Abstract
The blood–brain barrier (BBB) prevents the brain uptake of most pharmaceuticals. This property arises from the epithelial-like tight junctions within the brain capillary endothelium. The BBB is anatomically and functionally distinct from the blood–cerebrospinal fluid barrier at the choroid plexus. Certain small molecule drugs may cross the BBB via lipid-mediated free diffusion, providing the drug has a molecular weight <400 Da and forms <8 hydrogen bonds. These chemical properties are lacking in the majority of small molecule drugs, and all large molecule drugs. Nevertheless, drugs can be reengineered for BBB transport, based on the knowledge of the endogenous transport systems within the BBB. Small molecule drugs can be synthesized that access carrier-mediated transport (CMT) systems within the BBB. Large molecule drugs can be reengineered with molecular Trojan horse delivery systems to access receptor-mediated transport (RMT) systems within the BBB. Peptide and antisense radiopharmaceuticals are made brain-penetrating with the combined use of RMT-based delivery systems and avidin–biotin technology. Knowledge on the endogenous CMT and RMT systems expressed at the BBB enable new solutions to the problem of BBB drug transport.
Keywords: blood–brain barrier, cerebrospinal fluid, endothelium, receptors, vascular biology
Introduction
The blood–brain barrier (BBB) prevents entry into the brain of most drugs from the blood. The presence of the BBB makes difficult the development of new treatments of brain diseases, or new radiopharmaceuticals for neuroimaging of brain. All of the products of biotechnology are large molecule drugs that do not cross the BBB. While it is assumed that small molecules are freely transported across the BBB, ∼98% of all small molecules are not transported across the BBB.1 The lack of small molecule transport across the BBB is illustrated by the whole body autoradiogram in Figure 1A. The mouse was euthanized 5 minutes after the intravenous (IV) injection of [14C]-histamine, a small molecule of only 111 Da. Histamine rapidly enters the postvascular space of all organs of the body, with the exception of the brain and spinal cord. The anatomical site of the BBB is endothelial wall of the microvasculature in the brain.2 The density in the brain of the microvessels is shown at the light microscopic level for rat brain in Figure 1B, and at the electron microscopic level for the human brain in Figure 1C. The BBB is comprised of two membranes in series, the luminal and abluminal membranes of the brain capillary endothelium, which are separated by about 200 nm of endothelial cytoplasm. Owing to the presence of epithelial-like, high resistance tight junctions within the brain capillary endothelium, the intercellular pores that exist in the endothelial barriers in peripheral organs are absent in the endothelial barrier in the brain. In addition, there is minimal fluid-phase pinocytosis in brain capillary endothelium.2 The absence of paracellular or transcellular channels within the BBB means that molecules in the circulation gain access to brain ISF (interstitial fluid) via only one of the two mechanisms: (1) lipid-mediated free diffusion through the BBB or (2) carrier- or receptor-mediated transport (RMT) through the BBB.
Figure 1.
(A) Whole body autoradiogram of a mouse euthanized 5 minutes after the intravenous injection of [14C]-histamine.124 (B) India ink visualization of complexity of the microvasculature of the cortex of rat brain.125 (C) Vascular cast of the microvasculature of the human cerebellar cortex. The distance between capillaries in brain is about 40 μm.126
The BBB is the principal interface between blood and the ISF that bathes synaptic connections within the parenchyma of the brain. A separate barrier system in the brain is localized to the epithelial cells of the choroid plexus, which form the blood–cerebrospinal fluid (CSF) barrier (BCSFB). The choroid plexus is the principal interface between the blood and CSF that bathes the surface of the brain.
The BBB is the fundamental problem blocking progress in the development of new therapeutics for brain disorders, or the development of new radiopharmaceuticals for imaging brain. This review will discuss both the principals of BBB drug transport, and strategies for the reengineering of drugs to enable BBB transport. The classical approaches to drug delivery to the brain, transcranial drug delivery or small molecules, are discussed, and the limitations of these strategies are reviewed. The endogenous carrier-mediated transport (CMT) and RMT systems within the BBB are discussed, and strategies are reviewed for the reengineering of pharmaceuticals that penetrate the brain from blood via access of the CMT and RMT systems within the BBB.
Historical origins of modern concepts on brain drug transport
Drug distribution into CSF is frequently used as an indicator of BBB transport. This is a misconception that stems from the lumping of the BBB and BCSFB systems as a single brain barrier. In fact, the BCSFB is leaky compared with the BBB, and all molecules in blood enter the CSF at a rate inversely related to molecular weight (MW).3 The finding of drug penetration into CSF is expected for any molecule, and does not provide information on rates of drug penetration across the BBB at the brain capillary endothelium. Thus, drug distribution in CSF is not an index of BBB transport, but rather is simply a measure of transport across the choroid plexus at the BCSFB. The idea that CSF composition reflects BBB transport is 100 years old, and finds its origins in Goldman's vital dye experiments published in 1913.4 Before 1913, it was known that certain acidic vital dyes, such as trypan blue, are excluded from entry into the brain following peripheral administration, and this observation was explained, not within the context of a barrier between blood and brain, but rather because the brain lacked the ‘receptors' that bind the dye and sequester the molecule within the tissue. Goldman injected the trypan blue into the lumbar sac, or subdural space, of rabbits and observed vital dye staining of spinal cord and brain tissue, respectively.4 Thus, the brain did express vital dye binding sites. The failure to stain the brain following peripheral administration of the dye was hypothesized to be due to a ‘blut-gehirn schranke' or BBB. However, in 1913, it was believed that the pathway of nutrient flux from blood to brain took place across the choroid plexus. The anatomical site of the BBB was erroneously placed at the choroid plexus.4 The idea that nutrient flux from blood to brain occurred across the capillaries within the parenchyma of brain was not accepted in 1913.
By the 1930s, studies with brain-penetrating basic vital dyes showed that the dye entered the brain from blood across the walls of capillaries perfusing brain parenchyma.5 Broman6 and Friedemann5 clearly observed that the BBB was localized to the capillary wall in brain, and that drug entry into the CSF across the choroid plexus was an entirely separate problem from drug transport across the BBB. Indeed, in his 1942 review on the BBB, Friedemann5 stated that, ‘distribution between blood and CSF is an entirely different problem and remains outside the scope of this review.' Nevertheless, the idea that the BBB and BCSFB are functionally equivalent, and that the CSF and ISF are functionally equivalent, is perpetuated in neuroscience text books.7 These ideas form the basis for bypassing the BBB with intrathecal drug delivery to the brain. In 2013, or 100 years after the publication of Goldman's experiment defining the BBB, there are multiple ongoing clinical trials of intrathecal delivery to the human brain of drugs that do not cross the BBB.
Brain drug transport and cerebrospinal fluid
The brain has no lymphatic system. The CSF of the brain is produced at the choroid plexi of the ventricles, moves over the surface of the brain, and is absorbed into the general circulation across the arachnoid villi into the superior sagittal sinus of the venous bloodstream.8, 9 In the human brain, there is about 100 to 140 mL of CSF, and this entire volume is turned over completely every 4 to 5 hours, or 4 to 5 times per day.10 In the mouse brain, there is about 40 μL of CSF, and the entire volume is turned over every 2 hours, or about 12 times per day.11 Cerebrospinal fluid is a fluid compartment in rapid equilibrium with the blood, which is due to the rapid rate of bulk flow (convection) of CSF from brain to blood. Conversely, drug movement into brain tissue from the CSF flow tracts occurs via diffusion. The differential rates of convection and diffusion create the paradox that drug injected into the CSF distributes easily to blood and poorly to brain beyond the ependymal surface.
Poor Drug Transport from Cerebrospinal Fluid to Brain
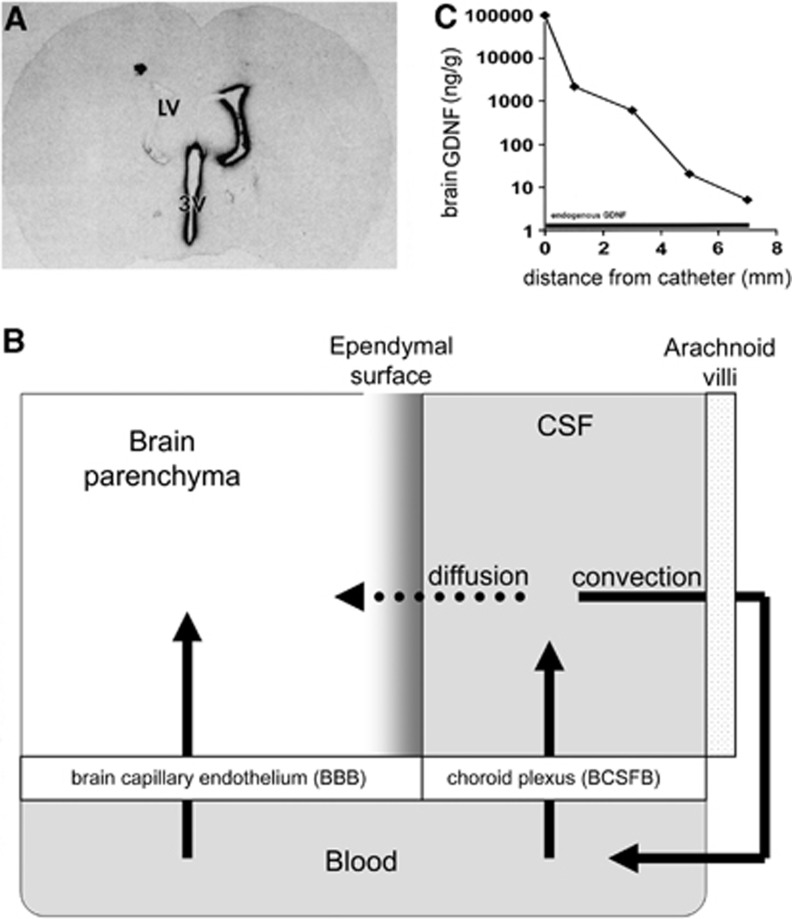
The poor distribution of drug into brain parenchyma is shown by the autoradiogram of rat brain prepared 20 hours after a single injection of [125I]-BDNF (brain-derived neurotrophic factor) into a lateral ventricle.12 The drug distributes only to the ependymal surface of brain ipsilateral to the ventricular injection (Figure 2A). From the lateral ventricle, the drug moves via bulk flow to the third ventricle (3 V), then to the fourth ventricle, then over the surface of the brain, where the drug undergoes exodus from the CSF space to the venous blood. In contrast to the relative rapid rate of bulk flow of CSF out of brain, the transport of drug from the CSF to brain tissue is slow, and limited by diffusion. Diffusion decreases with the square of the distance. The limited distribution into brain from the CSF is also observed for small molecules. The concentration of water-soluble small molecules in brain tissue at increasing distances from the CSF surface was measured in the primate brain following the intracerebroventricular (ICV) injection of the drug.13 Small molecule drug concentration in the brain decreased logarithmically with each mm of distance from the CSF surface. There was an ∼10-fold decrease in drug concentration of small molecule with each mm of distance removed from the brain surface. In the case of a lipid soluble small molecule, which can diffuse into brain cells, there is a 10-fold decrease in drug concentration with each 500 μm of distance into the brain.14 The logarithmic decrease in drug concentration in the brain removed from the CSF surface is even steeper for a large molecule drug, which has a lower diffusion coefficient. To achieve a therapeutic drug level in the brain at 5 mm removed from the CSF surface, it is necessary to inject into the CSF a dose of drug that generates at least a 5-log increase in CSF drug concentration. This approach may cover drug distribution in the mouse brain, where all parts of the brain are within 3 to 5 mm of the CSF surface. However, in the human brain, the distance between brain and the CSF surface is up to 50 mm, which precludes significant drug distribution into brain parenchyma from the CSF space. A side effect of the ICV injection of very high doses of drug is the exposure of the ependymal surface of the brain to potentially toxic concentrations of drug. The ICV injection of nerve growth factor in rats or monkeys15 or basic fibroblast growth factor in rats16 causes leptomeningeal changes with axonal sprouting and astrogliosis at the ependymal surface of the brain.
Figure 2.
(A) Film autoradiogram of rat brain section 20 hours after the injection of [125I]-BDNF (brain-derived neurotrophic factor) into the lateral ventricle (LV).12 (B) Solute distribution into brain parenchyma from the cerebrospinal fluid (CSF) compartment is limited by the slow rate of solute diffusion from the ependymal surface, relative to the rapid rate of bulk flow (convection) of CSF from the ventricles to the systemic circulation across the arachnoid villi. (C) Logarithmic decrease in the brain concentration of glial-derived neurotrophic factor (GDNF) in the brain of the Rhesus monkey relative to the distance from the intracerebral catheter used for brain drug delivery with convection-enhanced diffusion.127
In addition to the macrocirculation of CSF through the ventricular system, there is a CSF microcirculation through the Virchow-Robin compartment, which is formed by the perivascular space around penetrating cortical precapillary arterioles, which emanate from the pial vessels on the surface of the brain.17 However, the fluid flow via the microcirculation is quantitatively small compared with the CSF macrocirculation. The rate of flow of the CSF macrocirculation is 2 μL/min in the rat,8 whereas the rate of flow of the Virchow-Robin microcirculation is only 5% of the CSF flow rate, or 0.1 μL/min in the rat.18
Rapid Drug Transport from Cerebrospinal Fluid to Blood
Fishman and Christy19 observed nearly 50 years ago that an intrathecal injection of drug ‘is, in effect, equivalent to no more than a prolonged IV injection.' The rapid movement of CSF to blood means that drug injected into the CSF compartment will also rapidly move to the general bloodstream. This creates the paradox that drugs injected into the CSF may have a pharmacological effect within brain parenchyma, but only after transport of the drug from CSF to blood and then to brain across the BBB (Figure 2B). Working with barbiturates in dogs, Aird20 observed that the anesthesia was maintained at the same dose of drug, 0.2 mg per kg per minute, whether the barbiturate was infused by either the IV or the ICV route of administration. Drug injected into the CSF is rapidly distributed to blood, followed by entry into brain tissue from the blood via transport across the BBB. Drug concentration in the dialysate of an intracerebral dialysis fiber was measured following the ICV injection of drug; drug entry into the blood compartment was an obligatory intermediate step between the drug injection into the CSF and drug entry into the dialysis fiber placed within brain parenchyma.21 A secondary effect of the rapid movement of drug from CSF to blood is the finding of a pharmacological response following ICV injection, owing to the pharmacological activity of the drug in peripheral tissues. The effect of the neuropeptide, cholecystokinin, on feeding following ICV injection of the peptide is mediated via cholecystokinin transfer from CSF to blood, which enables activation of cholecystokinin receptors in the stomach.22
Transnasal Drug Delivery to Brain
The olfactory region of the submucous space of the nose is contiguous with the CSF flow tracts around the olfactory lobe. Therefore, intranasal drug administration could lead to direct delivery into the CSF providing (1) the drug is transportable across the nasal epithelium and (2) the drug is transportable across the arachnoid membrane separating the submucous space of the nose and the olfactory CSF compartment.23 The properties governing drug distribution from the nose to the olfactory CSF are similar to the properties determining drug transport across the BBB. A lipid soluble small molecule introduced into the nose would be expected to have some distribution into the olfactory CSF. However, a large molecule, or a water-soluble small molecule, would not enter olfactory CSF from the nose in the absence of local injury to the nasal epithelial barriers. Injury is induced in the nose following the intranasal instillation of volumes >100 μL/nares in humans.24 The volume that induces local injury in the mouse or rat nose is likely <10 μL/nares, and most studies of intranasal drug delivery to the brain in rodents use larger volumes, which may create nasal membrane disruption. There are species differences between humans and rodents with respect to the part of the nasal mucosa that corresponds to the olfactory region. Whereas the olfactory region of the nasal epithelium constitutes 50% of the nasal mucosa in the rat or mouse, the olfactory region is only 3% to 5% of the nasal mucosa in humans.25, 26 Consequently, it is difficult to detect drug movement into CSF following intranasal drug delivery in humans. When vitamin B12 or melatonin was administered to humans with small volumes, for example, 70 μL/nares, no drug distribution to CSF was observed following intranasal drug administration.24
Convection-Enhanced Diffusion
Drug delivery to the brain via the CSF is limited by the slow diffusion of drug from the ependymal surface of the brain (Figure 2A). So as to replace diffusion with bulk flow, drug delivery to the brain has been attempted with CED (convection-enhanced diffusion). In this approach, drug is placed in a peripheral reservoir connected to a pump, which is in line with a transcranial catheter. In theory, drug will penetrate the brain tissue by bulk flow, which should produce high drug levels in the brain at sites remote from the intracerebral catheter. In practice, drug concentrations in the brain decrease logarithmically with each mm of distance removed from the tip of the CED catheter in the brain. A logarithmic decrease in the brain concentration of GDNF (glial-derived neurotrophic factor) is observed following the CED infusion of the neurotrophin in the monkey brain,27 and these data are plotted in Figure 2C. Instead of a broad distribution of the drug into brain tissue beyond the catheter, which would be expected for a convection or bulk flow process, there is a logarithmic decrease in drug distribution in the brain, which is typical of a diffusion process. It would appear that the resistance of brain tissue reduces convection within the brain beyond the catheter tip. Similar to intrathecal drug delivery to the brain, the CED approach requires exposure of parts of brain proximal to the catheter to very high drug levels, to achieve therapeutic levels of drug at sites of brain distal to the catheter (Figure 2C).
Intrathecal or intracerebral drug delivery to the brain is limited by diffusion, which causes logarithmic gradients of drug concentration. In contrast, diffusion plays no role in the transvascular route of drug delivery to the brain. The distance between capillaries in brain is about 40 μm (Figure 1C). Even a large molecule antibody drug diffuses 40 μm within a second. Therefore, once a drug is delivered across the BBB from blood, there is instantaneous equilibration of drug throughout the entire brain volume. There are three mechanisms of trans-BBB transport of drug: (1) lipid-mediated transport of lipid soluble small molecules; (2) CMT transport of water-soluble small molecules that have an affinity for an endogenous BBB CMT system; and (3) RMT transport of large molecules that have a high affinity for an endogenous BBB RMT system.
Lipid-Mediated Transport of Small Molecules
Almost all drugs for the brain presently in clinical practice are lipid soluble small molecules with an MW <400 Da. These drugs fit the dual criteria for lipid-mediated free diffusion across the BBB, which are (1) MW <400 Da threshold and (2) high lipid solubility, which is equivalent to low hydrogen bonding. In practice, very few small molecule drug candidates for the brain fit these dual criteria, and do not cross the BBB. Of >6,000 drugs in the Comprehensive Medicinal Chemistry database, only 6% are active in the brain, and these drugs treat only a select group of brain diseases, comprised of affective disorders and insomnia.28 In another study, only 12% of all drugs were CNS active, but if affective disorders are excluded, only 1% of all drugs are active in the CNS.29 CNS active drugs form ≤7 hydrogen bonds with solvent water.30 These considerations indicate that the BBB transport of a small molecule drug candidate can be predicted based on the chemical structure of the drug, which allows for computation of (1) MW and (2) hydrogen bonding.
Molecular Weight Threshold
Small molecule drug permeation through lipid membranes demonstrates an MW threshold, which is not predicted by the classical Overton rules for solute diffusion in water.31, 32 Unlike diffusion through water, solute diffusion through lipid membranes is dependent on the molecular volume of the solute, which is generally proportional to solute MW or solute surface area. That is, when the size of the drug exceeds the volume threshold, then membrane permeation is less than predicted on the basis of the lipid solubility of the drug. The MW threshold of BBB drug transport of small molecules has been demonstrated previously in studies of drug penetration into the brain.33, 34 Blood–brain barrier permeation decreases 100-fold when the size of the drug is increased from an MW of 300 Da, which corresponds to a surface area of 50 square angstroms, to an MW of 450 Da, which corresponds to a surface area of 100 square angstroms.35 The molecular mechanism of the MW threshold phenomenon is consistent with the observation that transient water pores form in biological membranes, which are caused by the transient fluctuations in the conformation of the fatty acyl side chains of membrane lipids.36, 37, 38
Hydrogen Bonding
The lipid solubility of a drug may be determined by measurement of the 1-octanol lipid partition coefficient. However, an important parameter, which can be determined just from an inspection of the drug structure, is the hydrogen bonding of the drug and solvent water, which is 55 molar. Diamond and Wright39 reviewed the rules for determination of the number (N) of hydrogen bonds a solute forms in water, for example, N=2 for each hydroxyl, N=3 for each primary amine, and N=4 for each amide group. Stein40 observed that there is a 10-fold decrease in membrane permeation with each pair of hydrogen bonds on the solute, and this rule has been generally confirmed in studies of in vivo BBB transport of steroid hormones41 and oligopeptides.42 Consideration of the rules for hydrogen bonding show that even a simple dipeptide, glycylglycine, which forms eight hydrogen bonds with water, would have a very low BBB permeation. Conversely, if the glycylglycine was cyclized, the number of hydrogen bonds of the cyclic glycylglycine would be reduced to 4, and BBB transport would be predicted to increase by 100-fold for the cyclic form of the glycylglycine dipeptide, because glycine has no polar side chains.
When confronted with the problem of a water-soluble drug that does not penetrate the BBB, the medicinal chemist is asked to fix the problem by lipidizing the drug. This can be done by blocking hydrogen bond forming functional groups on the drug. For example, when the two hydroxyl groups of morphine are acetylated, to form heroin, the BBB penetration rate increases 100-fold.43 However, apart from the morphine/heroin example, there have been few successes in developing new drugs for human brain disorders by lipidization of a water-soluble drug. This is because the hydrogen blocking functional groups are rapidly hydrolyzed in vivo. If the hydrogen bond is blocked with a noncleavable group, then drug activity may be reduced. An alternative approach to the medicinal chemical modification of non-BBB-penetrating drugs is to focus on the endogenous CMT systems within the BBB. Instead of blocking hydrogen bonds, medicinal chemistry can be used to modify the structure of the drug to increase drug affinity for one of several endogenous carrier-mediated BBB transporters.
Carrier-mediated transport of small molecules
Discovery of Blood–Brain Barrier Carrier-Mediated Transport Systems
Physiology of blood–brain barrier carrier-mediated transport
The first evidence of saturable or CMT of a solute across the BBB was demonstrated by Crone44 for 𝒟-glucose using the indicator dilution technique, which is a venous sampling-carotid artery injection method. Subsequently, the Brain Uptake Index technique, a tissue sampling-carotid artery injection method developed by Oldendorf45, was used to characterize the saturable kinetics of BBB transport of multiple water-soluble metabolic substrates. The Michelis–Menten kinetics of BBB CMT for the major metabolic substrates were determined,46 and this analysis showed a wide range of affinity (Km) and maximal transport activity (Vmax) for the BBB nutrient transporters (Table 1).
Table 1. BBB carrier-mediated transporters.
| Carrier | Substrate | Genea product | Kmb (uM) | Vmaxb,c (nmol/min/g) | Ccapd (pmol/mgp) | Vmax:Cape (molecules per second) |
|---|---|---|---|---|---|---|
| Glucose | 𝒟-glucose | GLUT1 (SLC2A1) | 11,000±1,400 | 1,420±140 | 139±46 | 600 |
| Monocarboxylic acid | ℒ-lactic acid | MCT1 (SLC16A1) | 1,800±600 | 91±35 | 2.3±0.8 | 2,300 |
| Neutral amino acid | ℒ-phenyl-alanine | LAT1 (SLC7A5) | 26±6 | 22±4 | 0.43±0.09 | 3,000 |
| Basic amino acid | ℒ-arginine | CAT1 (SLC7A1) | 40±24 | 5±3 | 1.1±0.2 | 270 |
| Purine nucleoside | Adenosine | CNT2 (SLC28A2) | 25±3 | 0.75±0.08 | <0.14 | ND |
BBB, blood–brain barrier.
Solute carrier (SLC) group of transporter genes.
From Pardridge.47
Per gram wet brain in the rat.
From Uchida et al.48 Data reported per mg isolated human brain capillary protein.
Vmax/Ccap ratio is the transporter turnover number, which is the number of substrate molecules transported per second by a single transporter molecule, and is based on 0.14 mg capillary protein per gram wet brain; it is assumed that 50% of the total capillary transporter is expressed on the endothelial luminal membrane; nd=not determined.
Molecular biology of blood–brain barrier carrier-mediated transport
The methods of molecular biology were employed to identify the principal gene product responsible for BBB transport of a given class of metabolic substrates. The BBB glucose carrier, which also transports other hexoses, including mannose, galactose, deoxyglucose, or 3-0-methyl glucose,49 is the glucose transporter type 1 or GLUT1.50 The BBB phenylalanine carrier, which also transports over 10 other large neutral amino acids,51 and to a lesser extent small neutral amino acids, was identified as the large neutral amino-acid transporter type 1 or LAT1.52 The BBB arginine carrier, which also transports other cationic amino acids (lysine, ornithine),45 was shown to be the cationic amino-acid transporter type 1 or CAT1.53 The BBB lactate carrier, which also transports other monocarboxylic acids (pyruvate, and the ketone bodies, acetoacetate, and β-hydroxybutryyrate),54 was identified as the monocarboxylic acid transporter type 1 or MCT1.55 The BBB adenosine transporter, which also transports other purine nucleosides (guanosine, inosine), as well as the pyrimidine nucleoside, uridine,56 is the concentrative nucleoside transporter type 2 or CNT2 in the rat.57
Quantitation of blood–brain barrier transporter protein expression
Terasaki and colleagues58 determined the mass of transporters in isolated brain capillaries using the methods of LC–MS (liquid chromatography mass spectrometry). Capillaries were isolated from brain followed by digestion with trypsin. The tryptic peptides were separated and identified by LC–MS. Transporter-specific peptides were identified and quantitated by solid-phase synthesis of synthetic peptide standards. These peptide standards, which enable the quantification of the transporter expression, could be synthesized based on the known amino-acid sequences of the cloned transporters, as well as standard programs for predicting the sequence of tryptic peptides based on known amino-acid sequence of the target protein. The transporter concentrations in isolated human brain capillaries, Ccap, are listed in Table 2 for human GLUT1, MCT1, LAT1, and CAT1, and are compared with the transporter Vmax values determined in vivo in the rat. The comparison between rat Vmax values and human Ccap values was made, because the Ccap values are generally comparable for human and rodent brain capillaries.48
Table 2. Reengineering of protein drugs for BBB penetration as IgG Trojan horse fusion proteins.
|
Therapeutic class |
Protein therapeutic |
Target brain disease |
Animal models |
| Lysosomal enzymes | Iduronidase (IDUA) | MPS-I (Hurler's syndrome) | Rhesus monkey, Hurler mouse |
| Iduronate 2-sulfatase (IDS) | MPS-II (Hunter's syndrome) | Rhesus monkey | |
| Decoy receptors | Type II TNFα decoy receptor (TNFR) | Stroke neurodegeneration | Rhesus monkey, Mouse PD, Mouse stroke |
| Neurotrophins | Erythropoietin (EPO) | Stroke Neurodegeneration | Rhesus monkey, Mouse PD |
| Glial-derived neurotrophic factor (GDNF) | Neurodegeneration Stroke | Rhesus monkey, Mouse PD | |
| Therapeutic antibodies | Antiamyloid antibody (AAA) | Alzheimer's disease | Rhesus monkey, Mouse AD |
Transporter turnover number
The Vmax values are expressed per gram wet brain, whereas the Ccap values are expressed per mg of brain capillary protein. It is estimated that there is 0.17 mg capillary protein per gram brain, since there is 100 mg protein per gram brain, and the intracellular water volume of the brain capillary endothelial space, 1 μL/g,61 is 1/700 parts of the total water volume of brain. Based on this conversion, and assuming 50% of the capillary transporters are expressed on the luminal endothelial membrane, the Vmax/Ccap ratio can be computed, which is the transporter turnover number, or molecules of substrate transported by the carrier per second (Table 1). The estimated turnover numbers for BBB CMT systems range from 270 to 3,000 molecules/s. These calculations are approximations given the divergent in vitro and in vivo methodologies that were performed. Nevertheless, the estimated turnover rates approximate the turnover rate for other transporters, for example, the turnover number for the sucrose transporter (SUT1) is 500 molecules/s.62 To design drugs that access the CMT systems listed in Table 1, it is necessary to define the exact cellular location of the transporter at the brain microvasculature. A transporter identified in an isolated brain capillary preparation could be selectively expressed on the capillary endothelium, on the capillary pericyte, on smooth muscle cells of precapillary arterioles, or even on astrocyte endfeet, which remain adhered to the basement membrane of the isolated capillary. Following localization of the transporter to the capillary endothelium, it is necessary to determine whether the transporter is expressed on the luminal membrane, the abluminal membrane, or both. The cellular and subcellular expression of a BBB transporter is illustrated in the case of GLUT1 expression at the BBB.
Expression of GLUT1 in Brain Is Localized to the Blood–Brain Barrier
There are over 10 members of the sodium-independent GLUT glucose transporter gene family, and the first transporter identified, GLUT1, was cloned from human HepG2 liver cells using an antibody to the human erythrocyte glucose transporter.63 Cloning of the cDNA allowed for prediction of the amino-acid sequence and a two-dimensional representation of the transporter structure,63 which is shown in Figure 3A for the GLUT1 glucose transporter cloned from a bovine BBB cDNA library.64 Shortly after cloning from human liver cells, the same GLUT1 glucose transporter cDNA was cloned from a rat brain cDNA library, and the transporter was originally known as the brain-type glucose transporter.65 The GLUT1 glucose transporter was observed to be enriched at the brain microvasculature.66 However, studies of GLUT1 glucose transporter mRNA expression in the brain with the capillary depletion method showed that the GLUT1 glucose transporter was expressed in the brain only at the BBB, with little, if any, detection of GLUT1 transcript in mRNA devoid of capillary-derived mRNA.67 The GLUT1 Northern blot for capillary-depleted brain is illustrated in Figure 3B. The 2.9-kb GLUT1 glucose transporter mRNA was detected in Northern blotting of nonfractionated RNA from the brain (lane 1, left panel, Figure 3B). However, the GLUT1 glucose transporter mRNA was >95% removed with the capillary depletion method, which had no effect on the abundance of a common transcript, the 2.0-kb actin mRNA (Figure 3B). This finding indicated that GLUT1-mediated glucose transport at the BBB, but not at brain cells. The selective expression of the GLUT1 isoform at the BBB in the brain was subsequently confirmed with in situ hybridization, quantitative Western blotting,50 and quantitative immunogold electron microscopy.68 The GLUT1 glucose transporter is also expressed in other barrier structures in the brain, including the basolateral membrane of choroid plexus epithelium, and the apical membrane of certain regions of the ependymal epithelium.69
Figure 3.
(A) Predicted secondary structure of the bovine blood–brain barrier (BBB) GLUT1 glucose transporter is shown, which is formed by 12 transmembrane domains, a glycosylated extracellular loop between transmembrane domains 1 and 2, and intracellular amino and carboxyl termini.128 (B) Northern blot of mRNA derived from either total rabbit brain (lane 1) or capillary-depleted rabbit brain (lane 2) for either the GLUT1 glucose transporter or actin.71 (C) Electron microscopic immunogold study of human brain with a primary antiserum against the purified human erythrocyte glucose transporter, and a secondary antibody conjugated with 10 nm gold particles, shows abundant expression of immunoreactive GLUT1 glucose transporter on the luminal (L) and abluminal (AL) membranes of the capillary endothelium in human brain.70
The localization of the immunoreactive GLUT1 glucose transporter at the human BBB was demonstrated with electron microscopy using an antibody directed against the human erythrocyte GLUT1 glucose transporter and a secondary antibody conjugated with 10 nm gold particles.70 GLUT1 glucose transporters are observed on the endothelial plasma membranes, but not within the neuropil of human brain (Figure 3C). The expression of the GLUT1 glucose transporter on both luminal and abluminal membranes at the human BBB illustrates how these two endothelial membranes operate in series to regulate BBB transport (Figure 3C). GLUT1 is also detected on the plasma membrane of the human erythrocyte (Figure 3C). The abundance of GLUT1 within the human erythrocyte plasma membrane is said to be highest of all transporters. Yet, the abundance of GLUT1 glucose transporter, expressed as transporter molecules per micron of membrane length, is 2.6-fold higher on the luminal membrane of the human brain capillary endothelium relative to the human red cell membrane.70
The abundance of the GLUT1 glucose transporter, Ccap, at the microvasculature of human brain is high, relative to other transporters, 139±46 pmol/mg protein, as determined by LC–MS (Table 1). The Ccap value determined with LC–MS conforms to a previous estimate of the Ccap of GLUT1, 102±25 pmol/mg protein, determined with a cytochalasin B radioreceptor assay in isolated brain capillaries.71 The Ccap of the GLUT1 glucose transporter is nearly 100-fold higher than the Ccap for other transporters (Table 1), which is attributed to the sole derivation of energy in the brain from the combustion of glucose. Cerebral rates of glycolysis, 500 to 1,000 nmol per minute per gram, are over 100-fold greater than rates of amino-acid incorporation into brain proteins.46 Glucose supply in the brain is mediated solely via the activity of the BBB GLUT1 glucose transporter.
Drug Transport into Brain via Blood–Brain Barrier Carrier-Mediated Transporters
The characterization of the BBB GLUT1 glucose transporter exemplifies the extensive knowledge base on the molecular characteristics of a given BBB CMT system, and these systems have been recently cataloged with respect to the transporter abundance at the BBB.48 The cDNAs of almost all of the BBB CMT systems are available, and cell lines can be stably transfected with the transporter gene to enable high-throughput screening of drugs that have an acceptable affinity for a given BBB transporter. This knowledge base is not being exploited by the pharmaceutical industry for the discovery of drugs that cross the BBB on a given CMT system. However, there are historical examples of drugs that have an unexpected affinity for a BBB CMT system that may, or may not, be predicted on the basis of the drug structure. For example, ℒ-DOPA is an effective treatment for PD (Parkinson's disease), because this dopamine precursor is a large neutral amino acid that is transported across the BBB via the LAT1 neutral amino-acid carrier.51 ℒ-DOPA is a straightforward example of a large neutral amino-acid drug that would be predicted to have an affinity for LAT1. However, there are examples of other drugs that have an affinity for LAT1 that are unexpected. Gabapentin is a water-soluble drug that is active in the CNS because the drug crosses the BBB on the large neutral amino-acid transporter,72 which is LAT1. The affinity of gabapentin for LAT1 is unexpected, because gabapentin is a γ-amino acid, and LAT1 has an affinity only for α-amino acids. However, gabapentin is a cyclic form of a γ-amino acid, which places the amino and carboxyl groups in a conformation that mimics α-amino acids. Paraquat is N,N′-dimethyl-4,4′-bipyridinium, a widely used, highly water-soluble herbicide, which should not cross the BBB. However, paraquat is neurotoxic, which suggests that this double quaternary ammonium salt is BBB penetrating. Paraquat crosses the BBB via a process that is inhibited by valine, a large neutral amino acid,73 which suggests that paraquat is a substrate for LAT1. Melphalan is a polar alkylating agent used to treat brain cancer. Melphalan, or phenylalanine mustard, crosses the BBB via transport on the BBB large neutral amino-acid carrier.74
ℒ-DOPA, gabapentin, paraquat, and melphalan are examples of BBB delivery via LAT1 of drugs that have structures that mimic the endogenous substrate, neutral amino acids. An alternative approach is to conjugate a drug, which normally does not cross the BBB, with a CMT substrate, for example, glucose, that does cross the BBB. This was attempted with oligopeptides, which were conjugated with glucose. However, the glucose moiety of the glycopeptide no longer was transported via GLUT1.75 Instead, the structure of the CMT substrate must be retained following conjugation of the nontransportable drug. This is possible with ingenious uses of medicinal chemistry as applied to BBB structure–transport relationships. A novel example is the case where a nontransportable drug is coupled to the thiol group of ℒ-cysteine.76 Cysteine is a small neutral amino acid, which has a low affinity for LAT1.45 However, when the drug is conjugated to the thiol group of cysteine, the small neutral amino acid is converted into a large neutral amino acid, and significant BBB transport via LAT1 was observed.76 This case shows that a CMT substrate can be used as a drug carrier, providing the original structural characteristic of the substrate class for the targeted CMT system is retained.
Active Efflux Transporters and Enzymatic Blood–Brain Barrier
The active efflux transporters (AET) serve to mediate the asymmetric efflux of metabolites and drugs from the brain to blood. The classical AET system within the BBB is p-glycoprotein, which is a product of the MDR (multidrug resistance) gene, MDR1, also named the ABC (ATP-binding cassette) subfamily B member 1 (ABCB1). Apart from p-glycoprotein, there are numerous other members of the ABC gene family of transporters that are expressed at the BBB, and these have been quantified by Terasaki and coworkers.48 The AET systems may work in concert with enzymes expressed within the BBB, such as P450 enzymes.77 Both the AET systems, and the enzymatic barriers are subject to modulation. Blood–brain barrier p-glycoprotein activity is suppressed by vascular endothelial growth factor,78 and the CYP1A1 and CYP1B1 P450 enzymes are upregulated by environmental toxins, such as dioxin derivatives, which activate the aryl hydrocarbon receptor at the BBB.79 In addition to environmental toxins, or growth factors, BBB permeability may be altered by disease states, including aging.80
Receptor-mediated transport of protein therapeutics
Discovery of Blood–Brain Barrier Receptor-Mediated Transport Systems
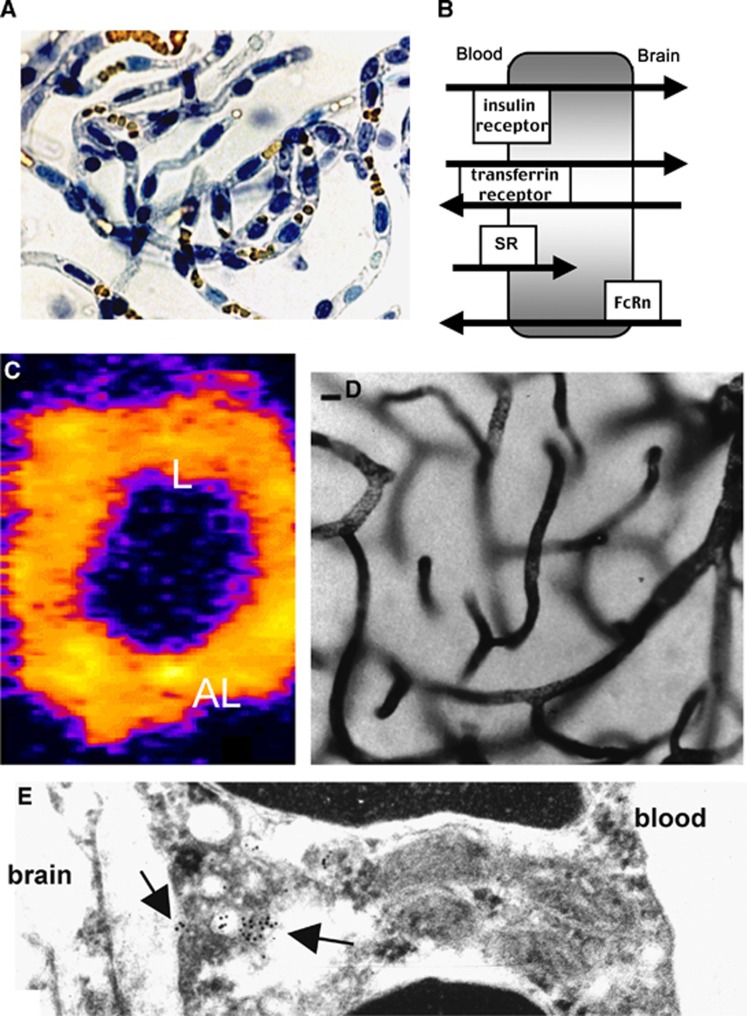
Insulin is present in the brain, but there is no insulin mRNA in the brain.81, 82 Therefore, insulin in the brain is of peripheral origin and derived from the blood. Insulin in the blood is transported across the human BBB via RMT on the endogenous BBB insulin receptor.83 Similarly, transferrin (Tf) in blood is transported via RMT across the BBB on the endogenous human BBB Tf receptor (TfR).84 There are separate BBB receptors for the insulin-like growth factors85 and leptin.86 The BBB peptide receptors on either the animal or human BBB were identified using isolated brain capillaries and radioreceptor methodology. The brain capillaries are purified from the brain with a mechanical homogenization procedure and capillaries freshly isolated from bovine brain are shown in Figure 4A. The BBB peptide receptors may mediate functionally different processes, including (1) transcytosis of ligand from blood to brain; (2) reverse transcytosis from brain to blood; or (3) only endocytosis into the brain capillary endothelium without net transport across the endothelial cell (Figure 4B). Insulin RMT across the BBB from blood to brain was demonstrated with carotid artery infusion and thaw mount autoradiography of the brain.87 Transferrin transport from the blood to brain was similarly shown by internal carotid artery infusion and autoradiography.88 Transferrin efflux from the brain to blood was demonstrated with the Brain Efflux Index method,89 as applied to large molecules.90 IgG molecules are asymmetrically transcytosed only in the brain to blood direction via a Fc receptor (FcR) on the BBB.91 Confocal microscopy of brain shows the principal FcR on the brain capillary is the neonatal FcR or FcRn.92 The BBB FcR does not mediate the transport of IgG molecules in the blood to brain direction. Some BBB receptors do not mediate transcytosis across the endothelial barrier, but only mediate endocytosis into the endothelial cell. Acetylated LDL (low-density lipoprotein) is a ligand for the scavenger receptor (SR) on the BBB, and this system mediates the endocytosis of acetyl LDL from blood, but not the net transcytosis across the BBB into the brain.93 Scavenger receptor-related receptors, such as the LDL-related protein receptor (LRP) type 1, similarly mediate only endocytosis of ligands into cells, and not transcytosis.94 Proteins targeting LRP1 are taken up by brain at reduced rates compared with proteins that target the transcytosing receptor systems. The brain uptake of melanotransferrin (p97) or angiopep-2, ligands that target LRP1, is only 0.2% to 0.3% of injected dose (ID)/g in the mouse,95, 96 which is 10-fold lower than the brain uptake of a monoclonal antibody (MAb) that targets the BBB TfR in the mouse.97
Figure 4.
(A) Light micrograph of freshly isolated bovine brain capillaries stained with trypan blue, which stains nuclei blue and luminal red blood cells yellow. The capillaries are isolated free of adjoining brain tissue. (B) Schematic diagram of different brain endothelial receptors, including the insulin receptor, the transferrin (Tf) receptor, the scavenger receptor (SR), which mediates only the endocytosis from blood into the endothelial compartment for ligands such as acetylated low density lipoprotein; and the neonatal Fc receptor (FcRn), which mediates the asymmetric transcytosis of IgG molecules selectively from brain to blood, but not blood to brain. (C) Confocal microscopy of isolated rat brain capillaries showing the expression of the Tf receptor on both luminal (L) and abluminal (AL) membranes of the endothelium.100 (D) Light micrograph of rat brain removed after a 10-minute carotid artery infusion of a conjugate of 5 nm gold and the mouse OX26 monoclonal antibody (MAb) against the rat TfR. The staining of the capillary compartment represents TfRMAb present in the intraendothelial compartment of the brain.101 (E) Electron microscopy of rat brain after perfusion with the conjugate of gold and TfRMAb shows the MAb concentrated in intraendothelial vesicles (intracellular arrow), as well as MAb molecules undergoing exocytosis from the endothelial compartment to the brain interstitial space (extracellular arrow).101
The BBB TfR mediates reverse transcytosis of apotransferrin in the brain to blood direction,90 in addition to influx of holo-transferrin from blood to brain.88 The reverse transcytosis is enabled, because the TfR is also expressed on the abluminal membrane of the capillary endothelium in brain. In one study, the abluminal TfR was not detected with preembedding immune labeling methods.98 However, abluminal receptors on the endothelium in the brain cannot be detected with preembedding methods.99 Using confocal microscopy of isolated rat brain capillaries, and the OX26 rat MAb against the rat TfR, it was possible to identify the TfR on both luminal and abluminal membranes of the brain capillary endothelium (Figure 4C).100 The pathway of transcytosis through the BBB via the TfR was examined with electron microscopy. The OX26 TfRMAb was conjugated with 5 nm gold and infused in the carotid artery of rats for 10 minutes followed by saline clearance of the brain vasculature and perfusion fixation with glutaraldehyde.101 At the light microscopic level, the TfRMAb entrapped within the capillary endothelium was detected with a silver enhancement technique as shown in Figure 4D. There is no visible reaction product in the brain extravascular space (Figure 4D), owing to the differences in intraendothelial and postendothelial volumes in the brain. The intraendothelial volume is 1 μL/g brain and the postendothelial volume is nearly 3-log orders higher, 700 μL/g brain. Therefore, as the TfRMAb exits the intraendothelial volume, the antibody concentration is diluted 700-fold, and the postvascular antibody in the brain cannot be detected with immune labeling methods. The rapid postvascular distribution into the brain via transport on the BBB TfR was demonstrated with the capillary depletion method and autoradiography.88 At the electron microscopic level, the OX26 TfRMAb is detected in endosomes within the capillary endothelium (intracellular arrow, Figure 4E), and antibody is observed exocytosing into the ISF on the brain side of the BBB (extracellular arrow, Figure 4E).
Blood–Brain Barrier Penetrating Genetically Engineered IgG Fusion Proteins
The presence of endogenous RMT systems within the BBB, such as the insulin receptor or the TfR, enables the reengineering of recombinant proteins as BBB-penetrating pharmaceuticals with the use of molecular Trojan horse (MTH) technology.59 An MTH is an endogenous peptide, or a peptidomimetic MAb, which traverses the BBB on a specific RMT system. Insulin, per se, as an MTH is not preferred, since insulin causes hypoglycemia. Tf, per se, is not a preferred MTH, because the plasma concentration of endogenous Tf, 25 μmol/L, is so high that the Tf-binding site on the BBB TfR is fully saturated by endogenous Tf. Alternatively, RMT-specific MTHs may be peptidomimetic MAbs that bind exofacial epitopes on the BBB receptor, which are spatially removed from the endogenous ligand binding site. Once bound to the RMT system on the BBB, the MTH undergoes RMT across the BBB (Figure 4). An MAb against the human insulin receptor, designated the HIRMAb, undergoes rapid transport across the BBB of the Rhesus monkey in vivo, and the brain uptake is 2% to 3% of ID/brain.102 The HIRMAb crossreacts with the insulin receptor of Old World primates, but does not react with the insulin receptor of New World primates, such as the squirrel monkey, or lower animals such as rodents. The murine HIRMAb has been genetically engineered as either a chimeric or humanized antibody.103 There is no known MAb against the mouse insulin receptor that can be used as a BBB MTH. For BBB delivery in the mouse, a genetically engineered chimeric MAb against the mouse TfR, designated the cTfRMAb, has been produced.104 With the genes encoding the heavy chain and the light chain of either the engineered HIRMAb or the cTfRMAb, IgG fusion proteins may be genetically engineered as bifunctional molecules, which both cross the BBB via RMT and exert pharmacological effects in brain, following transport into the brain.
An IgG-type MTH is also preferred for genetic engineering of MTH fusion proteins, because the protein therapeutic can be fused to the carboxyl terminus of the heavy chain of the IgG, as shown in Figure 5A. It is essential that the bifunctionality of the IgG fusion protein be retained, and that the ‘head' of the molecule retains high affinity (low nmol/L KD) binding to the BBB receptor, and that the ‘tail' of the IgG fusion protein retains the pharmacological activity of the original protein therapeutic. If the protein therapeutic is a neurotrophin, there must be retention of high affinity binding of the IgG-neurotrophin fusion protein to the cognate neurotrophin receptor in brain. If an IgG-decoy receptor fusion protein is engineered, there must be retention of high affinity binding to the target cytokine in the brain. If an IgG-lysosomal enzyme fusion protein is produced, the enzyme activity of the IgG-enzyme fusion protein must be comparable to the native enzyme. If the therapeutic molecule is also an antibody, then the problem is the genetic engineering of a bispecific antibody, where there is retention of high affinity binding for both the BBB receptor and the target antigen. This was accomplished by fusion of an ScFv (single chain Fv) form of the therapeutic antibody to the carboxyl terminus of the heavy chain of the IgG.105 This molecular design places the monomeric ScFv antibody in a dimeric configuration, which restores high avidity binding to the target antigen of a bivalent therapeutic antibody.
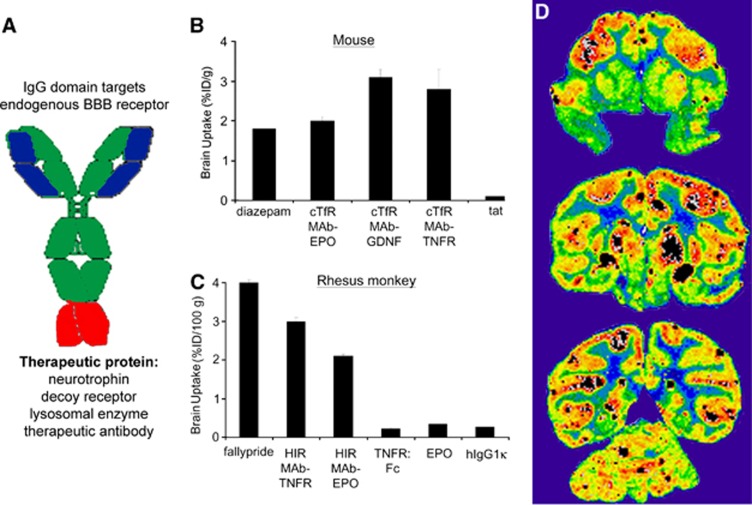
Figure 5.
(A) Structure of an IgG fusion protein formed by fusion of a therapeutic protein to the carboxyl terminus of the heavy chain of a blood–brain barrier (BBB) targeting monoclonal antibody (MAb). (B) Brain uptake in the mouse, expressed as % injected dose (ID)/g brain, is shown for diazepam,110 a cTfRMAb–EPO fusion protein,59 a cTfRMAb–GDNF fusion protein,59 a cTfRMAb–TNFR fusion protein,59 and the cationic import peptide, tat.129 (C) Brain uptake in the Rhesus monkey is expressed as % ID/100 g brain, since the size of the primate brain is 100 g. Brain uptake is shown for fallypride,111 a HIRMAb–TNFR fusion protein,109 a HIRMAb–EPO fusion protein,107 the TNFR alone,109 EPO alone,107 and a human IgG1κ isotype control antibody (hIgG1κ), which is confined to the vascular compartment.108 (D) Film autoradiogram of Rhesus monkey brain removed 2 hours after the intravenous (IV) injection of [125I]-HIRMAb–IDUA fusion protein, showing global distribution in brain with higher uptake in gray matter relative to white matter.130
If the high affinity for the target BBB receptor is retained following engineering of the IgG fusion protein, then the rate of transport of the fusion protein across the BBB will be comparable to the rate of transport of the MTH without a fusion partner. For the mouse, several cTfRMAb fusion proteins were engineered, expressed, and tested for in vivo brain uptake activity. The brain uptake of the cTfRMAb fusion protein of EPO (erythropoietin), GDNF, or the type II TNF-α (tumor necrosis factor-α) decoy receptor (TNFR), is high, 2% to 3% ID/g brain at 60 minutes after an IV injection (Figure 5B). In contrast, the brain uptake of a cationic import peptide, tat, is low, 0.1% ID/g (Figure 5B), and this low level of brain uptake is comparable to the brain uptake of an IgG that does not cross the BBB in the mouse.106 Without fusion to the MTH, there is no BBB transport of the EPO alone,107 the GDNF alone,108 or the TNFR alone.109 The brain uptake of the cTfRMAb fusion proteins in the mouse is comparable to the brain uptake of a lipid soluble small molecule such as diazepam (Figure 5B), which is 1.8% ID/g at 10 minutes after an IV injection.110 Therefore, the brain uptake of the MTH fusion proteins in the mouse is comparable to the brain uptake of lipid soluble small molecules. Similar findings are made in the primate. In the Rhesus monkey, the brain uptake of the HIRMAb fusion proteins is 2% to 3% ID/100 g brain (Figure 5C), which is comparable to the peak brain uptake in the Rhesus monkey of fallypride, a lipid soluble small molecule.111 The brain uptake in the Rhesus monkey is expressed per 100 g brain, because the weight of the brain in this species is 100 g. The brain uptake in the Rhesus monkey of the TNFR alone, or EPO alone, is low, about 0.2% ID/100 g brain. This level of brain uptake does not represent a low level of BBB penetration, but rather sequestration of the protein within the vascular compartment of brain. The primate brain uptake of an isotype IgG1 control antibody that does not cross the BBB is also 0.2% ID/100 g at 60 minutes after an IV injection (Figure 5C). The IgG fusion proteins undergo transcytosis through the BBB, and penetrate brain parenchyma. The global distribution within the brain of the fusion protein is shown by the brain scan of the Rhesus monkey at 2 hours after the IV injection of a fusion protein of the HIRMAb, and the human lysosomal enzyme, iduronidase (IDUA) (Figure 5D). The higher uptake in gray matter, as compared with white matter, is due to the higher vascular density in gray matter. The finding of transcytosis of the fusion proteins through the BBB and into brain parenchyma is corroborated by the in vivo CNS pharmacological effects of IV administration of the fusion proteins in mouse models of brain disease, including Hurler's syndrome, PD, Alzheimer's disease (AD), and stroke (Table 2).
Blood–brain barrier transport of peptide radiopharmaceuticals for neuroimaging
The number of potential peptide radiopharmaceuticals that could be used to image brain disorders is large. However, peptide radiopharmaceuticals for the brain are not developed, because these large molecules do not cross the BBB. Peptide radiopharmaceuticals can be reengineered for BBB transport with the combined use of MTH technology, avidin–biotin technology, and radiopharmaceutical technology. The peptide radiopharmaceutical can be conjugated to the IgG MTH via a high affinity avidin–biotin linker, as shown in Figure 6A. An MTH–avidin fusion protein has been genetically engineered both for the HIRMAb, for primate or human applications, and for the cTfRMAb, for mouse studies.112, 113 The IgG–avidin fusion protein is formulated in one vial. The monobiotinylated peptide radiopharmaceutical is formulated in a second vial. The two vials are mixed before IV administration. Owing to the very high affinity of avidin binding of biotin, there is instantaneous capture of the peptide radiopharmaceutical by the MTH (Figure 6A). The efficacy of this approach has been demonstrated in imaging models in both primates and rodents, using either the Aβ1–40 amyloid peptide of AD or epidermal growth factor (EGF) as the model peptide radiopharmaceutical.
Figure 6.
(A) Conjugate of an IgG–avidin fusion protein and a monobiotinylated peptide radiopharmaceutical. The biotin group on the peptide binds to the avidin domain of the fusion protein to form a high affinity linkage between the IgG molecular Trojan horse and the peptide radiopharmaceutical. (B, C) Scans of Rhesus monkey brain at 3 hours after the intravenous (IV) injection of either [125I, N-biotinyl]-Aβ1–40 alone (B), or [125I, N-biotinyl]-Aβ1–40 conjugated to the HIRMAb (monoclonal antibody) molecular Trojan horse via a streptavidin linker (C).115 (D) A rat brain tumor model was produced by the intracerebral injection of human U87 glioma cells in nude rats. Immunocytochemistry (ICC) of brain removed 16 days after tumor implantation with an antibody to the human EGFR shows abundant expression of the EGFR in the brain tumor.119 (E) Scan of rat brain removed 2 hours after the IV injection of [111 In-DTPA, Lys-biotinyl]–EGF conjugated to the TfRMAb molecular Trojan horse via a streptavidin linker. The section used for the film autoradiography was parallel to the section used for the ICC study in (D).119
The Aβ1–40 amyloid peptide is a potential peptide radiopharmaceutical for the neuroimaging of brain amyloid in AD, as this peptide avidly binds amyloid plaque in sections of AD autopsy brain.114 However, the Aβ1–40 amyloid peptide alone does not cross the BBB,113, 115 and the absence of BBB transport prevents the development of this peptide as a radiopharmaceutical for brain imaging in AD. The [N-biotinyl]-Aβ1–40 was radioiodinated and injected alone IV in the Rhesus monkey.115 No measureable brain uptake of the peptide was observed by brain scanning (Figure 6B). However, in a second Rhesus monkey, the [N-biotinyl]-Aβ1–40 was first conjugated to the HIRMAb via a streptavidin linker before IV injection of the HIRMAb/Aβ complex. Now, the peptide radiopharmaceutical rapidly penetrated the BBB in the primate (Figure 6C). Following reengineering with the MTH technology, the brain uptake of the Aβ1–40 amyloid peptide is comparable to the brain uptake of lipid soluble small molecule amyloid imaging agents. The brain uptake of the [N-biotinyl]-Aβ1–40 alone is very low in the mouse, 0.1% ID/g, but the brain uptake of the peptide increases to 2.1% ID/g following conjugation to the cTfRMAb–avidin fusion protein.113 This level of brain uptake of a peptide radiopharmaceutical is comparable to the brain uptake of a lipid soluble small molecule brain amyloid imaging agent, which is 2% to 3% ID/g in the mouse.116 The complex of the HIRMAb–avidin fusion protein and [N-biotinyl]-Aβ1–40 retains high affinity binding to the amyloid plaques in autopsy sections of AD brain.117 The [N-biotinyl]-Aβ1–40 can be radiolabeled with 124-iodine for positron emission tomography imaging of the brain. Alternatively, the [N-biotinyl]-Aβ1–40 can be conjugated with a chelator moiety and labeled with 111-indium118 for single photon emission computed tomography.
Epidermal growth factor is a potential peptide radiopharmaceutical for imaging brain cancers that overexpress the EGF receptor (EGFR). Epidermal growth factor was monobiotinylated and conjugated with diethylenediaminepentaacetic acid (DTPA) and radiolabeled with 111-indium. A rat model of human brain cancer was developed with the intracranial injection of human U87 glioma cells in nude rats.119 At autopsy, immunocytochemistry with an antibody against the human EGFR showed selective expression of the EGFR in the brain tumor (Figure 6D). IV injection of the [111In-DTPA, biotinyl]-EGF alone enabled no imaging of the brain tumor in vivo, because EGF does not cross the BBB.120 However, when the [111In-DTPA, biotinyl]-EGF was conjugated to the TfRMAb with a streptavidin linker, there was robust imaging of the EGFR-positive brain cancer following IV injection of the BBB-targeted peptide radiopharmaceutical (Figure 6E).
Blood–brain barrier transport of antisense radiopharmaceuticals for neuroimaging
Antisense radiopharmaceuticals are potential large molecule agents for imaging in brain the gene expression of sequence specific mRNA. However, antisense agents do not cross the BBB.121 An ideal antisense imaging agent is the peptide nucleic acid (PNA). Peptide nucleic acids are resistant to nucleases, as compared with phosphodiester-oligodeoxynucleotides (ODN), and have less nonspecific binding than phosphorothioate-ODNs. Peptide nucleic acid binding to target mRNAs does not trigger RNase H activity as is observed with ODNs.122 To evaluate BBB-targeted PNAs as antisense imaging agents for the brain, an intracranial RG-2 brain cancer model in rats was developed.123 Gene expression within the tumor was assessed for glial fibrillary acidic protein (GFAP) and caveolin-1α (CAV). The sequence of the GFAP-targeted and CAV-targeted PNAs is shown in Figure 7A. The amino terminus of the PNA was conjugated with DTPA for radiolabelling with111 In (Figure 7A). The amide backbone of the PNA contained a carboxyl terminal lysine (Lys) residue and the ɛ-amino group of the Lys residue was monobiotinylated (Figure 7A). Confocal microscopy showed the intracranial brain tumor underexpressed the GFAP protein and overexpressed the CAV protein (Figure 7B). Northern blot analysis showed the level of the GFAP and CAV mRNAs in the tumor paralleled the protein, as the GFAP mRNA was underexpressed, and the CAV mRNA was overexpressed.123 The biotin-GFAP-PNA or the biotin-CAV-PNA was conjugated to the TfRMAb via a streptavidin linker. Conjugation of the PNA to the MTH did not affect binding of the PNA to the target transcript. This was demonstrated with Northern blotting using the labeled PNA as the probe, and cloned GFAP or CAV RNA as the target.123 Following IV injection of either the [111In-DTPA, biotinyl]-GFAP PNA alone, or the [111In-DTPA, biotinyl]-CAV PNA alone, there was no imaging of brain, as these molecules do not cross the BBB. Following the IV injection of the [111In-DTPA, biotinyl]-GFAP PNA/TfRMAb conjugate, the nontumor brain was imaged, but there was no signal over the tumor (Figure 7C). Therefore, the in vivo brain scan with the targeted GFAP-PNA antisense radiopharmaceutical confirmed the in vitro finding of minimal expression of the GFAP mRNA in the glial tumor. Following the IV injection of the [111In-DTPA, biotinyl]–CAV PNA/TfRMAb conjugate, the tumor showed an intense signal, owing to overexpression of the CAV mRNA in the tumor (Figure 7D). The in vivo brain scans (Figures 7C and 7D) of the GFAP or CAV mRNAs with BBB-targeted antisense radiopharmaceuticals paralleled the immune detection of GFAP or CAV proteins in autopsy sections (Figure 7B), and the levels of the GFAP or CAV mRNAs in the tumor.123 Peptide and antisense radiopharmaceuticals have the potential to greatly expand the diagnostic capabilities of modern neuroimaging. However, these molecules need to be reformulated for BBB transport, and this is possible with the combined use of MTH and avidin–biotin technologies.
Figure 7.
(A) Structures are shown for model peptide nucleic acids (PNAs) with sequences that target either the rat glial fibrillary acidic protein (GFAP) mRNA or the rat caveolin-1α (CAV) mRNA.123 The methionine initiation codon sequence is underlined. (B) Confocal microscopy of brain from a rat with an intracranial RG-2 glioma is shown after double labeling with an antibody against GFAP (green channel) and an antibody against CAV (red channel).123 (C) Scan of brain removed from a rat with an intracranial RG-2 glioma at 1 hour after the intravenous (IV) injection of the [111In-DTPA, Lys-biotinyl]-GFAP PNA conjugated to the TfRMAb (monoclonal antibody) via a streptavidin linkage. The image over the tumor is of low intensity, as the RG-2 tumor does not express the GFAP mRNA.123 (D) Scan of brain removed from a rat with an intracranial RG-2 glioma at 6 hours after the IV injection of the [111In-DTPA, Lys-biotinyl]-CAV PNA conjugated to the TfRMAb via a streptavidin linkage. The image over the tumor is of high intensity, as the RG-2 tumor expresses abundant CAV mRNA.123
In summary, small molecule drugs may be reengineered to enable transport across the BBB via the CMT systems expressed in the brain capillary endothelium. Large molecule drugs, including recombinant proteins, and peptide and antisense radiopharmaceuticals may be delivered across the BBB with MTHs that target the endogenous RMT systems expressed within the brain capillary endothelium. The reengineering of pharmaceuticals for BBB transport is enabled with knowledge on the endogenous CMT and RMT systems within the BBB.
The author is a consultant to ArmaGen Technologies.
References
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta. 1987;163:319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Mott FW. The late Professor Edwin Goldmann's investigations on the central nervous system by vital staining. Br Med J. 1913;2:871–873. [PMC free article] [PubMed] [Google Scholar]
- Friedemann U. Blood-brain barrier. Physiol Rev. 1942;22:125–145. [Google Scholar]
- Broman T. The possibilities of the passage of substances from the blood to the central nervous system. Acta Psych Neurol. 1941;16:1–16. [Google Scholar]
- Rowland LP.Blood-brain barrier, cerebrospinal fluid, brain edema, and hydrocephalusIn: Kandel ER, Schwartz JH, (eds). Principles of Neural Science2nd ednElsevier Science Publishing: New York, NY; 1985837–844. [Google Scholar]
- Davson H. The cerebrospinal fluid. Handbook of Neurochemistry. 1969;2:23–48. [Google Scholar]
- Oldendorf WH. Cerebrospinal fluid formation and circulation. Prog Nucl Med. 1972;1:336–358. [PubMed] [Google Scholar]
- Cutler RW, Page L, Galicich J, Watters GV. Formation and absorption of cerebrospinal fluid in man. Brain. 1968;91:707–720. doi: 10.1093/brain/91.4.707. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Zirretta DK, Herndon RM. Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J Neurosci Methods. 1982;6:253–259. doi: 10.1016/0165-0270(82)90088-7. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther. 1975;195:73–83. [PubMed] [Google Scholar]
- Fung LK, Shin M, Tyler B, Brem H, Saltzman WM. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13:671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- Day-Lollini PA, Stewart GR, Taylor MJ, Johnson RM, Chellman GJ. Hyperplastic changes within the leptomeninges of the rat and monkey in response to chronic intracerebroventricular infusion of nerve growth factor. Exp Neurol. 1997;145:24–37. doi: 10.1006/exnr.1997.6448. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kinoshita A, Kohmura E, Sakaguchi T, Taguchi J, Kataoka K, et al. Basic fibroblast growth factor prevents thalamic degeneration after cortical infarction. J Cereb Blood Flow Metab. 1991;11:472–478. doi: 10.1038/jcbfm.1991.90. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M, Knopman DS. Developmental aspects of the intracerebral microvasculature and perivascular spaces: insights into brain response to late-life diseases. J Neuropathol Exp Neurol. 2011;70:1060–1069. doi: 10.1097/NEN.0b013e31823ac627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246:F835–F844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- Fishman RA, Christy NP. Fate of adrenal cortical steroids following intrathecal injection. Neurology. 1965;15:1–6. doi: 10.1212/wnl.15.1.1. [DOI] [PubMed] [Google Scholar]
- Aird RB. A study of intrathecal, cerebrospinal fluid-to-brain exchange. Exp Neurol. 1984;86:342–358. doi: 10.1016/0014-4886(84)90192-4. [DOI] [PubMed] [Google Scholar]
- de Lange EC, Danhof M, de Boer AG, Breimer DD. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994;666:1–8. doi: 10.1016/0006-8993(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Fiske SM, Durieux C, Derrien M, Roques BP. Centrally administered cholecystokinin suppresses feeding through a peripheral-type receptor mechanism. J Pharmacol Exp Ther. 1991;257:1076–1080. [PubMed] [Google Scholar]
- Kristensson K, Olsson Y. Uptake of exogenous proteins in mouse olfactory cells. Acta Neuropathol. 1971;19:145–154. doi: 10.1007/BF00688493. [DOI] [PubMed] [Google Scholar]
- Merkus P, Guchelaar HJ, Bosch DA, Merkus FW. Direct access of drugs to the human brain after intranasal drug administration. Neurology. 2003;60:1669–1671. doi: 10.1212/01.wnl.0000067993.60735.77. [DOI] [PubMed] [Google Scholar]
- Westin U, Piras E, Jansson B, Bergstrom U, Dahlin M, Brittebo E, et al. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci. 2005;24:565–573. doi: 10.1016/j.ejps.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Ajay BemisGW, Murcko MA. Designing libraries with CNS activity. J Med Chem. 1999;42:4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Bangham AD. Diffusion of small non-electrolytes across liposome membranes. Nature. 1972;236:173–174. doi: 10.1038/236173a0. [DOI] [PubMed] [Google Scholar]
- Lieb WR, Stein WD. Non-Stokesian nature of transverse diffusion within human red cell membranes. J Membr Biol. 1986;92:111–119. doi: 10.1007/BF01870701. [DOI] [PubMed] [Google Scholar]
- Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- van de Waterbeemd H, Camenisch G, Folkers G, Chretien JR, Raevsky OA. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J Drug Target. 1998;6:151–165. doi: 10.3109/10611869808997889. [DOI] [PubMed] [Google Scholar]
- Fischer H, Gottschlich R, Seelig A. Blood-brain barrier permeation: molecular parameters governing passive diffusion. J Membr Biol. 1998;165:201–211. doi: 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- Trauble H. The movement of molecules across lipid membranes: a molecular theory. J Membrane Biol. 1971;4:193–208. doi: 10.1007/BF02431971. [DOI] [PubMed] [Google Scholar]
- Hingson DJ, Diamond JM. Comparison of nonelectrolyte permeability patterns in several epithelia. J Membr Biol. 1972;10:93–135. doi: 10.1007/BF01867849. [DOI] [PubMed] [Google Scholar]
- Marrink SJ, Jahnig F, Berendsen HJ. Proton transport across transient single-file water pores in a lipid membrane studied by molecular dynamics simulations. Biophys J. 1996;71:632–647. doi: 10.1016/S0006-3495(96)79264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JM, Wright EM. Molecular forces governing non-electrolyte permeation through cell membranes. Proc R Soc Lond B Biol Sci. 1969;171:273–316. doi: 10.1098/rspb.1969.0022. [DOI] [PubMed] [Google Scholar]
- Stein WD. The Molecular Basis of Diffusion Across Cell Membranes. Academic Press: New York, NY; 1967. pp. 66–125. [Google Scholar]
- Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale EG, Ng KY, Burton PS, Borchardt RT. Hydrogen bonding potential as a determinant of the in vitro and in situ blood-brain barrier permeability of peptides. Pharm Res. 1994;11:412–419. doi: 10.1023/a:1018969222130. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965;181:103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Oldendorf WH. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977;28:5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol. 1988;28:25–39. doi: 10.1146/annurev.pa.28.040188.000325. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Oldendorf WH. Kinetics of blood-brain transport of hexoses. Biochim Biophys Acta. 1975;382:377–392. doi: 10.1016/0005-2736(75)90279-5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–18040. [PubMed] [Google Scholar]
- Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta. 1975;401:128–136. doi: 10.1016/0005-2736(75)90347-8. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci USA. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J, Wadhwani KC, Smith QR. Identification of the cationic amino acid transporter (System y+) of the rat blood-brain barrier. J Neurochem. 1993;60:1956–1959. doi: 10.1111/j.1471-4159.1993.tb13428.x. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem. 1979;33:439–445. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Oldendorf WH. Independent blood-brain barrier transport systems for nucleic acid precursors. Biochim Biophys Acta. 1975;394:211–219. doi: 10.1016/0005-2736(75)90259-x. [DOI] [PubMed] [Google Scholar]
- Li JY, Boado RJ, Pardridge WM. Cloned blood-brain barrier adenosine transporter is identical to the rat concentrative Na+ nucleoside cotransporter CNT2. J Cereb Blood Flow Metab. 2001;21:929–936. doi: 10.1097/00004647-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Uchida Y, Kubo Y, Terasaki T. Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: methodology, advantages, strategy, and prospects. J Pharm Sci. 2011;100:3547–3559. doi: 10.1002/jps.22612. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–292. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- Sumbria RK, Boado RJ, Pardridge WM.Brain protection from stroke with intravenous TNFα decoy receptor-Trojan horse fusion protein J Cereb Blood Flow Metab 2012. in press. [DOI] [PMC free article] [PubMed]
- Gjedde A, Christensen O. Estimates of Michaelis-Menten constants for the two membranes of the brain endothelium. J Cereb Blood Flow Metab. 1984;4:241–249. doi: 10.1038/jcbfm.1984.33. [DOI] [PubMed] [Google Scholar]
- Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, Poschet G, et al. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011;68:129–136. doi: 10.1111/j.1365-313X.2011.04672.x. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, et al. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. Molecular cloning of the bovine blood-brain barrier glucose transporter cDNA and demonstration of phylogenetic conservation of the 5′-untranslated region. Mol Cell Neurosci. 1990;1:224–232. doi: 10.1016/1044-7431(90)90005-o. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci USA. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier JS, Mueckler M, McCall AL, Lodish HF. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987;79:657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. The brain-type glucose transporter mRNA is specifically expressed at the blood-brain barrier. Biochem Biophys Res Commun. 1990;166:174–179. doi: 10.1016/0006-291x(90)91927-k. [DOI] [PubMed] [Google Scholar]
- Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci USA. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell CL, Yang J, Pardridge WM. GLUT1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. J Histochem Cytochem. 1992;40:193–199. doi: 10.1177/40.2.1552163. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Swartz BE. The human brain GLUT1 glucose transporter: ultrastructural localization to the blood-brain barrier endothelia. J Cereb Blood Flow Metab. 1994;14:106–112. doi: 10.1038/jcbfm.1994.15. [DOI] [PubMed] [Google Scholar]
- Dwyer KJ, Pardridge WM. Developmental modulation of blood-brain barrier and choroid plexus GLUT1 glucose transporter messenger ribonucleic acid and immunoreactive protein in rabbits. Endocrinology. 1993;132:558–565. doi: 10.1210/endo.132.2.8093876. [DOI] [PubMed] [Google Scholar]
- Wang Y, Welty DF. The simultaneous estimation of the influx and efflux blood-brain barrier permeabilities of gabapentin using a microdialysis-pharmacokinetic approach. Pharm Res. 1996;13:398–403. doi: 10.1023/a:1016092525901. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, et al. Carrier-mediated processes in blood--brain barrier penetration and neural uptake of paraquat. Brain Res. 2001;906:135–142. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Young D, Paxton JW, Finlay GJ, Wilson WR, Pardridge WM. Melphalan penetration of the blood-brain barrier via the neutral amino acid transporter in tumor-bearing brain. Cancer Res. 1992;52:138–143. [PubMed] [Google Scholar]
- Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides. 2001;22:2329–2343. doi: 10.1016/s0196-9781(01)00537-x. [DOI] [PubMed] [Google Scholar]
- Killian DM, Hermeling S, Chikhale PJ. Targeting the cerebrovascular large neutral amino acid transporter (LAT1) isoform using a novel disulfide-based brain drug delivery system. Drug Deliv. 2007;14:25–31. doi: 10.1080/10717540600559510. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, et al. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51:1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Rapid MillerDS. reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, et al. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol. 2009;77:897–909. doi: 10.1016/j.bcp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Zeevi N, Pachter J, McCullough LD, Wolfson L, Kuchel GA. The blood-brain barrier: geriatric relevance of a critical brain-body interface. J Am Geriatr Soc. 2010;58:1749–1757. doi: 10.1111/j.1532-5415.2010.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker GT, Studelska D, Harmon S, Burke W, O'Malley KL. Analysis of tyrosine hydroxylase and insulin transcripts in human neuroendocrine tissues. Brain Res Mol Brain Res. 1990;8:93–98. doi: 10.1016/0169-328x(90)90052-f. [DOI] [PubMed] [Google Scholar]
- Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci USA. 2004;101:2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36:892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Pardridge WM, Rosenfeld RG. Human blood-brain barrier insulin-like growth factor receptor. Metabolism. 1988;37:136–140. doi: 10.1016/s0026-0495(98)90007-5. [DOI] [PubMed] [Google Scholar]
- Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [125I]transferrin through the rat blood-brain barrier. Brain Res. 1995;683:164–171. doi: 10.1016/0006-8993(95)00363-u. [DOI] [PubMed] [Google Scholar]
- Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood-brain barrier. J Neurochem. 2001;76:1597–1600. doi: 10.1046/j.1471-4159.2001.00222.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–172. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem. 2002;81:203–206. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30:94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkan D, Pfeifer C, Vitalis TZ, Arthur G, Ujiie M, Chen Q, et al. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PLoS One. 2008;3:e2469. doi: 10.1371/journal.pone.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che C, Yang G, Thiot C, Lacoste MC, Currie JC, Demeule M, et al. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem. 2010;53:2814–2824. doi: 10.1021/jm9016637. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Lu JZ, Hui EK-W, Pardridge WM. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab Dispos. 2010;38:566–572. doi: 10.1124/dmd.109.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Fine RE, Sandra A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J Cell Sci. 1993;104 (Pt 2:521–532. doi: 10.1242/jcs.104.2.521. [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J Neurocytol. 1989;18:359–368. doi: 10.1007/BF01190839. [DOI] [PubMed] [Google Scholar]
- Huwyler J, Pardridge WM. Examination of blood-brain barrier transferrin receptor by confocal fluorescent microscopy of unfixed isolated rat brain capillaries. J Neurochem. 1998;70:883–886. doi: 10.1046/j.1471-4159.1998.70020883.x. [DOI] [PubMed] [Google Scholar]
- Bickel U, Kang YS, Yoshikawa T, Pardridge WM. In vivo demonstration of subcellular localization of anti-transferrin receptor monoclonal antibody-colloidal gold conjugate in brain capillary endothelium. J Histochem Cytochem. 1994;42:1493–1497. doi: 10.1177/42.11.7930531. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang YF, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng. 2007;96:381–391. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102:1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang YF, Zhang Y, Xia CF, Pardridge WM. Fusion antibody for Alzheimer's disease with bidirectional transport across the blood-brain barrier and abeta fibril disaggregation. Bioconjug Chem. 2007;18:447–455. doi: 10.1021/bc060349x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther. 2000;292:1048–1052. [PubMed] [Google Scholar]
- Boado RJ, Hui EK, Lu JZ, Pardridge WM. Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J Pharmacol Exp Ther. 2010;333:961–969. doi: 10.1124/jpet.109.165092. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Drug Metab Dispos. 2009;37:2299–2304. doi: 10.1124/dmd.109.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol. 2010;146:84–91. doi: 10.1016/j.jbiotec.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DJ, Sethy VH. Benzodiazepine concentrations in brain directly reflect receptor occupancy: studies of diazepam, lorezepam, and oxazepam. Psychopharmacology (Berl) 1990;102:373–378. doi: 10.1007/BF02244106. [DOI] [PubMed] [Google Scholar]
- Christian BT, Vandehey NT, Fox AS, Murali D, Oakes TR. Converse AK, et al. The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage. 2009;44:1334–1344. doi: 10.1016/j.neuroimage.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering, expression, and activity of a chimeric monoclonal antibody-avidin fusion protein for receptor-mediated delivery of biotinylated drugs in humans. Bioconjug Chem. 2008;19:731–739. doi: 10.1021/bc7004076. [DOI] [PubMed] [Google Scholar]
- Zhou QH, Lu JZ, Hui EK, Boado RJ, Pardridge WM. Delivery of a peptide radiopharmaceutical to brain with an IgG-avidin fusion protein. Bioconjug Chem. 2011;22:1611–1618. doi: 10.1021/bc200174x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JE, Stimson ER, Ghilardi JR, Allen CJ, Dahl CE, Whitcomb DC, et al. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci USA. 1992;89:5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Yang J, Pardridge WM. Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J Clin Invest. 1997;100:1804–1812. doi: 10.1172/JCI119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Cheng Y, Kimura H, Cui M, Kagawa S, Nishi R, et al. Novel 18F-labeled benzofuran derivatives with improved properties for positron emission tomography (PET) imaging of beta-amyloid plaques in Alzheimer's brains. J Med Chem. 2011;54:2971–2979. doi: 10.1021/jm200057u. [DOI] [PubMed] [Google Scholar]
- Sumbria RK, Boado RJ, Pardridge WM. Imaging amyloid plaque in Alzheimer's disease brain with a biotinylated Abeta peptide radiopharmaceutical conjugated to an IgG-avidin fusion protein. Bioconjug Chem. 2012;23:1318–1321. doi: 10.1021/bc3001744. [DOI] [PubMed] [Google Scholar]
- Kurihara A, Pardridge WM. Abeta(1-40) peptide radiopharmaceuticals for brain amyloid imaging: (111)In chelation, conjugation to poly(ethylene glycol)-biotin linkers, and autoradiography with Alzheimer's disease brain sections. Bioconjug Chem. 2000;11:380–386. doi: 10.1021/bc9901393. [DOI] [PubMed] [Google Scholar]
- Kurihara A, Pardridge WM. Imaging brain tumors by targeting peptide radiopharmaceuticals through the blood-brain barrier. Canc Res. 1999;54:6159–6163. [PubMed] [Google Scholar]
- Kurihara A, Deguchi Y, Pardridge WM. Epidermal growth factor radiopharmaceuticals: 111In chelation, conjugation to a blood-brain barrier delivery vector via a biotin-polyethylene linker, pharmacokinetics, and in vivo imaging of experimental brain tumors. Bioconjug Chem. 1999;10:502–511. doi: 10.1021/bc980123x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, Kang YS. Vector-mediated delivery of a polyamide (‘peptide') nucleic acid analogue through the blood-brain barrier in vivo. Proc Natl Acad Sci USA. 1995;92:5592–5596. doi: 10.1073/pnas.92.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H, Nielsen PE. Antisense properties of duplex- and triplex-forming PNAs. Nucleic Acids Res. 1996;24:494–500. doi: 10.1093/nar/24.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Wu D, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Imaging endogenous gene expression in brain cancer in vivo with 111In-peptide nucleic acid antisense radiopharmaceuticals and brain drug-targeting technology. J Nucl Med. 2004;45:1766–1775. [PubMed] [Google Scholar]
- Pardridge WM.Misconceptions about the blood-brain barrierIn: Pardridge WM, moderator. Blood-brain barrier: interface between internal medicine and brain. Ann Int Med 198610582–95. [DOI] [PubMed]
- Bar T.The vascular system of the cerebral cortex Adv Anat Embryol Cell Biol 198059I-VI,1–62. [DOI] [PubMed] [Google Scholar]
- Duvernoy H, Delon S, Vannson JL. The vascularization of the human cerebellar cortex. Brain Res Bull. 1983;11:419–480. doi: 10.1016/0361-9230(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18:157–167. doi: 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ.Molecular cloning and regulation of gene expression of blood-brain barrier glucose transporterIn: Pardridge WM, ed. The Blood-Brain Barrier: Cellular and Molecular Biology Raven Press: New York; 1993347–362. [Google Scholar]
- Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, et al. The pharmacokinetics of cell-penetrating peptides. Mol Pharm. 2010;7:2224–2231. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol Bioeng. 2008;99:475–484. doi: 10.1002/bit.21602. [DOI] [PubMed] [Google Scholar]