB206580 (10 μm). IL-6 and IL-8 protein secretion was determined in supernatant 8 h after irradiation. Results are ± S.E. (error bars) of three independent experiments done in triplicate. *, p < 0.05 versus control cells; #, p < 0.05 versus irradiated cells transfected with TGIF1 vector.
DISCUSSION
Radiation therapy is used to treat a wide range of solid tumors. Radiation-induced side effects are associated with acute and chronic activation of the endothelium in preclinical models (15) as well as in patients (22). The radiation-induced inflammatory response is common to irradiated tissues and involves endothelial cell activation and secretion of a panel of adhesion molecules, inflammatory mediators, and growth factors (23). Here, we show for the first time that TGIF1 plays a role in the radiation-induced proinflammatory phenotype of endothelial cells. Interestingly, this new role for TGIF1 is not restricted to ionizing radiation stress and is also true after exposure to TNF-α.
Recently, we demonstrated that TGIF1 plays a role in radiation-induced normal tissue damage by a Smad-independent mechanism (20). In vivo, TGIF1 genetic deficiency sensitizes mice to radiation-induced intestinal damage after total body or localized small intestinal radiation exposure. Unexpectedly, we show here that TGIF1 genetic deficiency is associated with reduced expression of proinflammatory cytokines, suggesting that increased death of TGIF1−/− mice after radiation exposure is not due to exacerbated proinflammatory acute tissue reaction. These results open new perspectives concerning the role of inflammation in radiation damages. Most of teams that work on radiation-induced damages consider as an output for treatment efficiency that reducing inflammation is a benefit. In the same way, in numerous radiation injury score systems the severity of inflammation is one parameter to affirm whether one therapeutic method is good or bad. Transgenic mouse models with reduced proinflammatory radiation response are not necessarily associated with tissue radioprotection. For example, SMAD3−/− mice are protected against radiation-induced skin injury despite a transient strong increase in neutrophil influx (24, 25). In the same way, we recently showed that radiation proctitis is attenuated in mast cell-deficient mice compared with WT mice and that it is associated with increased tissue neutrophil influx and expression of several inflammatory mediators immediately after radiation exposure (26). Altogether, these results highlight the complex balances between the beneficial and detrimental roles of inflammation in the kinetics of tissue wound healing. Clearly, the relationship between reduced inflammatory transcriptional response in TGIF1−/− mice and increased radiation sensitivity of these mice remains unclear, and more experiments are needed to explore the potential link.
Tgif1 mutation in mouse contributes to holoprosencephaly by a disruption of the sonic hedgehog homolog pathway (27). In a model of brain injury, proinflammatory signals via CD11b+ macrophages are necessary for sonic hedgehog homolog expression and astrocyte formation involved in the resolution of brain injury (28). In this case a positive role for proinflammatory signals during acute brain injury is underlined. The relationship between TGIF1 and sonic hedgehog homolog signaling in brain damages and recovery was never investigated, and the new role for TGIF1 in inflammation reported in the present work could open perspectives in the molecular pathogenesis of holoprosencephaly.
Interestingly, a computational approach identified TGIF1 as a putative regulator of macrophages activation in response to TLR stimulation (29), and TGIF1 was reported as essential to the TNF-α cytotoxic program (13). Moreover, TGIF1 can directly bind c-Jun and influence the cross-talk between the c-Jun N-terminal kinase (JNK) and Smad signaling pathways (30). So there is a growing body of evidence in the literature suggesting that TGIF1 could play a role in the inflammatory process. The role of TGIF1 in inflammation has never been studied, and here we report an increased TGIF1 immunoreactivity in inflamed tissues from patients treated with radiation therapy. We demonstrate that TGIF1 expression influences the proinflammatory phenotype of endothelial cells after irradiation or TNF-α exposure and that the p38 MAP kinase pathway is involved. MAPKs are serine/threonine-specific protein kinases that regulate various cellular activities including the inflammatory response following extracellular stimuli. Irradiation and TNF-α are known to activate MAPK pathways in endothelial cells (31, 32). Here, we report a novel cross-talk between p38 MAPK and TGIF1, although the molecular events involved remain unknown. p38α silencing limits TGIF1-dependent overexpression of proinflammatory cytokines, and TGIF1 silencing is associated with a decreased expression of the phosphorylated form of p38. Interestingly, c-Jun physically binds TGIF1 and stabilizes the TGIF1-Smad2 complex, and this interaction is critical for the repression of Smad2-dependent transcription (30). Further experiments are needed to explore the putative stabilization of TGIF1 by p38 or p38 by TGIF1. Moreover, Bartholin et al. showed that TGIF1 inhibits retinoid signaling by the interaction with the ligand-binding domain of the RXRα retinoid receptor (33). TGIF1 is an RXRα transcriptional co-repressor and represses RXR-dependent transcriptional responses. RXRα genetic deficiency in myeloid cells results in lower recruitment of leukocytes to sites of inflammation and a lower susceptibility to sepsis (34). In our experimental conditions we cannot exclude that the TGIF1-dependent transcriptional response of proinflammatory cytokines is linked to a cross-talk with retinoid signaling, and this is an attractive perspective of our work.
In conclusion, we report a new role for TGIF1 as a proinflammatory molecule and suggest that TGIF1 could be a potent therapeutic target in limiting the proinflammatory phenotype in the vascular compartment. However, in light of our previous work (20) and because anti-inflammatory strategies benefit are highly context-dependent, such therapies must be explored with caution.
This work was supported by the Institute for Radiological Protection and Nuclear Safety.

This article contains supplemental Table 1 and Figs. 1 and 2.
- TGIF1
- TG-interacting factor 1
- CXCL1
- chemokine (C-X-C motif) ligand 1
- Gy
- gray
- HUVEC
- human umbilical vein endothelial cell
- MAPKAPK-2
- MAPK-activated protein kinase 2
- MCP-1 (or CCL2)
- monocyte chemotactic protein 1
- MIP-2 (or CXCL-2)
- macrophage inflammatory protein 2
- RXR
- retinoid X receptor.
REFERENCES
- 1. Barnett G. C., West C. M., Dunning A. M., Elliott R. M., Coles C. E., Pharoah P. D., Burnet N. G. (2009) Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer 9, 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bentzen S. M. (2006) Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer 6, 702–713 [DOI] [PubMed] [Google Scholar]
- 3. Anscher M. S. (2010) Targeting the TGF-β1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 15, 350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin M., Lefaix J., Delanian S. (2000) TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 47, 277–290 [DOI] [PubMed] [Google Scholar]
- 5. Kruse J. J., Floot B. G., te Poele J. A., Russell N. S., Stewart F. A. (2009) Radiation-induced activation of TGF-β signaling pathways in relation to vascular damage in mouse kidneys. Radiat. Res. 171, 188–197 [DOI] [PubMed] [Google Scholar]
- 6. Milliat F., François A., Isoir M., Deutsch E., Tamarat R., Tarlet G., Atfi A., Validire P., Bourhis J., Sabourin J. C., Benderitter M. (2006) Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am. J. Pathol. 169, 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milliat F., Sabourin J. C., Tarlet G., Holler V., Deutsch E., Buard V., Tamarat R., Atfi A., Benderitter M., François A. (2008) Essential role of plasminogen activator inhibitor type-1 in radiation enteropathy. Am. J. Pathol. 172, 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scharpfenecker M., Kruse J. J., Sprong D., Russell N. S., Ten Dijke P., Stewart F. A. (2009) Ionizing radiation shifts the PAI-1/ID-1 balance and activates notch signaling in endothelial cells. Int. J. Radiat. Oncol. Biol. Phys. 73, 506–513 [DOI] [PubMed] [Google Scholar]
- 9. Itoh S., ten Dijke P. (2007) Negative regulation of TGF-β receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19, 176–184 [DOI] [PubMed] [Google Scholar]
- 10. Massagué J., Seoane J., Wotton D. (2005) Smad transcription factors. Genes Dev. 19, 2783–2810 [DOI] [PubMed] [Google Scholar]
- 11. Wotton D., Lo R. S., Lee S., Massagué J. (1999) A Smad transcriptional corepressor. Cell 97, 29–39 [DOI] [PubMed] [Google Scholar]
- 12. Wotton D., Lo R. S., Swaby L. A., Massagué J. (1999) Multiple modes of repression by the Smad transcriptional corepressor TGIF. J. Biol. Chem. 274, 37105–37110 [DOI] [PubMed] [Google Scholar]
- 13. Demange C., Ferrand N., Prunier C., Bourgeade M. F., Atfi A. (2009) A model of partnership co-opted by the homeodomain protein TGIF and the Itch/AIP4 ubiquitin ligase for effective execution of TNF-α cytotoxicity. Mol. Cell 36, 1073–1085 [DOI] [PubMed] [Google Scholar]
- 14. Paris F., Fuks Z., Kang A., Capodieci P., Juan G., Ehleiter D., Haimovitz-Friedman A., Cordon-Cardo C., Kolesnick R. (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 [DOI] [PubMed] [Google Scholar]
- 15. Wang J., Boerma M., Fu Q., Hauer-Jensen M. (2007) Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 13, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abderrahmani R., François A., Buard V., Tarlet G., Blirando K., Hneino M., Vaurijoux A., Benderitter M., Sabourin J. C., Milliat F. (2012) PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PloS One 7, e35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaugler M. H. (2005) A unifying system: does the vascular endothelium have a role to play in multi-organ failure following radiation exposure? BJR Suppl. 27, 100–10515975880 [Google Scholar]
- 18. Holler V., Buard V., Gaugler M. H., Guipaud O., Baudelin C., Sache A., Perez Mdel R., Squiban C., Tamarat R., Milliat F., Benderitter M. (2009) Pravastatin limits radiation-induced vascular dysfunction in the skin. J. Invest. Dermatol. 129, 1280–1291 [DOI] [PubMed] [Google Scholar]
- 19. Li M. O., Wan Y. Y., Sanjabi S., Robertson A. K., Flavell R. A. (2006) Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 [DOI] [PubMed] [Google Scholar]
- 20. Hneino M., François A., Buard V., Tarlet G., Abderrahmani R., Blirando K., Hoodless P. A., Benderitter M., Milliat F. (2012) The TGF-β/Smad repressor TG-interacting factor 1 (TGIF1) plays a role in radiation-induced intestinal injury independently of a Smad signaling pathway. PloS One 7, e35672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mar L., Hoodless P. A. (2006) Embryonic fibroblasts from mice lacking Tgif were defective in cell cycling. Mol. Cell. Biol. 26, 4302–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halle M., Gabrielsen A., Paulsson-Berne G., Gahm C., Agardh H. E., Farnebo F., Tornvall P. (2010) Sustained inflammation due to nuclear factor-κB activation in irradiated human arteries. J. Am. Coll. Cardiol. 55, 1227–1236 [DOI] [PubMed] [Google Scholar]
- 23. Molla M., Panes J. (2007) Radiation-induced intestinal inflammation. World J. Gastroenterol. 13, 3043–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flanders K. C., Ho B. M., Arany P. R., Stuelten C., Mamura M., Paterniti M. O., Sowers A., Mitchell J. B., Roberts A. B. (2008) Absence of Smad3 induces neutrophil migration after cutaneous irradiation: possible contribution to subsequent radioprotection. Am. J. Pathol. 173, 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flanders K. C., Sullivan C. D., Fujii M., Sowers A., Anzano M. A., Arabshahi A., Major C., Deng C., Russo A., Mitchell J. B., Roberts A. B. (2002) Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 160, 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blirando K., Milliat F., Martelly I., Sabourin J. C., Benderitter M., François A. (2011) Mast cells are an essential component of human radiation proctitis and contribute to experimental colorectal damage in mice. Am. J. Pathol. 178, 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taniguchi K., Anderson A. E., Sutherland A. E., Wotton D. (2012) Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway. PLoS Genet. 8, e1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amankulor N. M., Hambardzumyan D., Pyonteck S. M., Becher O. J., Joyce J. A., Holland E. C. (2009) Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 29, 10299–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsey S. A., Klemm S. L., Zak D. E., Kennedy K. A., Thorsson V., Li B., Gilchrist M., Gold E. S., Johnson C. D., Litvak V., Navarro G., Roach J. C., Rosenberger C. M., Rust A. G., Yudkovsky N., Aderem A., Shmulevich I. (2008) Uncovering a macrophage transcriptional program by integrating evidence from motif scanning and expression dynamics. PLoS Comp. Biol. 4, e1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pessah M., Prunier C., Marais J., Ferrand N., Mazars A., Lallemand F., Gauthier J. M., Atfi A. (2001) c-Jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity. Proc. Natl. Acad. Sci. U.S.A. 98, 6198–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou C. H., Chen S. U., Cheng J. C. (2009) Radiation-induced interleukin-6 expression through MAPK/p38/NF-κB signaling pathway and the resultant antiapoptotic effect on endothelial cells through Mcl-1 expression with sIL6-Rα. Int. J. Radiat. Oncol. Biol. Phys. 75, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 32. Kuldo J. M., Westra J., Asgeirsdóttir S. A., Kok R. J., Oosterhuis K., Rots M. G., Schouten J. P., Limburg P. C., Molema G. (2005) Differential effects of NF-κB and p38 MAPK inhibitors and combinations thereof on TNF-α- and IL-1β-induced proinflammatory status of endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 289, C1229–1239 [DOI] [PubMed] [Google Scholar]
- 33. Bartholin L., Powers S. E., Melhuish T. A., Lasse S., Weinstein M., Wotton D. (2006) TGIF inhibits retinoid signaling. Mol. Cell. Biol. 26, 990–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Núñez V., Alameda D., Rico D., Mota R., Gonzalo P., Cedenilla M., Fischer T., Boscá L., Glass C. K., Arroyo A. G., Ricote M. (2010) Retinoid X receptor α controls innate inflammatory responses through the up-regulation of chemokine expression. Proc. Natl. Acad. Sci. U.S.A. 107, 10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]