Abstract
Acetylcholine, the first neurotransmitter to be identified in the vertebrate frog, is widely distributed among the animal kingdom. The presence of a large amount of acetylcholine in the nervous system of cephalopods is well known from several biochemical and physiological studies. However, little is known about the precise distribution of cholinergic structures due to a lack of a suitable histochemical technique for detecting acetylcholine. The most reliable method to visualize the cholinergic neurons is the immunohistochemical localization of the enzyme choline acetyltransferase, the synthetic enzyme of acetylcholine. Following our previous study on the distribution patterns of cholinergic neurons in the Octopus vulgaris visual system, using a novel antibody that recognizes choline acetyltransferase of the common type (cChAT), now we extend our investigation on the octopus central brain mass. When applied on sections of octopus central ganglia, immunoreactivity for cChAT was detected in cell bodies of all central brain mass lobes with the notable exception of the subfrontal and subvertical lobes. Positive varicosed nerves fibers where observed in the neuropil of all central brain mass lobes.
Key words: invertebrate, cephalopod, choline acetyltransferase, neuron, immunohistochemistry.
Introduction
It is universally acknowledged that acetylcholine (ACh), the first neurotransmitter to be discovered,1 plays an important role in neurotransmission and is of great significance for the assessment of the neurochemical organization of the nervous system. The cholinergic system has been assumed to play various roles in motor function, but also in more complex functions, such as learning, in both vertebrates2–4 and invertebrates,5–7 and may modulate memory,8,9 responding to the visual and other sensory inputs in the cephalopod.10,11
The invertebrate central nervous system (CNS) is a good model to use to study the distribution of ACh and its associated enzymes, and the study of the cephalopod nervous system has played a crucial role in our understanding of the biochemical mechanism underlying cholinergic synaptic transmission.12–14
Cephalopods, such as octopus, squid and cuttlefish, are highly evolved invertebrates and have some of the largest and most complex nervous systems among the invertebrates, with processing and computational capabilities that in some tasks rival those of some vertebrates, including a marked ability to learn.15–18 Cephalopod CNS have been shown to contain the same major neurotransmitters that are found in the mammals' brains19 and it may be possible to use immunohistochemistry to define those areas of the cephalopod CNS that are analogous to neural regions in mammals.20,21
Biochemical information is available on the distribution of ACh within the CNS of cephalopods.10,19 There is already substantial evidence showing that the involvement of cholinergic neurotransmission in learning and memory processes appear to be differentially modulated in specific CNS areas of cuttlefish9 and that ACh performs an important physiological role in mediating neuronal signaling at synapses of the cuttlefish optic lobe and into the octopus statocyst sensory hair cells.22,23 The presence of ACh in the cephalopod nervous system, in particular the octopus optic lobe, and the activities of both acetyl-cholinesterase (AChE) and choline acetyl-transferase (ChAT), has been shown,10,24–30 but very little is known about the distribution of cholinergic structures.
Bacq and Mazza31 first reported the occurrence of ACh in the octopus and the activities of both ChAT and AChE, two ACh metabolizing enzymes. The wide distribution of ACh and AChE in the octopus nervous system indicates that, presumably, the central ganglia of this animal should also contain such large amounts of ACh, as these ganglia predominantly consist of cholinergic neurons.23 ChAT, the synthetic enzyme of ACh, has been widely used as a specific marker for cholinergic neurons. ChAT has been isolated from neuronal structures of both vertebrates and invertebrates. Successful purification of ChAT proteins from vertebrates led to the production of a number of specific ChAT antibodies that has made it possible to confirm by immunohistochemistry a detailed map of the cholinergic systems of mammals32–34 and non-mammalian vertebrates.35–39 A similar approach has been used to produce antibodies against purified ChAT from invertebrates, though this has been limited to animals such as flies,40,41 moths,42,43 and worms.44 Conversely, few data are available on ChAT immunoreactive (IR) structures in octopus because none of the available ChAT antibodies cross-react sufficiently with octopus ChAT, whose chemical structure at protein and mRNA levels remains unknown.
In our recent immunohistochemical study,28 we used a new antiserum45 to label ChAT of the common type (cChAT) in the optic lobe and peduncle complex of cephalopod mollusk Octopus vulgaris (Cuvier 1797). Western blot and immunoprecipitation analyses indicate that cChAT antiserum recognizes an octopus ChAT-like protein which is capable of producing ACh. Using this antiserum, we found cChAT immunoreactivity in the retina, optic lobe, and its neighboring peduncle complex, and IR cell bodies in the cell islands of the optic lobe medulla and the cortical layer of the posterior olfactory lobule. IR fibers and nerve terminals were also found in the plexiform layer of the deep retina, within the stroma of the optic gland, and the neuropils of the optic, peduncle, and olfactory lobe. In the present study, we used the same cChAT antiserum to extend our investigation to delineate the distribution pattern of cChAT-IR neuronal cells and fibers in the other parts of octopus central nervous system (CNS).
Organization of the central nervous system
The octopus CNS is made up of central and peripheral ganglia. The CNS includes central brain mass and optic lobes. The central brain mass is encased in a cartilaginous capsule which is penetrated by the esophagus. It is partitioned by the esophagus into a supra- and a subesophageal mass, which are bilaterally interconnected. Laterally and tightly connected to the distal boundaries of the central brain mass, the paired optic lobes are situated adjacent to the eyes. Both supra-esophageal and sub-esophageal masses are further subdivided into numerous, mostly bilateral, symmetrical brain lobes that are functionally differentiated.46 Each lobe is generally described as being composed of a peripheral region of cell layers (cortex) surrounding a neuropil consisting of fiber network.47,48
The magnocellular lobes lie laterally to the esophagus, linking the supra- and sub-esophageal regions, and the chromatophore lobes lie on the latero-dorsal side of the subesophageal mass. All lobes of the CNS lie inside a cartilaginous brain capsule except the optic lobe that lies adjacent to each eye outside the cartilage.
Materials and Methods
Tissue preparation
Specimens of octopus (Octopus vulgaris), each weighing approximately 1–3 kg, collected either from the Island Sea of Japan near Akashi or from the sea near the Straits of Messina (Sicily, Italy) were kept alive in cold seawater. Animals were killed under anesthesia by adding 2% tricaine (ethyl 3-aminobenzoate methane-sulfonate salt; MS-222, Sigma, St. Louis, MO, USA)49 to seawater at 15°C and used for immunohistochemical examination, as described elsewhere.28 Central brain mass was quickly dissected out. For Western blot analysis, the central nervous system was stored at -80°C until use. For immunohistochemistry, the tissues were fixed for 24 h in ice-cold 0.1 M phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde and 0.2% picric acid. After washing with PB, the tissues were immersed for at least 24 h in PB containing 15% sucrose at 4°C, embedded in PB containing 10% gelatin and frozen. The sections were cut into 30 µm-thick transverse sections using a cryostat (HM 505 E, Microm, Walldorf, Germany). Free-floating sections were collected in ice-cold PB containing 15% sucrose. Before immunohistochemical staining, the sections were incubated for at least 3 days in PB containing 0.9% NaCl (PBS) plus 0.3% Triton X-100 at 4°C to improve tissue permeability.
cChAT antibody
The production of cChAT antiserum has been described previously.45 The specificity of the antibody used to octopus cChAT-like molecules has been previously described.30 For Western blotting, aliquots of either cChAT primary antiserum or pre-immune serum of the rabbit which produced cChAT antiserum, each containing 100 µg of protein, were labeled using the Peroxidase Labeling Kit-NH2 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer's instructions.
Western-blot
Octopus central brain mass was homogenized with 10% (v/w) cold Tris-HCl (50 mM, pH 7.4) containing protease-inhibitor cocktail (P-2714, Sigma) using a Polytron device. After centrifugation at 12,000g for 20 min, the supernatant was collected as a crude extract of octopus central brain mass. Protein concentration was measured using a protein assay kit adapted from the method of Bradford (Bio-Rad Laboratories, Tokyo, Japan) with bovine serum albumin as a standard. Aliquots of the supernatant containing 25 µg of protein were electrophoresed on a 5–20% gradient dodecyl sulfate polyacrylamide gel under reductive conditions and then electro-transferred on polyvinylidene diflouride membrane as described previously.50 After transfer, membrane was fixed in 4% paraformaldehyde in 0.1 M Tris-HCl buffered saline (pH 7.4) (TBS) for 20 min at room temperature. After extensive washing in TBS, strips of the membrane were incubated for 1 h with 10% skim milk in TBS, then incubated overnight at 4°C either with the peroxidase labeled primary antiserum against cChAT or the peroxidase labeled pre-immune serum in an immunoreaction enhancer solution (Can Get Signal, Toyobo, Osaka, Japan). After extensive washing in TBS containing 0.5% tween 20, peroxidase labeled bound antibodies on membrane were directly visualized by reaction with enhanced chemiluminescence reagent (Chemi-Lumi One Super, Nacalaitesque Inc., Kyoto, Japan) and the signal emitted was recorded for 10 min using the Lumino-Image Analyzer LAS-4000 (Fujifilm, Tokyo, Japan).
Immunohistochemistry
To inactivate the endogenous peroxidase activity, the sections were pre-treated for 30 min at room temperature with PBS containing 0.3% Triton X-100, 0.1% sodium azide and 0.5% H2O2 and, to avoid non-specific binding of serum proteins, incubated for 30 min at room temperature with normal donkey serum 1:50 in PBS containing 0.3% Triton X-100 and 0.5% bovine serum albumin (BSA, Sigma). Serial sections were then incubated for 72 h at 4°C in the primary antibody solution rabbit anti cChAT (diluted 1:50,000). The sections were then incubated with a biotinylated donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, USA; diluted 1:1000) for 2 h at room temperature and then for 1 h at room temperature with avidin-biotin-peroxidase complex (ABC Elite, Vector Laboratories, Burlingame, USA; diluted 1:2000). PBS containing 0.3% Triton X-100 was used for diluting all the reagents and washing sections after each step. The localization of peroxidase activity was visualized by reacting the sections for 3 min at room temperature with a solution containing 0.04% 3–3' diaminobenzidine tetrahydrochloride (DAB; Fluka, Buchs, Switzerland), 0.4% nickel ammonium sulfate, and 0.003% H2O2 in 0.05M Tris-HCl buffer, pH 7.6 giving a dark blue granular precipitate. The stained sections were mounted on glass slides and air-dried. After staining, the sections were then dehydrated, cleared and coverslipped with Permount (Fisher Scientific, Pittsburg, PA, USA) for microscope observation (AX70 Provis, Olympus Optical, Tokyo, Japan). For control experiments, the primary antiserum was substituted with buffer or normal rabbit serum or primary antiserum was pre-absorbed overnight at +4°C with the antigen used for its production (2 µg/mL antiserum at working dilution). None of the control sections showed positive immuno-staining (Figure 1A).
Figure 1.
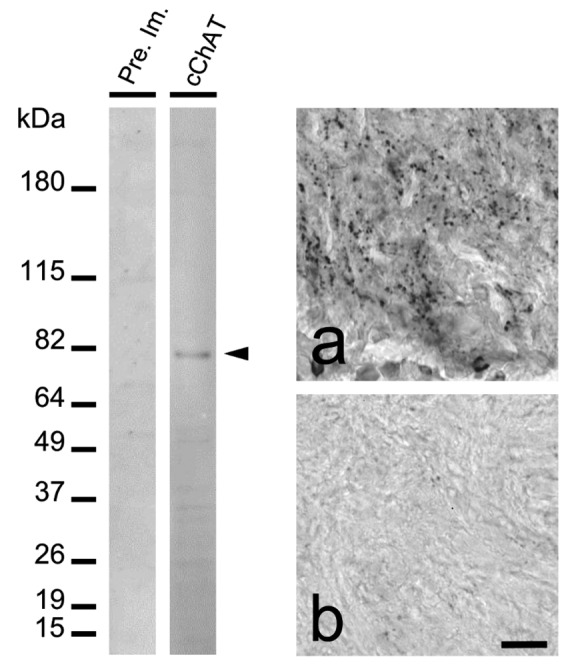
Western blot of crude protein extract (left panel) from the central brain mass of Octopus vulgaris revealed using peroxidase labeled pre-immune serum (left column) and peroxidase labeled cChAT (right column). A clear band at approximately 81-kDa (arrowhead) is recognized by cChAT antiserum. Molecular weight marker is Bench Mark Prestained Protein Ladder (Invitrogen, Carlsbad, CA, USA). cChAT immunoreactivity in the octopus pedal lobe (right panel) using cChAT antiserum (a) or cChAT antiserum pre-absorbed with antigenic peptide (b). Scale bar: 30 µm.
Image analysis for cells
We studied cChAT-positive neuronal cells on well-stained central brain mass sections randomly selected from 3 octopuses. We used a computer-assisted image analyzer equipped with a software tool (Hyperfocus, IAS 2000, Deltasistemi, Roma, Italy) to obtain high-resolution microphotographs and also to estimate the cell size of cChAT-positive somata. For pear-shaped somata we measured both their major and minor axes, and for round or ovoid somata we assessed only their mean axes. Cell size values are expressed as the mean ±SD.
Results
Western blot
We previously reported that a number of octopus tissue molecules reacted with secondary antibodies against anti-rabbit IgG used for detecting cChAT antibody on our Western blot after SDS-PAGE,30 resulting in non-specific signals. Then, in order to avoid the use of peroxidase labeled secondary antibodies, peroxidase labeled cChAT antiserum and peroxidase labeled pre-immune serum were used. In these conditions, a clearly stained band was observed on immunoblot of SDS-PAGE of crude extract from octopus central brain mass, while the pre-immune serum display no signals (Figure 1B). The molecular weight of the band is approximately 81-kDa which is similar to the size of octopus ChAT enzyme previously reported in optic lobe.30 It should be noted that a number of faint bands were observed but that these were considerably less intense than the major 81kDa. Next, we used sections of pedal lobe, which are normally highly immunoreactive to the cChAT antiserum (Figure 1A), and incubated them with the cChAT antiserum pre-absorbed with antigenic peptide (Figure 1B). A dramatic decrease in signal intensity was observed on the section incubated with cChAT antiserum pre-absorbed by the antigenic peptide, providing further confirmation of the cross-reaction of the rat cChAT antibody with the octopus cChAT in the tissue used for immunohistochemistry.
cChAT-immunoreactivity
Transverse sections of the octopus central brain mass has shown cChAT immunoreactivity in several lobes belonging to both the supraesophageal (frontal, subfrontal, buccal, vertical, subvertical and basal lobes) and subesophageal (brachial and pedal lobes) masses (Table 1). Geographically distinct specimens of octopus were examined but no difference in distribution of immunoreactivity in the central brain mass was found.
Table 1. Semi-quantitative analysis of cChAT immunoreactivity in transverse sections of the octopus central brain mass. Localization and relative abundance of cChAT-IR structures. Lobes are listed along the anterior-posterior axis. Frontal, subfontal, buccal, vertical, subvertical and basal are supraesophageal lobes; brachial and pedal are subesophageal lobes; magnocellular and chromatophore lobes lie laterally to the esophagus.
| Central brain mass lobes | Immunoreactive cell bodies | Immunoreactive fibers |
|---|---|---|
| Buccal | ||
| Superior | ++ (m) | +++ |
| Posterior | ++ (m) | ++ |
| Frontal | ||
| Superior | + (s) | ++ |
| Inferior | ++ (s) | +++ |
| Subfrontal | - | +++ |
| Magnocellular | ||
| Dorsal | ++ (s) | ++ |
| Ventral | ++ (s) ++ (l) | ++ |
| Brachial | ||
| Anterior | + (s) +++ (m) | + |
| Posterior | + (s) +++ (m) | + |
| Vertical | +/− (l) | +++ |
| Subvertical | - | +++ |
| Basal | ||
| Dorsal | - | +++ |
| Medial | +/− (s) | ++ |
| Lateral | +/− (s) | + |
| Chromatophore | +++ (s) +++(l) | ++ |
| Pedal | ++ (l) | +++ |
+++, Countless positive cells and /or fibers; ++, numerous positive cells and /or fibers; +, discrete number of positive cells and /or Fibers; +/− scattered positive cells and /or fibers; -, no positive cells and /or fibers. Cell sizes: s, small (5–15×5–15 µm); m, medium (20–30 ×20–30 µm); l, large (30–50×30–50 µm).
Frontal lobe
The frontal lobe can be divided into two main parts: superior and inferior frontal lobe. The superior frontal lobe can be further divided into median (the median superior frontal lobe, MSF) and lateral part. We found a discrete number of cChAT-IR small neurons (12.5±1.1 µm) in the MSF (Figure 2A). These IR cells come from the small cell layers surrounding the surface of the lobe. A network of IR fibers in the MSF, weaker than in the lateral superior frontal lobe (Figure 2A and B) was found. The inferior frontal lobe showed small cChAT-IR cells (12.5±1.7 µm) and an intensely IR fiber network in the neuropil. The neuropil of the frontal lobe showed two distinct zones: the outer and the inner zone. In both zones, irregularly tangled cChAT-IR fibers were found (Figure 2B).
Figure 2.
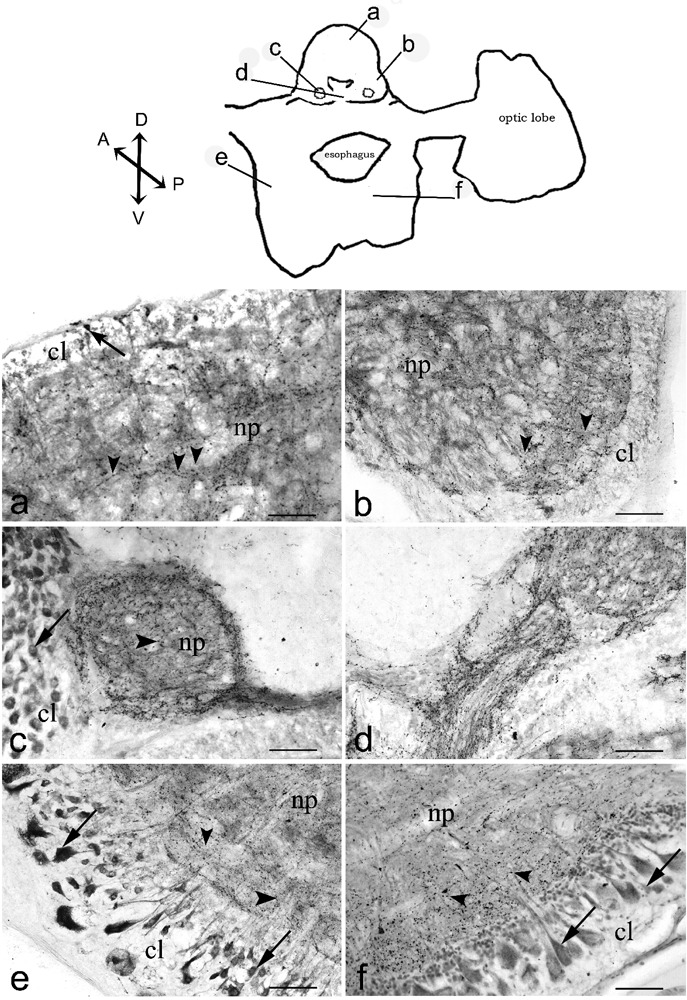
cChAT imunoreactivity in the octopus supra- and subesophageal . The diagram of transverse section (obtained by camera lucida) show the locations of magnified photomicrograph: median superior frontal lobe (a), lateral superior frontal lobe (b), posterior buccal lobe (c), posterior buccal commissure (d), ventral magnocellular lobe (e), brachial lobe (f). Arrows and arrowheads indicate immunoreactive cells and immunoreactive nerve fibers, respectively. Scale bars: 100 µm. cl, cellular layer; np, neuropil. Axis: D, dorsal; V, ventral; A, anterior; P, posterior.
Below MSF, in the neuropil of two subfrontal lobes, we observed an intensely cChAT-IR fiber network.
Buccal lobes
Buccal lobes include a superior buccal and two posterior buccal lobes. All buccal lobes appeared strongly cChAT-IR. The neuropil of the superior buccal lobe showed a dense network of intensely IR fibers surrounded by numerous medium sized (25±1.9 µm) cChAT-IR neurons. In the posterior buccal lobes, medium sized (25±2.2 µm) cChAT-IR neurons surrounded the neuropil (Figure 2C) where a ring of gathered cChAT-IR fibers surrounded an irregular tangle of small and large IR fibers. In the posterior buccal commissure, strongly cChAT-IR fibers were observed (Figure 2D).
Magnocellular lobes
In the cellular layer of both dorsal and ventral magnocellular lobes, several small cChAT-IR cells (7.5±1.1 µm) were found. In the ventral part, large pear shaped cChAT-IR cells (60±2.1×35±0.9 µm) were also found. cChAT-IR fibers were irregularly distributed throughout the neuropil (Figure 2E).
Brachial lobe
In the cellular layer of both anterior and posterior brachial lobes, numerous medium sized cChAT-IR pear shaped neurons (40±2.2×25±1.3 µm) were found together with small cells (12.5±0.8 µm). Irregularly tangled cChAT-IR fibers were observed in the neuropil (Figure 2F).
Vertical lobe
The vertical lobe, consisting of five gyri, occupies the top of the supraesophageal mass. The gyri communicate between each other by numerous tracts of fibers.48 The amacrine cells, whose fibers do not extend beyond the vertical lobe, were the most numerous in this lobe and had no cChAT-IR. Ventrally the gyri are connected with the subvertical lobe. Faint, large cChAT-IR neurons (52.5±2.4 µm) were observed between the amacrine cell layer and the neuropil. Intensely stained cChAT-IR fibers were found throughout the neuropil and in the connection with the subvertical lobe (Figure 3A). cChAT-IR fibers form a ring of surrounding cChAT-IR varicose fibers in the centre of the neuropil.
Figure 3.
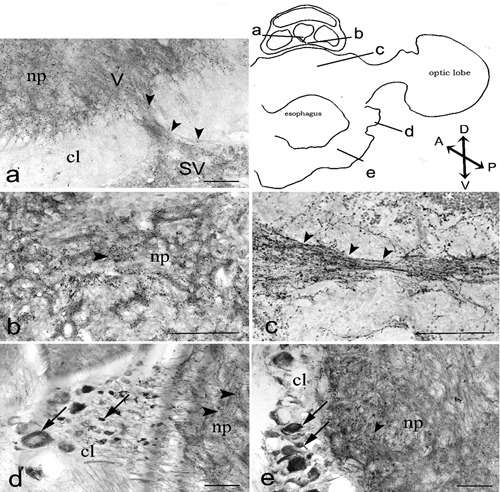
cChAT immunoreactivity in the octopus supra and suboesophageal masses. The diagram of transverse section (obtained by camera lucida) show the locations of magnified photomicrograph: vertical lobe, V and subvertical lobe, SV (a); subvertical lobe (b); dorsal basal lobe (c), chromatophore lobe (d), pedal lobe (e). Arrows and arrowheads indicate IR cells and IR nerve fibers, respectively. Scale bars 100 µm. cl, cellular layer; np, neuropil. Axis: D, dorsal, V, ventral; A, anterior; P, posterior.
In the subvertical lobe, numerous cChAT-IR fibers were observed but no cChAT-IR cells were found (Figure 3B).
Basal lobes
In the basal lobes, we can distinguish dorsal, median and lateral lobes. Throughout the basal lobes, a dense IR nerve fiber network was found, more intense than in the dorsal basal lobe (Figure 3C). Scattered small cChAT-IR cells (12.5±0.8 µm) were found in the cellular layer of the median and the lateral basal lobe.
Chromatophore lobes
Two numerous groups of cChAT-IR neurons were shown: large pear shaped (65±2.5×40±2.0 µm) and small (15±1.8 µm) neurons. The large neurons were regularly arranged at the periphery, while the smaller neurons were found in the inner side of the cell layer. A dense cChAT-IR rather regular fiber network was shown in the neuropil (Figure 3D).
Pedal lobes
Numerous large cChAT-IR pear shaped neurons (60±2.3×45±1.6 µm) were detected in the cellular layer together with small cells (20±1.8 µm) and the neuropil showed a dense cChAT-IR fiber network (Figure 3E).
Discussion
A great deal of information is available on the neuroanatomy and the functions of octopus CNS. Five functionally different areas can be distinguished: auxiliary memory centers, receptor analyzers, higher motor centers, lower motor centers and intermediate motor centers.48,51–56 We used histochemical procedures to describe and map the distribution of structures in the octopus central brain mass where cholinergic synaptic transmission is prevalent; this has not been described elsewhere. In agreement with previous data,48 our results demonstrate the presence of cholinergic structures in lobes which are involved in learning, memory and movement.
Examination of central brain mass sections gave us the opportunity to study cChAT - immunoreactive cell bodies and fibers and their distribution and intensity among the different lobes. Widespread cChAT-IR fibers were observed in the superior frontal, vertical and sub-vertical lobes that belong to auxiliary memory centers; these are areas important for establishing and storing visual or tactile memories. The MSF/vertical lobe system (MSF-V) in octopus is a brain region that has been known for over 50 years to be closely involved in learning and memory.57 The vertical lobe system also plays a part in tactile learning and is, together with the optic lobes, the main component of the visual learning system. In experiments in which the vertical lobe of Octopus vulgaris was lesioned, the ability to learn visual discriminations was drastically impaired58 and the disruption of the cholinergic systems in the higher motor centers interferes with both learning behaviors59 and memory recall.11
The vertical lobe contains two types of morphologically identified neurons: approximately 25 million small amacrine interneurons that synapse onto only approximately 65,000 large efferent cells.48,60,61 These neurons and their connections together with their afferent fibers from the MSF form an association matrix analogous to the vertebrate hippocampus.52 So the high numbers of interneurons suggest the importance of a large number of units to form a high redundancy of connections. As these features are found in both octopus MSF-V and in vertebrate hippocampus, it would appear that they are needed to create a large capacity for memory associations.62 Our immunohistochemical results support the hypothesis that cholinergic inputs from other brain structures convey modulator input signals into the vertical lobe, as do the acetylcholine fibers innervating the vertebrate hippocampus. Therefore, the organization of areas with comparable function in octopus and in mammals indicates that similar cellular organization has been retained between phylogenetically distinct animals with complex forms of learning. Furthermore, the vertical lobe of the octopus brain is also involved in complex forms of long-term learning and memory.58,63 In fact, Hochner et al.64 found that the octopus vertical lobe shows two different types of mechanisms for induction of long-term potentiation as in different parts of the vertebrate hippocampus. One type appears to depend on the postsynaptic response, while the other does not require a strong postsynaptic response for induction.64 Our results could suggest that, like serotonin,21 ACh may act as a neuromodulator in a reinforcing/reward signaling system. These findings reinforce the conclusion that the octopus vertical lobe and the vertebrate hippocampus seem to show a striking example of evolutionary convergence, so the CNS of cephalopods will improve our understanding of the evolution of such associative systems.65
Our observations of cChAT immunoreactivity in the subfrontal lobe are in agreement with its function as a memory store, confirming the putative involvement of cholinergic neuro-transmission in central brain learning processes.66 It is possible that cChAT-IR cell bodies and fibers may have an effect on memory storage. Indeed, available evidence suggests that the subfrontal lobe seems to be necessary for the performance of tactile discrimination and tactile memory.48,66,67
In the subfrontal lobe, cChAT-IR cell bodies were not detected. This suggests that the numerous cChAT-IR fibers observed in the neuropil have an extrinsic origin. It is possible that this bundle is composed of fibers originating from the inferior frontal lobe and from the posterior buccal lobes, where we clearly see cChAT-IR cell bodies. Some of them end here; others run on with the cerebral tracts to the subvertical lobes. It is presumed that the buccal tracts carry signals of taste from the mouth to the learning centers of the inferior frontal and superior frontal/vertical systems.44
A dense network of intensely stained fibers surrounded by numerous IR cell bodies was found in the superior buccal lobe. Together with the subfrontal lobe, the superior buccal lobe is a system concerned with tactile learning.44 A great number of cChAT-IR fibers and large IR neurons were found in the posterior buccal lobes, the center of learning chemotactile discrimination. They receive fibers from the arms and buccal mass, and they send fibers downwards to the arm nervous centers and backwards to the optic and superior frontal/vertical lobe systems.44 In octopus, it has been shown that nitric oxide neurotransmission is needed for both visual and chemotactile learning.68,69 On the other hand, ACh is known to regulate nitric oxide synthase expression in mammals as well as in snail, another mollusk.70,71 The observation here may suggest that regulation of nitric oxide by ACh may also occur in the chemotactile center of octopus.
The presence of numerous cChAT-IR fibers in the dorsal and median basal lobes, higher motor centers, agree with the involvement of cholinergic system also in movement.48 In fact, these lobes receive inputs from both the visual and gravitational (statocysts) systems, and have cerebellar-type effects on motor function.55 They form important cell stations on some of the main channels between the optic lobes and other receptor centers, and the intermediate and final motor cells of the subesophageal lobes. They also regulate movement, including attack. The setting-up of a memory representing association of a given situation with a shock is a property of the optic lobes shared with the basal lobe, but the persistence of the representation depends upon the presence of the vertical lobe.48,63 In the intermediate and in the lower motor centers our findings agree with the involvement of cholinergic system in movement.
Numerous neurons and intensely cChAT-IR fibers were seen in the chromatophore lobes. At a higher level, the chromatophores are controlled by the optic lobes that act on visual information and select specific motor programs. At a lower level, the chromatophores are controlled by the motoneurons in the chromatophore lobes that carry out the programs, so their activity or inactivity produces the patterning seen in the skin.72,73 Two different color classes of chromatophore motoneurons, that excite black chromatophores or yellow, orange and red chromatophores, are inhibited by descending cholinergic pathways derived from optic lobes.20,29,74,75 Our findings agree with previous investigation on AChE presence in the neuropil of all the chromatophore lobes and the optic lobes,29 and with cChAT immunoreactivity in the optic lobes.30 In the magnocellular lobes, we found many cChAT-IR cell bodies and fibers. The magnocellular lobes are involved in the escape behavior and receive fibers from the optic lobe; therefore, they are responsible for visual evoked activity.52 It has been postulated that visual inputs related to a potential predator, and thus likely to evoke an escape response, are communicated via identified pathways to the magnocellular lobes, so they are involved in direct motor responses.48
A great number of cChAT-IR neurons and intensely stained fibers were found in the pedal lobes. The pedal lobes, intermediate motor centers, integrate signals that result in various movements of the funnel, tentacles, head including movements of the eyes (oculomotor centre).47 In this context, it is interesting that Uemura et al.74 have reported the presence of serotonin-IR cells localized in some of the ganglia we had examined in our study. In the buccal lobes, they found large serotonin-IR cells,74 while we found medium sized cChAT-IR cells; so these are not the same cells. In the basal lobe, they visualized numerous serotonin-IR cells of various sizes,74 while we detected small, scattered cChAT-IR cells. In the chromatophore lobes, they have reported the presence both of some large and small serotonin-IR cells74 and we also found large and small cChAT-IR cells. In the pedal lobes, they have found some large serotonin-IR cells74 and we visualized numerous large cChAT-IR cells together with some small ones. Serotonin is a chemical mediator for the control of the change in color, in addition to the cholinergic innervation of the chromatophore system.25,74 Investigations still need to be carried out to determine whether cholinergic and serotonergic systems found in chromatophore and pedal lobes interact.
Cephalopods, a fascinating group of animals, are capable of producing a wide repertoire of behavior and provide an excellent invertebrate model for understanding the neural substrate of learning and memory, where the cholinergic system plays an important part. The roles of octopus brain cholinergic neurons in memory and motor function are reminiscent of those seen in higher vertebrates, including mammals.
Acknowledgments:
the authors wish to acknowledge the precious technical assistance given by Simone Sebastiano Iannacone during the experimental procedures.
This work was supported by a grant from the Sapienza University of Rome (2006–2008).
References
- 1.Blokland A. Acetylcholine: A neurotransmitter for learning and memory. Brain Res Rev. 1995;21:285–300. doi: 10.1016/0165-0173(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Ann Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Towards a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 4.Barraco DA, Eisenstein EM. Effects of pretraining administration of scopolamine on learning and retention in the cockroach, P americana. Pharmacol Biochem Behav. 1984;20:479–81. doi: 10.1016/0091-3057(84)90289-2. [DOI] [PubMed] [Google Scholar]
- 5.Mpitsos GJ, Murray TF, Creech HC, Barker DL. Muscarinic antagonist enhances one-trial food-aversion learning in the mollusc Pleurobranchea. Brain Res Bull. 1988;21:169–79. doi: 10.1016/0361-9230(88)90229-8. [DOI] [PubMed] [Google Scholar]
- 6.Gauthier M, Canolozano V, Zaoujal A, Richard D. Effects of intracranial injections of scopolamine on olfactory conditioning retrieval in the honeybee. Behav Brain Res. 1994;63:145–9. doi: 10.1016/0166-4328(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 7.Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellanger C, Dauphin F, Chichery M-P, Chichery R. Changes in cholinergic enzyme activities in the cuttlefish brain during memory formation. Physiol Behav. 2003;4–5:749–56. doi: 10.1016/s0031-9384(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 9.Bellanger C, Dauphin F, Belzunces LP, Chichery R. Parallel regional quantification of choline acetyltransferase and cholinesterase activity in the central nervous system of an invertebrate (Sepia officinalis) Brain Res Protoc. 1998;3:68–75. doi: 10.1016/s1385-299x(98)00023-3. [DOI] [PubMed] [Google Scholar]
- 10.Fiorito G, Agnisola C, d'Addio M, Valanzano A, Calamandrei G. Scopolamine impairs memory recall in Octopus vulgaris. Neurosci Lett. 1998;253:87–90. doi: 10.1016/s0304-3940(98)00611-9. [DOI] [PubMed] [Google Scholar]
- 11.Corteggiani E. Degree Diss. University of Paris, France, Dessaint; ed. Doullens: 1938. Contribution a I'etude de I'Acetylcholine Libre et Dissimulee sous Forme d'un Complexe dans le Cerveau. [Google Scholar]
- 12.Nachmansohn D, Meyerhof B. Relation between electrical changes during nerve activity and concentration of choline esterase. J Neurophysiol. 1941;4:348–61. doi: 10.1097/00004311-196806020-00022. [DOI] [PubMed] [Google Scholar]
- 13.Clarac F, Pearlstein E. Invertebrate preparations and their contribution to neurobiology in the second half of the 20th century. Brain Res Rev. 2007;54:113–61. doi: 10.1016/j.brainresrev.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Packard A. Cephalopods and fish: the limits of convergence. Biol Rev. 1972;47:241–307. [Google Scholar]
- 15.Williamson R, Chrachri A. Cephalopod neural networks. Neurosignals. 2004;13:87–98. doi: 10.1159/000076160. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon R, Messenger JB. Cambridge University Press; UK: 1996. Cephalopod behaviour. [Google Scholar]
- 17.Edelman DB, Baars BJ, Seth AK. Identifying hallmarks of consciousness in non-mammalian species. Conscious Cogn. 2005;14:169–87. doi: 10.1016/j.concog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Messenger JB. Neurotransmitters of cephalopods. Invert Neurosci. 1996;2:95–114. [Google Scholar]
- 19.Cornwell CJ, Messenger JB, Williamson R. Distribution of GABA-like immunoreactivity in the octopus brain. Brain Res. 1993;621:353–7. doi: 10.1016/0006-8993(93)90127-9. [DOI] [PubMed] [Google Scholar]
- 20.Shomrat T, Feinstein N, Klein M, Hochner B. Serotonin is a facilitatory neuromodulator of synaptic transmission and “reinforces” long-term potentiation induction in the vertical lobe of Octopus vulgaris. Neurosci. 2010;169:52–64. doi: 10.1016/j.neuroscience.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Nunes MA, Santos S, Cordeiro JM, Neves P, Silva VS, Sykes AV, et al. Acetylcholine release and choline uptake by cuttlefish (Sepia officinalis) optic lobe synaptosomes. Biol Bull. 2008;214:1–5. doi: 10.2307/25066654. [DOI] [PubMed] [Google Scholar]
- 22.Chrachri A, Williamson R. Cholinergic modulation of L-type calcium current in isolated sensory hair cells of the statocyst of octopus, Eledone cirrhosa. Neurosci Lett. 2004;1–2:90–4. doi: 10.1016/j.neulet.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 23.Florey E. Acetylcholine in invertebrate nervous systems. Can J Biochem Physiol. 1963;41:2619–26. [PubMed] [Google Scholar]
- 24.Loe PR, Florey E. The distribution of acetylcholine and cholinesterase in the nervous system and in innervated organs of Octopus dofleini. Comp Biochem Physiol. 1966;17:509–22. doi: 10.1016/0010-406x(66)90583-4. [DOI] [PubMed] [Google Scholar]
- 25.Florey E, Winesdorfer J. Cholinergic nerve endings in octopus brain. J Neurochem. 1968;15:169–77. doi: 10.1111/j.1471-4159.1968.tb06192.x. [DOI] [PubMed] [Google Scholar]
- 26.Barlow JJ. The distribution of acetylcholinesterase and catecholamines in the vertical lobe of Octopus vulgaris. Brain Res. 1971;35:304–7. doi: 10.1016/0006-8993(71)90624-x. [DOI] [PubMed] [Google Scholar]
- 27.Welsch F, Dettbarn WD. The subcellular distribution of acetylcholine, acetylcholinesterase and choline acetyltransferase in the optic lobes of the squid Loligo pealii. Brain Res. 1972;39:467–82. doi: 10.1016/0006-8993(72)90449-0. [DOI] [PubMed] [Google Scholar]
- 28.Tansey EM. Neurotransmitters in the cephalopod brain. Comp Biochem Physiol. 1979;64C:173–82. [Google Scholar]
- 29.D'Este L, Kimura S, Casini A, Matsuo A, Bellier JP, Kimura H, et al. First visualization of cholinergic cells and fibers by immunohistochemistry for choline acetyltransferase of the common type in the optic lobe and peduncle complex of Octopus vulgaris. J Comp Neurol. 2008;509:566–79. doi: 10.1002/cne.21761. [DOI] [PubMed] [Google Scholar]
- 30.Bacq ZM, Mazza F. Identification d'acetylcholine extraite des cellules ganglionnaires d'Octopus. C R Soc Biol Paris. 1935;120:246–7. [Google Scholar]
- 31.Kimura H, McGeer PL, Peng F, McGeer EG. Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science. 1980;208:1057–9. doi: 10.1126/science.6990490. [DOI] [PubMed] [Google Scholar]
- 32.Kimura H, McGeer PL, Peng JH, McGeer EG. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol. 1981;200:151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- 33.Eckenstein F, Thoenen H. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J. 1982;1:363–8. doi: 10.1002/j.1460-2075.1982.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson CD, Epstein ML. Monoclonal antibodies and polyvalent antiserum to chicken choline acetyltransferase. J Neurochem. 1986;46:968–76. doi: 10.1111/j.1471-4159.1986.tb13064.x. [DOI] [PubMed] [Google Scholar]
- 35.Medina L, Smeets WJ, Hoogland PV, Puelles L. Distribution of choline acetyltransferase immunoreactivity in the brain of the lizard Gallotia galloti. J Comp Neurol. 1993;331:261–85. doi: 10.1002/cne.903310209. [DOI] [PubMed] [Google Scholar]
- 36.Medina L, Reiner A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol. 1994;342:497–537. doi: 10.1002/cne.903420403. [DOI] [PubMed] [Google Scholar]
- 37.Marin O, Smeets WJ, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of anuran (Rana perezi, Xenopus laevis) and urodele (Pleurodeles waltl) amphibians. J Comp Neurol. 1997;382:499–534. doi: 10.1002/(sici)1096-9861(19970616)382:4<499::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Tavares NN, Hasson-Voloch A. Choline acetyltransferase from the electric organ of Electrophorus electricus (L.)-physicochemical characterization and immunochemical identification. Z Naturforsch. 1998;53:407–15. doi: 10.1515/znc-1998-5-617. [DOI] [PubMed] [Google Scholar]
- 39.Takagawa K, Salvaterra P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res. 1996;24:237–43. doi: 10.1016/0168-0102(95)00999-x. [DOI] [PubMed] [Google Scholar]
- 40.Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. J Comp Neurol. 2003;466:299–315. doi: 10.1002/cne.10867. [DOI] [PubMed] [Google Scholar]
- 41.Clark J, Meisner S, Torkkeli PH. Immunocytochemical localization of choline acetyltransferase and muscarinic ACh receptors in the antenna during development of the sphinx moth Manduca sexta. Cell Tissue Res. 2005;320:163–73. doi: 10.1007/s00441-004-1039-7. [DOI] [PubMed] [Google Scholar]
- 42.Torkkeli PH, Widmer A, Meisner S. Expression of muscarinic acetylcholine receptors and choline acetyltransferase enzyme in cultured antennal sensory neurons and non-neural cells of the developing moth Manduca sexta. J Neurobiol. 2005;62:316–29. doi: 10.1002/neu.20097. [DOI] [PubMed] [Google Scholar]
- 43.Duerr JS, Han H-P, Fields SD, Rand JB. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 2008;506:398–408. doi: 10.1002/cne.21551. [DOI] [PubMed] [Google Scholar]
- 44.Kimura S, Bellier JP, Matsuo A, Tooyama I, Kimura H. The production of antibodies that distinguish rat choline acetyltransferase from its splice variant product of a peripheral type. Neurochem Int. 2007;50:251–5. doi: 10.1016/j.neuint.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Nixon M, Young JZ. Oxford University Press; UK: 2003. The Brains and Lives of Cephalopods. [Google Scholar]
- 46.Boycott BB. The functional organization of the brain of the cuttlefish Sepia officinalis. Proc Roy Soc B. 1961;153:503–34. [Google Scholar]
- 47.Young JZ. Claredon Press; Oxford: 1971. The Anatomy of the Nervous System of Octopus vulgaris. [Google Scholar]
- 48.D'Este L, Casini A, Kimura S, Bellier JP, Ito E, Kimura H, et al. Immunohistochemical demonstration of cholinergic structures in central ganglia of the slug (Limax maximus, Limax valentianus) Neurochem Int. 2011;58:605–11. doi: 10.1016/j.neuint.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Frazier DT, Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid exon membranes. Eur J Pharmacol. 1975;33:313–7. doi: 10.1016/0014-2999(75)90175-2. [DOI] [PubMed] [Google Scholar]
- 50.Wells MJ. Chapman and Hall. London: John Wiley and Sons, NY, USA; 1978. The Octopus, Physiology and Behavior of an Advanced Invertebrate; pp. 217–327. [Google Scholar]
- 51.Young JZ. Computation in the learning-system of cephalopods. Biol Bull. 1991;180:200–8. doi: 10.2307/1542389. [DOI] [PubMed] [Google Scholar]
- 52.Abbott NJ, Williamson R, Maddock L. Oxford Science Publications; UK: 1995. Cephalopod Neurobiology. [Google Scholar]
- 53.Messenger JB. Multimodal convergence and the regulation of motor programs in cephalopods. Fortschr Zool. 1983;28:11–91. [Google Scholar]
- 54.Messenger JB. The peduncle lobe: a visuo-motor centre in octopus. Proc R Soc Lond B Biol Sci. 1967;167:225–51. doi: 10.1098/rspb.1967.0025. [DOI] [PubMed] [Google Scholar]
- 55.Zullo L, Sumbre G, Agnisola C, Flash T, Hochner B. Nonsomatotopic organization of the higher motor centers in octopus. Curr Biol. 2009;19:1632–6. doi: 10.1016/j.cub.2009.07.067. [DOI] [PubMed] [Google Scholar]
- 56.Boycott BB, Young JZ. The comparative study of learning. Symp Soc Exp Biol. 1950;4:432–53. [Google Scholar]
- 57.Fiorito G, Chichery R. Lesions of the vertical lobe impair visual discrimination learning by observation in Octopus vulgaris. Neurosci Lett. 1995;192:117–20. doi: 10.1016/0304-3940(95)11631-6. [DOI] [PubMed] [Google Scholar]
- 58.Halm MP, Chichery MP, Chichery R. The role of cholinergic networks of the anterior basal and inferior frontal lobes in the predatory behaviour of Sepia officinalis. Comp Biochem Physiol. 2002;132:267–74. doi: 10.1016/s1095-6433(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 59.Gray EG. The fine structure of the vertical lobe of octopus brain. Phil Trans Roy Soc Lond B. 1970;379:379–94. doi: 10.1098/rstb.1970.0040. [DOI] [PubMed] [Google Scholar]
- 60.Young JZ. Multiple matrices in the memory system of Octopus. In: Abbott JN, Williamson R, Maddock L, editors. Cephalopod neurobiology: neuroscience studies in squid, octopus and cuttlefish. Oxford University Press; 1995. pp. 431–43. [Google Scholar]
- 61.Hochner B, Shomrat T, Fiorito G. The octopus: A model for a comparative analysis of the evolution of learning and memory mechanisms. Biol Bull. 2006;210:308–17. doi: 10.2307/4134567. [DOI] [PubMed] [Google Scholar]
- 62.Boycott BB, Young JZ. A memory system in Octopus vulgaris. Lamarck Proc Roy Soc B. 1955;143:449–80. doi: 10.1098/rspb.1955.0024. [DOI] [PubMed] [Google Scholar]
- 63.Hochner B, Brown ER, Langella M, Shomrat T, Fiorito G. A learning and memory area in the Octopus brain manifests a vertebrate-like long-term potentiation. J Neurophysiol. 2003;90:3547–54. doi: 10.1152/jn.00645.2003. [DOI] [PubMed] [Google Scholar]
- 64.Graindorge N, Alves C, Darmaillacq AS, Chichery R, Dickel L, Bellanger C. Effects of dorsal and ventral vertical lobe electrolytic lesions on spatial learning and locomotor activity in Sepia officinalis. Behav Neurosci. 2006;120:1151–8. doi: 10.1037/0735-7044.120.5.1151. [DOI] [PubMed] [Google Scholar]
- 65.Wells MJ, Young JZ. Split brain preparations and touch learning in the octopus. J Exp Biol. 1965;43:565–79. doi: 10.1242/jeb.43.3.565. [DOI] [PubMed] [Google Scholar]
- 66.Wells MJ, Young JZ. The subfrontal lobe and touch learning in the octopus. Brain Res. 1975;92:103–21. doi: 10.1016/0006-8993(75)90530-2. [DOI] [PubMed] [Google Scholar]
- 67.Robertson JD, Bonaventura J, Kohm A, Hiscat M. Nitric oxide is necessary for visual learning in Octopus vulgaris. Proc Biol Sci. 1996;263:1739–43. doi: 10.1098/rspb.1996.0254. [DOI] [PubMed] [Google Scholar]
- 68.Robertson JD, Bonaventura J, Kohm AP. Nitric oxide is required for tactile learning in Octopus vulgaris. Proc Biol Sci. 1994;256:269–73. doi: 10.1098/rspb.1994.0080. [DOI] [PubMed] [Google Scholar]
- 69.Zaitseva OV, Markosova TG. Acetylcholine, nitric oxide and their possible colocalization in regulatory cells of the digestive system of gastropods. Dokl Biol Sci. 2008;421:248–50. doi: 10.1134/s001249660804008x. [DOI] [PubMed] [Google Scholar]
- 70.Roszer T, Józsa T, Szentmiklósi AJ, Bánfalvi G. Acetylcholine inhibits nitric oxide (NO) synthesis in the gastropod nervous system. Cell Tissue Res. 2009;336:325–35. doi: 10.1007/s00441-009-0764-3. [DOI] [PubMed] [Google Scholar]
- 71.Barlow JJ. Comparative biochemistry of the central nervous system. Symp Zool Soc Lond. 1977;38:325–46. [Google Scholar]
- 72.Tansey EM. Aminergic fluorescence in the cephalopod brain. Philos Transact R Soc Lond B. 1980;291:127–45. [Google Scholar]
- 73.Uemura T, Yamashita T, Haga C, Miyazaki N, Kondo H, Matsushita M. Localization of serotonin-immunoreactivity in the central nervous system of Octopus vulgaris by immunohistochemistry. Brain Res. 1987;406:73–86. doi: 10.1016/0006-8993(87)90770-0. [DOI] [PubMed] [Google Scholar]
- 74.Messenger JB. Cephalopod chromatophores: neurobiology and natural history. Biol Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. [DOI] [PubMed] [Google Scholar]


