Background: Stromal-epithelial interactions regulate mammary gland development and tumorigenesis.
Results: Targeted overexpression of adipocyte enhancer-binding protein (AEBP1) in stromal macrophages induces alveolar hyperplasia via up-regulation of NF-κB, TNFα, and hedgehog pathway components.
Conclusion: AEBP1 orchestrates the stromal-epithelial interactions via proinflammatory and hedgehog signaling.
Significance: This is a first report implicating AEBP1 in mammary gland hyperplasia with possible association to tumorigenesis.
Keywords: Breast Cancer, Inflammation, Macrophages, Mammary Gland, NF-κ B (NF-KB), Transgenic Mice, Tumor Necrosis Factor (TNF), Bone Marrow Transplantation, Hyperplasia, Sonic Hedgehog
Abstract
Disruption of mammary stromal-epithelial communication leads to aberrant mammary gland development and induces mammary tumorigenesis. Macrophages have been implicated in carcinogenesis primarily by creating an inflammatory microenvironment, which promotes growth of the adjacent epithelial cells. Adipocyte enhancer-binding protein 1 (AEBP1), a novel proinflammatory mediator, promotes macrophage inflammatory responsiveness by inducing NF-κB activity, which has been implicated in tumor cell growth and survival by aberrant sonic hedgehog (Shh) expression. Here, we show that stromal macrophage AEBP1 overexpression results in precocious alveologenesis in the virgin AEBP1 transgenic (AEBP1TG) mice, and the onset of ductal hyperplasia was accelerated in AEBP1TG mice fed a high fat diet, which induces endogenous AEBP1 expression. Transplantation of AEBP1TG bone marrow cells into non-transgenic (AEBP1NT) mice resulted in alveolar hyperplasia with up-regulation of NF-κB activity and TNFα expression as displayed in the AEBP1TG mammary macrophages and epithelium. Shh expression was induced in AEBP1TG macrophages and RAW264.7 macrophages overexpressing AEBP1. The Shh target genes Gli1 and Bmi1 expression was induced in the AEBP1TG mammary epithelium and HC11 mammary epithelial cells co-cultured with AEBP1TG peritoneal macrophages. The conditioned AEBP1TG macrophage culture media promoted NF-κB activity and survival signal, Akt activation, in HC11 cells, whereas such effects were abolished by TNFα neutralizing antibody treatment. Furthermore, HC11 cells displayed enhanced proliferation in response to AEBP1TG macrophages and their conditioned media. Our findings highlight the role of AEBP1 in the signaling pathways regulating the cross-talk between mammary epithelium and stroma that could predispose the mammary tissue to tumorigenesis.
Introduction
Chronic inflammation is often the perpetrator of mammary tumorigenesis via disruption of normal signaling between the ductal epithelium and stromal microenvironments (1, 2). The stromal microenvironment contains a heterogeneous population of cells such as fibroblasts, endothelium, adipocytes, and immune cells that can have a crucial impact on cancer development (3). In particular, stromal macrophages play an integral role in promoting inflammatory signaling leading to cancer development, progression, and metastasis (4, 5).
Nuclear factor-κ B (NF-κB) is a pivotal proinflammatory transcriptional regulator that is constitutively active in mammary tumors (6). NF-κB promotes aberrant cell proliferation, survival and invasion via secretion of various cytokines and chemokines (7). In the canonical pathway, NF-κB is a dimer of RelA (p65) and p105/50 that translocates to the nucleus and binds to the promoter regions of its target genes (8). However, NF-κB activity is diminished in quiescent cells when it is sequestered in the cytoplasm as it complexes with proteins of the inhibitor of NF-κB (IκB) family. NF-κB activity is induced by various signals that stimulate the multimeric IκB kinase complexes to phosphorylate IκB, triggering ubiquitin-mediated degradation by the proteasome (8).
NF-κB was recently shown to transcriptionally activate the mitogen sonic hedgehog (Shh)6 in inflammatory-stimulated macrophages (9). The hedgehog signaling pathway is a critical regulator of tissue morphogenesis, and it has been implicated in the development, progression, and metastasis of various types of cancer (9–11). Shh binds and inactivates the transmembrane receptor Patched (Ptch), which allows the activation of the membrane-bound hedgehog effector Smoothened (SMO). SMO activation prompts the release of the glioma-associated oncogene homolog (Gli) transcription factors to the nucleus (12), where they can activate Hh-responsive genes (e.g. Gli1, Ptch1, Hhip). Constitutive activation of hedgehog signaling is an essential pathway in cancer development as it up-regulates genes involved in proliferation (cyclins), apoptosis resistance (Bcl2), and epithelial-to-mesenchymal transition (Snail) (13–16). Although previous studies indicated that aberrant production of Shh originates from tumors themselves (9, 17), it has been suggested that Shh production from NF-κB-stimulated stromal macrophages is also essential for promoting tumor growth in a paracrine manner (18).
Adipocyte enhancer-binding protein 1 (AEBP1) is a novel proinflammatory mediator (19) that induces NF-κB activity via hampering IκBα inhibitory function (20). AEBP1 is expressed in many tissues, but it is most abundant in preadipocytes (21, 22) and macrophages (19, 20, 23), which are found in mammary stroma. We have recently demonstrated that AEBP1 is specifically localized in the stromal compartment of adult nulliparous mammary glands and that macrophage AEBP1 plays a critical role in mammary gland development (24). Interestingly, microarray analyses revealed that AEBP1 is up-regulated in the stroma of mammary tumors (25) and breast cancer cells (26). In this study we demonstrate that AEBP1-transgenic mice (AEBP1TG), with targeted AEBP1 overexpression in adipose tissue and macrophages (27), have a significantly higher incidence of alveolar hyperplasia and enhanced macrophage infiltration compared with non-transgenic mice (AEBP1NT). We also present compelling evidence suggesting that macrophage AEBP1 promotes mammary hyperplasia by sustaining a chronic inflammatory microenvironment associated with enhanced hedgehog and NF-κB signaling.
EXPERIMENTAL PROCEDURES
Animals
Generation of AEBP1TG (27) and AEBP1−/− (28) mice was previously described. Age-matched mice were kept on a 12-h light cycle in the Carleton Animal Care Facility at Dalhousie University where they were fed and watered ad libitum. Standard rodent chow and high fat diet (HFD; 45% of total calories from fat; D12451; Research Diets, New Brunswick, NJ) were used. All animal protocols have been approved by the Dalhousie University Animal Care committee. Mice were sacrificed by euthanasia using an overdose of sodium pentobarbital (Somnitol) and mammary glands were isolated for analysis.
Bone Marrow Transplantation
Bone marrow (BM) transplantation experiments were performed as previously described (29).
Whole Mount Analysis and Immunohistochemistry
Whole mount preparations of mammary glands were performed as previously described (24). Quantification of branching was performed by analyzing whole-mount preparations of mammary glands. Mammary gland sections were prepared as previously described (24). Consecutive serial sections were incubated overnight at 4°C with anti-F4/80 antibody (Abcam, Cambridge, MA), rat anti-mouse TROMA-1 (keratin 8) antibody (Developmental Studies Hybridoma Bank, University of Iowa, IA), or normal rat IgG. The immunoperoxidase staining was performed using Vectastain ABC kit (Vector Laboratories, Burlingame, CA). The signal was visualized using 3,3′-diaminobenzidine peroxidase substrate (Sigma).
Peritoneal Macrophage Isolation
Peritoneal macrophages were isolated as previously described (29). Briefly, mice were injected intraperitoneally with 3 ml of sterile 4% Brewer's thioglycollate broth medium (Sigma). Five days later mice were sacrificed, and peritoneal cells were isolated by lavage using high glucose Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% heat-inactivated fetal calf serum, 1% penicillin-streptomycin antibiotic mixture. Macrophage were further purified by adherence for ∼16 h at 37 °C, at which time media were replaced to remove non-adherent cells.
Isolation of Murine Mammary Epithelial Cells
Mammary gland digestion and isolation of mammary epithelial cells were performed as previously described (24). Briefly, Mammary gland tissue was excised, minced, and then digested in a collagenase/dispase (Sigma) solution at 37 °C for 2 h with shaking at 200 rpm. Mammary gland cells were collected by centrifugation, and mammary epithelial cells were isolated using the magnetic EasySep Mouse Epithelial Cell Enrichment Kit (EasySep, StemCell Technologies, BC, Canada) according to the manufacturer's protocols.
Cell Culture and Ligand Treatments
Peritoneal macrophages, RAW264.7 macrophages, and HC11 mammary epithelial cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For co-culture experiments, HC11 cells (0.6–1.0 × 105 per well) were seeded in 6-well tissue culture plates. The following day culture inserts (transparent/translucent PET membrane, 1-μm pore size; BD Biosciences) were seeded with AEBP1NT or AEBP1TG peritoneal macrophages (2.0–2.5 × 105) and co-cultured with HC11 cells for 48 h. HC11 cells were subsequently lysed for immunoblot analysis.
Cell Proliferation Assay
HC11 cells were seeded in 24-well co-culture combination plates at a density of 6.0–8.0 × 103 cells/well. After 24 h, AEBP1NT or AEBP1TG peritoneal macrophages were seeded in the upper chamber (1.0 × 104 cells/insert), and the growth of HC11 cells was monitored daily by hemocytometer cell counting. HC11 cells were also cultured with AEBP1NT or AEBP1TG peritoneal macrophage culture-conditioned media (24 h), and their growth was monitored by MTT reagent.
Transient Transfection of RAW264.7 Macrophages
RAW264.7 cells were transfected in 6-well plates (1.0 × 106 cells/well) using GenePorter3000 (Genlantis, San Diego, CA) in serum-free Opti-MEM media according to manufacturer's protocol. After 4 h of adding transfection reagent, an equal volume of Opti-MEM containing 10% FBS was added to each well. Cells were cultured under standard conditions for 24 h after treatment. The pRc/CMV-AEBP1 expression construct is derived from the pRc/CMV vector. Detailed plasmid construction is available upon request.
Morpholino Oligomer Construction and Treatments
Morpholino constructs were designed to target the translation initiation site of the murine AEBP1 transcript (22). AEBP1-morpholino (AEBP1-MO, 5′-TGT CCT CAA TGC GGT GTG ACT CCA T-3′) and the nonspecific control morpholino (CONT-MO, 5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′) were synthesized by Gene Tools (Philomath, OR). After removing media, complete DMEM medium with morpholino oligos (10 μm) was added to cells. Endo-Porter (6 μm, Gene Tools), a delivery reagent, was subsequently added to the cells, which were further incubated for 24 h.
Whole Cell and Nuclear Protein Extraction, Immunoblot Analysis, and Electrophoretic Mobility Gel Shift Assay (EMSA)
Whole cell and nuclear protein extraction of mammary glands, cell lines, and cells isolated from murine tissue as well as immunoblot analysis were performed as previously described (24). Nuclear and cytosolic fractions were verified using the markers c-myc and Akt, respectively, as previously described (20). Nuclear protein extracts (2 μg) were used for EMSA as previously described (20). The immunoblots were quantified based on densitometric analysis using the program ImageJ (www.rsb.info.nih.gov), normalized to actin, and represented as a bar graph.
Quantitative PCR Analysis
TRIzol reagent (Invitrogen) was used to extract RNA from cells according to manufacturer's instructions. Total RNA was purified from the aqueous phase of TRIzol extracts using the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized from total RNA (1 μg) using the QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer's instruction. Starting with 30 ng/μl of cDNA, the SYBR Green quantitative PCR master mix (Invitrogen) and the CFX96 real time PCR detection system (Bio-Rad) were used to amplify the genes of interest using the following primer sets: murine Gli1, 5′-TTC GTG TGC CAT TGG GGA GGT T-3′ and 5′-TCT TCA CGT GTT TGC GGA GCG A-3′; murine β-actin, 5′-GAC GGC CAG GTC ATC ACT AT-3′ and 5′-GAA AGG GTG TAA AAC GCA GC-3′. Relative gene expression was evaluated using the comparative Ct method (ΔΔCt method) (30) and normalized to β-actin expression.
Statistical Analysis
Data are expressed as the mean ± S.D. (or S.E. in some cases) of the indicated number of samples. Statistical significance was determined using Student's t test for unpaired observations. p < 0.05 (*) and p < 0.001 (**) are considered statistically significant.
RESULTS
AEBP1 Induces Mammary Epithelial Cell Hyperplasia with Increased Macrophage Infiltration into Mammary Gland
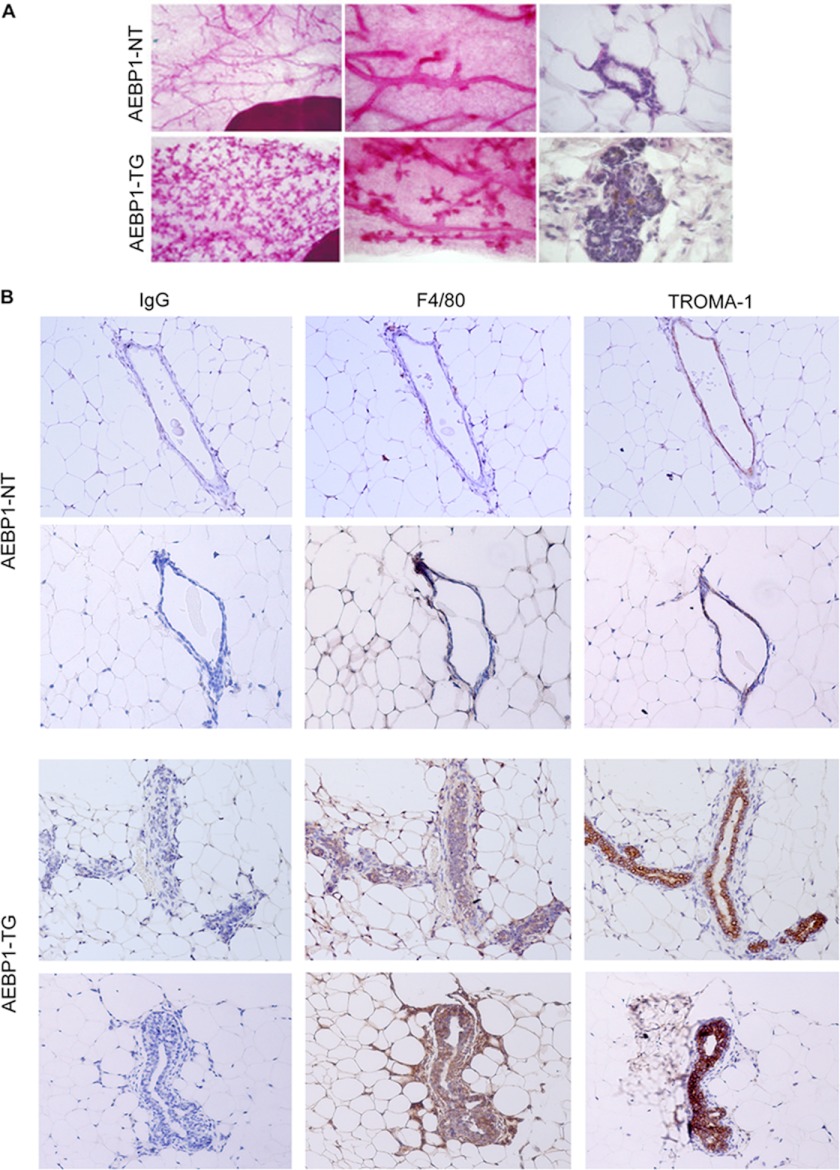
AEBP1 regulates inflammation by enhancing NF-κB activity in macrophages (20), resulting in up-regulation of proinflammatory chemokines and cytokines (19) reported to be involved in mammary tumorigenesis (3–5). AEBP1 is expressed in the stromal compartment of mammary gland (24). Aberrant up-regulation of stromal AEBP1 in the mammary gland may potentially promote tumorigenesis by inducing proinflammatory signals that result in aberrant proliferation of mammary epithelial cells. We tested this possibility using AEBP1TG mice with targeted AEBP1 overexpression in adipocytes (27) and macrophages (19). Whole mount analysis revealed that ∼30% of 30-week-old AEBP1TG females fed regular chow diet exhibit alveolar hyperplasia, whereas AEBP1NT females did not develop hyperplasia (Table 1; Fig. 1A). Experimental evidence suggests that a HFD challenge up-regulates AEBP1 expression in white adipose tissue (27), peritoneal macrophages (29), and mammary tissue (data not shown). We speculated that hyper-induction of AEBP1 in the AEBP1TG mice by HFD could further promote mammary epithelial cell hyperplasia. Remarkably, AEBP1TG females developed alveolar hyperplasia at a more dramatic rate when mice were fed HFD (Table 1). In as little as 7 weeks on HFD, the incidence of alveolar hyperplasia increased to ∼60% in 10-week-old AEBP1TG females. After 20 weeks of HFD feeding, 100% of 30-week-old AEBP1TG females exhibited alveolar hyperplasia. These results reveal that stromal overexpression of AEBP1 can cause aberrant proliferation of mammary epithelial cells, suggesting that AEBP1 and diet interplay to influence mammary epithelial cell growth in vivo.
TABLE 1.
Increased incidence of mammary hyperplasia in AEBP1TG mice
(n/n), the number of mice with alveolar hyperplasia per total number of mice.
| Diet | Age | AEBP1TG | AEBP1NT |
|---|---|---|---|
| Weeks | % | % | |
| Regular diet | 30 | 29 (2/7) | 0 (0/5) |
| HFD (20 weeks) | 30 | 100 (4/4) | 25 (1/4) |
| HFD (12 weeks) | 26 | 80 (4/5) | 20 (1/5) |
| HFD (7 weeks) | 10 | 60 (3/5) | 0 (0/4) |
FIGURE 1.
Stromal AEBP1 overexpression increases the incidence of mammary epithelial cell hyperplasia. A, shown are whole mount (left and middle panels) and histological (right panels) analyses of mammary glands from adult nulliparous AEBP1NT and AEBP1TG mice (n = 5–7). B, shown are representative paraffin-embedded sections of mammary glands from AEBP1TG (n = 4) and AEBP1NT (n = 3) mice fed a HFD for 22 weeks were stained with anti-F4/80, rat anti-mouse TROMA-1 (keratin 8) antibodies, or normal rat IgG and counterstained with hematoxylin.
AEBP1 is abundantly expressed in macrophages, and it promotes the expression of the proinflammatory chemokine Ccl2 (19), which is associated with tumor initiation and progression by promoting macrophage infiltration into the tumor site (5). Therefore, we examined whether AEBP1 regulates macrophage infiltration into mammary tissue. Immunohistochemical analysis using the macrophage marker F4/80 revealed that macrophages are recruited to the mammary epithelium, and they are associated with alveolar hyperplasia (Fig. 1B), similar to a reported mouse model of preneoplastic progression (4). Examination of epithelial structures in the mammary gland revealed a dramatic increase in the number of macrophages surrounding the ducts in AEBP1TG mice (Fig. 1B). Stromal macrophages appear to be intercalated into the mammary epithelium and in close proximity to the epithelial buds as observed with preneoplastic progression (4). Immunohistochemical analysis with the epithelial cell marker TROMA-1 (keratin 8) reveals multilayers of luminal epithelial cells and epithelial buds along the ducts of AEBP1TG mammary gland (Fig. 1B), a phenotype that is typically observed in mammary epithelial cell hyperplasia (4). We speculate that the increased rate of mammary epithelial cell hyperplasia in AEBP1TG mice by HFD feeding is mediated via AEBP1 up-regulation, leading to NF-κB activation, proinflammatory signaling, and macrophage infiltration.
AEBP1 Up-regulates NF-κB Activity in Mammary Gland
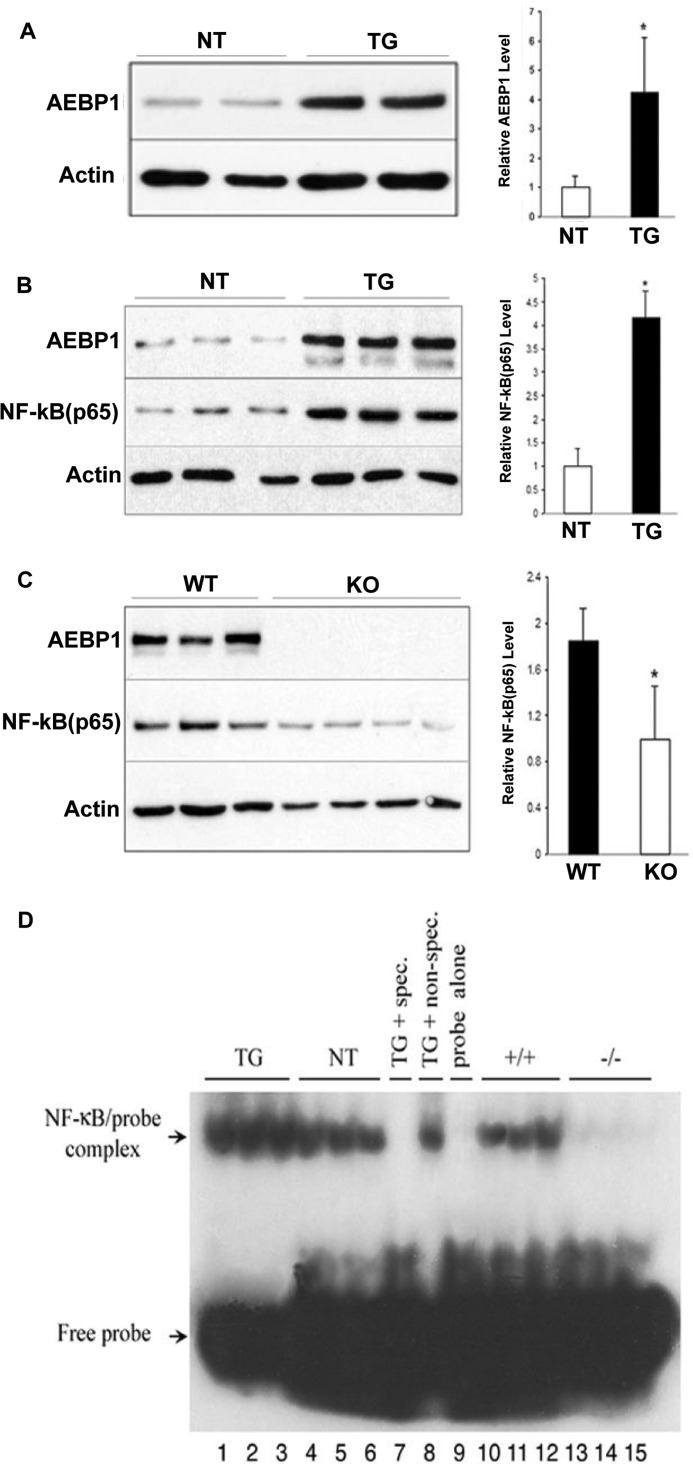
We previously reported that AEBP1 up-regulates NF-κB (p65) activity in macrophages (20). NF-κB transcriptional activity regulates several cytokines and chemokines implicated in cell growth, inflammation, apoptosis, transformation, and oncogenesis (31). Given that stromal AEBP1 overexpression promotes aberrant proliferation of mammary epithelial cells in vivo, we speculate that the proinflammatory function of AEBP1, which is mediated via NF-κB up-regulation, may play a pivotal role in mammary gland hyperplasia and potentially tumorigenesis. Using mammary glands isolated from AEBP1TG, AEBP1NT, AEBP1−/−, and AEBP+/+ mice, we examined whether AEBP1 influences NF-κB expression and activity in mammary glands. AEBP1TG mammary gland, which overexpresses AEBP1 by ∼4.5-fold (Fig. 2A), exhibits ∼4-fold higher levels of nuclear NF-κB (p65) compared with AEBP1NT mammary gland (Fig. 2B). Consistently, the nuclear NF-κB (p65) level in AEBP1−/− mammary gland is significantly lower than that in AEBP1+/+ mammary gland (Fig. 2C). To assess NF-κB transcriptional activity, nuclear proteins extracted from mammary glands were subjected to EMSA using 32P-labeled κB DNA binding consensus sequence. EMSA analysis indicates that NF-κB activity correlates with AEBP1 levels in the mammary gland (Fig. 2D). These results suggest that stromal AEBP1 overexpression may mediate proinflammatory signaling through augmented NF-κB activity in the mammary gland, ultimately promoting mammary epithelial cell hyperplasia.
FIGURE 2.
AEBP1 stimulates NF-κB activity in mammary gland. A, shown is a representative blot of AEBP1 expression in AEBP1TG and AEBP1NT mammary glands (n = 4). B and C, shown is a representative blot of nuclear NF-κB (p65) and AEBP1 levels in AEBP1TG, AEBP1NT, AEBP1+/+, and AEBP1−/− mammary glands (n = 3–4). Cytoplasmic (A) and nuclear (B and C) protein fractions were extracted and subjected to immunoblotting using β-actin level for normalization. Nuclear and cytosolic fractions were confirmed using the markers c-myc and Akt, respectively. D, nuclear protein extracts isolated from AEBP1TG, AEBP1NT, AEBP1+/+, and AEBP1−/− mammary glands (n = 3) were subjected to EMSA using 32P-labeled NF-κB probe. Nuclear protein extracts from AEBP1TG mammary glands were incubated with specific (unlabeled NF-κB probe) and nonspecific (unlabeled, unrelated probe) competitors to serve as positive controls. NF-κB probe alone served as a negative control.
AEBP1 Promotes TNFα Expression in Mammary Gland
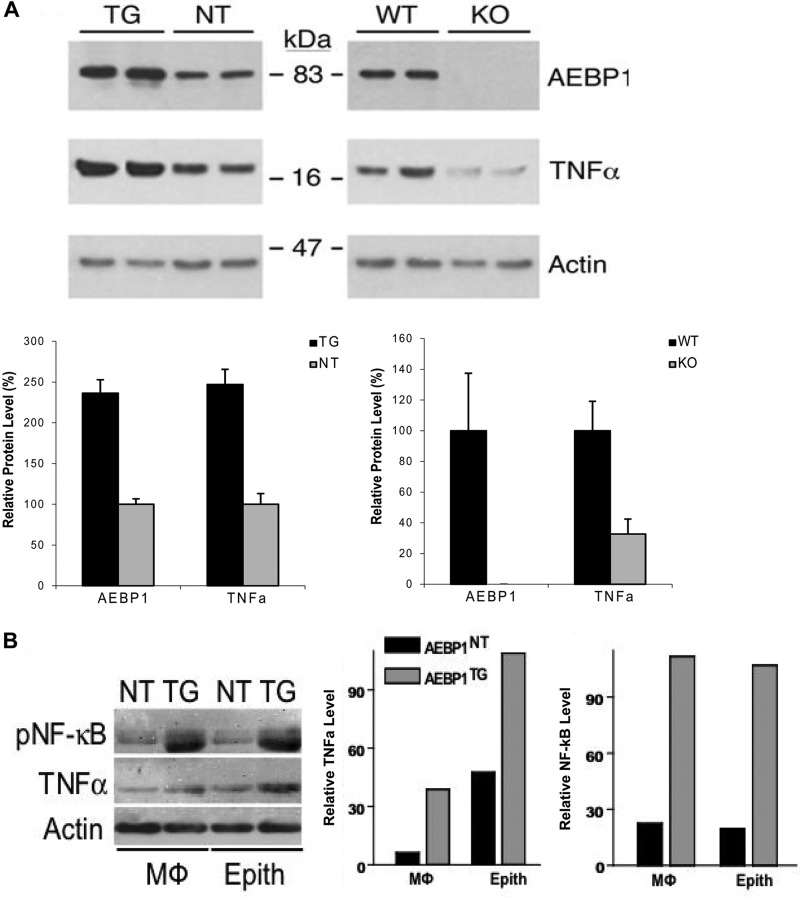
TNFα is a proinflammatory cytokine produced predominantly by infiltrative immune cells in an NF-κB-dependent manner (32). TNFα is integral in the initiation and progression of mammary tumors (33). Because AEBP1 enhances NF-κB activity in mammary gland, we anticipated that increased TNFα levels in the mammary gland of AEBP1TG mice mediate mammary hyperplasia and tumorigenesis. To examine this hypothesis, TNFα level in AEBP1TG, AEBP1NT, AEBP1−/−, and AEBP1+/+ mammary glands was assessed by immunoblot analysis. Compared with the AEBP1NT counterpart, TNFα level is significantly higher in AEBP1TG mammary gland (Fig. 3A). Conversely, TNFα expression is reduced in AEBP1−/− mammary gland compared with AEBP1+/+ control (Fig. 3A). Because immunohistochemical analysis indicated that stromal macrophages stain positive for AEBP1 (Fig. 1), we further analyzed CD11b+ macrophages isolated from AEBP1TG and AEBP1NT mammary glands to determine whether these macrophages were a significant cell source of TNFα. Expectedly, TNFα expression was ∼6-fold higher in AEBP1TG stromal macrophages compared with AEBP1NT counterparts (Fig. 3B), suggesting that stromal AEBP1 induces a proinflammatory microenvironment by up-regulating TNFα, promoting mammary tumorigenesis.
FIGURE 3.
AEBP1 promotes TNFα expression in mammary gland. A, shown is a representative blot of AEBP1 and TNFα expression in AEBP1TG, AEBP1NT, AEBP1+/+, and AEBP1−/− mammary glands (n = 3). B, shown is a representative blot of TNFα and p-NF-κB expression in macrophages and epithelial (Epith) cells isolated from pooled (n = 5) AEBP1TG and AEBP1NT mammary glands. For these data, whole cell protein extracts were obtained and subjected to immunoblotting using β-actin level for normalization.
Induced activation of NF-κB in the mammary epithelium by TNFα and other stimuli is pivotal to mammary tumor initiation and progression as it enhances cell proliferation, survival, and invasiveness (34). Because AEBP1 overexpression correlates with TNFα up-regulation in mammary gland, we anticipated that NF-κB activity and cytokine production to be enhanced in mammary epithelial cells. Indeed, AEBP1TG mammary epithelium displayed a 6-fold increase in NF-κB activity and a 2.5-fold increase in TNFα level compared with AEBP1NT control (Fig. 3B), suggesting that stromal AEBP1 may mediate mammary hyperplasia by inducing NF-κB activity in mammary epithelium via TNFα signaling.
Macrophage AEBP1 Regulates Mammary Epithelial Cell Growth
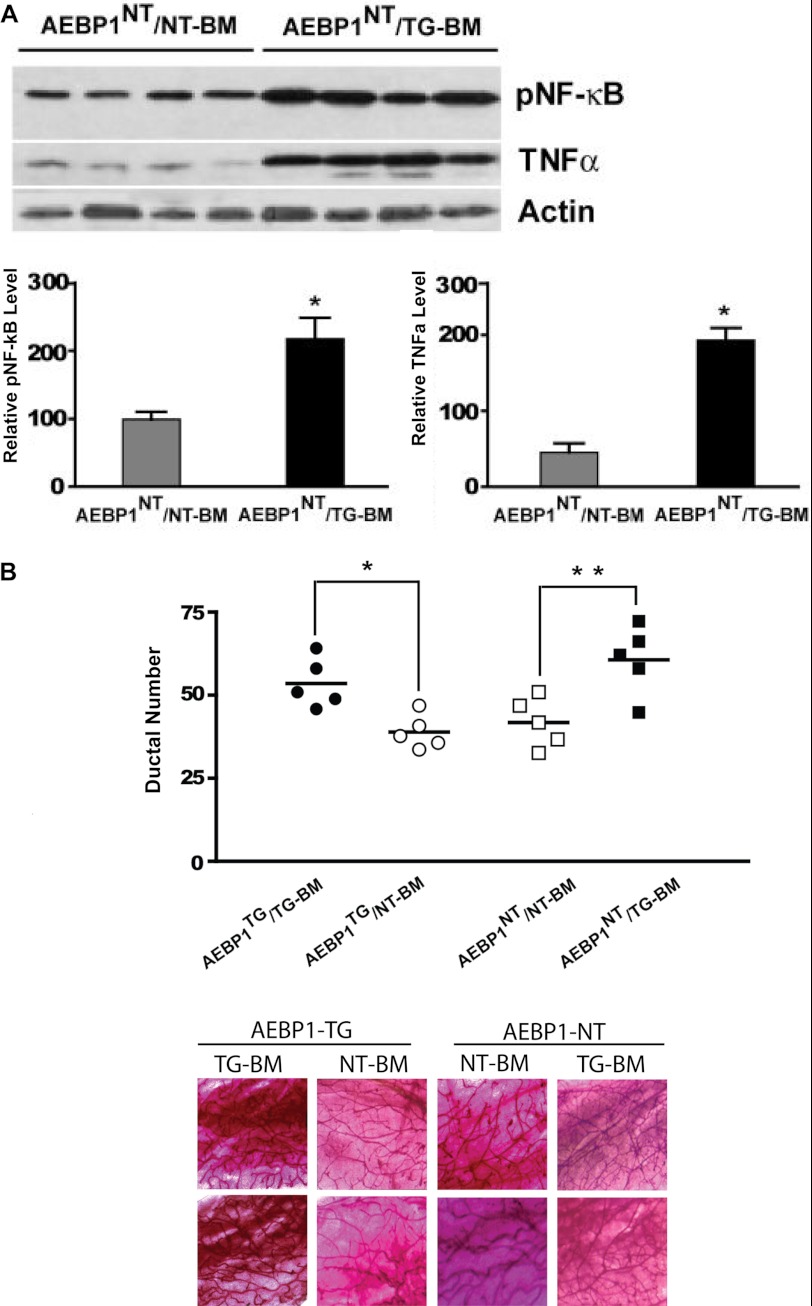
Although TNFα up-regulation in AEBP1TG macrophages suggests that AEBP1 overexpression is a key contributor to alveolar hyperplasia, it is plausible that ectopic expression of the AEBP1 transgene in adipocytes (27) may also contribute to mammary hyperplasia in AEBP1TG mice. To rule out this possibility, we performed BM transplantation experiments where BM cells from AEBP1TG and AEBP1NT mice were injected into γ-irradiated AEBP1NT recipients. Because stromal macrophages are derived from circulating monocytes, BM transplantation allows the repopulation of macrophages in the mammary gland after γ-irradiation (24), enabling us to specifically alter macrophage AEBP1 expression in the mammary stroma. Mammary glands from BM-chimeric mice were subjected to immunoblot analysis and ductal branch number quantification to further demonstrate that macrophage AEBP1 overexpression is primarily responsible for mammary epithelial cell hyperplasia. The adoptive transfer of AEBP1TG BM cells into AEBP1NT mice consequently resulted in up-regulation of NF-κB activity and TNFα expression in the mammary gland (Fig. 4A), concomitant with more extensive branching (Fig. 4B). Conversely, the adoptive transfer of AEBP1NT BM cells into AEBP1TG mice led to a significant reduction of ductal branch number (Fig. 4B). These findings suggest that stromal macrophage AEBP1 regulates mammary epithelial cell growth via modulation of TNFα signaling to induce NF-κB activity in the mammary epithelium.
FIGURE 4.
Macrophage AEBP1 stimulates NF-κB activation in mammary epithelial cells via TNFα. A, shown is a representative blot of p-NF-κB and TNFα expression in mammary gland of γ-irradiated chimeric mice. Whole cell protein extracts (n = 4) were obtained and subjected to immunoblotting using β-actin level for normalization. B, quantification of mammary ductal branching in γ-irradiated chimeric mice (n = 5) and representative images are shown.
AEBP1 Regulates Shh Signaling in Mammary Gland
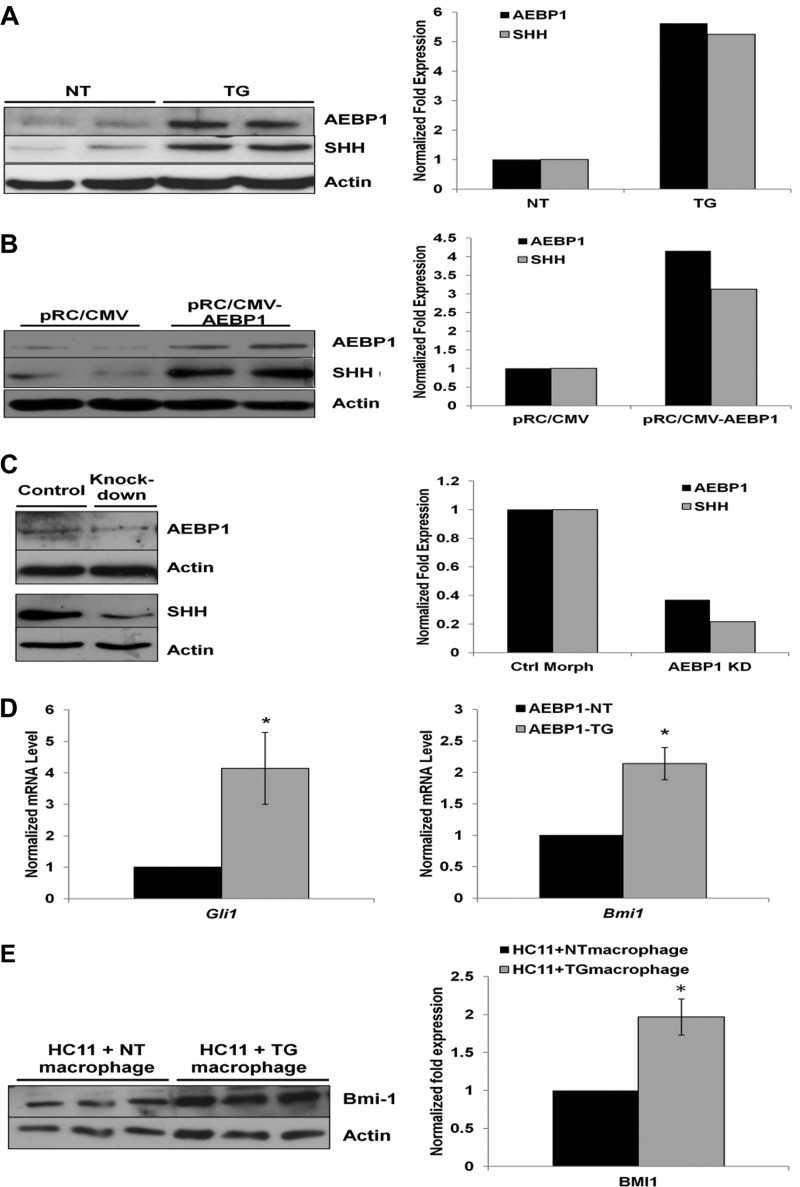
Because Shh expression is directly regulated by NF-κB in macrophages (9, 10), AEBP1 may manifest itself as a novel regulator of Shh signaling through its positive regulation of NF-κB activity. Indeed, Shh level was ∼3-fold higher in AEBP1TG peritoneal macrophages compared with AEBP1NT counterparts (Fig. 5A). Similarly, exogenous overexpression of AEBP1 (∼4-fold) in RAW264.7 macrophages resulted in an ∼3-fold increase in Shh expression (Fig. 5B). Conversely, Shh expression was decreased by ∼5-fold in peritoneal macrophages when the endogenous AEBP1 level was reduced (∼2.5-fold) by morpholino knockdown (Fig. 5C). We further investigated whether AEBP1 promotes hedgehog signaling in mammary gland by assessing the mRNA level of Gli1, a specific target of hedgehog signaling (35). Compared with AEBP1NT controls, mammary epithelial cells from AEBP1TG mice display a ∼4-fold increase in Gli1 mRNA levels (Fig. 5D).
FIGURE 5.
Macrophage AEBP1 up-regulates Shh signaling in mammary gland. A, shown is a representative blot of Shh and AEBP1 expression in AEBP1NT and AEBP1TG peritoneal macrophages (n = 2). B, shown is a representative blot of Shh and AEBP1 expression in RAW264.7 macrophages transfected with 0.5 μg of pRc/CMV or pRc/CMV-AEBP1 (n = 2). C, shown is a representative blot of Shh and AEBP1 expression in AEBP1NT peritoneal macrophages treated with control (Ctrl Morph) or knockdown (AEBP1 KD) morpholino (n = 1). D, Gli1 and Bmi1 mRNA levels in mammary epithelial cells (n = 3) from pooled AEBP1NT and AEBP1TG mammary glands (n = 4–5) are shown. E, shown is a representative blot of Bmi1 expression in HC11 cells (n = 3) cultured in the presence of pooled AEBP1NT or AEBP1TG peritoneal macrophages (n = 4–5). For the data shown, whole cell protein (A–C and E) and total RNA (D) extracts were obtained, and the expression of the indicated target proteins/genes was determined by immunoblotting or qRT-PCR. These results were normalized to β-actin levels.
Enhanced expression of Gli1 in AEBP1TG mammary epithelial cells suggests that AEBP1 modulates Shh signaling in macrophages. To examine whether macrophage AEBP1 modulates Shh signaling, we assessed the expression of the oncogene Bmi1 (36), another target of hedgehog signaling (37), in HC11 mammary epithelial cells co-cultured with peritoneal macrophages. HC11 cells co-cultured with AEBP1TG macrophages exhibited an ∼2-fold up-regulation of Bmi1 (Fig. 5E) compared with HC11 cells co-cultured with AEBP1NT macrophages, suggesting that AEBP1 promotes mammary hyperplasia not only by modulating TNFα signaling but also by regulating Shh signaling.
Stromal Macrophage AEBP1 Induces NF-κB and Akt Activation in Mammary Epithelial Cells via Paracrine TNFα Signaling Resulting in Increased Mammary Epithelial Cell Proliferation
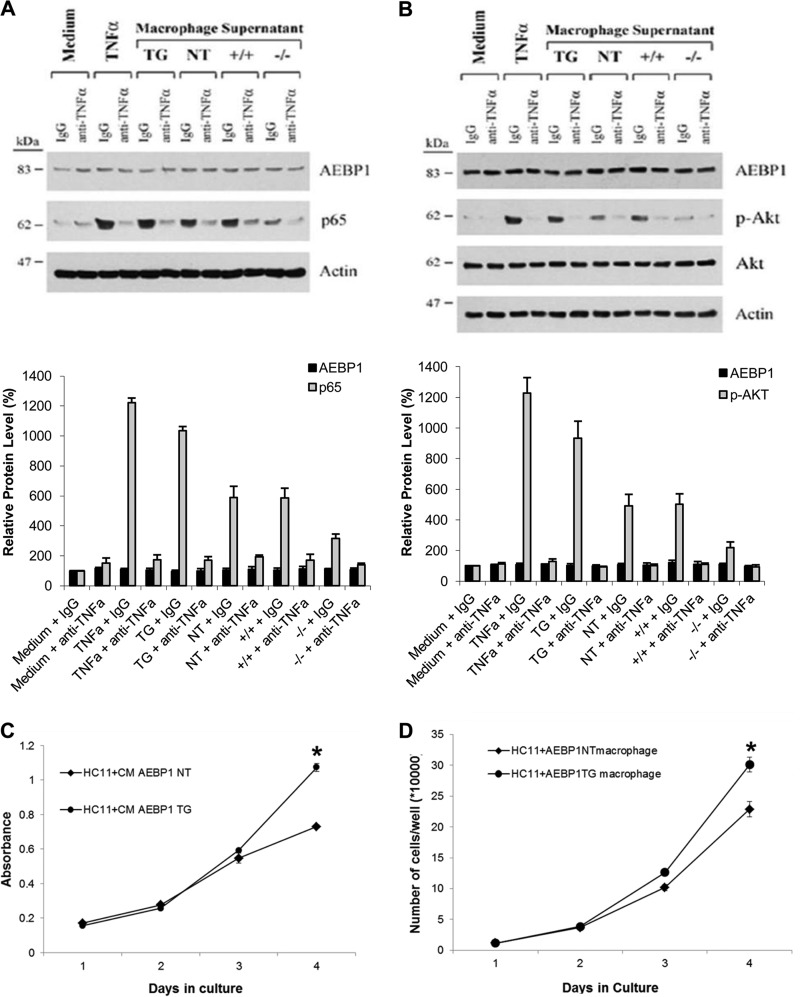
In addition to stimulating NF-κB activity, TNFα can also promote survival of mammary epithelial cells via Akt phosphorylation/activation (38). We evaluated the ability of macrophage-conditioned media to modulate NF-κB and Akt activity in HC11 mammary epithelial cells. HC11 cells were cultured in fresh medium supplemented with conditioned media of thioglycollate-elicited macrophages, and nuclear and cytoplasmic protein extracts were obtained and subjected to immunoblotting to assess NF-κB activity (pNF-κB) and Akt activity (pAkt), respectively. HC11 cells treated with AEBP1TG macrophage supernatant resulted in a significant increase in pNF-κB level compared with AEBP1NT control (Fig. 6A). Consistently, pNF-κB level is significantly lower in HC11 cells treated with AEBP1−/− macrophage supernatant compared with AEBP1+/+ control (Fig. 6A). In the presence of anti-TNFα blocking antibody, the induction of pNF-κB level was completely abolished (Fig. 6A). Furthermore, pAkt level was significantly higher in HC11 cells treated with AEBP1TG macrophage supernatant compared with AEBP1NT control (Fig. 6B). Conversely, pAkt was significantly reduced in HC11 cells treated with AEBP1−/− macrophage supernatant compared with AEBP1+/+ control (Fig. 6B). Again, the addition of anti-TNFα blocking antibody in the macrophage supernatants abolished the induction of pAkt level (Fig. 6B). These results suggest that macrophage AEBP1 modulates paracrine TNFα signaling to stimulate NF-κB and Akt activity in the mammary epithelial cells, promoting aberrant cell survival and proliferation. Because AEBP1 up-regulates various pro-proliferative factors such as TNFα, NF-κB, Shh, and Akt, we evaluated the effect of AEBP1TG and AEBP1NT macrophage co-culture on epithelial cell proliferation. AEBP1TG macrophage supernatants significantly increased HC11 cell proliferation (Fig. 6C). Similarly, and compared with AEBP1NT controls, co-culture with AEBP1TG peritoneal macrophages resulted in increased proliferation of HC11 cells (Fig. 6D), suggesting that macrophage AEBP1 causes aberrant epithelial cell proliferation potentially via synergistic action of TNFα, Akt, NF-κB, and Shh signaling, which stems from the macrophages.
FIGURE 6.
Stromal macrophage AEBP1 induces NF-κB and Akt activation in mammary epithelial cells via paracrine TNFα signaling, resulting in increased mammary epithelial cell proliferation. Mouse mammary epithelial HC11 cells were seeded (0.6 × 106) into 6-well plates. The next day HC11 cells were treated for 30 min with fresh medium, 10 ng/ml recombinant mouse TNFα protein (PeproTech), or 100 ml supernatants obtained from 0.2 × 106 AEBP1TG, AEBP1NT, AEBP1+/+, and AEBP1−/− peritoneal macrophages (32-week-old mice, fed HFD starting at 3 weeks of age) cultured in fresh medium for 12 and 48 h. Treatment was carried out in the presence of 1 μg/ml normal goat IgG (Santa Cruz Biotechnology) or goat anti-mouse TNFα antibody (R&D Systems). A representative blot of nuclear (A) and cytoplasmic (B) protein extracts (n = 3) were obtained and subjected to immunoblotting using β-actin level for normalization. C, AEBP1NT or AEBP1TG macrophage supernatants were applied to cultured HC11 mammary epithelial cells, and cell proliferation was assessed by MTT assay. D, macrophages (upper chamber) were co-cultured with HC11 cells (lower chamber) for 4–5 days, and cell growth was monitored by trypan blue counting.
DISCUSSION
Chronic inflammation is a common denominator in cancer progression of various tissues (1, 2). In the mammary gland, chronic inflammation stimulates aberrant epithelial cell proliferation and survival, consequently promoting tumor initiation and progression (3–6). AEBP1 is a critical proinflammatory mediator (19, 20, 39, 40) expressed in the mammary stroma (24). AEBP1 up-regulates NF-κB activity in macrophages via IκBα inhibition in conjunction with enhanced proinflammatory profile (19, 20). TNFα promotes the growth of normal and transformed mammary epithelial cells and mammary tumors in vivo by inducing NF-κB activity (32, 33). AEBP1 expression in mammary gland correlates with up-regulation of NF-κB activity, TNFα expression, and increased macrophage infiltration. Akt activity is also stimulated by a proinflammatory microenvironment in mammary tumors and has a strong correlation with breast cancer survival rate (38). We demonstrate that mammary epithelial cells cultured in the presence of AEBP1TG and AEBP1−/− macrophage culture media exhibit significantly increased and decreased NF-κB and Akt activity, respectively. Furthermore, inhibiting macrophage-derived TNFα signaling hinders AEBP1 ability to induce NF-κB and Akt activity. These findings suggest that stromal overexpression of AEBP1 influences the initial stage of mammary tumorigenesis by promoting paracrine proinflammatory signaling, resulting in aberrant survival and proliferation of the ductal epithelium, subsequently leading to alveolar hyperplasia.
Our findings also present AEBP1 as a novel regulator of Shh signaling, a pathway that is critically involved in tumorigenesis, angiogenesis, and epithelial-mesenchymal transition (9, 17, 18, 41). Our findings demonstrate that AEBP1 expression correlates positively with Shh expression in macrophages, and it is conceivable that AEBP1 up-regulates Shh expression through regulation of NF-κB activity (18). In addition, cellular cholesterol levels are essential for regulating the processing of the hedgehog precursor proteins (42). Interestingly, AEBP1 regulates cholesterol homeostasis in macrophages by transcriptional repression of cholesterol efflux genes including liver X receptor α (LXRα) (19, 39). By depleting cellular cholesterol levels, LXRα inhibits Shh signaling possibly through decreasing the level of its cholesterol-dependant cleavage and post-translational modification (43). The effect of AEBP1 in regulating cholesterol homeostasis via LXRα repression may enhance cholesterol-dependent processing of Shh, presenting AEBP1 as a novel dual regulator of hedgehog signaling via mediating Shh expression and cholesterol-dependent cleavage/post-translational modification.
Studies have demonstrated a link between chronic inflammation and enhanced hedgehog signaling (9, 18). Our findings indicate that paracrine TNFα and Shh signaling from stromal macrophages to mammary epithelial cells induces phosphoinositide 3-kinase (PI3K)/Akt and Shh-Gli signaling pathways, respectively. PI3K-dependent Akt activation promotes hedgehog activity (44) by antagonizing PKA-mediated Gli-inactivation. In our study macrophage AEBP1 enhances Akt activation in mammary epithelial cells via TNFα, which may also potentiate the effects of hedgehog signaling. This suggests that macrophage AEBP1 promotes mammary hyperplasia through the synergistic effect of the PI3K/Akt and hedgehog pathways that promote oncogenic activity of the Gli transcription factors in mammary epithelium. We propose that macrophage AEBP1 plays a role as a critical mammary stromal factor that stimulates the NF-κB and Akt survival signals in mammary epithelial cells via enhanced TNFα secretion from stromal macrophages. Furthermore, we speculate that increased Shh expression in macrophage overexpressing AEBP1 may cause up-regulation of Gli1 and Bmi1, genes known to be induced by the hedgehog signaling pathway, in the mammary epithelial cells.
Our findings focus on the role of macrophage AEBP1 in promoting mammary epithelial cell hyperplasia through TNFα and Shh signaling. However, these signals are also involved in tumor progression and metastasis (18, 43, 45–47), which may implicate AEBP1 as a critical factor in breast cancer progression. Indeed, xenograft experiments indicated that growth of 4T1 mammary tumor cells in NOD/SCID mice was significantly promoted by co-injected AEBP1TG macrophages.7 Interestingly, AEBP1 is not expressed in normal mammary epithelial cells (24), yet its expression is strongly induced in the hyperplastic epithelium of AEBP1TG mammary gland.8 Furthermore, AEBP1 expression is induced in mammary epithelial cells and breast cancer cells treated with TNFα,9 suggesting that epithelial AEBP1 induction may be a critical step in aberrant mammary epithelial cell proliferation. Several recent studies indicate that AEBP1 is also induced in malignant breast cells (26), transgenic mouse probasin-Neu induced prostate cancer (48), primary glioblastoma multiforme (49), and primary breast and colorectal cancers.10 This prompts further investigation of the mechanism of epithelial AEBP1 induction that underlines the growth, invasiveness, and metastasis of tumors.
Acknowledgments
We thank Chris Webber, Giban Ray, Hong Ma, Xuefang Pan, Grace D. Wu, and Dalila Ouchellouche for their technical assistance. Recombinant mouse TNFα protein (PeproTech) and goat anti-mouse TNFα antibody (R&D Systems) were kindly provided by Dr. Jean Marshall (Dalhousie University).
This work was supported by Canadian Institute of Health Research Grant MOP-57675 and Canadian Breast Cancer Research Alliance Grant 019454 (to H. S. R.).
O. Bogachev, A. G. Bharadwaj, R. W. Holloway, and H.-S. Ro, unpublished information.
R. W. Holloway, O. Bogachev, and H.-S. Ro, unpublished information.
G. D. McCluskey and H.-S. Ro, unpublished information.
X. Hu, Shanghai Jiao Tong University, personal communication.
- Shh
- sonic hedgehog
- HFD
- high fat diet
- Gli
- glioma-associated oncogene homolog
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- LXRα
- liver X receptor α
- TG
- transgenic
- NT
- non-transgenic.
REFERENCES
- 1. Coussens L. M., Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clevers H. (2004) At the crossroads of inflammation and cancer. Cell 118, 671–674 [DOI] [PubMed] [Google Scholar]
- 3. Wiseman B. S., Werb Z. (2002) Stromal effects on mammary gland development and breast cancer. Science 296, 1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwertfeger K. L., Rosen J. M., Cohen D. A. (2006) Mammary gland macrophages. Pleiotropic functions in mammary development. J. Mammary Gland Biol. Neoplasia 11, 229–238 [DOI] [PubMed] [Google Scholar]
- 5. Qian B. Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L. R., Kaiser E. A., Snyder L. A., Pollard J. W. (2011) CCL2 recruits inflammatory monocytes to facilitate breast tumor metastasis. Nature 475, 222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhat-Nakshatri P., Sweeney C. J., Nakshatri H. (2002) Identification of signal transduction pathways involved in constitutive NF-κB activation in breast cancer cells. Oncogene 21, 2066–2078 [DOI] [PubMed] [Google Scholar]
- 7. Baud V., Karin M. (2009) Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 8, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden M. S., Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 9. Nakashima H., Nakamura M., Yamaguchi H., Yamanaka N., Akiyoshi T., Koga K., Yamaguchi K., Tsuneyoshi M., Tanaka M., Katano M. (2006) Nuclear factor-κB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 66, 7041–7049 [DOI] [PubMed] [Google Scholar]
- 10. Kasperczyk H., Baumann B., Debatin K. M., Fulda S. (2009) Characterization of sonic hedgehog as a novel NF-κB target gene that promotes NF-κB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 23, 21–33 [DOI] [PubMed] [Google Scholar]
- 11. Cui W., Wang L. H., Wen Y. Y., Song M., Li B. L., Chen X. L., Xu M., An S. X., Zhao J., Lu Y. Y., Mi X. Y., Wang E. H. (2010) Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci. 101, 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz i Altaba A. (1999) The works of GLI and the power of Hedgehog. Nat. Cell Biol. 1, E147–E148 [DOI] [PubMed] [Google Scholar]
- 13. Duman-Scheel M., Weng L., Xin S., Du W. (2002) Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature 417, 299–304 [DOI] [PubMed] [Google Scholar]
- 14. Bigelow R. L., Chari N. S., Unden A. B., Spurgers K. B., Lee S., Roop D. R., Toftgard R., McDonnell T. J. (2004) Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J. Biol. Chem. 279, 1197–1205 [DOI] [PubMed] [Google Scholar]
- 15. Pola R., Ling L. E., Silver M., Corbley M. J., Kearney M., Blake Pepinsky R., Shapiro R., Taylor F. R., Baker D. P., Asahara T., Isner J. M. (2001) The morphogen Sonic hedgehog is an indirect angiogenic agent up-regulating two families of angiogenic growth factors. Nat Med 7, 706–711 [DOI] [PubMed] [Google Scholar]
- 16. Li X., Deng W., Nail C. D., Bailey S. K., Kraus M. H., Ruppert J. M., Lobo-Ruppert S. M. (2006) Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene 25, 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan L., Pepicelli C. V., Dibble C. C., Catbagan W., Zarycki J. L., Laciak R., Gipp J., Shaw A., Lamm M. L., Munoz A., Lipinski R., Thrasher J. B., Bushman W. (2004) Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 145, 3961–3970 [DOI] [PubMed] [Google Scholar]
- 18. Yamasaki A., Kameda C., Xu R., Tanaka H., Tasaka T., Chikazawa N., Suzuki H., Morisaki T., Kubo M., Onishi H., Tanaka M., Katano M. (2010) Nuclear factor κB-activated monocytes contribute to pancreatic cancer progression through the production of Shh. Cancer Immunol. Immunother. 59, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majdalawieh A., Zhang L., Fuki I. V., Rader D. J., Ro H. S. (2006) Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation. Proc. Natl. Acad. Sci. U.S.A. 103, 2346–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majdalawieh A., Zhang L., Ro H. S. (2007) Adipocyte enhancer-binding protein-1 promotes macrophage inflammatory responsiveness by up-regulating NF-κB via IκBα negative regulation. Mol. Biol. Cell 18, 930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim S. W., Muise A. M., Lyons P. J., Ro H. S. (2001) Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J. Biol. Chem. 276, 10199–10206 [DOI] [PubMed] [Google Scholar]
- 22. Ro H. S., Kim S. W., Wu D., Webber C., Nicholson T. E. (2001) Gene structure and expression of the mouse adipocyte enhancer-binding protein. Gene 280, 123–133 [DOI] [PubMed] [Google Scholar]
- 23. Majdalawieh A., Ro H. S. (2009) LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int. J. Biochem. Cell Biol. 41, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 24. Zhang L., Reidy S. P., Bogachev O., Hall B. K., Majdalawieh A., Ro H. S. (2011) Lactation defect with impaired secretory activation in AEBP1-null mice. PLoS One 6, e27795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farmer P., Bonnefoi H., Anderle P., Cameron D., Wirapati P., Wirapati P., Becette V., André S., Piccart M., Campone M., Brain E., Macgrogan G., Petit T., Jassem J., Bibeau F., Blot E., Bogaerts J., Aguet M., Bergh J., Iggo R., Delorenzi M. (2009) A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 15, 68–74 [DOI] [PubMed] [Google Scholar]
- 26. Grigoriadis A., Mackay A., Reis-Filho J. S., Steele D., Iseli C., Stevenson B. J., Jongeneel C. V., Valgeirsson H., Fenwick K., Iravani M., Leao M., Simpson A. J., Strausberg R. L., Jat P. S., Ashworth A., Neville A. M., O'Hare M. J. (2006) Establishment of the epithelial-specific transcriptome of normal and malignant human breast cells based on MPSS and array expression data. Breast Cancer Res. 8, R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L., Reidy S. P., Nicholson T. E., Lee H. J., Majdalawieh A., Webber C., Stewart B. R., Dolphin P., Ro H. S. (2005) The role of AEBP1 in gender-specific diet-induced obesity. Mol Med 11, 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ro H. S., Zhang L., Majdalawieh A., Kim S. W., Wu X., Lyons P. J., Webber C., Ma H., Reidy S. P., Boudreau A., Miller J. R., Mitchell P., McLeod R. S. (2007) Adipocyte enhancer binding protein 1 modulates adiposity through effects on pre-adipocyte survival and differentiation. Obesity 15, 288–302 [DOI] [PubMed] [Google Scholar]
- 29. Bogachev O., Majdalawieh A., Pan X., Zhang L., Ro H. S. (2011) AEBP1, a novel macrophage proinflammatory mediator, overexpression promotes and ablation attenuates atherosclerosis in ApoE−/− and LDLR−/− mice. Mol. Med 17, 1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 31. Ghosh S., Karin M. (2002) Missing pieces in the NF-κB puzzle. Cell 109, S81–S96 [DOI] [PubMed] [Google Scholar]
- 32. Baud V., Karin M. (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11, 372–377 [DOI] [PubMed] [Google Scholar]
- 33. Warren M. A., Shoemaker S. F., Shealy D. J., Bshar W., Ip M. M. (2009) Tumor necrosis factor deficiency inhibits mammary tumorigenesis and a tumor necrosis factor neutralizing antibody decreases mammary tumor growth in neu/erbB2 transgenic mice. Mol. Cancer Ther. 8, 2655–2663 [DOI] [PubMed] [Google Scholar]
- 34. Karin M. (2006) Nuclear factor-κB in cancer development and progression. Nature 441, 431–436 [DOI] [PubMed] [Google Scholar]
- 35. Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development. Paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 36. Wang X., Venugopal C., Manoranjan B., McFarlane N., O'Farrell E., Nolte S., Gunnarsson T., Hollenberg R., Kwiecien J., Northcott P., Taylor M. D., Hawkins C., Singh S. K. (2012) Sonic hedgehog regulates Bmi-1 in human medulloblastoma brain tumor-initiating cells. Oncogene 31, 187–199 [DOI] [PubMed] [Google Scholar]
- 37. Maertens G. N., El Messaoudi-Aubert S., Racek T., Stock J. K., Nicholls J., Rodriguez-Niedenführ M., Gil J., Peters G. (2009) Several distinct polycomb complexes regulate and co-localize on the INK4a tumor suppressor locus. PLoS One 4, e6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prueitt R. L., Boersma B. J., Howe T. M., Goodman J. E., Thomas D. D., Ying L., Pfiester C. M., Yfantis H. G., Cottrell J. R., Lee D. H., Remaley A. T., Hofseth L. J., Wink D. A., Ambs S. (2007) Inflammation and IGF-I activate the Akt pathway in breast cancer. Int J. Cancer 120, 796–805 [DOI] [PubMed] [Google Scholar]
- 39. Majdalawieh A., Ro H. S. (2010) PPARγ1 and LXRα face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1. Nucl. Recept. Signal. 8, e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Majdalawieh A., Ro H. S. (2010) Regulation of IκBα Function and NF-κB Signaling. AEBP1 is a novel proinflammatory mediator in macrophages. Mediators Inflamm. 2010, 823821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoo Y. A., Kang M. H., Lee H. J., Kim B. H., Park J. K., Kim H. K., Kim J. S., Oh S. C. (2011) Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 71, 7061–7070 [DOI] [PubMed] [Google Scholar]
- 42. Guy R. K. (2000) Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc. Natl. Acad. Sci. U.S.A. 97, 7307–7312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim W. K., Meliton V., Park K. W., Hong C., Tontonoz P., Niewiadomski P., Waschek J. A., Tetradis S., Parhami F. (2009) Negative regulation of Hedgehog signaling by liver X receptors. Mol. Endocrinol. 23, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riobó N. A., Lu K., Ai X., Haines G. M., Emerson C. P., Jr. (2006) Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 4505–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taipale J., Beachy P. A. (2001) The Hedgehog and Wnt signaling pathways in cancer. Nature 411, 349–354 [DOI] [PubMed] [Google Scholar]
- 46. Soria G., Ofri-Shahak M., Haas I., Yaal-Hahoshen N., Leider-Trejo L., Leibovich-Rivkin T., Weitzenfeld P., Meshel T., Shabtai E., Gutman M., Ben-Baruch A. (2011) Inflammatory mediators in breast cancer. Coordinated expression of TNFα and IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 11, 130–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neumark E., Sagi-Assif O., Shalmon B., Ben-Baruch A., Witz I. P. (2003) Progression of mouse mammary tumors. MCP-1-TNFα cross-regulatory pathway and clonal expression of promalignancy and antimalignancy factors. Int. J. Cancer 106, 879–886 [DOI] [PubMed] [Google Scholar]
- 48. Li Z., Szabolcs M., Terwilliger J. D., Efstratiadis A. (2006) Prostatic intraepithelial neoplasia and adenocarcinoma in mice expressing a probasin-Neu oncogenic transgene. Carcinogenesis 27, 1054–1067 [DOI] [PubMed] [Google Scholar]
- 49. Reddy S. P., Britto R., Vinnakota K., Aparna H., Sreepathi H. K., Thota B., Kumari A., Shilpa B. M., Vrinda M., Umesh S., Samuel C., Shetty M., Tandon A., Pandey P., Hegde S., Hegde A. S., Balasubramaniam A., Chandramouli B. A., Santosh V., Kondaiah P., Somasundaram K., Rao M. R. (2008) Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin. Cancer Res. 14, 2978–2987 [DOI] [PubMed] [Google Scholar]