Abstract
Beta-catenin is a major protein in the Wnt signalling pathway. Although it has been studied in various types of carcinoma, little is known about its expression in mesenchymal tumours. In this study 41 specimens of a variety of mesenchymal childhood tumours were compared to 24 samples of the corresponding adult tumours to assess the diagnostic value of nuclear β-catenin expression using tissue microarray-based immunohistochemistry. Similar to adult sarcoma and fibromatosis, β-catenin was not expressed in the majority of childhood sarcomas, and its nuclear translocation was detected in paediatric fibromatosis; non-negligible levels of nuclear staining in other tumour types demonstrate Wnt pathway activation in mesenchymal neoplasms of childhood and adolescence.
Key words: beta-catenin, Wnt pathway, immunohistochemistry, paediatric mesenchymal tumours.
Introduction
The Wnt signalling pathway is highly conserved in the animal kingdom.1 The Wnt genes encode a large family of secreted Wnt proteins that act as extracellular signalling factors. Wnt genes participate in several cellular activities such as determination of cell fate, proliferation, apoptosis, migration and differentiation, both during embryo development and in adult homeostasis.2 A critical factor in each of these processes is the intracellular concentration of β-catenin,3 a multi-functional protein that acts in the Wnt signalling pathway to modulate transcription of specific target genes; β-catenin is at the centre of the Wnt pathway and is the key arm of Wnt signalling.
When the Wnt pathway is in resting state, β-catenin is phosphorylated by glycogen synthase kinase 3beta (GSK3-β) in a protein complex (cadherin adhesion complex, CAC) that also includes casein kinase 1, adenomatous polyposis coli (APC) and axin.4 In the absence of Wnt signals, β-catenin concentration is kept low via the degradation complex involving GSK3-β GSK3-β, axin, APC, and β-TrCP/Slimb and via the ubiquitin proteolytic pathway.5
In the presence of Wnt genes, Wnt binding to Frizzled (Fz) results in activation of Dishevelled (Dsh), which inhibits the activity of GSK3-β, resulting in de-phosphorylation and stabilization of β-catenin. When not degraded via the proteolytic pathway, β-catenin collects just outside the nucleus in the form of a cytoplasmic pool of free signalling molecules.5 Here, stable β-catenin interacts with members of the T-cell factor/lymphocyte enhancer factor (TCF/LEF) family of transcription factors and is relocated to the nucleus as a β-catenin/Lef/Tcf complex which, in turn, stimulates expression of downstream target genes involved in cell-cycle progression, cancer stemness, neoangiogenesis and tissue invasion (e.g. myc, cyclin D1, TNF-α, SF1, Notch1, MYCBP, survivin).6 Although its role in the creation and maintenance of epithelial stability by regulating cell growth and cell-cell adhesion is well documented, recent evidence suggests that β-catenin plays a range of important functions in various aspects of cell biology, including control of polarization, differentiation, stemness, stem-cell renewal and cell motility.7–10 The phrase epithelial-to-mesenchymal transition (EMT) describes a process where epithelial cells lose their characteristic epithelial polarity, disassemble cell-cell junctions, assume a fibroblastoid mesenchymal morphology, and become more migratory. Although the notion of EMT and its role in tumour development and/or progression are still controversial, it is well known that β-catenin has the potential to exert a strong effect on cell phenotype and behaviour.11
Deregulation and constitutive activation of the Wnt/β-catenin pathway have been seen to lead to various forms of cancer. It has been noted that if any of the four proteins in the degradation complex (GSK3-β, axin, APC, and β-TrCP/Slimb) is mutated, uncontrolled intracellular concentrations of β-catenin almost always lead to cancer.12 Previous works have shown that CTNNB1, the β-catenin gene, APC and axin are frequently mutated in different types of human epithelial cancers as well as in colorectal, gastric, liver and pancreatic cancer.13 Previous research has shown that nuclear β-catenin expression in >50% of neoplastic mesenchymal cells can be considered as a surrogate marker of its mutation.14 In a study of adult mesenchymal neoplasms increasing the cut-off point from 25% to 50% of stained cells did not significantly increase specificity.15 In the present study we used both cut-offs to assess the specificity and sensitivity of this parameter. Although the relevance of Wnt signalling in epithelial malignancies is well established, its role in mesenchymal tumours remains largely unexplored. A correlation has been demonstrated between Wnt signalling via β-catenin and early osteoblast differentiation, because β-catenin signalling plays a direct role in BMP2-mediated signal transduction.16 Nuclear β-catenin signalling has also been observed in fibromatosis and desmoid tumours.17–25 Finally, by mediating development of and/or commitment to the mesenchymal programme, Wnt signalling seems to have an important role in mesenchymal tumorigenesis. In this study 41 specimens of a variety of mesenchymal childhood tumours were compared to 24 samples of the corresponding adult tumours to assess the diagnostic value of nuclear β-catenin expression using tissue microarray-based immunohistochemistry (TMA-IHC).
Materials and Methods
Patient selection and informed consent
Upon approval of the study by the Ethics Committees of all Institutions 41 paediatric mesenchymal tumour samples of different histologi-cal types were selected from the electronic archives of the Pathological Anatomy Section of Pausillipon Paediatric Oncological Hospital, Napoli, Italy; their clinical and pathological characteristics are summarized in Table 1. Twenty-four corresponding adult sarcomas selected from the electronic archives of the E.E. Franco Section of Pathological Anatomy, University of Bari, Italy (listed in Table 2) were used for comparison to the paediatric lesions. The written informed consent of parents/relatives or patients was obtained for the paediatric and adult cases, respectively. The histopathological diagnosis was reviewed by two expert pathologists from the Surgical Science Department, Section of Pathological Anatomy (University of Foggia, Italy).
Table 1. β-catenin expression in 41 paediatric soft-tissue tumours as evaluated by tissue microarray-based immunohistochemistry.
| Case | Age | Anatomical site | Histopathological diagnosis | Membranous/cytoplasmic β-catenin (%) | Nuclear β-catenin (%) |
|---|---|---|---|---|---|
| 1 | 11 ys | Subcutaneous breast | Nodular fasciitis | 100% M/C | 0 |
| 2 | 11 ys | Arm | Nodular fasciitis | 100% C and 5% M | <25% |
| 3 | 2 ys | Neck | Nodular fasciitis | 80% M/C | 0 |
| 4 | 8 ys | Paravertebral | Nodular fasciitis | 95% M/C | 5% |
| 5 | 10 ys | Eyebrow | Nodular fasciitis | 95% M/C | 5% |
| 6 | 8 ys | Retroauricular | Nodular fasciitis | 100% M/C | 0 |
| 7 | 1 ys | Glabella (subcutaneous) | Nodular fasciitis | 100% M/C | 80–100% |
| 8 | 10 ys | Neck skin | Dermatomyofibroma | 100% M | 0 |
| 9 | 4 ms | Vulva | Myofibroma | 100% M | <5% |
| 10 | 9 ms | Tongue | Myofibroma | 95% M/C | 5% |
| 11 | 11 ys | Auriche | Myofibroma | <25% M/C | <25% |
| 12 | 1 m | Parietal skin | Myofibroma | <25% C | 0 |
| 13 | 5 ys | Supraclavicular | Myofibroma | 20% C | 80% |
| 14 | 1 m | Paravertebral | Myofibromatosis | 25% M/C | <10% |
| 15 | 1 m | Paravertebral | Myofibromatosis | 100% C | 25% |
| 16 | 9 ms | Arm | Lipofibromatosis | 5–10% M/C | 0 |
| 17 | 4 ys | Palm | Lipofibromatosis | 25% C | 0 |
| 18 | 5 ys | Toe | Fibromatosis | 75% M/C | 0 |
| 19 | 11 ys | Paravertebral | Fibromatosis | 75% M/C | >25% |
| 20 | 2 ys | Finger | Fibromatosis | 70% C | 25–50% |
| 21 | 9 ys | Hip | Fibromatosis | 100% C | 100% |
| 22 | 14 ys | Abdomen | Fibromatosis | 100% M/C | 25–50% |
| 23 | 6 ys | Gluteus | Fibromatosis | 100% C | 100% |
| 24 | 15 ys | Abdomen | Fibromatosis | 100% C | 75% |
| 25 | 3 ys | Jaw | Fibromatosis | 0 | <10% |
| 26 | 11 ms | Toe | Fibromatosis | 50% C | 0 |
| 27 | 9 ys | Maxillary sinus | Myxoma | 100% C | 0 |
| 28 | 2 ys | Toe | Hypertrophic scar | 100% M | 0 |
| 29 | 7 ys | Chest skin | Hypertrophic scar | 0 | 0 |
| 30 | 8 ys | Lumbar skin | Hypertrophic scar | <5% C | 0 |
| 31 | 13 ys | Skin | Hypertrophic scar | 100% M | 0 |
| 32 | 9 ys | Chest skin | Keloid | 100% C | 70% |
| 33 | 8 ys | Arm skin | Keloid | 0 | 5% |
| 34 | 9 ys | Skin | Keloid | 100% C | 25% |
| 35 | 11 ys | Auriche | Keloid | 100% M | 0 |
| 36 | 11 ys | Preauricular subcutaneous tissue | Fibrosarcoma | 100% M/C | <10% |
| 37 | 5 ys | Subcutaneous scalp | Fibrosarcoma | 100% C | 40% |
| 38 | 2 ds | Abdomen | Fibrosarcoma | 90% C | 10% |
| 39 | 2 ys | Subcutaneous | Fibrosarcoma | 20% M/C | 0 |
| 40 | 2 ys | Bladder | Rhabdomyosarcoma | 80% M/C | 5% |
| 41 | 17 ys | Hip | Giant cell leiomyosarcoma | 100% M/C | 10% |
Ys, years; m/ms, month/s; ds, days; C, cytoplasmic; M, membranous; M/C, mixed membranous and cytoplasmic. Mean spot percentage of positive cells. Staining intensity was graded from + (faint) to +++ (strong).
Table 2. Beta-catenin expression in 24 adult soft-tissue lesions as evaluated by tissue microarray-based immunohistochemistry.
| Case | Age (years) | Site | Histopathological diagnosis | Membranous/cytoplasmic β-catenin (%) | Nuclear β-catenin (%) |
|---|---|---|---|---|---|
| 1 | 59 | Subcutis | Angiosarcoma | 50% C | 0 |
| 2 | 64 | Subcutis | Angiosarcoma | >25% M | 0 |
| 3 | 59 | Subcutis | Angiosarcoma | >25% C | 0 |
| 4 | 74 | Subcutis | Angiosarcoma | 100% M/C | 0 |
| 5 | 64 | Subcutis (thigh) | Liposarcoma | <25% C | <5% |
| 6 | 72 | Kidney | Myxoid liposarcoma | 60% C | 25% |
| 7 | 56 | Subcutis (buttock) | Liposarcoma | 15% C | 0 |
| 8 | 76 | Subcutis (abdomen) | Myxoid liposarcoma | 100% M | 0 |
| 9 | 76 | Subcutis | Liposarcoma | 50% M/C | 0 |
| 10 | 54 | Retroperitonaeum | Liposarcoma | 50% C | 5% |
| 11 | 74 | Subcutis | Clear cell sarcoma | 100% C | 0 |
| 12 | 70 | Subcutis (arm) | Pleomorphic sarcoma | 80% C | 10% |
| 13 | 69 | Nose | Sarcoma | 10% M | 0 |
| 14 | 57 | Retroperitoneum | Fibrosarcoma | 100% M/C | 0 |
| 15 | 75 | Paravesical region | High-grade fibrosarcoma | 100% C | 0 |
| 16 | 70 | Subcutis (arm) | Sarcoma | 100% M/C | 5% |
| 17 | 47 | Kidney | Sarcoma | 50% C | 0 |
| 18 | 80 | Subcutis | Synovial sarcoma | 95% M/C | 0 |
| 19 | 35 | Iliac region | Leiomyosarcoma | 100% C | <10% |
| 20 | 90 | Subcutis | Leiomyosarcoma | 100% M/C | 0 |
| 21 | 43 | Retroperitonaeum | Leiomyosarcoma | 40% C | 0 |
| 22 | 90 | Subcutis | Leiomyosarcoma | 100% M/C | 0 |
| 23 | 83 | Subcutis (back) | Leiomyosarcoma | 100% M/C | 0 |
| 24 | 84 | Abdomen | Rhabdomyosarcoma | 100% M/C | 0 |
C, cytoplasmic; M, membranous; M/C, mixed membranous and cytoplasmic. Mean spot percentage of positive cells. Staining intensity was graded from + (faint) to +++ (strong).
Tissue microarray-based immunohistochemistry
For TMA construction, areas of interest rich in non-necrotic tumour cells were identified on corresponding haematoxylin and eosin-stained sections and marked on the source paraffin block. A 3-mm-thick core of the source block was placed in the recipient master block of the EZ-TMA MANUAL TISSUE MICROARRAY® kit (IHC World, LLC, Woodstock, MD, USA). Three cores from different areas of the same tissue block were arrayed for each case. Then 4-µm-thick serial sections were obtained from the block for IHC analysis. IHC was performed with the standard linked streptavidin-biotin horseradish peroxidase technique (LSAB-HRP) using the best β-catenin antibody protocol obtained in our laboratory. Briefly, endogenous peroxidase activity was quenched by treatment with 3% H2O2 for 10 min. Non-specific antibody binding was blocked by treatment with normal horse/goat serum [diluted 1:20 in phosphate buffered saline (PBS), 0.1% bovine serum albumin (BSA)]. Sections were irradiated (5 min × 3) in capped polypropylene slide-holders with citrate buffer (pH 6.0), using a microwave oven (750 W) to unmask antigen sites. A monoclonal antibody that detects nuclear, cytoplasmic and membranous β-catenin (clone 6B3, Cell Signaling Techno logy, Beverly, MA, USA) was applied on sections at 1:150 dilution and incubated overnight at 4°C in a moist chamber. Immunocomplexes were detected by incubation with the secondary antibody and then with streptavidin-peroxidase complexes for 15 min each at room temperature (LSAB2/HRP kit, DAKO, Glostrup, Denmark). After rinsing in 3 PBS changes the immunoreaction was visualized by incubating sections in 0.1% 3,3'-diaminobenzidine and 0.02% hydrogen peroxide solution (DAB substrate kit, Vector Laboratories, Burlingame, CA, USA) for 4 min. Finally sections were lightly counterstained with Mayer's haematoxylin (Histolab Products AB, Goteborg, Sweden) and mounted on GVA mount (Zymed, Laboratories, San Francisco, CA, USA).
Colon adenocarcinoma carrying a β-catenin mutation was used as the positive control. Negative controls were obtained by omitting the primary antibody. The mean spot percentage of positive cells was assessed independently by two observers. β-catenin staining intensity was rated from + (faint) to +++ (strong), and localization rated N, nuclear, C, cytoplasmic; M, membranous; M/C, mixed membranous (predominant) and cytoplasmic; C/M, mixed cytoplasmic (predominant) and membranous. We chose staining > 25% of neoplastic cells rather than any staining (>0%) as the cut-off for our study.14 However, since previous research15 indicates that raising it from 25–50% did not significantly increase specificity, we applied both cutoff values (Table 3).
Table 3. Ability of nuclear β-catenin expression to differentiate paediatric fibromatosis from fibrosarcoma.
| Proportion of tumour cells exhibiting nuclear β-catenin expression | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Cut-off (25%) | 85.7% | 62.5% | 66.6% | 83.3% |
| Cut-off (50%) | 75% | 45.4% | 33.3% | 83.3% |
PPV, positive predictive value; NPV, negative predictive value.
Results
Nuclear staining
The relative frequency of nuclear β-catenin expression in the two sets of tumours is reported in Table 4. Benign paediatric myxoma and lipofibromatosis never exhibited nuclear β-catenin expression. Similarly to the immunohistochemical profile of adult sarcoma and fibromatosis, β-catenin was not expressed in the majority of infantile sarcomas, and its nuclear translocation was detected in the fibromatosis specimens. In addition, nuclear expression was demonstrated in other types of mesenchymal tumours. Comparison of the two sets of mesenchymal neoplasms highlighted the following proportions of tumour cells exhibiting nuclear β-catenin expression.
Table 4. Analysis of relative β-catenin N/M/C (nuclear, membranous/cytoplasmic) frequency in paediatric and adult mesenchymal tumours.
| Paediatric mesenchymal tumours | No. cases | N β-catenin (>50%)* | N β-catenin (>25%)* | M/C β-catenin (≥50%) |
|---|---|---|---|---|
| Nodular fasciitis | 7 | 1/7 | 1/7 | 7/7 |
| (14%) | (14%) | (100%) | ||
| Myofibroma/Dermatomyofibroma/Myofibromatosis | 8 | 2/8 | 1/8 | 4/8 |
| (25%) | (12.5%) | (50%) | ||
| Fibromatosis, adult-type | 5 | 5/5 | 3/5 | 5/5 |
| 100% | 60% | (100%) | ||
| Fibromatosis, infantile-type | 4 | 1/4 | 0/4 | 3/4 |
| (25%) | (0) | (75%) | ||
| Lipofibromatosis | 2 | 0/2 | 0/2 | 0/2 |
| (0) | (0) | (0) | ||
| Myxoma | 1 | 0/1 | 0/1 | 1/1 |
| (0) | (0) | (100%) | ||
| Keloid/Hypertrophic scar | 8 | 2/8 | 1/8 | 5/8 |
| (25%) | (12.5%) | (62.5%) | ||
| Fibrosarcoma | 4 | 1/4 | 1/4 | 3/4 |
| (25%) | (25%) | (75%) | ||
| Rhabdo/leiomyosarcoma | 2 | 0/2 | 0/2 | 2/2 |
| (0) | (0) | (100%) |
| Adult mesenchymal tumours | No. cases | N β-catenin (>25%) | N β-catenin (>5%) | M/C β-catenin (≥50%) |
|---|---|---|---|---|
| Angiosarcoma | 4 | 0/4 | 0/4 | 2/4 |
| (0%) | (0%) | (50%) | ||
| Liposarcoma | 6 | 1/6 | 1/6 | 4/6 |
| (16%) | (16%) | (66.6%) | ||
| Sarcoma (clear cell and synovial) | 2 | 0/2 | 0/2 | 2/2 |
| (0) | (0) | (100%) | ||
| Fibrosarcoma | 6 | 0/6 | 1/6 | 5/6 |
| (0%) | (16.6%) | (83.3%) | ||
| Leiomyosarcoma/Rhabdomyosarcoma | 6 | 0/6 | 1/6 | 5/6 |
| (0%) | (16%) | (83.3%) |
Nuclear staining evaluated using two different cut-offs from the literature.
Adipose tissue tumours: nuclear staining >25% was detected only in 1/6 cases (16.6%) of adult liposarcoma.
Fibroblastic/myofibroblastic/fibrohistiocytic tumours:
Benign lesions: nuclear staining >50% (80%) was observed in 1/7 cases (14.2%) of paediatric nodular fasciitis and in 1/10 cases (10%) of myofibroma/myofibromatosis. The single case of paediatric dermatomyofibroma showed no nuclear β-catenin staining.
Lesions with intermediate grade of malignancy: nuclear staining >50% (75%) was seen in 3/9 cases (33.3%) of paediatric fibromatosis (Figure 1 A–D); both specimens of paediatric lipofibromatosis showed no nuclear immunopositivity.
Malignant lesions: nuclear staining >25% (40%) was detected in 1/4 cases (25%) of paediatric fibrosarcoma (16.6%) as opposed to 10% of tumour cells seen in 1/6 cases of adult fibrosarcoma.
Figure 1.
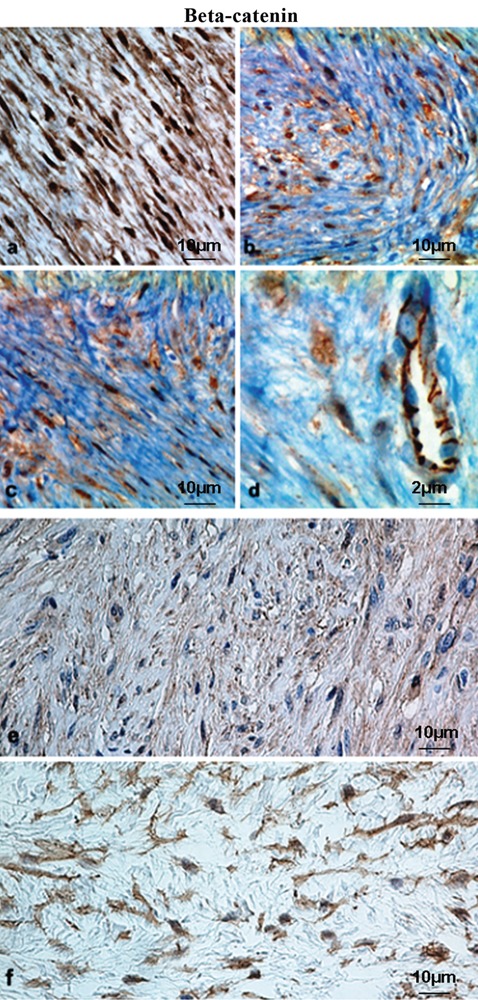
Immunohistochemical expression of β-catenin in paediatric mesenchymal tumours. a, b, c (× 400, scale bars: 10 µm) and d (× 600, scale bar 2 µm), representative case of fibromatosis showing strong and diffuse nuclear-cytoplasmic staining compared to membrane staining of endothelial cells; e (× 400, scale bar: 10 µm), representative case of infantile digital fibromatosis showing faint cytoplasmic staining; f, representative case of fibromyxoid sarcoma with moderate cytoplasmic expression (LSAB-HRP, nuclear counterstaining with haematoxylin); scale bar: 10 µm.
Smooth muscle tumours: 10% of tumour cells were positive in 1/5 cases of adult leiomyosarcoma and in the single case of paediatric leiomyosarcoma.
Skeletal muscle tumours: nuclear staining was 5% in the single case of paediatric rhabdomyosarcoma and none of the 6 adult tumours.
Vascular tumours: all cases of adult angiosarcoma were negative for nuclear staining. Tumours of uncertain differentiation: the single cases of synovial sarcoma and clear cell sarcoma were immunonegative for nuclear β-catenin. Keloid/hypertrophic scar: nuclear staining >50% (70%) was observed in 1/4 cases (25%) of paediatric keloid, whereas all 3 cases of hypertrophic scar were negative.
Membrane and cytoplasmic staining
Immunohistochemical β-catenin localization was also evaluated as relative membrane/ cytoplasmic expression (Figure 1 E–F); such mixed staining patterns were more frequent and the proportion of stained tumour cells was greater in nearly all childhood and adult tumours.
Discussion
Although the relevance of Wnt signalling in epithelial malignancies is well established,26 there is limited knowledge on its role in soft-tissue tumours. Wnt signalling via β-catenin has been seen to play a role in early proliferation and differentiation of human connective tissue progenitor cells,27 and β-catenin deregulation has been implicated in the inherited predisposition to fibromatosis and in the pathogenesis of sporadic desmoid-type fibromatosis.17–25
Nuclear immunoreactivity for β-catenin is a useful adjunct to the diagnosis of adult desmoid-type fibromatosis, many types of which exhibit mutations in the APC/β-catenin (Wnt) pathway.17–25 Some studies have examined β-catenin expression as a prognostic marker of desmoid tumours, others have sought an association between expression levels and underlying molecular/genetic alterations. A recent paper found that desmoids exhibiting increased nuclear β-catenin expression had a significantly higher recurrence rate than those lacking it.28 Sequencing of CTNNB1 exon 3 disclosed the presence of one of three specific mutation types of CTNNB1 (41A, 45F, and 45P) in 85% of desmoid tumour cases. In the same study decreased rather than increased intensity of nuclear β-catenin staining was associated with a more aggressive disease phenotype and with the CTNNB1 45F mutation, which independently correlated with a greater tendency for recurrence.29 Nuclear expression of β-catenin may also distinguish mesenteric fibromatosis from gastrointestinal stromal tumours.30 Examination of its expression in a variety of paediatric fibroblastic and myofibroblastic lesions found high-level expression in 42% of usual-type or deep fibromatoses.31
Montgomery and co-workers found that superficial and deep fibromatoses are genetically distinct32 and concluded that the different clinical manifestations despite the morphological resemblance may partly be due to the different genetic background, since superficial fibromatoses lack β-catenin and APC gene mutations. In our study, a representative case of infantile digital fibromatosis (superficial) displayed faint cytoplasmic staining, but no evidence of nuclear translocation (Figure 1E).
Our data confirm that high proportions of cells exhibiting nuclear β-catenin staining are found in deep adult-type fibromatoses arising in children, although this happens less frequently than in adult fibromatosis. This suggests that a subset of adult-type fibromatoses in childhood shares similar tumorigenesis mechanisms with adults ones. We chose staining >25% of cells rather than any staining (>0%) as the cut-off for our study; however, application of a cut-off of >25% or >50% of cells would not have significantly affected the major findings of our study. This cut-off appears to be optimal for diagnostic purposes because it maximizes the number of cases of fibromatosis considered as truly positive. Among the paediatric tumours 12/41 cases (29.7%), half of which were fibromatoses, expressed high-level nuclear β-catenin staining; among the fibrous tumours fibromatosis was the only other tumour type showing significant high-level staining (6/9 cases, 66.66%). Only one of the four cases of fibrosarcoma (25%) showed high-level staining. High-level staining (>25%) proved to be the cut-off that best differentiated fibromatosis from fibrosarcoma, with a sensitivity of 85.71%, a specificity of 62.5%, a positive predictive value of 66.66% and a negative predictive value of 83.33%. Non-negligible levels cytoplasmic/nuclear β-catenin staining in mesenchymal tumours other than fibromatosis/ fibrosarcoma demonstrate Wnt pathway activation also in these neoplasms. In particular, accumulation of nuclear β-catenin has been demonstrated in synovial sarcoma,33 osteosarcoma,34 liposarcoma, malignant fibrous histiocytoma35 and high-grade sarcoma with high proliferative index.36
Low-level β-catenin staining was seen in a variety of tumour types in our material; of the 41 paediatric neoplasms 29 (70.7%) exhibited low-level positivity, whereas among the 24 adult tumours high-level nuclear staining was detected only in one liposarcoma (4.16%). In contrast high- or low-level membrane and/or cytoplasmic staining was seen across all soft-tissue tumour subtypes, both in adult and in paediatric patients (Figure 1F).
Detection of high-level nuclear β-catenin expression in a single case of paediatric fibrosarcoma was intriguing. The tumour was diagnosed as sclerosing epithelioid fibrosarcoma (SEF), a rare variant of fibrosarcoma, originally described in 1995 by Meis-Kindblom et al.37 Despite a comparatively bland appearance and low mitotic activity, SEF is capable of local recurrence and distant metastasis years after surgical removal. Although in the study of Bhattacharya et al. the tumour stained negative for nuclear β-catenin,38 in our experience their antibody is not as capable of detecting nuclear β-catenin as the one used in our study. Detection of high levels of nuclear β-catenin in SEF is interesting, because according the original observation the epithelioid cell can be considered as dedifferentiated, and in this situation the term means mesenchymal to epithelial transition (MET).39 β-catenin is an important gene located at the crossroads between MET and EMT.40
The present study of soft-tissue tumours was devised to examine the specificity and sensitivity of nuclear β-catenin expression in the diagnosis of paediatric mesenchymal tumours. Our findings show that high-level nuclear expression is seen in a narrow subset of such tumours. Among these neoplasms a pathogenic role for β-catenin is best supported for paediatric fibromatosis, a heterogeneous group of diagnostically challenging lesions, especially on biopsy. Our data indicate that nuclear β-catenin is a sensitive marker for fibromatosis and that high-level staining is specific for this tumour type, contributing to the differential diagnosis with fibrosarcoma. Finally, our findings suggest that the differential diagnosis of infantile fibromatosis from low-grade fibrosarcoma can be achieved according to the flow-chart reported in Figure 2: nuclear β-catenin positivity in >25% of tumour cells is suggestive of fibromatosis, and strong nuclear staining in >50% of cells is highly suggestive of fibromatosis; CTNNB1 mutation analysis is mandatory when nuclear staining is between 10% and 50%; finally, nuclear staining in ≤10% of cells favours a diagnosis of low-grade fibrosarcoma.
Figure 2.
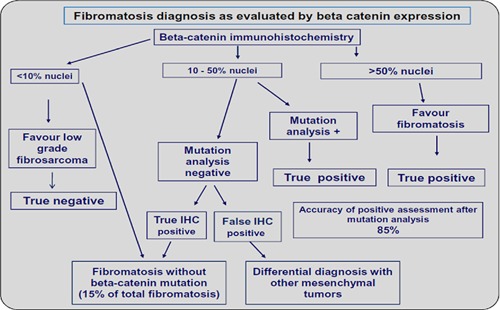
Diagnosis of fibromatosis as evaluated by β-catenin immunohistochemical expression. As per the current findings, the differential diagnosis of infantile fibromatosis from low-grade fibrosarcoma can be achieved as: nuclear β-catenin positivity in >25% of tumour cells is suggestive of fibromatosis, and strong nuclear staining in >50% of cells is highly suggestive of fibromatosis; CTNNB1 mutation analysis is mandatory when nuclear staining is between 10% and 50%; nuclear staining in ≤10% of cells favours a diagnosis of low-grade fibrosarcoma.
Although these findings are by no means conclusive they suggest that β-catenin may be a useful tool in histopathological differential diagnosis. Further investigation of larger series should focus on β-catenin expression and on manipulation of its pathway to pinpoint its role in mesenchymal cell differentiation and proliferation.
Acknowledgements:
the authors are grateful to Dr. Maria Carmela Pedicillo and Dr. Simona Cagiano for their valuable technical assistance.
References
- 1.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 2.Miller JR. The Wnts. Genome Biol. 2001;3:reviews 3001.1–3001.15. [Google Scholar]
- 3.Brown JD, Moon RT. Wnt signaling: why is everything so negative? Curr Opin Cell Biol. 1998;10:182–7. doi: 10.1016/s0955-0674(98)80140-3. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–45. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 5.Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/Beta-catenin and Wnt/Ca+2 pathways. Oncogene. 1999;18:7860–72. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 6.Novak A, Dedhar S. Signaling through Beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–37. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 8.Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem. 2007;282:31703–12. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- 9.Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, Moon RT, Kaufman DS. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–31. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harington KJ, Syrigos KN. The role of E-Cadherin-Catenin complex: more than an intercellular glue? Ann Surgic Oncol. 2000;7:783–88. doi: 10.1007/s10434-000-0783-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–76. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H, Bienz M. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–20. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 13.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 14.Hasegawa T, Yokoyama R, Matsuno Y, Shimoda T, Hirohashi S. Prognostic significance of histologic grade and nuclear expression of beta-catenin in synovial sarcoma. Hum Pathol. 2001;32:257–63. doi: 10.1053/hupa.2001.22764. [DOI] [PubMed] [Google Scholar]
- 15.Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, et al. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 16.Bain G, Müller T, Wang X, Papkoff J. Activated Beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participated in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor) Am J Pathol. 1998;153:709–14. doi: 10.1016/s0002-9440(10)65614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giarola M, Wells D, Mondini P, Pilotti S, Sala P, Azzarelli A, et al. Mutations of adenomatous polyposis coli (APC) gene are uncommon in sporadic desmoid tumours. Br J Cancer. 1998;78:582–7. doi: 10.1038/bjc.1998.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent mutations in the beta-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res. 1998;10:591–94. [PubMed] [Google Scholar]
- 21.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18:6615–20. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 22.De Wever I, Dal Cin P, Fletcher CD, Mandahl N, Mertens F, Mitelman F, et al. Cytogenetic, clinical, and morphologic correlations in 78 cases of fibromatosis: a report from the CHAMP Study Group. CHromosomes And Morphology. Mod Pathol. 2000;13:1080–5. doi: 10.1038/modpathol.3880200. [DOI] [PubMed] [Google Scholar]
- 23.Couture J, Mitri A, Lagace R, Smits R, Berk T, Bouchard HL, et al. A germline mutation at the extreme 3' end of the APC gene results in a severe desmoid phenotype and is associated with overexpression of beta-catenin in the desmoid tumor. Clin Genet. 2000;57:205–12. doi: 10.1034/j.1399-0004.2000.570306.x. [DOI] [PubMed] [Google Scholar]
- 24.Shitoh K, Konishi F, Iijima T, Ohdaira T, Sakai K, Kanazawa K, et al. A novel case of a sporadic desmoid tumour with mutation of the beta catenin gene. J Clin Pathol. 1999;52:695–6. doi: 10.1136/jcp.52.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurbuz AK, Giardiello FM, Petersen GM, Krush AJ, Offerhaus GJ, Booker SV, et al. Desmoid tumours in familial adenomatous polyposis. Gut. 1994;35:377–81. doi: 10.1136/gut.35.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaus A, Birchmeier W. Wnt signaling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 27.Hoover BA, Muschler G. Beta-catenin mediated Wnt signaling as a marker for characterization of human bone marrow-derived connective tissue progenitor. The Journal of Young Investigators. 2005:12–12. Available from: http://www.jyi.org/research/re.php?id=190.
- 28.Gebert C, Hardes J, Kersting C, August C, Supper H, Winkelmann W, et al. Expression of b-catenin and p53 are prognostic factors in deep aggressive fibromatosis. Histopathol. 2007;50:491–7. doi: 10.1111/j.1365-2559.2007.02619.x. [DOI] [PubMed] [Google Scholar]
- 29.Lazar AJF, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Tumorigenesis and Neoplastic Progression. Specific Mutations in the β-Catenin Gene (CTNNB1) Correlate with Local Recurrence in Sporadic Desmoid Tumors. Am J Pathol. 2008;173:1518–27. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26:1296–301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Thway K, Gibson S, Ramsay A, Sebire NJ. Beta-catenin expression in pediatric fibroblastic and myofibroblastic lesions: a study of 100 cases. Pediatr Devel Pathol. 2009;12:292–6. doi: 10.2350/08-07-0506.1. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod Pathol. 2001;14:695–701. doi: 10.1038/modpathol.3880374. [DOI] [PubMed] [Google Scholar]
- 33.Saito T, Oda Y, Sakamoto A, Tamiya S, Kinukawa N, Hayashi K, et al. Prognostic value of the preserved expression of the E-cadherin and catenin families of adhesion molecules and of beta-catenin mutations in synovial sarcoma. J Pathol. 2000;192:342–50. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH705>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, et al. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002;102:338–42. doi: 10.1002/ijc.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamoto A, Oda Y, Adachi T, Saito T, Tamiya S, Iwamoto Y, et al. Beta-catenin dedifferentiated liposarcoma and malignant fibrous histiocytoma. Arch Pathol Lab Med. 2002;126:1071–8. doi: 10.5858/2002-126-1071-CAAGMI. [DOI] [PubMed] [Google Scholar]
- 36.Kuhnen C, Herter P, Muller O, Muehlberger T, Krause L, Homann H, et al. Beta-catenin in soft-tissue sarcomas: expression is related to proliferative activity in high-grade sarcomas. Mod Pathol. 2000;13:1005–13. doi: 10.1038/modpathol.3880181. [DOI] [PubMed] [Google Scholar]
- 37.Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol. 1995;19:979–93. doi: 10.1097/00000478-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, et al. Nuclear beta-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29:653–9. doi: 10.1097/01.pas.0000157938.95785.da. [DOI] [PubMed] [Google Scholar]
- 39.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Mini-Review. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 40.Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, et al. β-Catenin and TGF β signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene. 2004;23:2672–80. doi: 10.1038/sj.onc.1207416. [DOI] [PubMed] [Google Scholar]


