Abstract
The aim of this study was to identify immunoreactive neuropeptide Y (NPY) and calcitonin gene-related peptide (CGRP) neurons in the autonomic and sensory ganglia, specifically neurons that innervate the rat temporomandibular joint (TMJ). A possible variation between the percentages of these neurons in acute and chronic phases of carrageenan-induced arthritis was examined. Retrograde neuronal tracing was combined with indirect immunofluorescence to identify NPY-immunoreactive (NPY-IR) and CGRP- immunoreactive (CGRP-IR) neurons that send nerve fibers to the normal and arthritic temporomandibular joint. In normal joints, NPY-IR neurons constitute 78±3%, 77±6% and 10±4% of double-labeled nucleated neuronal profile originated from the superior cervical, stellate and otic ganglia, respectively. These percentages in the sympathetic ganglia were significantly decreased in acute (58±2% for superior cervical ganglion and 58±8% for stellate ganglion) and chronic (60±2% for superior cervical ganglion and 59±15% for stellate ganglion) phases of arthritis, while in the otic ganglion these percentages were significantly increased to 19±5% and 13±3%, respectively. In the trigeminal ganglion, CGRP-IR neurons innervating the joint significantly increased from 31±3% in normal animals to 54±2% and 49±3% in the acute and chronic phases of arthritis, respectively. It can be concluded that NPY neurons that send nerve fibers to the rat temporomandibular joint are located mainly in the superior cervical, stellate and otic ganglia. Acute and chronic phases of carrageenan-induced arthritis lead to an increase in the percentage of NPY-IR parasympathetic and CGRP-IR sensory neurons and to a decrease in the percentage of NPY-IR sympathetic neurons related to TMJ innervation.
Key words: trigeminal ganglion, otic ganglion, superior cervical ganglion, arthritis, temporomandibular joint.
Introduction
Temporomandibular disorders (TMDs) constitute a heterogenic group of pathological conditions that induce pain in the TMJ and/or masticatory muscles.1,2 TMDs are classified in myofascial pain, internal derangement and arthritis (degenerative/inflammatory joint disorders) in the TMJ.3 Several modulators participate in the pathophysiology of TMDs, such as those from the extracellular matrix, cytokines, growth factors and neuromediators.4–10 In the last decade, increasing evidences have shown the influence of peptidergic innervation in the evolution of degenerative/inflammatory joint disorders.11–16 However, some aspects need to be clarified concerning the effects of arthritis in neuropeptide expression in sensory and autonomic ganglia involved with TMJ innervation. The rat temporomandibular joint (TMJ) is a richly innervated orofacial territory, supplied with sensory nerve endings derived mainly from the trigeminal ganglion and autonomic nerve endings from the superior cervical, stellate and otic ganglia.17–21 Axons innervating the TMJ contain several neuropeptides, among which are the NPY and the CGRP.22–26 NPY is a 36 amino acid sequence related to the pancreatic polypeptide family. It is synthesized in the rat peripheral nervous system, mainly by autonomic sympathetic and parasympathetic neurons.27,28 CGRP is a 37 amino acid peptide formed by alternative splicing of calcitonin gene primary transcript in the nervous system; with intense expression in sensory primary neurons.29,30
It has been reported that there is an increase in concentrations of NPY and CGRP in the synovial fluid of arthritic joints, such as human knee and TMJ as well as rat TMJ.12,13,31–34 Several studies have focused on CGRP immunohistochemical expression in sensory neurons that send axons to the knee and lumbar facet joints during arthritis.35–39 On the other hand, NPY expression in autonomic neurons has not been analyzed during arthritis. It is not clear, however, whether the neuropeptide increase in the TMJ synovial fluid is due to an increased release from a limited number of axons or whether, as part of the inflammatory response, more neurons are recruited for the production of neuropeptides. The present study combined retrograde neuronal tracing and immunohistochemistry methods to reveal the percentages and morphometric characteristics of CGRP immunoreactive sensory neurons and NPY immunoreactive autonomic neurons that innervate normal or arthritic rat TMJ.
Materials and Methods
Surgical procedures
The experimental protocols were previously approved by the local Institutional Committee for Research and Animal Care. All efforts were made to reduce the number of animals and to minimize suffering.
Thirty male Wistar rats (Rattus novergicus) weighing 150–200 g were anesthetized by intra-muscular injection of ketamine (80 mg/kg, Francotar® - Virbac, São Paulo, Brazil) and xylazine (10 mg/kg, Rompun® - Bayer, São Paulo, Brazil). The left supradiscal articular space of the TMJ was surgically exposed through a cavity made in the zygomatic process of the temporal bone, and one compact crystal cylinder of fluorescent retrograde neuronal tracer, fast blue [(trans-1-(5-amidino-2-benzofuranyl)-2-(6-amidino-2-indolyl)ethylene-2HCl], (EMS-Polyloy, GmbH, Gross-Ulmstadt, Germany) was deposited. This solid cylinder was prepared as described by Casatti et al. (1999).19 After deposition of the neuronal tracer, the cavity was sealed with gutta percha (Odhacan, Herpo dental Products Ltda, Rio de Janeiro, Brazil). The temporal muscle was repositioned and the skin sutured. The animals were subsequently divided into three groups: group 1 (n=15), control group, animals were submitted to intra-articular deposition of retrograde neuronal tracer alone, 15 days prior to euthanasia; group 2 (n=15), intra-articular deposition of retrograde neuronal tracer 15 days prior to euthanasia, plus a 2 mg pellet of carrageenan (Sigma Chemical Co., St. Louis, MO, USA), 48 h prior to euthanasia, to induce an acute inflammatory response in the TMJ and; group 3 (n=15), intra-articular deposition of retrograde neuronal tracer and carrageenan 15 days prior to euthanasia, to induce a chronic inflammatory response in the TMJ.
Tissue preparation
Fifteen days after deposition of the retrograde neuronal tracer, the animals were anesthetized with an intraperitoneal overdose of sodium pentobarbital and transcardiacally perfused through the aorta with 100 mL of 0.9% saline, followed by 700 mL of 4% formaldehyde fixative solution (Sigma) in 0.1M phosphate buffered saline (Sigma), pH 7.4 at 4°C. The trigeminal, superior cervical, stellate and otic ganglia were removed and post-fixed for 5 hours in the same fixative solution containing 20% (wt/v) sucrose at 4°C (Sigma). Frozen sections of 15 µm (superior cervical, stellate and otic ganglia) or 30 µm (trigeminal ganglion) were cut on a freezing microtome (Jung SM 2000R, Leika Instruments GmbH, Nussloch, Germany), and submitted to the indirect immunofluorescence method.
Immunohistochemistry
Half of the animals from each group were used for NPY immunolabeling, and the other half was used for CGRP immunolabeling. The sections were rinsed twice in 0.1 M potassium phosphate-buffered saline pH 7.4, for 20 min at room temperature. Sections were incubated in a solution of potassium phosphate-buffered saline containing primary polyclonal antibodies raised in rabbit to CGRP (1:2000, Chemicon International Inc., Temecula, CA, USA) or NPY (1:2000, Chemicon), Triton X-100 (0.3%) (Sigma) and normal goat serum (dilution 1:200, Colorado Serum Company, Denver, CO, USA), for 24 h at 4°C. Subsequently, sections were rinsed twice in potassium phosphate-buffered saline, followed by incubation for 1 h at room temperature with biotinylated secondary antibody raised in goat against rabbit IgG (1:250, Vector Laboratories Inc., Burlingame, CA, USA), rinsed again in potassium phosphate-buffered saline and then incubated for 1 h at room temperature with Cy3 conjugated streptavidin (1:500, Amersham Pharmacia Biotech, Buckinghamshire, England). The sections were again rinsed twice in potassium phosphate-buffered saline, mounted on chrome-gelatin-coated slides, air-dried at room temperature and coverslipped with buffered glycerol solution.
Control procedures
In order to observe whether leakage of the retrograde neuronal tracer to adjacent tissues had occurred, the protocol described by Casatti et al. (1999) was followed.19 The TMJs were removed, postfixed overnight at 4°C in the same fixative solution (4% formaldehyde in 0.1 M phosphate buffered saline, pH 7.4), and decalcified in a solution of 10% sodium citrate (Sigma) and 25% formic acid (Sigma) for 2 weeks at 4°C. Following cryoprotection in 0.1 M potassium phosphate-buffered saline containing 20% sucrose, frontal sections of 30 µm were cut on a freezing microtome, mounted on chrome-gelatin-coated slides, air-dried at room temperature and coverslipped with buffered glycerol solution. To verify the histopathological conditions of the TMJs, additional decalcified TMJs from all experimental groups were submitted to routine histological procedures (paraffin embedding). The histological sections (5 µm) were stained with hematoxylin and eosin, and observed under light microscope. The contralateral superior cervical ganglion was collected to test for possible nonspecific labeling of autonomic perikarya caused by leakage of the retrograde neuronal tracer into the blood stream.
Control experiments were carried out by omission of the primary antibody and adsorption test to check the specificity of the immunostaining. There was no NPY- or CGRP-IR in the ganglia studied when both tests were applied.
Morphometric and statistical analysis
The counting method used was the central profiles count.40 Values were obtained by counting the total number of fast blue labeled perikarya with visible nuclei in the sections as well as the percentage of those perikarya that exhibited immunoreactivity to CGRP or NPY (double-labeled perikarya). To validate our quantification method, some ganglia were used to perform a serial reconstruction method by comparing sequential sections in series. This allowed double-labeled perikarya with visible nuclei to be identified from section to section. It was found that only 3% of the double-labeled perikarya were counted twice, and it happened every time a perikaryon was sliced directly through the middle of the nucleus. As the mean diameter of the perikarya was 24 µm (and the nucleus diameter even smaller) most perikarya with visible nuclei were within the 15 µm (superior cervical, stellate and otic ganglia) or 30 µm (trigeminal ganglion) sections. If a nucleus was sliced in its periphery, two adjacent sections would show double-labeled perikarya with different nuclear volumes. In this case, the neuron would be counted only once, since the double-labeled perikaryal profile with the largest nuclear volume showed a visible nucleus, while in the double-labeled perikaryal profile with the smallest nuclear volume, the image of the nucleus was obscured by the intense fluorescence present in the cytoplasm. Since our counting method does not represent the absolute number of labeled perikarya, we have referred to labeled perikarya as labeled perikaryal profiles.40 Despite this counting method resulting in a 3% overestimation, this percentage is acceptable as the deviation is quite insignificant from real numbers of cells. Similar biases should occur during counting of double-labeled perikaryal profile in control and experimental groups, as there are no significant differences in the perikaryal diameters in autonomic ganglia. In contrast, there was a discrete difference in the mean diameter of perikarya from the trigeminal ganglion among the experimental groups; however, the histological sections were 30 µm thick, reducing the possibility of double counting of labeled perikarya.
The investigator was blinded to groups, and in some cases the material was counted by two individuals, providing highly reproducible results. To calculate the perikaryal diameter, perikaryal profiles were observed by epifluorescence microscopy (400× magnification) and drawn using a camera lucida. To confirm the perikaryal outline, a concomitant analysis was made by phase contrast microscopy. The diameters were determined by computer-aided measurement (Sigmascan, Jandel Scientific Software, San Rafael, CA, USA). Only perikaryal profiles with visible nucleolus, visible by simultaneous employment of the epifluorescence microscope and Nomarsky were selected for morphometric analyses. The Kruskal-Wallis nonparametric ANOVA test and Dunn's multiple comparisons test were used, considering P<0.05 as significant. Statistical analysis was applied to all available data.
Microscopic observation
Fluorescent perikarya labeled with fast blue were observed on an epifluorescence photomicroscope (Optiphot 2, Nikon, Shinagawa-ku, Tokyo, Japan) using a UV-2A filter (excitation wave length of 330–380 nm). Immunofluorescence was observed with a G-2A filter (510–560 nm) to reveal Cy3. The results were photographed with Ilford HP5 plus film (400 ASA).
Results
Control procedures
Histological analysis of the TMJ showed that the neuronal tracer was incorporated into the connective tissue throughout the supradiscal compartment, mainly in the synovial membrane, and did not leak into adjacent territories. The contralateral superior cervical ganglion did not reveal labeled nucleated neuronal profiles, indicating that systemic leakage was not a significant source of error.
Observation of hematoxylin and eosin stained TMJ sections showed, in group 1 (control group), a normal histological pattern with only a few macrophages in restricted areas of the subsynovial tissue (Figure 1 A, B); however not characterizing an active inflammatory process. In group 2, there was an intense inflammatory infiltration composed of mononuclear cells and polymorphonuclear neutrophils located in the subsynovial tissue and mainly in the supradiscal articular space (Figure 1 C, D), validating the acute phase of the inflammatory process. In group 3, there was a moderate inflammatory infiltration composed of mononuclear cells, distributed throughout all TMJ regions. The subsynovial connective tissue was infiltrated by lymphocytes, plasma cells and mainly macrophages, but the supradiscal articular space had few immune cells (Figure 1 E, F), validating the chronic phase of the inflammatory process. In both inflammatory groups (2 and 3), the presence of inflammatory cells was restricted to the TMJ compartment, and did not spread to the surrounding periosteum, muscles or parotid gland.
Figure 1.
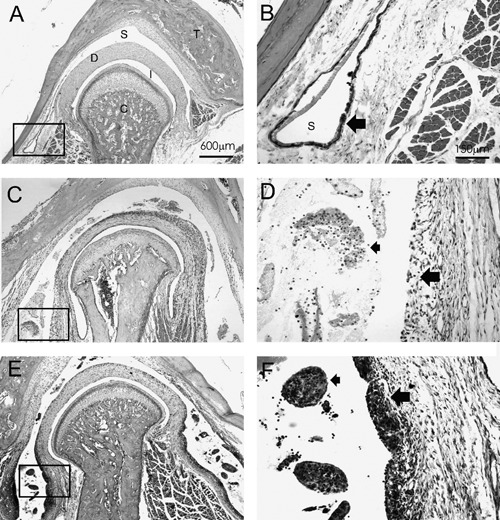
Frontal sections of temporomandibular joints and adjacent tissues from experimental groups stained with haematoxylin and eosin (H&E) and observed in light microscopy. A) Low magnification of joint from normal group (group 1); note overall absence of inflammatory process in supradiscal articular space. B) Higher magnification of rectangle in A, with synovial membrane adjacent to supradiscal space; observe slight thickness (large arrow) of normal synovial membrane and absence of immune cells in articular space. C) Low magnification of joint in acute phase of inflammation induced by carrageenan; note presence of intense inflammatory infiltration in enlarged supradiscal space. D) Higher magnification of rectangle in C, observe that synovial membrane does not reveal typical intima layer (large arrow) due to intense influx of immune cells, composed of mononuclear and polymorphonuclear neutrophils (small arrow) in supradiscal space. E) Low magnification of joint in chronic phase of inflammation induced by carrageenan; note presence of condensed immune cells in enlarged supradiscal space and thick synovial membrane. F) higher magnification of rectangle in E; observe that inflammatory infiltrated is formed of lymphocytes, plasma cells and, primarily, macrophages (small arrow); intima layer of synovial membrane is very thick (large arrow). Areas identified within photographs: C, mandibular condyle; D, articular disc; I, infradiscal articular space; S, supradiscal articular space; T, temporal bone. In A, C and E, scale bar = 600 µm. In B, D and F, scale bar = 150 µm.
NPY immunoreactivity in group 1
Fast blue-labeled nucleated neuronal profiles were observed in all ganglia studied, but only the autonomic ganglia (superior cervical, stellate and otic ganglia) showed NPY-IR in the cell bodies (Figure 2 A, B). In the superior cervical ganglion, a mean value of 138±11 fast blue-labeled nucleated neuronal profiles were observed per ganglion, which were diffusely distributed tthroughout the ganglion. Out of these fast blue-labeled nucleated neuronal profiles, 78±3% also showed NPY-IR (Figure 2 A, B; Figure 3). The mean diameter was 24 µm (ranging from 17 to 35 µm), exhibiting unimodal distribution, obtained by measuring 176 double-labeled nucleated neuronal profiles randomly chosen from different superior cervical ganglia from control group animals (Figure 4). The stellate ganglion contained 11±5 fast blue-labeled nucleated neuronal profiles per ganglion, of which 77±6% also showed NPY-IR (Figure 3). The mean diameter of these double-labeled nucleated neuronal profiles was 28 µm (ranging from 20 to 39 µm), obtained by measuring 40 double-labeled nucleated neuronal profiles randomly chosen from different stellate ganglia of the control group (Figure 4). The otic ganglion showed 37±11 fast blue-labeled nucleated neuronal profiles per ganglion, of which 10±4% were NPY-IR (Figure 3), with a mean diameter of 22 µm (ranging from 16 to 25 µm) obtained by measuring 16 double-labeled nucleated neuronal profiles randomly chosen from different otic ganglia of the control group (Figure 5). In the trigeminal ganglion, no double-labeled nucleated neuronal profiles were found.
Figure 2.
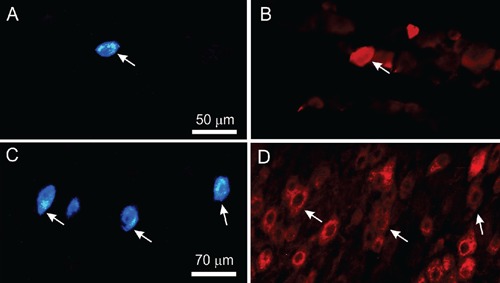
Left: fluorescence photomicrographs of labeled nucleated neuronal profiles in superior cervical (A) and trigeminal (C) ganglia after fast blue deposition in supradiscal articular space of rat temporomandibular joint. Right: photomicrographs of same sections showing NPY (B) and CGRP (D) immunoreactive nucleated neuronal profiles after immunostaining with Cy3 conjugated streptavidin. Arrows indicate nucleated neuronal profiles retrogradely labelled with fast blue and displaying NPY-IR or CGRP-IR. Left and right photomicrographs sets are from identical sections viewed through separate filters. Scale bar = 50 µm (A and B) and 70 µm (C and D).
Figure 3.
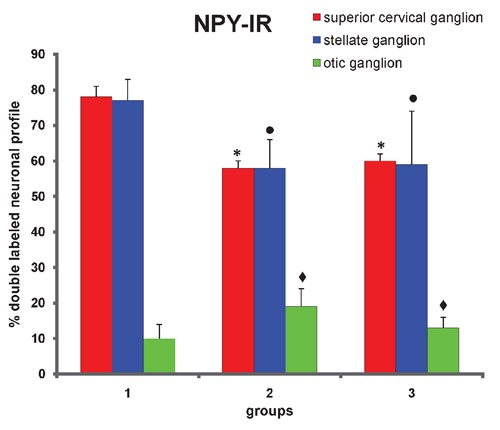
Proportion of NPY-IR nucleated neuronal profiles that innervate rat temporomandiular joint from superior cervical, stellate and otic ganglia. Group 1 represents animals with normal joint (normal group). Group 2 represents animals 48 hours after carrageenan deposition in joint (acute inflammation). Group 3 represents animals 15 days after carrageenan deposition in joint (chronic inflammation). Data are expressed as mean±SEM Kruskal-Wallis non-parametric ANOVA test and Dunn's multiple comparisons test were used to analyze differences between groups. Values of P<0.05 were considered significant. *control group vs chronic and acute inflammation groups, considering superior cervical ganglion, P<0.01. •control group vs chronic and acute inflammation groups, considering stellate ganglion, P<0.05. ✦control group vs chronic and acute inflammation groups, considering otic ganglion, P<0.05.
Figure 4.
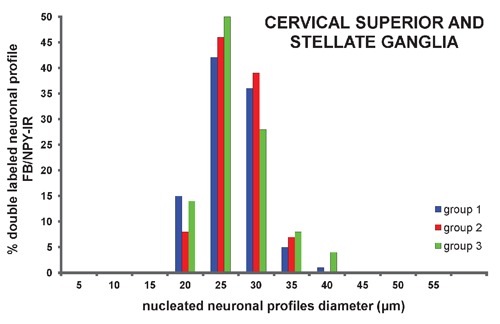
Percentage of double labeled neuronal profiles (FB/NPY-IR) distributed according to perikaryal diameter of neurons from cervical superior and stellate ganglia.
Figure 5.
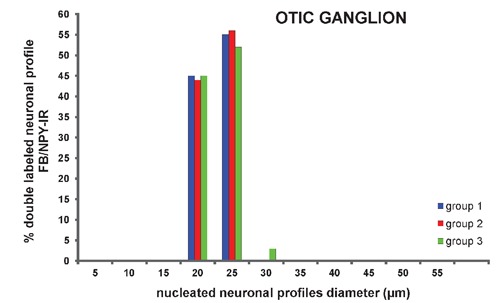
Percentage of double labeled neuronal profiles (FB/NPY-IR) distributed according to perikaryal diameter of neurons from otic ganglion.
NPY immunoreactivity in groups 2 and 3
In all the autonomic ganglia studied, there were no statistically significant changes in the number of fast blue-labeled nucleated neuronal profiles among the different groups.
In the superior cervical ganglion, the percentage of double-labeled nucleated neuronal profiles in group 2 (acute phase of inflammation, 58±2%) was lower than in group 1 (78±3%) (P<0.01) (Figure 3). However, no significant difference was observed in the mean diameter of double-labeled nucleated neuronal profiles between groups 1 (24 µm, ranging from 17 to 35 µm) and 2 (24 µm, ranging from 17 to 36 µm), obtained by measuring 259 double-labeled nucleated neuronal profiles randomly chosen from different superior cervical ganglia of group 2 (Figure 4).
In group 3 (chronic phase of inflammation), the percentage of double-labeled nucleated neuronal profiles (60±2%) was higher than in group 2 (58±2%), but still lower than in the control group (P<0.01) (Figure 3). In group 3, no significant difference was observed in the mean diameter of double-labeled nucleated neuronal profiles (mean diameter of 24 µm, ranging from 14 to 34 µm), obtained by measuring 186 double-labeled nucleated neuronal profiles randomly chosen from different superior cervical ganglia of group 3 (Figure 4).
In the stellate ganglion, the percentage of double-labeled nucleated neuronal profiles was significantly (P<0.05) lower in groups 2 (58±8%) and 3 (59±15%) than in group 1 (77±6%) (Figure 3). No significant difference was observed in the mean diameter, obtained by measuring 15 and 13 double-labeled nucleated neuronal profiles randomly chosen from different stellate ganglia of groups 2 (mean diameter of 28 µm, ranging from 21 to 41 µm) and 3 (mean diameter of 27 µm, ranging from 22 to 40 µm), respectively (Figure 4). In the otic ganglion, the percentage of double-labeled nucleated neuronal profiles was significantly (P<0.05) higher in group 2 (19±5%) and 3 (13±3%) than in group 1 (10±4%) (Figure 3). No significant difference was observed in the mean diameter, obtained by measuring 21 and 18 double-labeled nucleated neuronal profiles randomly chosen from different otic ganglia of groups 2 (mean diameter of 21 µm, ranging from 17 to 24 µm) and 3 (mean diameter of 22 µm, ranging from 17 to 26 µm), respectively (Figure 5).
Finally, in the trigeminal ganglion, no double-labeled nucleated neuronal profiles were observed in groups 2 and 3.
CGRP immunoreactivity in groups 1, 2 and 3
In the trigeminal ganglion, a mean value of 206±83 nucleated neuronal profiles per ganglion were labeled with fast blue, and most of these neurons were located in the dorsoposterolateral portion of the trigeminal ganglion and in the maxillo-mandibular intersection. There were no statistically significant changes in the number of fast blue-labeled nucleated neuronal profiles between different groups.
Out of the fast blue-labeled nucleated neuronal profiles, 31±3% were also immunoreactive to CGRP in group 1 (Figure 2 C, D; Figure 6). The percentage of double-labeled nucleated neuronal profiles was higher (P<0.001) in groups 2 (54±2%) and 3 (49±3%) than in group 1 (Figure 6). The mean diameter was 26 µm (ranging from 13 to 47 µm) with a unimodal distribution, obtained by measuring 154 double-labeled nucleated neuronal profiles randomly chosen from different trigeminal ganglia of the control group (Figure 7). The mean diameter of double-labeled nucleated neuronal profiles in groups 2 (mean diameter of 33 µm, ranging from 21 to 49 µm) and 3 (mean diameter of 33 µm, ranging from 20 to 51 µm) were higher than in group 1 (P<0.05), and these values were obtained by measuring 86 and 106 double-labeled nucleated neuronal profiles randomly chosen from different trigeminal ganglia of groups 2 and 3, respectively (Figure 7).
Figure 6.
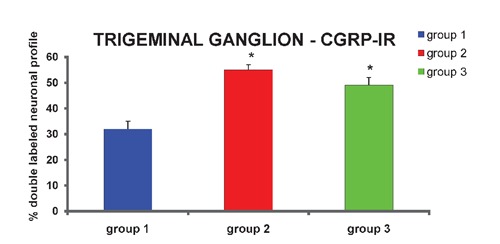
Proportion of CGRP-IR nucleated neuronal profiles that innervate rat temporomandibular joint from trigeminal ganglion. Group 1 represents animals with normal joint (normal group). Group 2 represents animals 48 h after carrageenan deposition in joint (acute inflammation). Group 3 represents animals 15 days after carrageenan deposition in joint (chronic inflammation). Data are expressed as mean±SEM. Kruskal-Wallis non-parametric ANOVA test and Dunn's multiple comparisons test were used to analyze differences between groups. Values of P<0.05 were considered significant. *control group vs chronic and acute inflammation groups, P<0.001.
Figure 7.
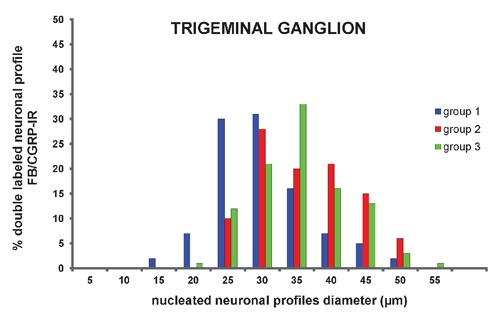
Percentage of double labeled neuronal profiles (FB/CGRP-IR) distributed according to perikaryal diameter of neurons from trigeminal ganglion.
Discussion
The modulatory role of neuropeptides in TMJ arthritis has been investigated over the past few years. The analysis of joint fluid has been used to correlate neuropeptide concentrations with clinical signs and symptoms of TMJ disease.21,32,34,41,42 High levels of neuropeptides have been found in the synovial fluid from patients with temporomandibular joint (TMJ) inflammatory diseases and from experimentally-induced TMJ arthritis in rats.12,13,21,34 Among these neuropeptides are the calcitonin gene-related peptide (CGRP) and the neuropeptide Y (NPY), both having vasoregulatory functions and originating in sensory and autonomic ganglia, respectively. The autonomic ganglia that supply NPY fibers to the TMJ have been investigated, however, the morphometric characteristics of these perikarya have not been determined.18,43 It is still not known whether during TMJ inflammation there is a modulation in the NPY or CGRP expression in neuronal profiles that send nerve fibers to the rat TMJ. Using a fluorescent retrograde neuronal tracing technique combined with immunofluorescence, the present study found that NPY immunoreactive fibers in the TMJ have sympathetic and parasympathetic origin. Also, there was a variation in the percentage of autonomic NPY and sensory CGRP neurons that send nerve fibers to the TMJ during the inflammatory process induced by carrageenan.
Technical considerations
Fast blue fluorescent neuronal retrograde tracer has been used in studies aiming to identify efferent autonomic and afferent sensory neurons that send nerve fibers to synovial joints, such as knee joint and temporomandibular joint.19,35,44–46
The choice of fast blue as a fluorescent neuronal tracer allowed us to submit ganglia sections to the immunofluorescence technique to identify neuropeptides present in nucleated neuronal profiles. Another important aspect was the efficient neuronal labeling and its stability without leaking from neurons in long-term experiments.47,48 In the present study, fast blue was deposited in the rat TMJ fifteen days before euthanasia. This long post-surgery period was adopted as carrageenan was also deposited in the TMJ in order to obtain a chronic inflammatory process in the TMJ during euthanasia (group 3).
The retrograde neuronal tracer deposition method demonstrated to be efficient in avoiding leakage to adjacent tissues, such as muscle, parotid gland and adjacent ganglia, as previously reported by Casatti et al. (1999).19
NPY immunoreactive neuronal profiles sending nerve fibers to the normal temporomandibular joint
It was observed in this study that most sympathetic neurons (78%) from the superior cervical ganglion that innervate the rat TMJ showed NPY-IR. Kido et al. (2001) has also observed a dense NPY innervation in the rat TMJ originating from superior cervical ganglion.26 Since a similar approach has not been made in any other joint, comparisons were made with previous studies involving the innervation of other orofacial territories. There are available data showing the existence of different targets for NPY immunoreactive fibers of sympathetic and parasympathetic origin. Experiments using retrograde neuronal tracing technique combined with immunohistochemistry to identify NPY immunoreactive fibers in the rat submandibular and parotid glands and porcine parotid glands showed that the NPY sympathetic innervation is confined to the blood vessels of the glands, while periacinar NPY fibers have a parasympathetic origin.49–51
In the rabbit ear, NPY-IR neurons of small diameter from the superior cervical ganglion project primarily to arterial vessels.52 It was found that NPY perikarya from superior cervical ganglion projecting to the TMJ have small mean diameter (mean, 24±0.3 µm). Thus, it is possible that of superior cervical ganglion NPY perikarya may be sending nerve fibers mainly to arterial blood vessels. Regarding the parasympathetic innervation of the rat TMJ, it was shown for the first time that 10% of retrogradely labeled neurons in the otic ganglion showed NPY-IR. However, very little is known about the possible functions of parasympathetic innervation in the TMJ. In a previous study, it was observed that the removal of the rat superior cervical ganglion resulted in complete absence of NPY fibers in the TMJ.26 These data are not in accordance with our findings, since the otic ganglion is also a putative source of NPY innervation to the rat TMJ. In addition, we found that sympathetic denervation of the rat TMJ did not eliminate all NPY innervations, remaining this peptidergic innervation in the largest arterial vessels in the TMJ (data not published). It is important to mention that a putative neurotransmitter molecule detected in cell bodies of a given population of neurons is not necessarily transported to their terminals in concentrations detectable by immunohistochemical methods in epifluorescence or light microscopy. For instance, NPY has been detected by immunohistochemistry in the rat ciliary ganglion but was undetectable in nerve terminals of the iris and ciliary body of sympathectomized rats.50
Regarding the trigeminal ganglion, our results are in agreement with previous studies showing no NPY immunoreactive perikarya in the normal rat.53,54
NPY immunoreactive neuronal profiles sending nerve fibers to arthritic temporomandibular joint
In the present work, a significant decrease in the percentage of double-labeled nucleated neuronal profiles in the superior cervical ganglion from animals with acute and chronic TMJ inflammation was found, comparing with the control group (Figure 3). A possible explanation for this result could be an increased transport of large dense core vesicles, reducing neuropeptides storage in some perikarya to an undetectable level by immunohistochemistry. In line with this idea, there are studies showing a decreased density of NPY immunoreactive fibers in the human rheumatoid synovium, as well as an increase in the NPY concentration, during arthritis, in the synovial fluid of rat and human TMJ.12,14,55 In addition, some studies show that NPY is released under conditions of elevated neuronal activity.56 Prolongated sympathetic stimulation over 1 h decreases by 58% the content of NPY in sympathetic fibers distributed in the spleen.57 Studies show that slow velocity of axonal transport of NPY results in a complete resupply of the nerve terminal with this neuropeptide at around eleven days.57,58 On the other hand, a diminished synthesis of NPY during arthritis may also be a putative explanation for the decreased number of double-labeled neuronal profiles found in the present study. Inflammatory factors such as the leukemia inhibitory factor, generally produced during the onset of the inflammatory process, could be altering the NPY expression in superior cervical ganglion neurons.59,60 Thus, if neuronal NPY synthesis is decreased during arthritis, the increase of NPY levels in the synovial fluid from arthritic rat and human TMJ may have a non-neuronal origin, since immune cells and endothelium are able to synthesize NPY.12,14,61–63 The stress hypothesis should not be neglected. Several studies showed that chronic or acute arthritis resulted in hypothalamus-pituitary-adrenal axis oscillations, identified by elevated circulating corticosterone levels.64–66 NPY plasma levels also rise in response to stress conditions and the release of the peptide proved to be mainly from noradrenergic nerve endings.67,68 However, this does not appear to be the case in the present study, since significant alterations in NPY concentration were found mainly in the synovial fluid and not in the plasma from rats submitted to carrageenan-induced arthritis.13
Regarding the otic ganglion, we observed that there was a slight increase in the number of NPY double-labeled neuronal profiles during acute and chronic inflammation. However, no data is available regarding a possible modulatory role of parasympathetic innervation during arthritis in any other joint, although its putative influence has already been suggested.69
No double-labeled neuronal profiles were seen in the trigeminal ganglion from animals with acute and chronic TMJ inflammation, in agreement with a study focusing on inflammatory induction in the orofacial territory.70
CGRP immunoreactive neuronal profiles sending nerve fibers to normal and arthritic temporomandibular joint
This study showed that TMJ inflammation induced by carrageenan increases the percentage of CGRP-IR double-labeled neuronal profiles in the ipsilateral trigeminal ganglion, both during acute and chronic inflammation.
Our findings are in agreement with a study that adopted the same double-labeling method, but in neurons from the dorsal root ganglia innervating the cat knee joint.35 In this previous study, unilateral joint inflammation induced by intra-articular injection of kaolin and carrageenan increased the proportion of double-labeled neurons from 42% to 52% in the control and experimental groups, respectively, in the first thirty-two hours.35 Inflammation induced by complete Freund's adjuvant injection in the L5–L6 facet joint of the rat also caused a significant increase in the percentage of CGRP neurons in the dorsal root ganglia L1 (17% to 50%) and L2 (24% to 39%), seven days after inoculation.36 Similarly, it was noted a significant increase in the total number of CGRP immunoreactive neurons and in CGRP mRNA expression in neurons of dorsal root ganglia after adjuvant inoculation.38,71 The overall CGRP concentration was also increased in the trigeminal ganglion 28 days after adjuvant deposition in the rat TMJ.15,72 Therefore, the literature shows that CGRP modulation during arthritis is evident in the onset of the inflammatory process and continues for a long period, in accordance with the present data.
The triggering mechanisms for the up-regulation of CGRP synthesis in the neuron are likely to involve the presence of the nerve growth factor. It was observed that in carrageenan-induced arthritis, nerve growth factor concentration in the synovial membrane of the rat knee and ankle joints were significantly increased.73,74 In addition, injection of nerve growth factor into the skin of the rat ear induced an increase in the percentage of trigeminal ganglion neurons expressing CGRP.75 Besides the nerve growth factor, other proinflammatory substances such as the cytokine leukemia inhibitory factor and interleukin-1 could be up-regulating CGRP expression. The leukemia inhibitory factor is overexpressed by synoviocytes of arthritic patients, and is retrogradely transported by the great majority of CGRP-LI sensory neurons from the rat L4 and L5 dorsal root ganglia in vivo.60,76,77 Interleukin-1 deposition in the rat TMJ induced an increase of CGRP in the synovial fluid.78 Thus, these data support the hypothesis that a reciprocal interaction between neural and immune systems occurs in the synovium, during joint inflammation.
In the morphometric analysis, the mean diameter of double-labeled neuronal profiles (fast blue and CGRP) in the trigeminal ganglion of animals from the inflamed groups was significant larger (33 µm) compared to the control group (26 µm). The analysis of these data shows that out of the neuronal profiles innervating the TMJ with 35 to 45 µm, a very low percentage express CGRP in normal conditions. However, when the joint is inflamed, an increased percentage of these neuronal profiles (35 to 45 µm) begin to express CGRP, consequently increasing the mean diameter of double-labeled neuronal profiles. In a study performed in the cat knee joint, no statistically significant difference in the mean diameter of CGRP neurons innervating normal or inflamed joint was noted.35 On the other hand, a statistically significant (P<0.01) increase in the mean diameter of CGRP neurons in the dorsal root ganglia was observed after inflammation, induced by injection of complete Freund's adjuvant in the rat L5–L6 lumbar facet joint.36
In summary, the present study demonstrated that most NPY-IR neurons projecting to the normal rat TMJ are of sympathetic origin, with a minor contribution from neurons located in the parasympathetic otic ganglion. During the acute and chronic phases of unilateral carrageenan-induced TMJ inflammation, the percentage of NPY-IR neuronal profiles that send nerve fibers to the rat TMJ in the superior cervical ganglion decreased while in the otic ganglion it increased, when compared with the controls. On the other hand, an increase in the number of neurons expressing CGRP in the trigeminal ganglion during acute and chronic inflammation of the rat TMJ was observed. Although, NPY and CGRP have vasoregulatory functions in the joints, they have distinct origins and are differently expressed during arthritis in the rat TMJ, as detected by immunohistochemistry.
Acknowledgments:
this study was supported by a research grant (# 98/16464-4; 1999/12629-1) from FAPESP (The State of São Paulo Research Foundation, SP, Brasil) and a fellowship grant from CAPES (the Federal Agency of Support and Evaluation of Postgraduate Education, Brasil). We thank Karina Vieira Casatti for reading the manuscript.
References
- 1.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 2.Herb K, Cho S, Stiles MA. Temporomandibular joint pain and dysfunction. Curr Pain Headache Rep. 2006;10:408–14. doi: 10.1007/s11916-006-0070-7. [DOI] [PubMed] [Google Scholar]
- 3.Lobbezoo F, Drangsholt M, Peck C, Sato H, Kopp S, Svensson P. Topical review: new insights into the pathology and diagnosis of disorders of the temporomandibular joint. J Orofac Pain. 2004;18:181–91. [PubMed] [Google Scholar]
- 4.Kubota E, Kubota T, Matsumoto J, Shibata T, Murakami KI. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg. 1998;56:192–8. doi: 10.1016/s0278-2391(98)90868-0. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Kondoh T, Fukuda M, Yamazaki Y, Toyosaki T, Suzuki R. Proinflammatory cytokines detectable in synovial fluids from patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:135–41. doi: 10.1016/s1079-2104(98)90415-2. [DOI] [PubMed] [Google Scholar]
- 6.Alstergren P. Cytokines in temporo-mandibular joint arthritis. Oral Dis. 2000;6:331–4. doi: 10.1111/j.1601-0825.2000.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 7.Tesser-Viscaíno SA, Denadai-Souza A, Teixeira SA, Ervolino E, Cruz-Rizzolo RJ, Costa SK, et al. Putative antinociceptive action of nitric oxide in the caudal part of the spinal trigeminal nucleus during chronic carrageenan-induced arthritis in the rat temporomandibular joint. Brain Research. 2009;302:85–96. doi: 10.1016/j.brainres.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Loreto C, Almeida LE, Migliore MR, Caltabiano M, Leonardi R. TRAIL, DR5 and caspase 3-dependent apoptosis in vessels of diseased human temporomandibular joint disc. An immunohistochemical study. Eur J Histochem. 2010;54:e40–e40. doi: 10.4081/ejh.2010.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto T, Inayama M, Tojyo I, Kiga N, Fujita S. Expression of hyaluronan synthase 3 in deformed human temporomandibular joint discs: in vivo and in vitro studies. Eur J Histochem. 2010;54:e50–e50. doi: 10.4081/ejh.2010.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiga N, Tojyo I, Matsumoto T, Hiraishi Y, Shinohara Y, Makino S, et al. Expression of lumican and fibromodulin following interleukin-1 beta stimulation of disc cells of the human temporomandibular joint. Eur J Histochem. 2011;55:e11–e11. doi: 10.4081/ejh.2011.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmlund A, Ekblom A, Hansson P, Lind J, Lundeberg T, Theodorsson E. Concentrations of neuropeptides substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid of the human temporomandibular joint. A correlation with symptoms, signs and arthroscopic findings. Int J Oral Maxillofac Surg. 1991;20:228–31. doi: 10.1016/s0901-5027(05)80181-x. [DOI] [PubMed] [Google Scholar]
- 12.Appelgren A, Appelgren B, Eriksson S, Kopp S, Lundeberg T, Nylander M, et al. Neuropeptides in temporomandibular joints with rheumatoid arthritis: a clinical study. Scand J Dent Res. 1991;99:519–21. doi: 10.1111/j.1600-0722.1991.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 13.Lundeberg T, Alstergren P, Appelgren A, Appelgren B, Carleson J, Kopp S, et al. A model for experimentally induced temperomandibular joint arthritis in rats: effects of carrageenan on neuropeptide-like immunoreactivity. Neuropeptides. 1996;30:37–41. doi: 10.1016/s0143-4179(96)90052-9. [DOI] [PubMed] [Google Scholar]
- 14.Carleson J, Alstergren P, Appelgren A, Appelgren B, Kopp S, Srinivasan GR, et al. Effects of adjuvant on neuropeptide-like immunoreactivity in experimentally induced temporomandibular arthritis in rats. Arch Oral Biol. 1996;41:705–12. doi: 10.1016/s0003-9969(96)00027-1. [DOI] [PubMed] [Google Scholar]
- 15.Carleson J, Bileviciute I, Theodorsson E, Appelgren B, Appelgren A, Yousef N, et al. Effects of adjuvant on neuropeptide-like immunoreactivity in the temporomandibular joint and trigeminal ganglia. J Orofac Pain. 1997;11:195–9. [PubMed] [Google Scholar]
- 16.Kopp S. The influence of neuropeptides, serotonin, and interleukin 1beta on temporomandibular joint pain and inflammation. J Oral Maxillofac Surg. 1998;56:189–91. doi: 10.1016/s0278-2391(98)90867-9. [DOI] [PubMed] [Google Scholar]
- 17.Widenfalk B, Wiberg M. Origin of sympathetic and sensory innervation of the temporomandibular joint. A retrograde axonal tracing study in the rat. Neurosci Lett. 1990;109:30–5. doi: 10.1016/0304-3940(90)90533-f. [DOI] [PubMed] [Google Scholar]
- 18.Uddman R, Grunditz T, Kato J, Sundler F. Distribution and origin of nerve fibers in the rat temporomandibular joint capsule. Anat Embryol. 1998;197:273–82. doi: 10.1007/s004290050137. [DOI] [PubMed] [Google Scholar]
- 19.Casatti CA, Frigo L, Bauer JA. Origin of sensory and autonomic innervation of the rat temporomandibular joint: a retrograde axonal tracing study with the fluorescent dye fast blue. J Dent Res. 1999;78:776–83. doi: 10.1177/00220345990780031001. [DOI] [PubMed] [Google Scholar]
- 20.Sessle BJ. Trigeminal central sensitization. Rev Analgesia. 2005;8:85–102. [Google Scholar]
- 21.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa H, Wakisaka S, Matsuo S, Akai M. Peptidergic innervation of the temporomandibular disk in the rat. Experientia. 1989;45:303–4. doi: 10.1007/BF01951817. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu S, Kido MA, Kiyoshima T, Tanaka T. Postnatal development of substance P-, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactive nerve fibres in the synovial membrane of the rat temporomandibular joint. Arch Oral Biol. 1996;41:749–59. doi: 10.1016/s0003-9969(96)00067-2. [DOI] [PubMed] [Google Scholar]
- 24.Haeuchi Y, Matsumoto K, Ichikawa H, Maeda S. Immunohistochemical demonstration of neuropeptides in the articular disk of the human temporomandibular joint. Cells Tissues Organs. 1999;164:205–11. doi: 10.1159/000016660. [DOI] [PubMed] [Google Scholar]
- 25.Kido MA, Kiyoshima T, Kondo T, Ayasaka N, Moroi R, Terada Y, et al. Distribution of substance P and calcitonin gene-related peptide-like immunoreactive nerve fibers in the rat temporomandibular joint. J Dent Res. 1993;72:592–8. doi: 10.1177/00220345930720030701. [DOI] [PubMed] [Google Scholar]
- 26.Kido MA, Zhang JQ, Muroya H, Yamaza T, Terada Y, Tanaka T. Topography and distribution of sympathetic nerve fibers in the rat temporomandibular joint: immunocytochemistry and ultrastructure. Anat Embryol. 2001;203:357–66. doi: 10.1007/s004290100163. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg JM, Terenius L, Hökfelt T, Martling CR, Tatemoto K, Mutt V, et al. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116:477–80. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- 28.Leblanc GG, Trimmer BA, Landis SC. Neuropeptide Y-like immunoreactivity in rat cranial parasympathetic neurons: coexistence with vasoactive intestinal peptide and choline acetyltransferase. Proc Natl Acad Sci. 1987;84:3511–5. doi: 10.1073/pnas.84.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–4. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 30.Ju G, Hökfelt T, Brodin E, Fahrenkrug J, Fischer JA, Frey P, et al. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatotastin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987;247:417–31. doi: 10.1007/BF00218323. [DOI] [PubMed] [Google Scholar]
- 31.Larsson J, Ekblom A, Henriksson K, Lundeberg T, Theodorsson E. Concentration of substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand J Rheumatol. 1991;20:326–35. doi: 10.3109/03009749109096808. [DOI] [PubMed] [Google Scholar]
- 32.Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special consideration to rheumatoid arthritis. J Orofac Pain. 1995;9:215–25. [PubMed] [Google Scholar]
- 33.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Covariation of neuropeptide Y, calcitonin gene-related peptide, substance P and neurokinin A in joint fluid from patients with temporomandibular joint arthritis. Arch Oral Biol. 1995;40:127–35. doi: 10.1016/0003-9969(94)00141-w. [DOI] [PubMed] [Google Scholar]
- 34.Kopp S. Neuroendocrine, immune, and local responses related to temporomandibular disorders. J Orofac Pain. 2001;15:9–28. [PubMed] [Google Scholar]
- 35.Hanesch U, Heppelmann B, Schmidt RF. Quantification of cat's articular afferents containing calcitonin gene-related peptide or substance P innervating normal and acutely inflamed knee joints. Neurosci Lett. 1997;223:105–8. doi: 10.1016/s0304-3940(97)00645-9. [DOI] [PubMed] [Google Scholar]
- 36.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Phenotypic inflammation switch in rats shown by calcitonin gene-related peptide immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints. Spine. 2001;26:1009–13. doi: 10.1097/00007632-200105010-00005. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa T, Miyagi M, Ohtori S, Aoki Y, Ozawa T, Doya H, et al. Characteristics of sensory DRG neurons innervating the lumbar facet joints in rats. Eur Spine J. 2005;14:559–64. doi: 10.1007/s00586-004-0834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staton PC, Wilson AW, Bountra C, Chessell IP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–9. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira-Gomes J, Adães S, Sarkander J, Castro-Lopes JM. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum. 2010;62:3677–85. doi: 10.1002/art.27713. [DOI] [PubMed] [Google Scholar]
- 40.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Kaneyama K, Segami N, Sato J, Fujimura K, Nagao T, Yoshimura H. Prognostic factors in arthrocentesis of the temporomandibular joint: Comparison of brdykinin, leucotriene B4, prostaglandin E2, and substance P level in synovial fluid between successful and unsuccessful cases. J Oral Maxillofac. 2007;65:242–47. doi: 10.1016/j.joms.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 42.Bouloux GF. Temporomandibular joint pain and synovial fluid analysis: a review of the literature.Review. J Oral Maxillofac Surg. 2009;67:2497–504. doi: 10.1016/j.joms.2009.04.103. [DOI] [PubMed] [Google Scholar]
- 43.Tahmasebi-Sarvestani A, Tedman R, Goss AN. The influence of experimentally induced osteoarthrosis on articular nerve fibers of the sheep temporomandibular joint. J Orofac Pain. 2001;15:206–17. [PubMed] [Google Scholar]
- 44.Bentivoglio M, Kuypers HG, Catsman-Berrevoets CE, Loewe H, Dann O. Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett. 1980;18:25–30. doi: 10.1016/0304-3940(80)90208-6. [DOI] [PubMed] [Google Scholar]
- 45.Hanesch U, Heppelmann B. A simple method for a specific retrograde labelling of dorsal root and sympathetic ganglion cells innervating the knee joint of the cat. J Neurosci Methods. 1995;63:55–9. doi: 10.1016/0165-0270(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 46.Denadai-Souza A, Cenac N, Casatti CA, Câmara PR, Yshii LM, Costa SK, et al. PAR(2) and temporomandibular joint inflammation in the rat. J Dent Res. 2010;89:1123–8. doi: 10.1177/0022034510375284. [DOI] [PubMed] [Google Scholar]
- 47.Puigdellívol-Sánchez A, Prats-Galino A, Ruano-Gil D, Molander C. Efficacy of the fluorescent dyes Fast Blue, Fluoro-Gold, and Diamidino Yellow for retrograde tracing to dorsal root ganglia after subcutaneous injection. J Neurosci Methods. 1998;86:7–16. doi: 10.1016/s0165-0270(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 48.Puigdellívol-Sánchez A, Valero-Cabré A, Prats-Galino A, Navarro X, Molander C. On the use of fast blue, fluoro-gold and diamidino yellow for retrograde tracing after peripheral nerve injury: uptake, fading, dye interactions, and toxicity. J Neurosci Methods. 2002;115:115–27. doi: 10.1016/s0165-0270(01)00532-5. [DOI] [PubMed] [Google Scholar]
- 49.Luebke JI, Wright LL. Characterization of superior cervical ganglion neurons that project to the submandibular glands, the eyes, and the pineal gland in rats. Brain Res. 1992;589:1–14. doi: 10.1016/0006-8993(92)91155-8. [DOI] [PubMed] [Google Scholar]
- 50.Leblanc GG, Landis SC. Target specificity of neuropeptide Y-immunoreactive cranial parasympathetic neurons. J Neurosci. 1988;8:146–55. doi: 10.1523/JNEUROSCI.08-01-00146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojtkiewicz J, Juranek JK, Kowalski I, Bladowski M, Całka J, Majewski M. Immunohistochemical characterization of superior cervical ganglion neurons supplying porcine parotid salivary gland. Neurosci Lett. 2011;500:57–62. doi: 10.1016/j.neulet.2011.05.242. [DOI] [PubMed] [Google Scholar]
- 52.Morris JL, Zhu BS, Gibbins IL, Blessing WW. Subpopulations of sympathetic neurons project to specific vascular targets in the pinna of the rabbit ear. J Comp Neurol. 1999;412:147–60. [PubMed] [Google Scholar]
- 53.Wakisaka S, Takikita S, Sasaki Y, Kato J, Tabata MJ, Kurisu K. Cell size-specific appearance of neuropeptide Y in the trigeminal ganglion following peripheral axotomy of different branches of the mandibular nerve of the rat. Brain Res. 1993;620:347–50. doi: 10.1016/0006-8993(93)90179-q. [DOI] [PubMed] [Google Scholar]
- 54.Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 55.Mapp PI, Kidd BL, Gibson SJ, Terry JM, Revell PA, Ibrahim NB, et al. Substance P, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37:143–53. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- 56.Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–79. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 57.Lundberg JM, Rudehill A, Sollevi A, Fried G, Wallin G. Co-release of neuropeptide Y and noradrenaline from pig spleen in vivo: importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience. 1989;28:475–86. doi: 10.1016/0306-4522(89)90193-0. [DOI] [PubMed] [Google Scholar]
- 58.D'Hooge R, De Deyn PP, Verzwijvelen A, De Block J, De Potter WP. Storage and fast transport of noradrenaline, dopamine beta-hydroxylase and neuropeptide Y in dog sciatic nerve axons. Life Sci. 1990;47:1851–9. doi: 10.1016/0024-3205(90)90288-3. [DOI] [PubMed] [Google Scholar]
- 59.Freidin M, Dougherty M, Kessler JA. Cell density regulates neuropeptide Y expression in cultured sympathetic neurons. Brain Res. 1993;615:135–40. doi: 10.1016/0006-8993(93)91124-b. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. J Neuroimmunol. 1994;51:53–61. doi: 10.1016/0165-5728(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 62.Bracci-Laudiero L, Aloe L, Stenfors C, Tirassa P, Theodorsson E, Lundberg T. Nerve growth factor stimulates production of neuropeptide Y in human lymphocytes. Neuroreport. 1996;7:485–8. doi: 10.1097/00001756-199601310-00026. [DOI] [PubMed] [Google Scholar]
- 63.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–95. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 64.Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol. 2001;13:905–11. doi: 10.1046/j.1365-2826.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- 65.Lightman SL, Windle RJ, Ma XM, Harbuz MS, Shanks NM, Julian MD, et al. Hypothalamic-pituitary-adrenal function. Arch Physiol Biochem. 2002;110:90–3. doi: 10.1076/apab.110.1.90.899. [DOI] [PubMed] [Google Scholar]
- 66.del Rey A, Wolff C, Wildmann J, Randolf A, Hahnel A, Besedovsky HO, et al. Disrupted brain-immune system-joint communication during experimental arthritis. Arthritis Rheum. 2008;58:3090–9. doi: 10.1002/art.23869. [DOI] [PubMed] [Google Scholar]
- 67.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–35. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 68.Bernet F, Dedieu JF, Laborie C, Montel V, Dupouy JP. Circulating neuropeptide Y (NPY) and catecholamines in rat under resting and stress conditions. Arguments for extra-adrenal origin of NPY, adrenal and extra-adrenal sources of catecholamines. Neurosci Lett. 1998;250:45–8. doi: 10.1016/s0304-3940(98)00454-6. [DOI] [PubMed] [Google Scholar]
- 69.Jorgensen C, Sany J. Modulation of the immune response by the neuroendocrine axis in rheumatoid arthritis. Clin Exp Rheumatol. 1994;12:435–41. [PubMed] [Google Scholar]
- 70.Itotagawa T, Yamanaka H, Wakisaka S, Sasaki Y, Kato J, Kurisu K, et al. Appearance of neuropeptide Y-like immunoreactive cells in the rat trigeminal ganglion following dental injuries. Arch Oral Biol. 1993;38:725–8. doi: 10.1016/0003-9969(93)90013-c. [DOI] [PubMed] [Google Scholar]
- 71.Calzà L, Pozza M, Zanni M, Manzini CU, Manzini E, Hökfelt T. Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization study. Neuroscience. 1998;82:575–89. doi: 10.1016/s0306-4522(97)00272-8. [DOI] [PubMed] [Google Scholar]
- 72.Spears R, Dees LA, Sapozhnikov M, Bellinger LL, Hutchins B. Temporal changes in inflammatory mediator concentrations in an adjuvant model of temporomandibular joint inflammation. J Orofac Pain. 2005;19:34–40. [PubMed] [Google Scholar]
- 73.Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int. 1992;12:213–6. doi: 10.1007/BF00302155. [DOI] [PubMed] [Google Scholar]
- 74.Wu Z, Nagata K, Iijima T. Immunohistochemical study of NGF and its receptors in the synovial membrane of the ankle joint of adjuvant-induced arthritic rats. Histochem Cell Biol. 2000;114:453–9. doi: 10.1007/s004180000222. [DOI] [PubMed] [Google Scholar]
- 75.Amann R, Sirinathsinghji DJ, Donnerer J, Liebmann I, Schuligoi R. Stimulation by nerve growth factor of neuropeptide synthesis in the adult rat in vivo: bilateral response to unilateral intraplantar injections. Neurosci Lett. 1996;203:171–4. doi: 10.1016/0304-3940(95)12287-7. [DOI] [PubMed] [Google Scholar]
- 76.Lotz M, Moats T, Villiger PM. Leukemia inhibitory factor is expressed in cartilage and synovium and can contribute to the pathogenesis of arthritis. J Clin Invest. 1992;90:888–96. doi: 10.1172/JCI115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson SW, Vernallis AB, Heath JK, Priestley JV. Leukaemia inhibitory factor is retrogradely transported by a distinct population of adult rat sensory neurons: co-localization with trkA and other neurochemical markers. Eur J Neurosci. 1997;9:1244–51. doi: 10.1111/j.1460-9568.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 78.Carleson J, Alstergren P, Appelgren A, Appelgren B, Kopp S, Theodorsson E, et al. A model for the study of experimentally induced temporomandibular arthritis in rats: the effect of human recombinant interleukin-1 alpha on neuropeptide-like immunoreactivity. J Orofac Pain. 1996;10:9–14. [PubMed] [Google Scholar]


