Abstract
The thalamus has been found to be activated during the early phase of moderate hypoglycemia. Here, we tested the hypothesis that this region is less activated during hypoglycemia in subjects with type 1 diabetes (T1DM) and hypoglycemia unawareness relative to controls. Twelve controls (5 F/7 M, age 40±14 years, body mass index 24.2±2.7 kg/m2) and eleven patients (7 F/4 M, age 39±13 years, body mass index 26.5±4.4 kg/m2) with well-controlled T1DM (A1c 6.8±0.4%) underwent a two-step hyperinsulinemic (2.0 mU/kg per minute) clamp. Cerebral blood flow (CBF) weighted images were acquired using arterial spin labeling to monitor cerebral activation in the midbrain regions. Blood glucose was first held at 95 mg/dL and then allowed to decrease to 50 mg/dL. The CBF image acquisition during euglycemia and hypoglycemia began within a few minutes of when the target blood glucose values were reached. Hypoglycemia unaware T1DM subjects displayed blunting of the physiologic CBF increase that occurs in the thalamus of healthy individuals during the early phase of moderate hypoglycemia. A positive correlation was observed between thalamic response and epinephrine response to hypoglycemia, suggesting that this region may be involved in the coordination of the counter regulatory response to hypoglycemia.
Keywords: diabetes, glucose, hypoglycemia, imaging
Introduction
Hypoglycemia induces an ordered and complex neurohormonal response that ensures the recovery of blood glucose to the normal physiologic range. This response involves the detection of the decrease in blood glucose, which in turn elicits the activation of the sympathetic nervous system and secretion of hormones like epinephrine and glucagon that increase plasma glucose by enhancing endogenous glucose production and reducing insulin-mediated glucose uptake.
The identification of the brain regions involved in glucose sensing and the coordination of the counter regulatory (CR) response in humans has relied on the use of imaging techniques like magnetic resonance imaging (MRI) or positron emission tomography (PET) to detect changes in neuronal activity based on the indirect outcomes of the effects of neuronal activation on hemodynamic and metabolic parameters. Teves et al1 found that the thalamus and medial prefrontal cortex, along with the right globus pallidus, the right orbital frontal cortex, the right sensory motor cortex, and the periaqueductal gray region were activated during hypoglycemia in healthy human participants. Others have found the hypothalamus to be activated in response to modest hypoglycemia using functional MRI.2, 3 More recently, the dynamics of cerebral activation during the induction of and recovery from hypoglycemia was examined by Teh et al4 who found that at different times during the experimental period, activation could be observed in various brain areas, including the thalamic pulvinar, anterior cingulate cortex, the bilateral anterior insula, ventral striatum and pituitary, and the posterior parahippocampal gyrus in healthy human subjects.
The CR hormone response is altered in patients with type 1 diabetes (T1DM) and recurrent hypoglycemia. In such individuals, hypoglycemia-induced glucagon secretion disappears and each episode of hypoglycemia reduces the glucose level necessary to trigger the release of the other CR hormones during subsequent episodes.5 As a result, the patients develop hypoglycemia unawareness, the clinical situation where adrenergic symptoms do not develop before neuroglycopenia. The lack of symptoms (hypoglycemia unawareness), reduced glycemic thresholds for CR hormonal secretion, and lower hormonal responses characterize the so-called hypoglycemia-associated autonomic failure (HAAF). The neural substrates that contribute to the development of hypoglycemia unawareness and HAAF remain uncertain. While some studies show less activation during hypoglycemia in several brain areas such as the amygdala and orbitofrontal cortex in patients with T1DM and hypoglycemia unawareness,6 others have found thalamic activation to be greater during hypoglycemia in human models of HAAF.7 In both studies, hypoglycemia-induced epinephrine secretion was noted to be reduced in the HAAF group, but direct correlation with regional activation was not reported.
Overall, the evidence reported so far in the literature suggests that, among the different areas that are involved in the response to hypoglycemia, mainly midbrain regions such as the hypothalamus, thalamus, and amygdala seem to have altered responses in models of HAAF7 or in patients with T1DM.2, 6 The aim of our study was to evaluate regional midbrain activation in response to moderate hypoglycemia in subjects with T1DM and hypoglycemia unawareness and compare these responses with those of healthy controls. Cerebral activations were detected based on changes in regional cerebral blood flow (CBF) as measured with noninvasive arterial spin labeling MRI techniques. Such MRI methods are widely used for neuroimaging studies, and provide CBF measurements that are consistent with those obtained with positron emission tomography.8 Since the thalamus has been shown to be activated in healthy subjects during paradigms of experimental hypoglycemia similar to the ones used in the present study,1, 4 we hypothesize that hypoglycemia unaware subjects with T1DM will have less activation in this region relative to healthy controls as a manifestation of their impaired response to experimental hypoglycemia. To gain insights into the role activated regions may play in supporting the CR response, we also correlated the magnitude of activation with the CR hormonal response measured during hypoglycemia.
Materials and methods
Subjects
Twenty-three subjects between 18 and 65 years of age completed the study after giving informed consent using procedures approved by the Institutional Review Board: Human Subjects Committee of the University of Minnesota. Eleven subjects had T1DM with hypoglycemia unawareness verified by Cox questionnaire.9 The remaining 12 subjects were healthy controls. The average age and body mass index of the healthy controls were similar to the group of subjects with diabetes (subject characteristics are summarized in Table 1). Four additional subjects participated in the study, but did not provide complete data due to excessive head movements or MRI artifacts. Exclusion criteria for both groups of subjects included history of stroke, seizures, neurosurgical procedures, arrhythmias, and use of drugs that can alter glucose metabolism (other than insulin for the patients with diabetes). Subjects also met requirements for a study in the magnet, which includes weight <300 lbs and the absence of metallic substances in their body. Subjects with diabetes had good glycemic control, as evidenced by a hemoglobin A1c of <8.0% in the last 3 months. One subject in the control group and one subject in the diabetic group were being treated for hypertension. They were both taking a low-dose â-blocker. In addition, two subjects in the diabetic group were on medication to protect their kidneys, but were not diagnosed with hypertension (one subject on Lisinopril and one on Losartan).
Table 1. Subject characteristics.
| Healthy control | T1DM | |
|---|---|---|
| Female/male | 5/7 | 7/4 |
| Age (years) | 40±14 | 39±13 |
| BMI (kg/m2) | 24.2±2.7 | 26.5±4.4 |
| HbA1c (%) | 6.8±0.4 | |
| Duration of diabetes (years) | 26±13 |
BMI, body mass index; T1DM, type 1 diabetes.
Data presented as number or as mean±s.d.
Magnetic Resonance Imaging Parameters
The MRI measurements were performed using a 3.0 Tesla Siemens Trio scanner (Siemens, Erlangen, Germany). Radiofrequency was transmitted with the scanner body coil, while signal was received with a 12-channel receive head coil. Subjects were asked to lie as still as possible in the magnet during the study, to maintain their eyes closed, and to avoid any intense thinking during the acquisition. After initial acquisition of scout images, high-resolution anatomical images were acquired with a T1-weighted MPRAGE sequence, with the following parameters: 256 × 256 mm2 field of view, 160 slices, 1 mm isotropic resolution, repetition time=2,150 ms, echo time=2.47 ms, inversion time=1,000 ms, for a total scan time of 5 minutes.
For CBF measurements, we used a technique of arterial spin labeling called Q2TIPS as described in Luh et al.10 A volume of 15 transverse slices 3.5 mm thick, with 1 mm interslice gap, was positioned around the midbrain regions, covering an area of ∼7 cm in the sagittal direction. Other relevant experimental parameters were matrix size=64 × 64, field of view=192 × 192 mm2 (except two cases where the field of view was increased to 220 × 220 mm2), repetition time=3,000 ms, echo time=12 ms, inversion time 1=700 ms, inversion time 2=1,800 ms, labeling width=10 cm, labeling gap=1.5 cm. The number of repetitions was 201, for an acquisition time of ∼10 minutes. The first acquired volume represented the M0 volume and was measured without preparation scans. The even/odd volumes were the ‘label' and ‘control' volumes, respectively. This choice of parameters guaranteed sufficient signal to noise in the region of interest, while minimizing the influence on the MRI contrast induced from the signal receive profile of the head coil, as well as from the arterial transit time through the imaged slices. For convenience, we will refer to these perfusion-weighted arterial spin labeling images simply as CBF images.
General Protocol
Subjects arrived at the Center for Magnetic Resonance Research in the morning after an overnight fast. Subjects with T1DM were instructed to manage their diabetes during the day before the study in such a way as to minimize the amount of subcutaneous insulin present during the experiment while maintaining adequate control. On arrival, an intravenous catheter was placed antegrade in a forearm for subsequent infusions and a catheter was placed retrograde in the lower leg for blood sampling. The leg used for blood sampling was wrapped in heated towels and hot packs to arterialize the venous blood to minimize the impact of transcapillary transport on glucose measurements.11 As a result, blood glucose levels could be clamped at the level of glycemia seen in the precapillary circulation.
Two baseline samples for glucose and CR hormones including glucagon, epinephrine, norepinephrine, cortisol, and growth hormone were drawn 30 and 35 minutes after the placement of the last intravenous catheter. After obtaining baseline samples, intravenous infusions of insulin (2.0 mU/kg per minute), potassium phosphate (4 mEq/h), and glucose (20% dextrose) were begun. Samples for blood glucose were collected every 5 minutes for measurement of glucose on a nearby Analox machine (Analox Instruments, Lunenburg, MA, USA) and used to alter the glucose infusion rate to maintain blood glucose at 95 mg/dL. Subjects were then placed into the 3-Tesla magnet. Blood glucose was maintained at 95 mg/dL while the MRI data were collected according to the protocol detailed above to obtain high-resolution anatomical images along with one set of CBF images.
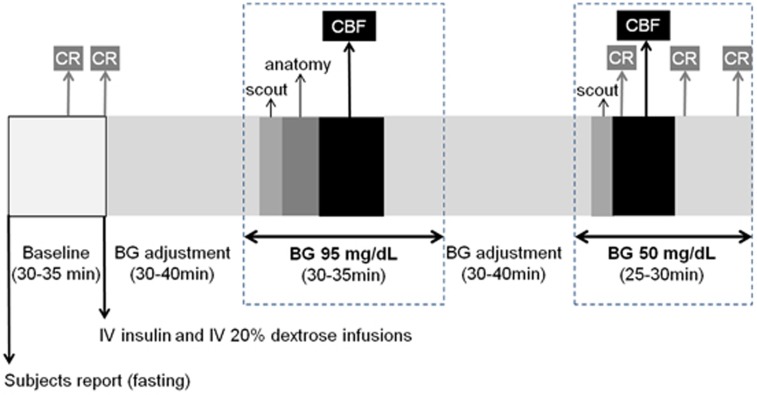
After collection of data in the euglycemic condition, the patient table was pulled out from the scanner without losing the reference position. The glucose infusion was discontinued and then restarted at a lower rate to allow blood glucose to decrease and stabilize at 50 mg/dL. As soon as blood glucose reached the target level of 50 mg/dL, the patient table was repositioned inside the scanner, the localization was quickly checked with new scouting images, and one set of CBF images was again collected. Blood glucose was maintained at 50 mg/dL for the duration of the hypoglycemia study and for an extra 10 to 15 minutes. During the hypoglycemic period, blood samples were collected every 10 minutes (for a total of 3) for subsequent measurements of glucagon, epinephrine, norepinephrine, cortisol, and growth hormone. The overall experimental protocol is summarized in Figure 1.
Figure 1.
Diagram of the experimental protocol, highlighting the number and timing of the measurements of counter regulatory (CR) hormones along with the MRI measurements (details are in the text). Blood samples for CR measurements were drawn 5 minutes apart at baseline and 10 minutes apart during hypoglycemia. Other blood samples were drawn every 5 minutes during the entire duration of the study to monitor glucose levels (not indicated in the diagram). Measurements of cerebral blood flow (CBF) were performed within a few minutes of blood glucose reaching the target level. Scout, anatomy, and CBF refer to the different MRI acquisitions performed during the study. BG, blood glucose; IV, intravenous.
Postprocessing and Statistical Analysis
The CBF images were calculated on-line, and were subsequently coregistered off-line on the subject's anatomical reference using the software Brain Voyager QX (Brain Innovation, Maastricht, The Netherlands). The anatomical reference of each subject was then normalized on Tailarach space, and the CBF images were resampled on the same template.
Parametric maps representing the difference in the signal intensity (SI) of the CBF images for hypoglycemia minus euglycemia, relative to euglycemia (here named ΔSI (%)), were calculated for each subject and were then averaged within groups. Maps of t-scores were additionally generated with the aim of identifying brain areas where (1) the within-person CBF response to hypoglycemia (i.e., ΔSI (%)) differed from zero, separately in the group of controls and in the group of hypoglycemia unaware subjects with T1DM (two-sided one-sample t-test on ΔSI (%) for each group separately, Figure 2); (2) the within-person CBF response to hypoglycemia differed significantly between controls and hypoglycemia unaware subjects with T1DM (two-sided unpaired t-test on the control minus patient group difference in ΔSI (%), Figure 3). For both within-group and between-group comparisons, thresholding of the maps retained only those voxels exceeding the uncorrected t-quantile (with degrees of freedom appropriate for the comparison), and then among those remaining retained only voxels within a cluster of size at least 20. Finally, average values of CR hormones were calculated separately from the two measurements at baseline and the three measurements during hypoglycemia for each subject. The average values of CR hormones during hypoglycemia were compared between groups using unpaired two-sided t-tests (Table 1), and were also correlated with the average ΔSI (%) calculated in the thalamus of each subject as outlined in the common normalized Tailarach space (Figure 4). Notably, identifying regions of interest on the common normalized brain of subjects is less prone to operator-dependent biases than hand-drawing regions of interest on a single subject basis. The statistical analyses were performed using custom-written codes in Matlab Version 7.1 (The MathWorks Inc., Natick, MA, USA).
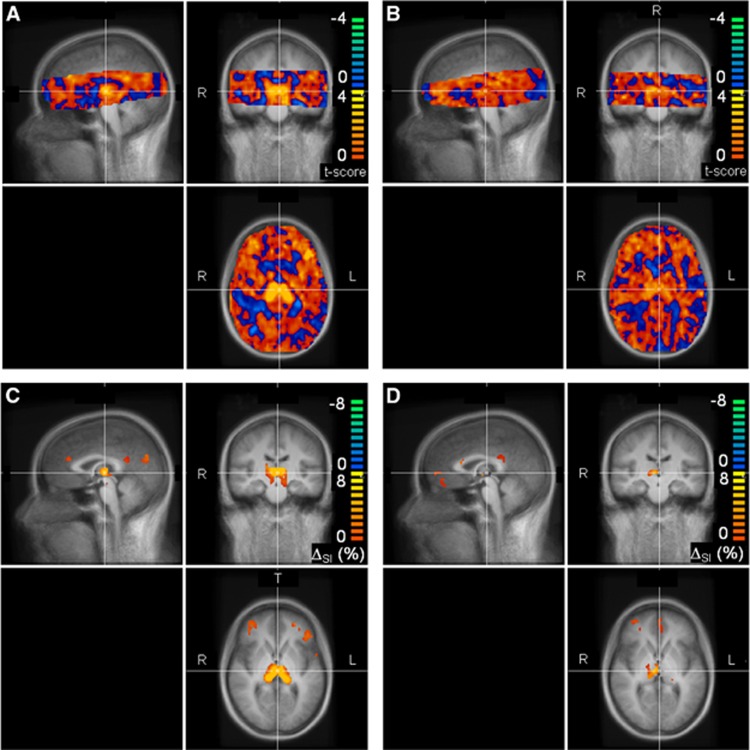
Figure 2.
Cerebral blood flow (CBF) responses to hypoglycemia (ΔSI (%)) in 12 healthy controls (A, C) and 11 hypoglycemia unaware subjects with type 1 diabetes (B, D). T-scores evaluating where ΔSI (%) differed from zero are shown in (A) and (B) for controls and diabetes patients, respectively. Only voxels common to all subjects and belonging to the brain are shown; red to yellow colors (blue to green colors) indicate larger (smaller) CBF in hypoglycemia versus euglycemia. Values of ΔSI (%) are displayed in (C) and (D) for controls and diabetes patients, respectively, only in those brain areas where differences occurred at a significance level of P<0.02, uncorrected for multiple comparisons. Maps in (A) to (D) are superimposed on the mean T1-weighted images of the Tailarach-normalized brain calculated from the 23 subjects. Sagittal, coronal, and transverse views are shown in the top left, top right, and bottom right section of each panel, respectively. SI, signal intensity.
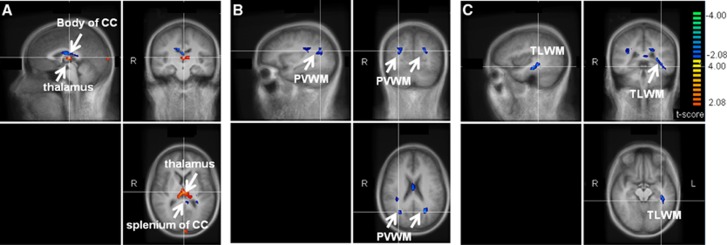
Figure 3.
Comparisons of ΔSI (%) induced by hypoglycemia in 12 healthy controls versus 11 hypoglycemia unaware subjects with type 1 diabetes, displayed on different brain sections (A to C). Images show the t-score maps calculated in the overlapping brain volume between controls and patients, as detailed in Figure 2. Parametric maps are superimposed on the mean T1-weighted images of the Tailarach-normalized brain calculated from the 23 subjects. Colors identify brain regions where the hypoglycemia-induced ΔSI (%) were different between controls and hypoglycemia unaware subjects with type 1 diabetes at a significance level of P<0.05, uncorrected for multiple comparisons. Red to yellow colors (blue to green colors) indicate larger (smaller) ΔSI (%) responses of controls as compared with patients. Sagittal, coronal, and transverse views are shown in the top left, top right, and bottom right section of each panel, respectively. R and L indicate right and left, respectively. While larger ΔSI (%) were observed in the thalamus of controls versus patients, smaller ΔSI (%) were observed in the splenium and body of the corpus callosum (CC), in the periventricular white matter (PVWM), and in temporal lobe white matter (TLWM). SI, signal intensity.
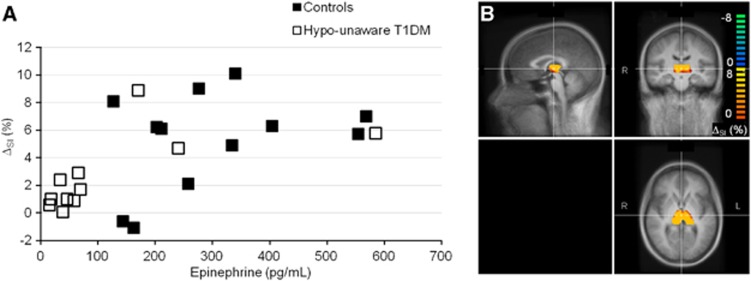
Figure 4.
(A) Correlation between the average ΔSI (%) induced by hypoglycemia in the thalamus and the average epinephrine response during hypoglycemia. The average ΔSI (%) was calculated in each subject from the thalamic region as shown in (B). Data in (B) are from one representative healthy control, superimposed on the mean T1-weighted images of the Tailarach-normalized brain calculated from the 23 subjects. Sagittal, coronal, and transverse views are shown in the top left, top right, and bottom right section of (B). The thalamic region was outlined once on the Tailarach space, and then applied on the normalized brain of each subject. The correlation was r=0.572, with a significance level of P=0.0043. SI, signal intensity; T1DM, type 1 diabetes.
Laboratory Analyses
Blood samples obtained during the hypoglycemia protocol were sent to Vanderbilt DRTC core laboratory for analysis. Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography (Dionex, formerly ESA Inc., Sunnyvale, CA, USA). Plasma growth hormone and cortisol were measured by radioimmunoassay (Diagnostic Products Corporation, Inc.). Plasma glucagon was measured by radioimmunoassay (modified Millipore, Billerica, MA, USA).
Results
The hypoglycemia unaware group and the control group had similar gender, age, and body mass index distributions (Table 1). The two groups attained the same glycemia targets during the euglycemic and hypoglycemic conditions (Table 2).
Table 2. Plasma glucose and counter regulatory hormones during euglycemia and hypoglycemia; hormone and glucose levels were averaged within person during each glycemic condition.
|
Healthy controls |
Hypoglycemia unaware subjects with type 1 diabetes |
Group comparison during hypoglycemia |
|||
|---|---|---|---|---|---|
| Euglycemia | Hypoglycemia | Euglycemia | Hypoglycemia | P* | |
| Glucose (mg/dL) | 92±6 | 49±3 | 91±7 | 51±3 | 0.20 |
| Epinephrine (pg/mL) | 19±6 | 278±134 | 23±8 | 121±168 | 0.01 |
| Norepinephrine (pg/mL) | 231±162 | 257±86 | 237±111 | 251±113 | 0.89 |
| Glucagon (pg/mL) | 55±16 | 90±20 | 57±30 | 50±33 | 0.002 |
| Cortisol (μg/dL) | 14±4 | 24±5 | 21±13 | 22±12 | 0.58 |
| Growth hormone (ng/mL) | 5±3 | 8±4 | 7±6 | 12±12 | 0.20 |
Data presented as within-group mean±s.d. P* values by two-sided unpaired t-test of mean values during hypoglycemia for healthy controls versus hypoglycemia unaware subjects with type 1 diabetes.
Cerebral blood flow was altered in several brain regions in response to hypoglycemia in both groups of subjects (Figure 2). Statistically significant hypoglycemia-induced CBF increases occurred in the thalamus of healthy controls (Figure 2C), but were generally smaller and less extensive in the hypoglycemia unaware subjects with T1DM (Figure 2D). Cerebral blood flow was also observed to increase in the medial prefrontal and orbitofrontal cortices (partially visible in our experimental set-up) of healthy subjects (Figure 2C), as reported by others.1, 4
The hypoglycemia-induced CBF changes (i.e., ΔSI (%)) in the thalamus of healthy controls were significantly greater as compared with the changes in the hypoglycemia unaware subjects with T1DM (P<0.02, Figure 3A). In addition, controls were noted to have a significant smaller change in CBF in the periventricular and left temporal lobe white matter, as well as in the splenium and body of the corpus callosum relative to subjects with diabetes (P<0.02, Figures 3A to 3C); however, the changes in such areas had lower statistical significance than in the thalamus, and most importantly lost statistical significance when using a cluster size of >80 rather than >20 (data not shown). Notably, cluster sizes of >90 pixels have been consistently used in previous studies similar to ours.1, 4, 6, 7, 12
Average epinephrine and glucagon levels were significantly higher during hypoglycemia in healthy controls as compared with subjects with T1DM and hypoglycemia unawareness (P=0.01 and 0.002, respectively), while other CR hormones were not (Table 2). A significant correlation was observed between hypoglycemia-induced thalamic activation and the within-person average epinephrine response (r=0.572, P=0.0043, Figure 4) during hypoglycemia. A trend toward a positive correlation was noted between the thalamic and growth hormone response during hypoglycemia (r=0.393, P=0.0636). No significant relationships were observed between hypoglycemia-induced thalamic activation and norepinephrine, glucagon, and cortisol responses (r=0.361, P=0.0905; r=0.324, P=0.1311; r=0.357, P=0.0941, respectively).
Discussion
In this experiment, we found that hypoglycemia-induced thalamic activation was blunted in subjects with T1DM and hypoglycemia unawareness as compared with healthy control subjects. We also observed a reduced activation in left temporal lobe white matter, splenium and body of the corpus callosum of controls as compared with patients. While the involvement of these white matter areas is potentially interesting, the significance of the difference between the groups disappeared in such regions when a large cluster equal to that of other investigators was used for analysis,1, 4, 6, 7, 12 in contrast to what observed in the thalamus. This observation raises questions about the robustness of the findings in the white-matter regions. Alternatively, they could reflect the microstructural changes known to be present in the white matter in T1DM.13 Finally, we found that the magnitude of thalamic activation significantly correlated with hypoglycemia-induced epinephrine secretion. These observations support the hypothesis that thalamic activation has a critical role in the detection of hypoglycemia and the coordination of the sympathetic CR response necessary to restore normoglycemia. The thalamus is one of the most heterogeneous structures of the brain, serving a variety of functions.14, 15 Notably, the thalamus has been recognized to be involved in modulating the states of consciousness, alertness, and awareness. These considerations are suggestive (yet not conclusive) that the blunted thalamic responses observed during hypoglycemia in patients with T1DM and hypoglycemia unawareness are ascribed to hypoglycemia unawareness itself rather than diabetes.
Identification of the brain regions involved in the CR response to hypoglycemia has been the focus of much research. Using PET, Teves et al1 were the first to find that hypoglycemia activated the thalamus along with the medial prefrontal cortex, right globus pallidus, the right orbital frontal cortex, the right sensory motor cortex, and the periaqueductal gray region in healthy human participants. Others have found the hypothalamus,3 the anterior cingulate cortex, the bilateral anterior insula, ventral striatum and pituitary, and the posterior parahippocampal gyrus4 all to show activation at some point during the creation of and recovery from experimental hypoglycemia in healthy human subjects.
Imaging methods have also been used to understand if changes in hypoglycemia-induced cerebral activation might explain why some patients with T1DM develop hypoglycemia unawareness and HAAF, but our results are the first to show the importance of blunted hypoglycemia-induced thalamic activation in such patients. In other work, reduced hypoglycemia-induced activations were identified in the amygdala and orbitofrontal cortex of hypoglycemia unaware subjects relative to hypoglycemia aware subjects.6 However, changes in thalamic activation were not observed, in contrast to the findings of the present study. One possible reason for this discrepancy might originate from the different methodologies used to detect brain activation, which monitored either glucose consumption rates in the study by Dunn et al6 or CBF in our study. Since diabetes can be associated with microvascular complications, altered neurovascular couplings cannot be excluded, thus implying that metabolic and hemodynamic measures of neuronal activation might not be equivalent. However, it is worth noting that impaired neurovascular couplings have not been proven so far in diabetes. Another critical factor to consider in understanding why there is discrepancy in the identification of brain regions activated in response of hypoglycemia is the timing of data acquisition. As Teh et al4 shown, different regions are activated at different time points and at different levels of glycemia during the development of and recovery from hypoglycemia. In the present study, data collection was initiated a few minutes after subjects reached a blood glucose of 50 mg/dL and then continued for 10 minutes, a design that is similar to that reported by Teves et al1 whose results are most like our own. Others have noted hypothalamic activation during the onset of hypoglycemia at glucose values >60 mg/dL both in controls3 and in subjects with diabetes,2 a glucose level not included in the current protocol. Dunn et al6 found reduced activation in the amygdala and orbitofrontal cortex in subjects with T1DM and hypoglycemia unawareness relative to hypoglycemia aware subjects, but their observations reflect data collected and then summed over a 60-minute period, a time period that exceeds our own. Since cerebral activation to any stimulus is likely to be a dynamic process dependent on the magnitude and duration of the stimulus, it will be important for future investigations to identify when data are collected in the course of exposure.
More recently, Arbelaez et al7 found that hypoglycemia-induced activity in the thalamus was greater in healthy controls after HAAF induction than before. The CR hormone response to hypoglycemia was also blunted after induction of HAAF, and they proposed that recurrent hypoglycemia leading to HAAF might enhance the inhibitory output from the thalamus during subsequent hypoglycemia which in turn might be responsible for the loss of the CR hormone response. These results are very different from those presented herein. One obvious difference is that we studied subjects with T1DM and hypoglycemia unawareness and compared them with healthy volunteers, whereas Arbelaez et al7 studied controls before and after the induction of HAAF. While there appears to be general acceptance that subjecting healthy humans and animals to recurrent hypoglycemia creates HAAF like that experienced in patients with T1DM who are continually exposed to recurrent hypoglycemia,16, 17 it may be that the induction protocol used by Arbelaez et al7 did not successfully induce all the changes noted in patients with HAAF. Another obvious difference is that we studied patients with long-standing diabetes, while Arbelaez et al7 studied normal volunteers. Without a control group of hypoglycemia aware subjects with T1DM we cannot determine if the differences noted between our subject groups were due to diabetes or hypoglycemia unawareness, but it is interesting to note that those patients with T1DM who showed activation of the thalamus during hypoglycemia had an epinephrine response more like controls than like the patients with a blunted thalamic response. It is possible that these patients did not have hypoglycemia unawareness despite having been categorized as such by our screening questionnaire, which suggests that the differences seen in thalamic activation during hypoglycemia are due to difference in HAAF and not in diabetes. Still, it could be argued that the differences between patients and controls observed in this study could be ascribed to microvascular complications inherent to the disease, rather than to different responses of brain activity to hypoglycemia. However this possibility is highly unlikely, because we did not observe any significant differences in the CBF images acquired in baseline euglycemic conditions between hypoglycemia unaware subjects with T1DM and healthy controls, not in the thalamus, nor in any other brain region covered by our experimental protocol. Also differences of heart rates in response to hypoglycemia between healthy controls and patients with T1DM and hypoglycemia unawareness cannot explain our findings, as there is no available evidence supporting the notion that the CBF in the thalamus is correlated with the heart rate or blood pressure.
In our experiments, we also found the existence of a statistically significant relationship between hypoglycemia-induced thalamic activation and the epinephrine response to the decrease in blood glucose. To better define this relationship and to ultimately parse out the exact role of thalamic activation in HAAF, studies of CR in human subjects in whom the thalamic region has been manipulated (i.e., by previously implanted devices) or in animal models where it is ablated will be necessary.
In summary, we observed that hypoglycemia-induced thalamic activation and epinephrine responses were reduced in subjects with T1DM and hypoglycemia unawareness relative to healthy controls. Our observations suggest that alterations in this region contribute to the pathogenesis of HAAF in patients with diabetes.
Acknowledgments
The authors are grateful for the participation of their research volunteers and for the support of the nursing personnel to conduct the studies.
The authors declare no conflict of interest.
Footnotes
This work was supported by NIH grants R01 NS035192 and DK62440 (both to ERS), and 2 T32 DK7203 (NT and AM), in addition to a grant from the American Diabetes Association (ERS and LEE). This project was also supported by the NIH National Center for Research Resources (P41 RR008079, 5M01 RR0400, 1UL1 RR033183 and KL2 RR033182, last one to SM) and the National Institute of Biomedical Imaging and Bioengineering (P41 EB015894). Additional CMRR funding is from Minnesota Medical Foundation and NIH P30 NS057091.
References
- Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA. 2004;101:6217–6221. doi: 10.1073/pnas.0307048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen G, Simonson DC, Bolo NR, Driscoll A, Weinger K, Raji A, Theberge J, Renshaw PF, Jacobson AM. Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2008;93:1450–1457. doi: 10.1210/jc.2007-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58:448–452. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh MM, Dunn JT, Choudhary P, Samarasinghe Y, Macdonald I, O'Doherty M, Marsden P, Reed LJ, Amiel SA. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage. 2010;53:584–592. doi: 10.1016/j.neuroimage.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592–3601. doi: 10.2337/diabetes.54.12.3592. [DOI] [PubMed] [Google Scholar]
- Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness. Diabetes. 2007;56:2766–2773. doi: 10.2337/db07-0666. [DOI] [PubMed] [Google Scholar]
- Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–475. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Wieckowska M, Meyer E, Pike GB. Cerebral blood flow measurement using fMRI and PET: a cross-validation study. Int J Biomed Imaging. 2008. p. 516359. [DOI] [PMC free article] [PubMed]
- Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Seaquist ER. Comparison of arterialized venous sampling from the hand and foot in the assessment of in vivo glucose metabolism. Metabolism. 1997;46:1364–1366. doi: 10.1016/s0026-0495(97)90245-6. [DOI] [PubMed] [Google Scholar]
- Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-D-glucose PET study. Diabetologia. 2005;48:2080–2089. doi: 10.1007/s00125-005-1900-6. [DOI] [PubMed] [Google Scholar]
- Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. Thalamic contributions to attention and consciousness. Conscious Cogn. 1995;4:172–193. doi: 10.1006/ccog.1995.1024. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes. 2003;52:605–613. doi: 10.2337/diabetes.52.3.605. [DOI] [PubMed] [Google Scholar]
- Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50:1911–1917. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]