Summary
Background
Clinical outcomes after major surgery are poorly described at the national level. Evidence of heterogeneity between hospitals and health-care systems suggests potential to improve care for patients but this potential remains unconfirmed. The European Surgical Outcomes Study was an international study designed to assess outcomes after non-cardiac surgery in Europe.
Methods
We did this 7 day cohort study between April 4 and April 11, 2011. We collected data describing consecutive patients aged 16 years and older undergoing inpatient non-cardiac surgery in 498 hospitals across 28 European nations. Patients were followed up for a maximum of 60 days. The primary endpoint was in-hospital mortality. Secondary outcome measures were duration of hospital stay and admission to critical care. We used χ2 and Fisher's exact tests to compare categorical variables and the t test or the Mann-Whitney U test to compare continuous variables. Significance was set at p<0·05. We constructed multilevel logistic regression models to adjust for the differences in mortality rates between countries.
Findings
We included 46 539 patients, of whom 1855 (4%) died before hospital discharge. 3599 (8%) patients were admitted to critical care after surgery with a median length of stay of 1·2 days (IQR 0·9–3·6). 1358 (73%) patients who died were not admitted to critical care at any stage after surgery. Crude mortality rates varied widely between countries (from 1·2% [95% CI 0·0–3·0] for Iceland to 21·5% [16·9–26·2] for Latvia). After adjustment for confounding variables, important differences remained between countries when compared with the UK, the country with the largest dataset (OR range from 0·44 [95% CI 0·19–1·05; p=0·06] for Finland to 6·92 [2·37–20·27; p=0·0004] for Poland).
Interpretation
The mortality rate for patients undergoing inpatient non-cardiac surgery was higher than anticipated. Variations in mortality between countries suggest the need for national and international strategies to improve care for this group of patients.
Funding
European Society of Intensive Care Medicine, European Society of Anaesthesiology.
Introduction
More than 230 million major surgical procedures are undertaken worldwide each year.1 For most patients, risks of surgery are low and yet evidence increasingly suggests that complications after surgery are an important cause of death.2–5 About 10% of patients undergoing surgery in the UK are at high risk of complications, accounting for 80% of postoperative deaths.2–4 If this rate is applicable worldwide, up to 25 million patients undergo high-risk surgical procedures each year, of whom 3 million do not survive until hospital discharge. Patients who develop complications but survive to leave hospital often have reduced functional independence and long-term survival.5–8
Despite obvious differences in procedure-related and patient-related mortality risks, most surgical patients use one care pathway, sharing standard facilities for preoperative assessment, anaesthesia, operating rooms, post-anaesthetic recovery, and hospital wards. This approach is adequate for most patients but might not meet the needs of the small number of patients at high risk of complications and death. In the USA, evidence of variations in postoperative mortality within health-care systems suggest the potential to implement measures that improve patient outcomes.9 Low rates of admission to critical care for patients at high risk of complications undergoing non-cardiac surgery are of particular concern,2–4 and might be affected by international differences in the provision of critical care.10,11 With high volumes of surgery undertaken, even a low rate of avoidable harm will be associated with many preventable deaths.
International comparative data might provide important insights into delivery of health care for surgical patients. However, little or no data are available describing provision of care or outcomes for unselected surgical patients. The objective of the European Surgical Outcomes Study (EuSOS) was to describe mortality rates and patterns of critical care resource use for patients undergoing non-cardiac surgery across several European nations.
Methods
Study design and participants
We did this European cohort study between 0900 h (local time) on April 4, 2011, and 0859 h on April 11, 2011. All adult patients (older than 16 years) admitted to participating centres for elective or non-elective inpatient surgery commencing during the 7 day cohort period were eligible for inclusion. Patients undergoing planned day-case surgery, cardiac surgery, neurosurgery, radiological, or obstetric procedures were excluded because these patients receive care within separate, dedicated pathways. Participating hospitals (appendix pp 11–68) were a voluntary convenience sample, identified through membership of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology and by direct approach from national study coordinators. Ethics requirements differed by country. In Denmark, centres were exempt from ethics approval because this study was deemed to be a clinical audit. In all other nations formal ethics approval was applied for and given. In Finland alone we were required to obtain written informed consent from individual patients.
Procedures
Local investigators were supported by national coordinators and via a website that provided key documentation, including the protocol and guidance on study procedures. We obtained data describing perioperative care facilities once for each hospital at the beginning of the study. We collected data describing consecutive patients with paper case record forms, which we made anonymous before entering the information onto a secure internet-based electronic case record form (OpenClinica, Boston, MA, USA). We completed an operating theatre case report form for each eligible patient who we then followed up until hospital discharge for data describing hospital stay, admission to critical care, and in-hospital mortality. We completed a critical care case record form to capture data describing the first admission to critical care for any individual patient at any time during the follow-up period. Example case record forms are available from the study website.
We selected patient-level variables on the basis that they were objective, routinely collected for clinical reasons, could be transcribed with a high level of accuracy, and would be relevant to a risk adjustment model in most patients. We censored critical care and hospital discharge data at 60 days after surgery. We assessed data for completeness and then checked for plausibility and consistency with prospectively defined ranges.12
The primary endpoint was in-hospital mortality. Secondary outcome measures were duration of hospital stay and admission to critical care.
Statistical analysis
Our aim was to recruit as many participating hospitals as possible and to recruit every eligible patient in those hospitals. We anticipated that a minimum sample size of 20 000 patients would enable a precise estimate of mortality. This sample size was also expected to provide a sufficient number of events (>200) for construction of a robust logistic regression model for mortality.
We used SPSS (version 19.0) for data analysis. Categorical variables are presented as number (%) and continuous variables as mean (SD) when normally distributed or median (IQR) when not. We used χ2 and Fisher's exact tests to compare categorical variables and the t test or the Mann-Whitney U test to compare continuous variables. Significance was set at p<0·05. We constructed several binary logistic regression models to identify factors independently associated with hospital mortality and to adjust for differences in confounding factors between countries. These included a one-level model and a hierarchical two-level generalised linear mixed model, with patients being at the first level and hospital at the second. Factors were entered into the model based on their univariate relation to outcome (p<0·05). All factors were biologically plausible with a sound scientific rationale and a low rate of missing data. The results of the model are reported as adjusted odds ratios (OR) with 95% CI. We assessed the models through sensitivity analyses with three random (disjoint) subsamples of countries and a fourth sample removing all patients from the largest country in the dataset (the UK). We explored all possible interacting factors and examined how they might have affected the final results.
This study is registered with ClinicalTrials.gov, number NCT01203605.
Role of the funding source
The study was funded by the European Society of Intensive Care Medicine and the European Society of Anaesthesiology who appointed an independent steering committee (appendix p 11), who were responsible for study design, conduct, and data analysis. Members of the steering committee had full access to the study data and were solely responsible for interpretation of the data, drafting and critical revision of the report, and the decision to submit for publication.
Results
We collected data describing patients undergoing in-patient surgery in 498 hospitals across 28 European nations. Median number of operating theatres in each hospital was 15 (IQR 10–22) and median number of critical care beds was 19 (9–40). Data were returned for 46 985 cases of which 446 were removed having been identified as duplicates or having missing critical care or mortality data, leaving 46 539 for analysis (figure 1). A median number of 83 (39–125) patients were included per hospital and 1045 (455–1732) per country. 281 (56%) hospitals were affiliated to a university, recruiting 31 132 patients (68% of total, appendix p 2).
Figure 1.
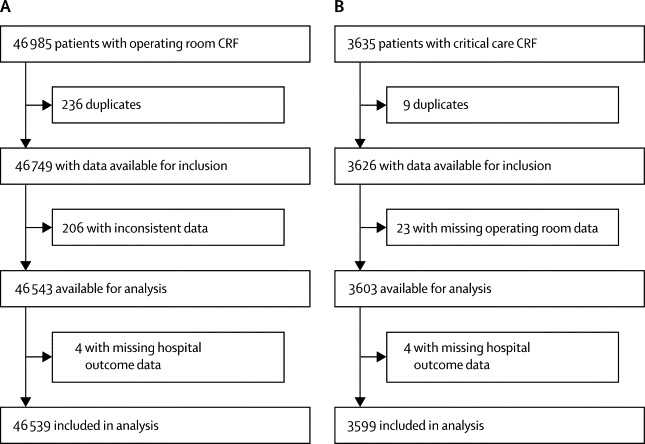
Study profile
(A) All patients. (B) Patients admitted to critical care. CRF=case report form.
Table 1 shows baseline data for all patients. Overall crude mortality was 4·0% and the median duration of hospital stay was 3·0 days (IQR 1·0–7·0). Prevalence of comorbid disease, grade of surgery, crude mortality rates, duration of hospital stay, and number of critical care admissions differed substantially between countries (table 2, appendix p 2). Table 2 shows unadjusted OR for hospital mortality by country. 3599 patients (8%) were admitted to critical care at some point during hospital stay, of whom 2555 (71%) had planned admissions (figure 2). Median stay in critical care was 1·2 days (0·9–3·6). 1358 patients who died were not admitted to critical care at any stage after surgery (73% of all deaths). 506 patients (14%) admitted to critical care died before hospital discharge, of whom 218 (43%) died after the first admission to critical care was complete.
Table 1.
Description of cohort
| All patients (n=46 539) | Died in hospital (n=1864) | Survived to hospital discharge (n=44 657) | Odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| Age (years) | 56·7 (18·5) | 61·0 (18·7) | 56·6 (18·5) | 1·01 (1·01–1·02) | <0·0001 | |
| Men | 22 607 | 968 | 21 629 | 1·15 (1·05–1·26) | 0·003 | |
| Present smoker | 9872 | 363 | 9503 | 0·90 (0·80–-1·01) | 0·07 | |
| ASA score | ||||||
| 1 | 11 642 | 362 | 11 280 | Reference | ·· | |
| 2 | 21 582 | 633 | 20 944 | 0·94 (0·83–1·07) | 0·36 | |
| 3 | 11 574 | 539 | 11 025 | 1·51 (1·32–1·73) | <0·0001 | |
| 4 | 1559 | 279 | 1277 | 6·75 (5·71–7·97) | <0·0001 | |
| 5 | 90 | 49 | 41 | 35·61 (23·23–54·59) | <0·0001 | |
| Grade of surgery | ||||||
| Minor | 12 041 | 431 | 11 608 | Reference | ·· | |
| Intermediate | 22 231 | 741 | 21 483 | 0·93 (0·82–1·05) | 0·22 | |
| Major | 12 170 | 685 | 11 476 | 1·59 (1·40–1·80) | <0·0001 | |
| Urgency of surgery | ||||||
| Elective | 35 049 | 1129 | 33 908 | Reference | ·· | |
| Urgent | 8923 | 483 | 8436 | 1·71 (1·52–1·91) | <0·0001 | |
| Emergency | 2557 | 249 | 2303 | 3·20 (2·77–3·70) | <0·0001 | |
| Surgical specialty | ||||||
| Orthopaedics | 12 214 | 468 | 11 744 | 1·02 (0·84–1·24) | 0·85 | |
| Breast | 1500 | 43 | 1456 | 0·76 (0·53–1·07) | 0·12 | |
| Gynaecology | 3972 | 115 | 3857 | 0·76 (0·59–0·99) | 0·04 | |
| Vascular | 2376 | 140 | 2233 | 1·61 (1·26–2·05) | 0·0001 | |
| Upper gastrointestinal | 2228 | 155 | 2071 | 1·88 (1·48–2·39) | 0·0001 | |
| Lower gastrointestinal | 4972 | 284 | 4683 | 1·54 (1·25–1·91) | 0·0001 | |
| Hepato-biliary | 2247 | 113 | 2134 | 1·35 (1·04–1·74) | 0·025 | |
| Plastic or cutaneous | 2432 | 73 | 2356 | 0·79 (0·59–1·06) | 0·12 | |
| Urology | 4881 | 144 | 4737 | 0·78 (0·61–0·99) | 0·042 | |
| Kidney | 463 | 9 | 454 | 0·51 (0·26–1·01) | 0·05 | |
| Head and neck | 5640 | 174 | 5466 | 0·82 (0·65–1·03) | 0·09 | |
| Other | 3463 | 132 | 3329 | Reference | ||
| Laparoscopic surgery | 5510 | 160 | 5350 | 0·69 (0·59–0·82) | <0·0001 | |
| Comorbid disorder | ||||||
| Cirrhosis | 498 | 65 | 433 | 3·64 (2·79–4·76) | <0·0001 | |
| Congestive heart failure | 2154 | 166 | 1985 | 2·10 (1·78–2·48) | <0·0001 | |
| COPD | 5162 | 244 | 4912 | 1·21 (1·05–2·48) | 0·008 | |
| Coronary artery disease | 6274 | 387 | 5881 | 1·73 (1·54–1·94) | <0·0001 | |
| Diabetes (taking insulin) | 2081 | 135 | 1939 | 1·73 (1·44–2·07) | <0·0001 | |
| Diabetes (not taking insulin) | 3495 | 147 | 3348 | 1·05 (0·88–1·24) | 0·61 | |
| Metastatic cancer | 2173 | 155 | 2017 | 1·91 (1·61–2·27) | <0·0001 | |
| Stroke | 2006 | 120 | 1884 | 1·57 (1·30–1·90) | <0·0001 | |
Data are mean (SD) or n unless otherwise specified. Odds ratios were constructed for in-hospital mortality with univariate binary logistic regression analysis. ASA=American Society of Anesthesiologists. COPD=chronic obstructive pulmonary disease.
Table 2.
Relation between country and in-hospital mortality
| Number of patients | Median days in hospital (IQR) | Number admitted to critical care | Percentage admitted to critical care (95% CI) | Number died in hospital | Percentage died in hospital (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Belgium | 1486 | 3·0 (1·0–6·0) | 136 | 9·2% (7·7–10·6) | 47 | 3·2% (2·3–4·1) | 0·89 (0·65–1·21) | 1·65 (0·81–3·40) | 0·17 |
| Croatia | 1767 | 4·0 (2·0–7·0) | 166 | 9·4% (8·0–10·8) | 131 | 7·4% (6·2–8·6) | 2·17 (1·77–2·67) | 1·89 (0·94–3·80) | 0·07 |
| Cyprus | 45 | 1·0 (1·0–3·0) | 0 | 0 | 1 | 2·2% (0·0–6·7) | 0·62 (0·09–4·48) | 0·82 (0·04–16·70) | 0·90 |
| Czech Republic | 434 | 4·0 (2·0–9·0) | 21 | 4·8% (2·8–6·9) | 10 | 2·3% (0·9–3·7) | 0·64 (0·34–1·21) | 1·30 (0·23–7·46) | 0·77 |
| Denmark | 1000 | 2·0 (1·0–5·0) | 36 | 3·6% (2·4–4·8) | 32 | 3·2% (2·1–4·3) | 0·90 (0·62–1·29) | 1·16 (0·52–2·61) | 0·72 |
| Estonia | 727 | 3·0 (1·0–6·0) | 51 | 7·0% (5·2–8·9) | 11 | 1·5% (0·6–2·4) | 0·42 (0·23–0·76) | 0·60 (0·16–2·28) | 0·45 |
| Finland | 1071 | 2·0 (1·0–5·0) | 43 | 4·0% (2·8–5·6) | 21 | 2·0% (1·1–2·8) | 0·54 (0·35–0·85) | 0·44 (0·19–1·05) | 0·06 |
| France | 2278 | 3·0 (1·0–6·0) | 132 | 5·8% (4·8–6·8) | 73 | 3·2% (2·5–3·9) | 0·90 (0·70–1·16) | 1·36 (0·72–2·56) | 0·34 |
| Germany | 5284 | 4·0 (2·0–9·0) | 611 | 11·6% (10·7–12·4) | 133 | 2·5% (2·1–2·9) | 0·70 (0·57–0·86) | 0·85 (0·50–1·43) | 0·54 |
| Greece | 1803 | 3·0 (2·0–7·0) | 63 | 3·5% (2·7–4·3) | 65 | 3·6% (2·7–4·5) | 1·01 (0·78–1·33) | 1·20 (0·66–2·16) | 0·55 |
| Hungary | 621 | 4·0 (2·0–7·0) | 44 | 7·1% (5·1–9·1) | 20 | 3·2% (1·8–4·6) | 0·90 (0·57–1·43) | 1·23 (0·43–3·50) | 0·69 |
| Iceland | 162 | 2·0 (1·0–4·0) | 15 | 9·3% (4·8–13·8) | 2 | 1·2% (0·0–3·0) | 0·34 (0·08–1·37) | 0·47 (0·07–3·41) | 0·46 |
| Ireland | 856 | 3·0 (1·0–6·0) | 66 | 7·7% (5·9–9·5) | 55 | 6·4% (4·8–8·1) | 1·86 (1·39–2·49) | 2·61 (1·30–5·27) | 0·007 |
| Italy | 2673 | 3·0 (2·0–7·0) | 200 | 7·5% (6·5–8·5) | 141 | 5·3% (4·4–6·1) | 1·51 (1·24–1·84) | 1·70 (0·97–2·97) | 0·06 |
| Latvia | 302 | 4·0 (2·0–8·0) | 19 | 6·3% (3·5–9·1) | 65 | 21·5% (16·9–26·2) | 7·44 (5·55–9·97) | 4·98 (1·22–20·29) | 0·025 |
| Lithuania | 375 | 3·0 (2·0–5·0) | 14 | 3·7% (1·8–5·7) | 10 | 2·7% (1·0–4·3) | 0·74 (0·39–1·40) | 1·21 (0·21–6·95) | 0·83 |
| Netherlands | 1627 | 3·0 (1·0–6·0) | 126 | 7·7% (6·4–9·0) | 32 | 2·0% (1·3–2·7) | 0·55 (0·38–0·78) | 0·63 (0·28–1·41) | 0·26 |
| Norway | 689 | 3·0 (1·0–6·0) | 31 | 4·5% (3·0–6·1) | 10 | 1·5% (0·6–2·4) | 0·40 (0·21–0·75) | 0·51 (0·17–1·49) | 0·22 |
| Poland | 397 | 5·0 (2·0–7·5) | 8 | 2·0% (0·6–3·4) | 71 | 17·9% (14·1–21·7) | 5·91 (4·48–7·79) | 6·92 (2·37–20·27) | 0·0004 |
| Portugal | 1489 | 3·0 (1·0–7·0) | 103 | 6·9% (5·6–8·2) | 61 | 4·1% (3·1–5·1) | 1·16 (0·88–1·53) | 1·43 (0·72–2·83) | 0·31 |
| Romania | 1298 | 5·0 (3·0–8·0) | 209 | 16·1% (14·1–18·1) | 88 | 6·8% (5·4–8·2) | 1·97 (1·55–2·51) | 3·19 (1·61–6·29) | 0·001 |
| Serbia | 85 | 5·0 (3·0–7·0) | 1 | 1·2% (0·0–3·5) | 2 | 2·4% (0·0–5·6) | 0·65 (0·16–2·67) | 1·06 (0·11–10·04) | 0·96 |
| Slovakia | 1156 | 3·0 (2·0–7·0) | 22 | 1·9% (1·1–2·7) | 129 | 11·2% (9·3–13·0) | 3·41 (2·76–4·20) | 2·15 (0·91–5·07) | 0·08 |
| Slovenia | 518 | 3·0 (1·0–7·0) | 13 | 2·5% (1·2–3·9) | 15 | 2·9% (1·5–4·3) | 0·81 (0·48–1·37) | 1·12 (0·30–4·22) | 0·86 |
| Spain | 5433 | 3·0 (1·0–7·0) | 677 | 12·5% (11·6–13·3) | 208 | 3·8% (3·3–4·3) | 1·08 (0·91–1·28) | 1·39 (0·89–2·18) | 0·15 |
| Sweden | 1314 | 2·0 (1·0–6·0) | 42 | 3·2% (2·2–4·2) | 24 | 1·8% (1·1–2·6) | 0·50 (0·33–0·77) | 0·58 (0·23–1·49) | 0·26 |
| Switzerland | 1019 | 4·0 (2·0–8·0) | 79 | 7·8% (6·1–9·4) | 20 | 2·0% (1·1–2·8) | 0·54 (0·35–0·86) | 0·86 (0·25–2·97) | 0·81 |
| UK | 10 630 | 2·0 (1·0–6·0) | 671 | 6·3% (5·9–6·8) | 378 | 3·6% (3·2–3·9) | 1·00 | ·· | ·· |
Odds ratios (OR) referenced against the UK and adjusted for age, American Society of Anesthesiologists' score, urgency of surgery, grade of surgery (minor, intermediate, major), surgical specialty, and the presence of either metastatic disease or cirrhosis in a two-level binary logistic regression model (with patient at the first level and hospital at the second).
Figure 2.
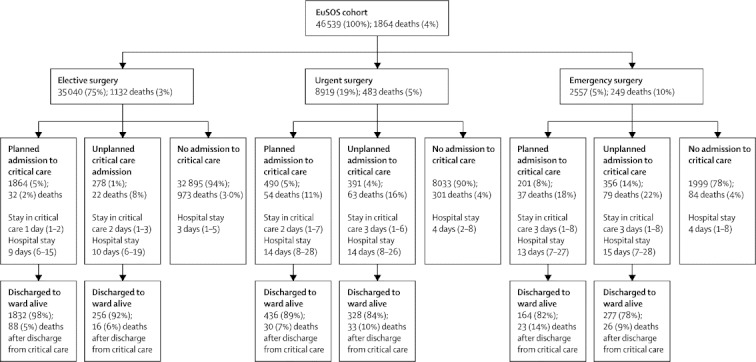
Planned and unplanned admission to a critical-care unit according to urgency of surgery
Data are n (%) or median (IQR). We collected data describing the first critical care admission for any individual patient. The data presented do not describe readmission to critical care. Because of incomplete data for admission planning, 19 admissions to critical care are not presented in this figure. EuSOS=European Surgical Outcomes Study. Elective=not immediately life saving; planned within months or weeks. Urgent=planned surgery within hours or days of the decision to operate. Emergency=as soon as possible; no delay to plan care; ideally within 24 h.
We explored variables associated with hospital mortality in a univariate analysis, the findings of which were much the same as for a sensitivity analysis of different subsets of the database (table 1, appendix pp 3–4). We then constructed several binary logistic regression models to adjust for baseline differences that might explain the unadjusted OR for individual countries (table 2). We developed both single-level and multilevel models (appendix pp 5–8) with variables that were significant in the univariate analysis. The point estimates for the OR did not differ greatly between the one-level and two-level models, but the hierarchical model consistently provided a more conservative estimate of country effects across the sensitivity tests (appendix p 9).
We constructed a further model including all significant interacting factors (appendix p 10). Since this increased model complexity did not substantially change the country-level estimates, we report results of the more parsimonious two-level model without interactions (figure 3). Factors that were independently associated with mortality and that we therefore used to adjust for baseline confounders were: country where surgery was done, urgency of surgery, grade of surgery, surgical procedure category, age, American Society of Anesthesiologists (ASA) score, metastatic disease, and cirrhosis (appendix pp 7–8). We entered ASA score rather than the Lee Revised Cardiac Index because, although the two were highly correlated, less data describing ASA score were missing.
Figure 3.
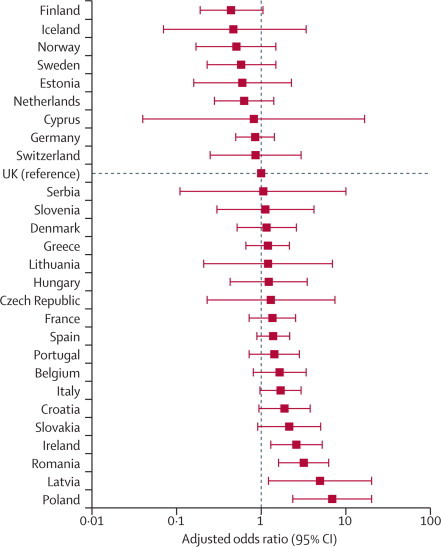
Adjusted odds ratio for death in hospital after surgery for each country
With the UK study population as the reference category, we identified higher unexplained rates of mortality in Poland, Romania, Latvia, and Ireland (table 2, figure 3).
Discussion
This international prospective study has provided data for a population of more than 46 000 unselected patients undergoing inpatient surgery from 28 European countries. 4% of included patients died before hospital discharge, which was a higher mortality rate than expected.2,3,6,13–16 We identified substantial differences in crude and risk adjusted mortality rates between countries. When compared with the UK, the recorded mortality rates for Poland, Latvia, Romania, and Ireland were higher even after adjustment for all identified confounding variables. This pattern could relate to cultural, demographic, socioeconomic, and political differences between nations, which might affect population health and health-care outcomes.
A major strength of our study was the large number of consecutive unselected patients enrolled in a multicentre and multinational setting. A vigorous approach to follow-up for missing and incomplete data provided a high-quality dataset for analysis. The dataset allowed us to explore probable prognostic factors and to adjust crude mortality rates to describe differences in outcomes between countries. Our analysis identified several factors associated with increased mortality. These findings suggest that surgery-related and patient-related factors interact to increase mortality risk. Only two comorbid disease categories were identified as independent variables. This finding probably arose because the ASA score was designed to describe the severity of coexisting medical disease.
Evidence suggests that critical-care-based cardiorespiratory interventions can improve outcomes among high-risk surgical patients.17–21 However, in our study, only 5% of patients underwent a planned admission to critical care with a median stay of about 1 day. Unplanned admissions to critical care were associated with higher mortality rates than were planned admissions. Remarkably, most patients who died (73%) were not admitted to critical care at any stage after surgery. Of patients who died after admission to critical care, 43% did so after the initial episode was complete and the patient had been discharged to a standard ward. These findings suggest a systematic failure in the process of allocation of critical care resources. This notion is consistent with previous reports of a failure to rescue deteriorating surgical patients with a detrimental effect on patient outcomes22 and the high incidence of myocardial injury in the days after surgery.23 For some patients with a poor prognosis, postoperative admission to critical care might have been deemed inappropriate—eg, after palliative surgery for disseminated malignancy. However, our data suggest these cases are few in number (<5% of patients had malignancy, table 1). Meanwhile other investigators have challenged the suggestion that patients should be offered surgery when the standard of postoperative care is unlikely to be adequate for their needs.2 The low rate of admission to critical care prevents any detailed comparison of this resource between nations. Further research is needed to better understand whether early admission to critical care can improve survival after major surgery.
Despite the large sample size, our study might not be truly representative of current practice across Europe because only a small proportion of European hospitals took part. Although in some countries the patient sample was large enough to show national practice, the high proportion of patients enrolled in university hospitals in other countries suggests a degree of selection bias. In particular, our data might not show the true surgical case-mix and standards of care in countries with a small number of participating hospitals. Although we planned to enrol every eligible patient undergoing surgery during the study period, we cannot be sure of the exact proportion of eligible patients included. Nonetheless, assuming the volume of surgery during the cohort week is typical of the participating hospitals, these centres undertake more than 2·3 million inpatient surgical procedures each year, which is 1% of the estimated volume of surgery taking place worldwide.1 Whether truly representative or not, our findings clearly describe a large cross-section of health care in Europe.
Some of our findings might be indicative of limitations of commonly used risk-adjustment variables with unexpected patterns of survival across categories for both ASA score and grade of surgery. This finding could result from the poor ability of clinicians to discriminate between the less severe categories of these variables. Random partitioning of the countries into three equal groups and repetition of the modelling exercise showed much the same results with regards to the OR of the relevant effect factors, showing some stability of the risk adjustment in subsets of countries. This stability was further confirmed in more complex models that included interactions between variables for which none of the interactions with the country factor contributed significantly to prediction. We identified other interactions that did significantly contribute to prediction but we did not record a substantial change in country effects when estimated from the extended model including these interactions. We therefore decided to use the simpler of the hierarchical models for the final analysis because our aim had been to construct a parsimonious model that practising clinicians would easily understand.
As far as we are aware, this was the first large, prospective, international assessment of surgical outcomes (panel). In some countries, data are available that describe survival after specific procedures such as vascular, joint replacement, or bowel cancer surgery.24–26 However, these audits are poorly representative of overall national surgical populations because high-risk patients are under-represented. The few previous estimates suggest an overall mortality for unselected inpatient surgery of between 1% and 2%,2,3,6,13–16 but these values are representative of only a few health-care systems. In a previous study13 of national registry data from the Netherlands, 30 day mortality was reported as 1·85%, which is much the same as the crude hospital mortality of 2% for this country in the EuSOS study. In the UK, a prospective investigation2 with a very similar methods to EuSOS identified a postoperative critical care admission rate of 6·7%, which is much the same as to the value of 6% for EuSOS in the UK.2 However, 30 day mortality was 1·6% compared with 3·6% for 60 day in-hospital mortality for UK patients in EuSOS. Reports from nations outside Europe describe 30 day mortality rates from 1·3% to 2·0%.6,14,15
Panel. Research in context.
Systematic review
We searched Medline for original research from the past 10 years describing mortality rates in large unselected national and international populations of patients undergoing non-cardiac surgery. We used the search terms “surgery”, “mortality”, and “complications” and widened our search to include retrospective analyses of health-care registries and prospective epidemiological studies. Publications were screened by title and then by abstract for relevance to the objectives of our study. Additionally, coinvestigators in various European nations searched for publicly available registry analyses reporting mortality rates for unselected populations of surgical patients. We identified seven large national studies2,3,6,13–16 describing mortality rates for the population of interest, three of which involved prospective data collection. No studies were identified that provided international comparative data. The last search was done on June 15, 2012.
Interpretation
As far as we are aware, this was the first large prospective international epidemiological study of unselected non-cardiac surgical patients and as such it provides a new perspective on mortality after surgery. A few national reports describe mortality rates from 1·3% to 2·0%.2,3,6,13–16 In our study, the overall crude mortality rate of 4% was higher than anticipated. We identified important variations in risk-adjusted mortality rates between nations, and critical care resources did not seem to be allocated to patients at greatest risk of death. Our findings raise important public health concerns about the provision of care for patients undergoing surgery in Europe.
Previous investigators have described the differences in provision of health services across Europe, in particular numbers of critical care beds.10,11 The reported seven-times greater provision of critical care beds for Germany than for the UK is likely to affect rates of admission to critical care and postoperative outcomes.10,11,27 This finding is in keeping with our present data that show a greater rate of admission to critical care after surgery in Germany than in the UK. Other studies have shown that fewer than a third of high-risk non-cardiac surgical patients are admitted to critical care after surgery in the UK despite high mortality rates,2–4 which is consistent with the results of our study; across Europe 73% of surgical patients who died were never admitted to critical care. This situation contrasts with perioperative care for cardiac surgical patients who by definition have severe comorbid disease and undergo major body cavity surgery followed by routine admission to critical care with mortality rates of less than 2%.28 Several reasons could explain why outcomes for cardiac and non-cardiac surgical patients differ but the quality of perioperative care is likely to be among the most important. The heath-care community increasingly recognises the importance of the entire perioperative care pathway including preoperative assessment, optimisation of coexisting medical disease, integrated care pathways relevant to the surgical procedure, WHO surgical checklists, advanced haemodynamic monitoring during surgery, early admission to critical care, acute pain management and critical-care outreach services, and hospital discharge planning together with the primary care physician.20,21 Routine audit and reporting of data for clinical outcomes has also proved a highly effective instrument for improvement of the quality of perioperative care.29
Our findings suggest both the need and potential to implement measures to improve postoperative outcomes. In addition to further research in this discipline, the root causes of this problem could be better understood through increased use of high-quality registries designed to capture robust data describing quality of care and clinical outcomes for surgical patients. This step would require increased funding for this specific area of health services research. The high mortality rate after surgery might be modified by changes in the organisation of care.20
Acknowledgments
Acknowledgments
This study was funded by the European Society of Intensive Care Medicine and the European Society of Anaesthesiology. RP is a National Institute for Health Research (UK) Clinician Scientist.
Contributors
All authors were involved in the design and conduct of the study. Data collection and collation was done by the members of the EuSOS study group. AR, RM, and PB did the data analysis with input from RP. The report was drafted by RP and AR and revised following critical review by all authors.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Weiser TG, Regenbogen SE, Thompson KD. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Findlay G, Goodwin A, Protopappa K, Smith N, Mason M. Knowing the risk: a review of the peri-operative care of surgical patients. National Confidential Enquiry into Patient Outcome and Death; London: 2011. [Google Scholar]
- 3.Pearse RM, Harrison DA, James P. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63:695–700. doi: 10.1111/j.1365-2044.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- 5.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 7.Head J, Ferrie JE, Alexanderson K, Westerlund H, Vahtera J, Kivimaki M. Diagnosis-specific sickness absence as a predictor of mortality: the Whitehall II prospective cohort study. BMJ. 2008;337:a1469. doi: 10.1136/bmj.a1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30:1615–1619. doi: 10.1200/JCO.2011.40.3568. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 10.Wunsch H, Angus DC, Harrison DA. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36:2787–2793. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012 doi: 10.1007/s00134-012-2627-8. published online July 10. [DOI] [PubMed] [Google Scholar]
- 12.Metnitz PG, Moreno RP, Almeida E. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31:1336–1344. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112:1105–1115. doi: 10.1097/ALN.0b013e3181d5f95c. [DOI] [PubMed] [Google Scholar]
- 14.Yu PC, Calderaro D, Gualandro DM. Non-cardiac surgery in developing countries: epidemiological aspects and economical opportunities—the case of Brazil. PLoS One. 2010;5:e10607. doi: 10.1371/journal.pone.0010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glance LG, Lustik SJ, Hannan EL. The surgical mortality probability model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255:696–702. doi: 10.1097/SLA.0b013e31824b45af. [DOI] [PubMed] [Google Scholar]
- 16.Canet J, Gallart L, Gomar C. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 18.Squadrone V, Coha M, Cerutti E. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 19.Pearse RM, Belsey JD, Cole JN, Bennett ED. Effect of dopexamine infusion on mortality following major surgery: individual patient data meta-regression analysis of published clinical trials. Crit Care Med. 2008;36:1323–1329. doi: 10.1097/CCM.0b013e31816a091b. [DOI] [PubMed] [Google Scholar]
- 20.Pearse RM, Holt PJ, Grocott MP. Managing perioperative risk in patients undergoing elective non-cardiac surgery. BMJ. 2011;343:d5759. doi: 10.1136/bmj.d5759. [DOI] [PubMed] [Google Scholar]
- 21.Pearse RM, Ackland GL. Perioperative fluid therapy. BMJ. 2012;344:e2865. doi: 10.1136/bmj.e2865. [DOI] [PubMed] [Google Scholar]
- 22.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 23.Devereaux PJ, Chan MT, Alonso-Coello P. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 24.Clement ND, Jenkins PJ, Brenkel IJ, Walmsley P. Predictors of mortality after total knee replacement: a ten-year survivorship analysis. J Bone Joint Surg Br. 2012;94:200–204. doi: 10.1302/0301-620X.94B2.28114. [DOI] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Bentrem DJ, Feinglass JM. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26:4626–4633. doi: 10.1200/JCO.2007.15.6356. [DOI] [PubMed] [Google Scholar]
- 26.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Care quality commission. Heart surgery in the United Kingdom. 2012. http://heartsurgery.cqc.org.uk/Survival.aspx (accessed June 6, 2012).
- 29.Rowell KS, Turrentine FE, Hutter MM, Khuri SF, Henderson WG. Use of national surgical quality improvement program data as a catalyst for quality improvement. J Am Coll Surg. 2007;204:1293–1300. doi: 10.1016/j.jamcollsurg.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


